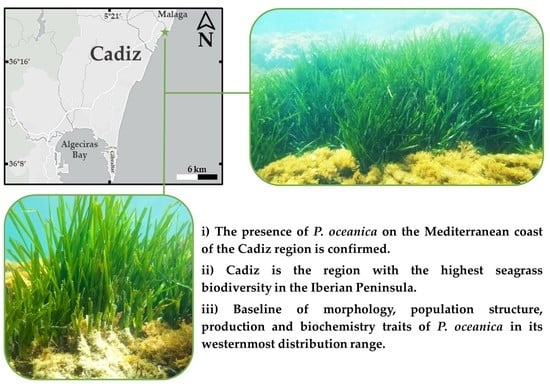
Plant and Meadow Structure Characterisation of Posidonia oceanica in Its Westernmost Distribution Range
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area


2.2. Morphometric Analyses
2.3. Population Analyses
2.4. Production Analyses
2.5. Biochemical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costanza, R.; d’Arge, R.; De Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Nordlund, L.M.; Jackson, E.L.; Nakaoka, M.; Samper-Villarreal, J.; Beca-Carretero, P.; Creed, J.C. Seagrass ecosystem services–What’s next? Mar. Pollut. Bull. 2018, 134, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lloréns, J.L.; Vergara, J.J.; Olivé, I.; Mercado, J.M.; Conde-Álvarez, R.; Pérez-Ruzafa, Á.; Figueroa, F.L. Autochthonous seagrasses. In Mediterranean Sea: Its History and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: Berlin, Germany, 2014; pp. 137–158. [Google Scholar]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G.; et al. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef] [PubMed]
- Marbà, N.; Duarte, C.M. Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Glob. Chang. Biol. 2009, 16, 2366–2375. [Google Scholar] [CrossRef]
- Pazzaglia, J.; Santillán-Sarmiento, A.; Ruocco, M.; Dattolo, E.; Ambrosino, L.; Marín-Guirao, L.; Procaccini, G. Local environment modulates whole-transcriptome expression in the seagrass Posidonia oceanica under warming and nutrients excess. Environ. Pollut. 2022, 303, 119077. [Google Scholar] [CrossRef]
- Marbà, N.; Díaz-Almela, E.; Duarte, C.M. Mediterranean seagrass (Posidonia oceanica) loss between 1842 and 2009. Biol. Conserv. 2014, 176, 183–190. [Google Scholar] [CrossRef]
- Moreno, D.; Luque, A.A.; Templado, J. Las praderas de Posidonia oceanica. Distribución en Andalucía. In Praderas Y Bosques Marinos de Andalucía; Luque, A.A., Templado, J., Eds.; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2004; pp. 60–63. [Google Scholar]
- Ruiz, J.M.; Guillén, J.E.; Ramos-Segura, A.; Otero, M.M. Atlas de Praderas Marinas de España; Instituto Español de Oceanografía: Madrid, Spain, 2015. [Google Scholar]
- Urra, J.; Mateo, A.; Marina, P.; Rueda, J.L.; García-Raso, J.E. First records of Posidonia oceanica flowering at its westernmost distributional limit (Malaga, Alboran Sea). Bot. Mar. 2011, 54, 101–104. [Google Scholar] [CrossRef]
- García-Gómez, J.C. Estudio comparado de las tanatocenosis y biocenosis malacológicas del Estrecho de Gibraltar y áreas próximas. Iberus 1983, 3, 75–90. [Google Scholar]
- Sánchez-Moyano, J.E.; Estacio, F.J.; García-Adiego, E.M.; García-Gómez, J.C. Las praderas submarinas de la Bahía de Algeciras. Evolución histórica y planes para su restauración y conservación. Almoraima 1998, 19, 173–180. [Google Scholar]
- Shaw, E. The sea-grass meadows of Gibraltar. Alcctoris 1993, 8, 66–69. [Google Scholar]
- Bull, J.C.; Kenyon, E.J.; Edmunds, D.; Cook, K.J. Recent loss of Gibraltar seagrasses. Bot. Mar. 2010, 53, 89–91. [Google Scholar] [CrossRef]
- Clausen, K.; Krause-Jensen, D.; Olesen, B.; Marbà, N. Seasonality of eelgrass biomass across gradients in temperature and latitude. Mar. Ecol. Prog. Ser. 2014, 506, 71–85. [Google Scholar] [CrossRef]
- Ito, M.; Lin, H.; O’Connor, M.; Nakaoka, M. Large-scale comparison of biomass and reproductive phenology among native and non-native populations of the seagrass Zostera japonica. Mar. Ecol. Prog. Ser. 2021, 675, 1–21. [Google Scholar] [CrossRef]
- Azcárate-García, T.; Beca-Carretero, P.; Cara-Ortega, C.L.; Villamayor, B.; Cosnett, E.; Bermejo, R.; Hernández, I.; Brun, F.G.; Stengel, D.B. Seasonal plant development and meadow structure of Irish and southern Spanish seagrass populations. Aquat. Bot. 2022, 183, 103569. [Google Scholar] [CrossRef]
- Bennett, S.; Wernberg, T.; Joy, B.A.; De Bettignies, T.; Campbell, A. Central and rear-edge populations can be equally vulnerable to warming. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Roca, G.; Alcoverro, T.; Krause-Jensen, D.; Balsby, T.; van Katwijk, M.; Marbà, N.; Santos, R.; Arthur, R.; Mascaró, O.; Fernández-Torquemada, Y.; et al. Response of seagrass indicators to shifts in environmental stressors: A global review and management synthesis. Ecol. Indic. 2016, 63, 310–323. [Google Scholar] [CrossRef]
- Pazzaglia, J.; Reusch, T.B.; Terlizzi, A.; Marín-Guirao, L.; Procaccini, G. Phenotypic plasticity under rapid global changes: The intrinsic force for future seagrasses survival. Evol. Appl. 2021, 14, 1181–1201. [Google Scholar] [CrossRef]
- Beca-Carretero, P.; Azcárate-García, T.; Teichberg, M.; Patra, P.; Feroze, F.; González, M.J.; Medina, I.; Winters, G. Predicted warming intensifies the negative effects of nutrient increase on tropical seagrass: A physiological and fatty acid approach. Ecol. Indic. 2022, 142, 109184. [Google Scholar] [CrossRef]
- Chefaoui, R.M.; Duarte, C.M.; Serrão, E.A. Dramatic loss of seagrass habitat under projected climate change in the Mediterranean Sea. Glob. Chang. Biol. 2018, 24, 4919–4928. [Google Scholar] [CrossRef]
- Vargas-Yáñez, M.; Plaza, F.; García-Lafuente, J.; Sarhan, T.; Vargas, J.; Vélez-Belchi, P. About the seasonal variability of the Alboran Sea circulation. J. Mar. Syst. 2002, 35, 229–248. [Google Scholar] [CrossRef]
- Shaltout, M.; Omstedt, A. Recent sea surface temperature trends and future scenarios for the Mediterranean Sea. Oceanologia 2014, 56, 411–443. [Google Scholar] [CrossRef]
- Chefaoui, R.M.; Duarte, C.M.; Serrão, E.A. Palaeoclimatic conditions in the Mediterranean explain genetic diversity of Posidonia oceanica seagrass meadows. Sci. Rep. 2017, 7, 2732. [Google Scholar] [CrossRef] [PubMed]
- Peralta, G.; Pérez-Lloréns, J.L.; Hernández, I.; Brun, F.; Vergara, J.J.; Bartual, A.; Gálvez, J.A.; García, C.M. Morphological and physiological differences between two morphotypes of Zostera noltii Hornem. from the south-western Iberian Peninsula. Helgol. Mar. Res. 2000, 54, 80–86. [Google Scholar] [CrossRef]
- Peralta, G.; Godoy, O.; Egea, L.; de los Santos, C.B.; Jiménez-Ramos, R.; Lara, M.; Brun, F.; Hernández, I.; Olivé, I.; Vergara, J.; et al. The morphometric acclimation to depth explains the long-term resilience of the seagrass Cymodocea nodosa in a shallow tidal lagoon. J. Environ. Manag. 2021, 299, 113452. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M. Allometric scaling of seagrass form and productivity. Mar. Ecol. Prog. Ser. Oldend. 1991, 77, 289–300. [Google Scholar] [CrossRef]
- Via, J.D.; Sturmbauer, C.; Schönweger, G.; Sötz, E.; Mathekowitsch, S.; Stifter, M.; Rieger, R. Light gradients and meadow structure in Posidonia oceanica: Ecomorphological and functional correlates. Mar. Ecol. Prog. Ser. 1998, 163, 267–278. [Google Scholar] [CrossRef]
- Gnisci, V.; de Martiis, S.C.; Belmonte, A.; Micheli, C.; Piermattei, V.; Bonamano, S.; Marcelli, M. Assessment of the ecological structure of Posidonia oceanica (L.) Delile on the northern coast of Lazio, Italy (central Tyrrhenian, Mediterranean). Ital. Bot. 2020, 9, 1–19. [Google Scholar] [CrossRef]
- Marbà, N.; Duarte, C.; Cebrián, J.; Gallegos, M.; Olesen, B.; Sand-Jensen, K. Growth and population dynamics of Posidonia oceanica on the Spanish Mediterranean coast:elucidating seagrass decline. Mar. Ecol. Prog. Ser. 1996, 137, 203–213. [Google Scholar] [CrossRef]
- Balestri, E.; Cinelli, F.; Lardicci, C. Spatial variation in Posidonia oceanica structural, morphological and dynamic features in a northwestern Mediterranean coastal area: A multi-scale analysis. Mar. Ecol. Prog. Ser. 2003, 250, 51–60. [Google Scholar] [CrossRef]
- Fernández-Torquemada, Y.; Díaz-Valdés, M.; Izquierdo-Muñoz, A.; Sánchez-Lizaso, J.L.; Ramos-Esplá, A.A. Spatial and Temporal Variability of Posidonia oceanica Monitoring Indicators, Valencian Community, Spain. Water 2020, 12, 3235. [Google Scholar] [CrossRef]
- Urra, J.; Ramírez, Á.M.; Marina, P.; Salas, C.; Gofas, S.; Rueda, J.L. Highly diverse molluscan assemblages of Posidonia oceanica meadows in northwestern Alboran Sea (W Mediterranean): Seasonal dynamics and environmental drivers. Estuar. Coast. Shelf Sci. 2013, 117, 136–147. [Google Scholar] [CrossRef]
- Micheli, C.; Cupido, R.; Lombardi, C.; Belmonte, A.; Peirano, A. Changes in Genetic Structure of Posidonia oceanica at Monterosso al Mare (Ligurian Sea) and Its Resilience Over a Decade (1998–2009). Environ. Manag. 2012, 50, 598–606. [Google Scholar] [CrossRef]
- Rigo, I.; Paoli, C.; Dapueto, G.; Pergent-Martini, C.; Pergent, G.; Oprandi, A.; Montefalcone, M.; Bianchi, C.N.; Morri, C.; Vassallo, P. The Natural Capital Value of the Seagrass Posidonia oceanica in the North-Western Mediterranean. Diversity 2021, 13, 499. [Google Scholar] [CrossRef]
- Olesen, B.; Enríquez, S.; Duarte, C.M.; Sand-Jensen, K. Depth-acclimation of photosynthesis, morphology and demography of Posidonia oceanica and Cymodocea nodosa in the Spanish Mediterranean Sea. Mar. Ecol. Prog. Ser. 2002, 236, 89–97. [Google Scholar] [CrossRef]
- Buia, M.C.; Gambi, M.C.; Dappiano, M. Seagrass systems. Biol. Mar. Mediterr. 2004, 10, 133–183. [Google Scholar]
- Ruiz, J.M.; Romero, J. Effects of disturbances caused by coastal constructions on spatial structure, growth dynamics and photosynthesis of the seagrass Posidonia oceanica. Mar. Pollut. Bull. 2003, 46, 1523–1533. [Google Scholar] [CrossRef]
- Peirano, A.; Damasso, V.; Montefalcone, M.; Morri, C.; Bianchi, C.N. Effects of climate, invasive species and anthropogenic impacts on the growth of the seagrass Posidonia oceanica (L.) Delile in Liguria (NW Mediterranean Sea). Mar. Pollut. Bull. 2005, 50, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, P.; Lorenti, M.; Buia, M.C.; Mazzella, L. Temporal Dynamics and Biomass Partitioning in Three Adriatic Seagrass Species: Posidonia oceanica, Cymodocea nodosa, Zostera marina. Mar. Ecol. 2002, 23, 51–67. [Google Scholar] [CrossRef]
- Tsirika, A.; Skoufas, G.; Haritonidis, S. Seasonal and bathymetric variations of epiphytic macroflora on Posidonia oceanica (L.) Delile leaves in the National Marine Park of Zakynthos (Greece). Mar. Ecol. 2007, 28, 146–153. [Google Scholar] [CrossRef]
- Drew, E.A. Factors affecting photosynthesis and its seasonal variation in the seagrasses Cymodocea nodosa (Ucria) Aschers, and Posidonia oceanica (L.) Delile in the mediterranean. J. Exp. Mar. Biol. Ecol. 1978, 31, 173–194. [Google Scholar] [CrossRef]
- Lee, K.-S.; Park, S.R.; Kim, Y.K. Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review. J. Exp. Mar. Biol. Ecol. 2007, 350, 144–175. [Google Scholar] [CrossRef]
- West, R. Depth-related structural and morphological variations in an Australian Posidonia seagrass bed. Aquat. Bot. 1990, 36, 153–166. [Google Scholar] [CrossRef]
- Pace, M.; Borg, J.A.; Galdies, C.; Malhotra, A. Influence of wave climate on architecture and landscape characteristics of Posidonia oceanica meadows. Mar. Ecol. 2017, 38, e12387. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Jiménez, C.; Viñegla, B.; Pérez-Rodríguez, E.; Aguilera, J.; Flores-Moya, A.; Altamirano, M.; Lebert, M.; Häder, D.P. Effects of solar UV radiation on photosynthesis of the marine angiosperm Posidonia oceanica from southern Spain. Mar. Ecol. Prog. Ser. 2002, 230, 59–70. [Google Scholar] [CrossRef]
- Giovannetti, E.; Lasagna, R.; Montefalcone, M.; Bianchi, C.N.; Albertelli, G.; Morri, C. Inconsistent responses to substratum nature in Posidonia oceanica meadows: An integration through complexity levels? Chem. Ecol. 2008, 24, 83–91. [Google Scholar] [CrossRef]
- Pergent, G.; Romero, J.; Pergent-Martini, C.; Mateo, M.; Boudouresque, C.-F. Primary production, stocks and fluxes in the Mediterranean seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 1994, 106, 139–146. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Rico-Raimondino, V.; Pergent, G. Primary production of Posidonia oceanica in the Mediterranean Basin. Mar. Biol. 1994, 120, 9–15. [Google Scholar] [CrossRef]
- Marbà, N.; Duarte, C.M.; Holmer, M.; Martínez, R.; Basterretxea, G.; Orfila, A.; Jordi, A.; Tintoré, J. Effectiveness of protection of seagrass (Posidonia oceanica) populations in Cabrera National Park (Spain). Environ. Conserv. 2002, 29, 509–518. [Google Scholar] [CrossRef]
- Alcoverro, T.; Cerbiān, E.; Ballesteros, E. The photosynthetic capacity of the seagrass Posidonia oceanica: Influence of nitrogen and light. J. Exp. Mar. Biol. Ecol. 2001, 261, 107–120. [Google Scholar] [CrossRef]
- Gacia, E.; Duarte, C.M.; Middelburg, J.J. Carbon and nutrient deposition in a Mediterranean seagrass (Posidonia oceanica) meadow. Limnol. Oceanogr. 2002, 47, 23–32. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Pergent, G.; Monnier, B.; Boudouresque, C.-F.; Mori, C.; Valette-Sansevin, A. Contribution of Posidonia oceanica meadows in the context of climate change mitigation in the Mediterranean Sea. Mar. Environ. Res. 2021, 165, 105236. [Google Scholar] [CrossRef] [PubMed]
- Greve, T.M.; Binzer, T. Which factors regulate seagrass growth and distribution. In European Seagrasses: An Introduction to Monitoring and Management; Borum, J., Duarte, C.M., Krausen-Jensen, D., Greve, T.M., Eds.; M and MS Project: Brussels, Belgium, 2004; pp. 19–23. [Google Scholar]
- García-Gómez, J.C.; Sempere-Valverde, J.; González, A.R.; Martínez-Chacón, M.; Olaya-Ponzone, L.; Sánchez-Moyano, E.; Ostalé-Valriberas, E.; Megina, C. From exotic to invasive in record time: The extreme impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the strait of Gibraltar. Sci. Total Environ. 2020, 704, 135408. [Google Scholar] [CrossRef] [PubMed]
- Casal-Porras, I.; Zubía, E.; Brun, F.G. Dilkamural: A novel chemical weapon involved in the invasive capacity of the alga Rugulopteryx okamurae in the Strait of Gibraltar. Estuar. Coast. Shelf Sci. 2021, 257, 107398. [Google Scholar] [CrossRef]
| Leaf (L) | |||||||
|---|---|---|---|---|---|---|---|
| Descriptors | L-1 | L-2 | L-3 | L-4 | L-5 | L-6 | Leaf Average |
| Leaf length (cm) | 18.2 ± 5.1 | 23.5 ± 6.4 | 27.6 ± 5.2 | 21.5 ± 3.7 | 13.7 ± 4.0 | 4.3 ± 1.9 | 21.0 ± 2.9 * |
| Leaf width (cm) | 1.1 ± 0.02 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.0 | 1.0 ± 0.03 |
| Leaf thickness (mm) | 0.51 ± 0.06 | 0.38 ± 0.05 | 0.32 ± 0.05 | 0.23 ± 0.03 | 0.18 ± 0.01 | 0.15 ± 0.01 | 0.31 ± 0.03 |
| Leaf biomass (mg DW) | 109.0 ± 31.9 | 141.9 ± 40.2 | 167.9 ± 32.6 | 129.7 ± 23.1 | 80.6 ± 25.1 | 22.1 ± 12.0 | 108.5 ± 47.2 * |
| Shoot leaf biomass (mg DW shoot−1) | - | - | - | - | - | - | 633.0 ± 77.1 |
| Shoot leaves (leaves shoot−1) | - | - | - | - | - | - | 6 ± 1 |
| Growth rate (cm day−1 shoot−1) | - | - | - | - | - | - | 0.7 ± 0.2 |
| Biomass production (mg DW day−1 shoot−1) | - | - | - | - | - | - | 4.3 ± 1.2 |
| Leaf production (leaf day−1 shoot−1) | - | - | - | - | - | - | 0.03 ± 0.01 |
| Patch Size | Shoot Density | AG Biomass | LAI | |
|---|---|---|---|---|
| Patch | (m2) | (Shoots m−2) | (kg DW m−2) | (m2 m−2) |
| 1 | 10.0 | 408 ± 42 | 0.26 ± 0.03 | 4.4 ± 1.2 |
| 2 | 17.6 | 475 ± 20 | 0.30 ± 0.01 | 5.8 ± 0.8 |
| 3 | 20.2 | 425 ± 20 | 0.27 ± 0.01 | 5.3 ± 1.8 |
| 4 | 5.2 | 408 ± 31 | 0.26 ± 0.02 | 3.8 ± 0.5 |
| 5 | 5.7 | 467 ± 31 | 0.30 ± 0.02 | 4.8 ± 1.1 |
| 6 | 1.0 | - | - | - |
| 7 | 0.5 | - | - | - |
| 8 | 0.5 | - | - | - |
| 9 | 0.5 | - | - | - |
| Mean | 9.8 ± 6.5 * | 437 ± 42 | 0.28 ± 0.03 | 4.8 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azcárate-García, T.; Beca-Carretero, P.; Brun, F.G. Plant and Meadow Structure Characterisation of Posidonia oceanica in Its Westernmost Distribution Range. Diversity 2023, 15, 101. https://doi.org/10.3390/d15010101
Azcárate-García T, Beca-Carretero P, Brun FG. Plant and Meadow Structure Characterisation of Posidonia oceanica in Its Westernmost Distribution Range. Diversity. 2023; 15(1):101. https://doi.org/10.3390/d15010101
Chicago/Turabian StyleAzcárate-García, Tomás, Pedro Beca-Carretero, and Fernando G. Brun. 2023. "Plant and Meadow Structure Characterisation of Posidonia oceanica in Its Westernmost Distribution Range" Diversity 15, no. 1: 101. https://doi.org/10.3390/d15010101
APA StyleAzcárate-García, T., Beca-Carretero, P., & Brun, F. G. (2023). Plant and Meadow Structure Characterisation of Posidonia oceanica in Its Westernmost Distribution Range. Diversity, 15(1), 101. https://doi.org/10.3390/d15010101