Diversity of Seed Flavan-3-Ols in Croatian Native Grapevine Cultivars (Vitis vinifera L.) Grown in Coastal Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Samples
2.2. Sample Preparation and Extraction
2.3. HPLC Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Seed Content
3.2. Flavan-3-Ols in Grape Seeds
3.3. Prediction of Flavan-3-Ols Yield in Conventional Production
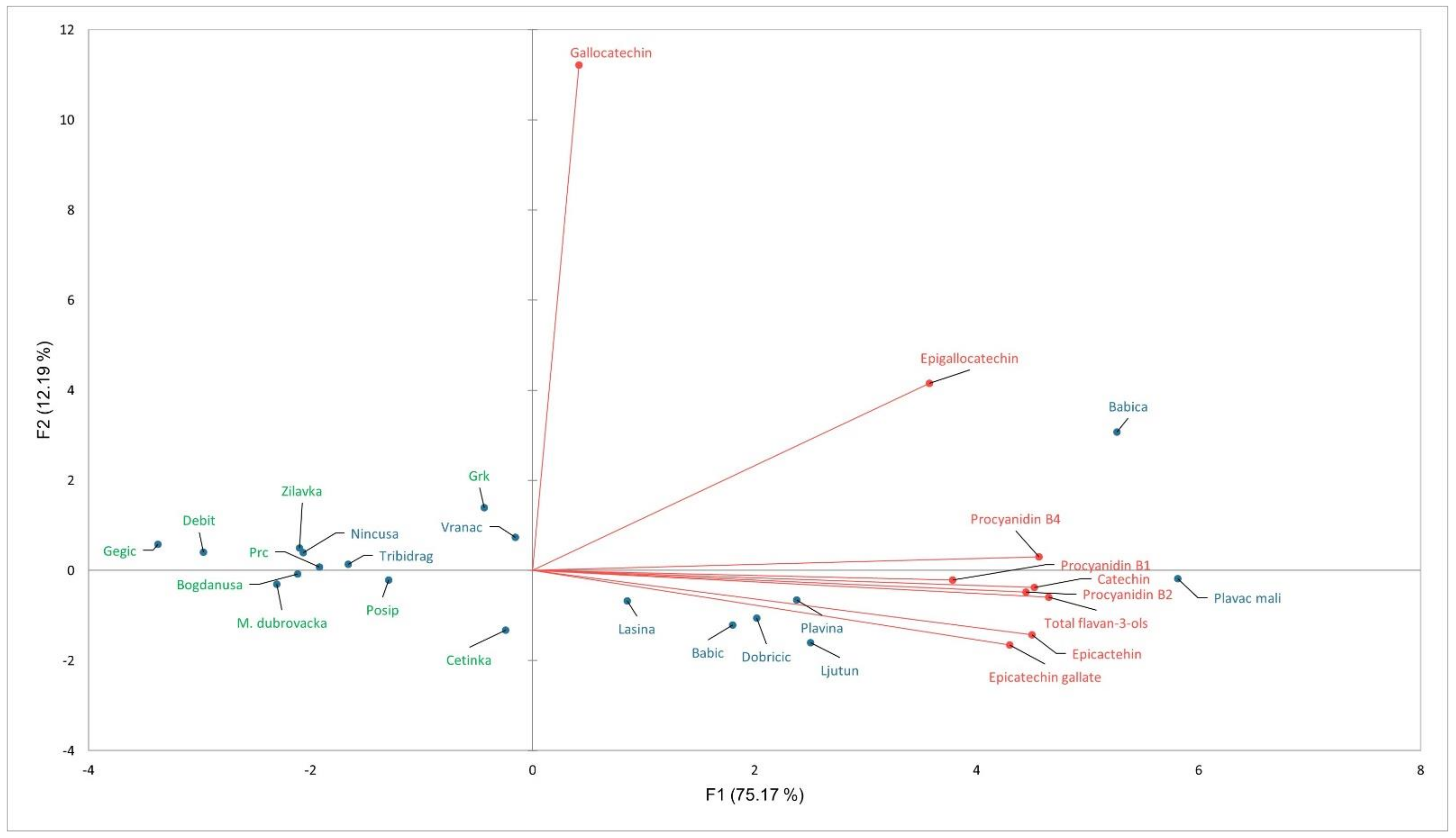
3.4. PCA Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maletic, E.; Karoglan Kontic, J.; Pejic, I.; Preiner, D.; Zdunic, G.; Bubola, M.; Stupic, D.; Andabaka, Z.; Markovic, Z.; Simon, S.; et al. Green Book; Indigenous Grapevine Varieties of Croatia; Ministarstvo Zaštite Okoliša i Prirode, Državni Zavod za Zaštitu Prirode: Zagreb, Croatia, 2015. [Google Scholar]
- Soares, S.; Brandao, E.; Mateus, N.; de Freitas, V. Sensorial properties of red wine polyphenols: Astringency and bitterness. Crit. Rev. Food Sci. Nutr. 2017, 57, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Šikuten, I.; Štambuk, P.; Andabaka, Ž.; Tomaz, I.; Marković, Z.; Stupić, D.; Maletić, E.; Kontić, J.K.; Preiner, D. Grapevine as a Rich Source of Polyphenolic Compounds. Molecules 2020, 25, 5604. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, J.; Pohoryl, J.E.; Kakuda, Y. Polyphenolics in Grape Seeds—Biochemistry and Functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Zerbib, M.; Mazauric, J.P.; Meudec, E.; Le Guerneve, C.; Lepak, A.; Nidetzky, B.; Cheynier, V.; Terrier, N.; Saucier, C. New flavanol O-glycosides in grape and wine. Food Chem. 2018, 266, 441–448. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef]
- Trad, M.; Le Bourvellec, C.; Ben Hamda, H.; Renard, C.; Harbi, M. Flavan-3-ols and procyanidins in grape seeds: Biodiversity and relationships among wild and cultivated vines. Euphytica 2017, 213, 12. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Brzezinski, M.; Park, E.; Sandhu, A.; Xiao, D.; Edirisinghe, I. A Selective Role of Dietary Anthocyanins and Flavan-3-ols in Reducing the Risk of Type 2 Diabetes Mellitus: A Review of Recent Evidence. Nutrients 2019, 11, 841. [Google Scholar] [CrossRef]
- Campos, E.M.; Jakobs, L.; Simon, M.C. Antidiabetic Effects of Flavan-3-ols and Their Microbial Metabolites. Nutrients 2020, 12, 1592. [Google Scholar] [CrossRef]
- Rodriguez-Perez, C.; Garcia-Villanova, B.; Guerra-Hernandez, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef]
- Rousserie, P.; Rabot, A.; Geny-Denis, L. From Flavanols Biosynthesis to Wine Tannins: What Place for Grape Seeds? J. Agric. Food Chem. 2019, 67, 1325–1343. [Google Scholar] [CrossRef]
- Curko, N.; Ganic, K.K.; Gracin, L.; Dapic, M.; Jourdes, M.; Teissedre, P.L. Characterization of seed and skin polyphenolic extracts of two red grape cultivars grown in Croatia and their sensory perception in a wine model medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef]
- Pajović-Šćepanović, R.; Wendelin, S.; Forneck, A.; Eder, R. Suitability of flavan-3-ol analysis to differentiate grapes from Vranac, Kratošija and Cabernet Sauvignon (Vitis vinifera L.) grown in Montenegro. Aust. J. Grape Wine Res. 2019, 25, 376–383. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Sustainability of Wine Production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- Coelho, J.P.; Filipe, R.M.; Robalo, M.P.; Stateva, R.P. Recovering value from organic waste materials: Supercritical fluid extraction of oil from industrial grape seeds. J. Supercrit. Fluids 2018, 141, 68–77. [Google Scholar] [CrossRef]
- Baiano, A. An Overview on Sustainability in the Wine Production Chain. Beverages 2021, 7, 15. [Google Scholar] [CrossRef]
- Dimic, I.; Teslic, N.; Putnik, P.; Kovacevic, D.B.; Zekovic, Z.; Sojic, B.; Mrkonjic, Z.; Colovic, D.; Montesano, D.; Pavlic, B. Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.B.; Romani, A.; Lampe, A.; Nicoli, S.F.; Gabrielli, P.; et al. Grape Seeds: Chromatographic Profile of Fatty Acids and Phenolic Compounds and Qualitative Analysis by FTIR-ATR Spectroscopy. Foods 2020, 9, 10. [Google Scholar] [CrossRef]
- Amin, R.A.; Edris, S.N. Grape Seed Extract as Natural Antioxidant and Antibacterial in Minced Beef. PSM Biol. Res. 2017, 2, 89–96. [Google Scholar]
- Aybastier, O.; Dawbaa, S.; Demir, C. Investigation of antioxidant ability of grape seeds extract to prevent oxidatively induced DNA damage by gas chromatography-tandem mass spectrometry. J. Chromatogr. B 2018, 1072, 328–335. [Google Scholar] [CrossRef]
- Libera, J.; Latoch, A.; Wojciak, K.M. Utilization of Grape Seed Extract as a Natural Antioxidant in the Technology of Meat Products Inoculated with a Probiotic Strain of LAB. Foods 2020, 9, 103. [Google Scholar] [CrossRef]
- Rajakumari, R.; Volova, T.; Oluwafemi, O.S.; Kumar, S.R.; Thomas, S.; Kalarikkal, N. Grape seed extract-soluplus dispersion and its antioxidant activity. Drug Dev. Ind. Pharm. 2020, 46, 1219–1229. [Google Scholar] [CrossRef]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-de la Paz, S. Grape (Vitis vinifera L.) Seed Oil: A Functional Food from the Winemaking Industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L.; Stupic, D.; Preiner, D.; Asperger, D.; Kontic, J.K. Solid-liquid Extraction of Phenolics from Red Grape Skins. Acta Chim. Slov. 2016, 63, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, I.; Maslov, L. Simultaneous Determination of Phenolic Compounds in Different Matrices using Phenyl-Hexyl Stationary Phase. Food Anal. Meth. 2016, 9, 401–410. [Google Scholar] [CrossRef]
- Preiner, D.; Kontić, J.K.; Šimon, S.; Marković, Z.; Stupić, D.; Maletić, E. Intravarietal Agronomic Variability in Croatian Native Vitis vinifera L. Cultivar Grk with Female Flower and Seedless Berries. Am. J. Enol. Vitic. 2012, 63, 291–295. [Google Scholar] [CrossRef]
- McRae, J.M.; Schulkin, A.; Kassara, S.; Holt, H.E.; Smith, P.A. Sensory Properties of Wine Tannin Fractions: Implications for In-Mouth Sensory Properties. J. Agric. Food Chem. 2013, 61, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Watrelot, A.A.; Heymann, H.; Waterhouse, A.L. Red Wine Dryness Perception Related to Physicochemistry. J. Agric. Food Chem. 2020, 68, 2964–2972. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Food. 2020, 67, 11. [Google Scholar] [CrossRef]
- Pantelic, M.M.; Dabic Zagorac, D.; Davidovic, S.M.; Todic, S.R.; Beslic, Z.S.; Gasic, U.M.; Tesic, Z.L.; Natic, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Mateus, N.; Marques, S.; Gonçalves, A.C.; Machado, J.M.; De Freitas, V. Proanthocyanidin Composition of Red Vitis vinifera Varieties from the Douro Valley during Ripening: Influence of Cultivation Altitude. Am. J. Enol. Vitic. 2001, 52, 115–121. [Google Scholar]
- Mattivi, F.; Vrhovsek, U.; Masuero, D.; Trainotti, D. Differences in the amount and structure of extractable skin and seed tannins amongst red grape varieties. Aust. J. Grape Wine Res. 2009, 15, 27–35. [Google Scholar] [CrossRef]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.L.C.; Gascuena, J.M.; Romero, E.G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Popov, M.; Hejtmankova, A.; Kotikova, Z.; Stralkova, R.; Lachman, J. Content of flavan-3-ol monomers and gallic acid in grape seeds by variety and year. Vitis 2017, 56, 45–48. [Google Scholar] [CrossRef]
- De Freitas, V.A.P.; Glories, Y.; Monique, A. Developmental changes of procyanidins in grapes of red Vitis vinifera varieties and their composition in respective wines. Am. J. Enol. Vitic. 2000, 51, 397–403. [Google Scholar]
- Fuleki, T.; Ricardo-da-Silva, J.M. Catechin and procyanidin composition of seeds from grape cultivars grown in Ontario. J. Agric. Food Chem. 1997, 45, 1156–1160. [Google Scholar] [CrossRef]
- De Freitas, V.A.P.; Glories, Y. Concentration and compositional changes of procyanidins in grape seeds and skin of white Vitis vinifera varieties. J. Sci. Food Agric. 1999, 79, 1601–1606. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Zhao, D.; Simon, J.E.; Wu, Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nutr. 2020, 60, 597–625. [Google Scholar] [CrossRef]
| Red Cultivars | White Cultivars |
|---|---|
| Babica | Bogdanusa |
| Babic | Cetinka |
| Dobricic | Debit |
| Lasina | Gegic |
| Ljutun | Grk |
| Nincusa | Malvazija dubrovacka |
| Plavac mali | Posip |
| Plavina | Prc |
| Tribidrag | |
| Vranac | Zilavka |
| Variety | Color | Mass Cluster (g) | Average Mass 1 Berry (g) | % Seeds in Berry | % Seeds in Bunch |
|---|---|---|---|---|---|
| Babica | Red | 329.62 ± 91.66 abcd * | 2.49 ± 0.65 abcde | 2.00 ± 0.71 d | 1.95 ± 0.63 d |
| Babic | Red | 422.66 ± 92.83 abc | 3.65 ± 1.15 a | 2.00 ± 0.70 d | 1.90 ± 0.56 d |
| Dobricic | Red | 235.52 ± 86.38 bcd | 1.77 ± 0.22 cdefg | 2.90 ± 1.41 bcd | 2.80 ± 1.41 bcd |
| Lasina | Red | 302.86 ± 85.63 abcd | 2.35 ± 0.35 abcde | 8.00 ± 3.26 a | 7.70 ± 3.48 a |
| Ljutun | Red | 165.53 ± 27.52 d | 1.34 ± 0.09 efg | 2.50 ± 0.84 cd | 2.40 ± 0.84 bcd |
| Nincusa | Red | 343.06 ± 32.31 abcd | 3.27 ± 0.61 ab | 2.10 ± 0.56 d | 2.05 ± 0.63 d |
| Plavac mali | Red | 174.91 ± 49.83 cd | 1.99 ± 0.52 abcdefg | 2.10 ± 0.55 d | 2.10 ± 0.55 d |
| Plavina | Red | 177.33 ± 50.54 cd | 1.59 ± 0.08 defg | 5.60 ± 1.27 ab | 5.30 ± 1.27 ab |
| Tribidrag | Red | 166.86 ± 31.39 d | 1.09 ± 0.33 fg | 7.40 ± 2.26 a | 7.15 ± 2.19 a |
| Vranac | Red | 232.04 ± 55.04 bcd | 1.68 ± 0.73 defg | 5.35 ± 0.35 abc | 5.10 ± 0.28 abc |
| Bogdanusa | White | 294.33 ± 80.76 abcd | 3.10 ± 1.12 abc | 2.45 ± 0.91 cd | 2.30 ± 0.84 cd |
| Cetinka | White | 477.25 ± 190.77 a | 2.93 ± 0.53 abcd | 3.15 ± 0.77 bcd | 3.10 ± 0.84 bcd |
| Debit | White | 433.91 ± 162.02 ab | 2.32 ± 0.33 abcdef | 2.75 ± 0.49 bcd | 2.65 ± 0.49 bcd |
| Gegic | White | 276.06 ± 94.15 abcd | 1.80 ± 0.41 cdefg | 2.20 ± 0.28 d | 2.10 ± 0.28 d |
| Grk | White | 349.79 ± 32.31 abcd | 2.28 ± 0.53 abcde | 1.93 ± 0.56 d | 0.58 ± 0.32 d |
| M. dubrovacka | White | 143.07 ± 25.88 d | 0.83 ± 0.26 g | 7.00 ± 2.83 a | 6.45 ± 2.47 a |
| Posip | White | 244.40 ± 2.19 bcd | 2.19 ± 0.37 abcdef | 2.65 ± 1.20 bcd | 2.55 ± 1.20 bcd |
| Prc | White | 218.99 ± 37.75 cd | 2.06 ± 0.65 abcdefg | 2.20 ± 0.42 d | 2.10 ± 0.42 d |
| Zilavka | White | 223.38 ± 54.90 cd | 1.98 ± 0.79 bcdefg | 2.85 ± 0.49 bcd | 2.75 ± 0.35 bcd |
| Pr > F | 0.036 | 0.025 | 0.001 | 0.001 |
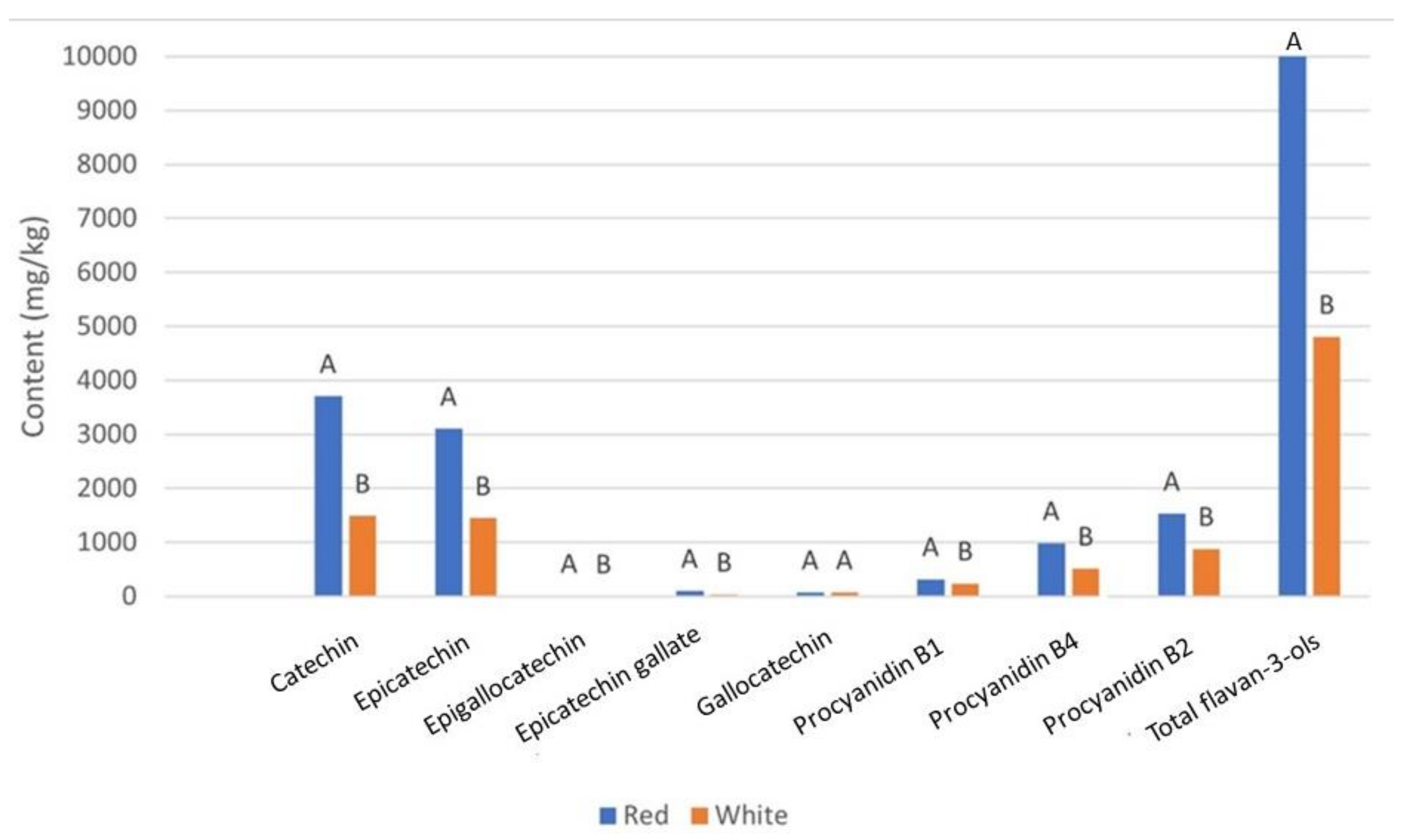
| Variety | Color | Catechin | Epicatechin | Epigallocatechin | Epicatechin Gallate | Gallocatechin | Procyanidin B1 | Procyanidin B4 | Procyanidin B2 | Total Flavan-3-Ols |
|---|---|---|---|---|---|---|---|---|---|---|
| Babica | Red | 6233.13 ± 2548.51 b | 4257.02 ± 850.86 b | 38.31 ± 10.24 b | 164.31 ± 79.00 a | 155.07 ± 105.49 a | 401.80 ± 95.91 a | 1400.60 ± 433.39 a | 2084.89 ± 576.48 ab | 14,953.29 ± 4697.78 b |
| Babic | Red | 3491.26 ± 506.59 de | 3967.44 ± 513.66 bc | 0.00 ± 0.00 cd | 157.19 ± 45.86 ab | 53.38 ± 10.10 cde | 343.56 ± 77.40 abc | 951.16 ± 167.56 bcd | 1901.80 ± 181.63 abc | 11,095.50 ± 1276.97 c |
| Dobricic | Red | 4206.28 ± 1783.25 cd | 3623.46 ± 1657.45 bcd | 6.64 ± 16.26 cd | 130.01 ± 69.21 ab | 50.91 ± 4.12 cde | 287.62 ± 112.17 bcdef | 1062.37 ± 392.24 bc | 1620.28 ± 707.27 cde | 11,127.40 ± 4754.21 c |
| Lasina | Red | 3049.24 ± 726.43 de | 3342.35 ± 835.11 bcd | 12.11 ± 20.96 c | 100.70 ± 89.94 bc | 54.31 ± 6.52 cde | 328.80 ± 38.86 abcd | 814.38 ± 242.17 cde | 1660.74 ± 360.22 bcde | 9607.69 ± 2237.02 cd |
| Ljutun | Red | 4135.25 ± 329.79 cd | 3745.28 ± 705.22 bc | 0.00 ± 0.00 d | 147.77 ± 55.75 ab | 42.67 ± 9.44 de | 358.59 ± 34.98 abc | 1052.81 ± 139.98 bc | 1789.39 ± 172.40 abcd | 11,455.87 ± 871.41 c |
| Nincusa | Red | 833.80 ± 462.01 gh | 1296.58 ± 516.26 hij | 0.00 ± 0.00 d | 48.36 ± 14.35 cdef | 80.72 ± 19.73 bcd | 234.07 ± 86.44 efgh | 452.04 ± 117.72 ghi | 930.35 ± 261.51 gh | 4024.24 ± 1459.56 fgh |
| Plavac mali | Red | 8124.22 ± 221.37 a | 5224.38 ± 100.15 a | 53.68 ± 1.19 a | 138.37 ± 8.12 ab | 52.63 ± 1.41 cde | 392.91 ± 10.73 a | 1539.43 ± 26.51 a | 2207.81 ± 37.12 a | 17,966.52 ± 399.24 a |
| Plavina | Red | 4945.50 ± 566.45 c | 3144.57 ± 651.87 cde | 0.00 ± 0.00 d | 137.08 ± 57.03 ab | 66.85 ± 3.33 cde | 373.59 ± 56.79 ab | 1162.97 ± 216.21 b | 1447.45 ± 233.43 def | 11,473.41 ± 1772.70 c |
| Tribidrag | Red | 1417.59 ± 684.75 fgh | 1718.51 ± 793.86 ghij | 0.00 ± 0.00 d | 14.85 ± 20.64 ef | 72.55 ± 5.68 cde | 234.38 ± 98.56 efgh | 649.95 ± 248.69 defg | 1003.64 ± 371.61 fgh | 5271.73 ± 2282.51 efg |
| Vranac | Red | 1757.09 ± 603.27 fg | 1654.93 ± 509.09 ghij | 7.22 ± 17.67 cd | 49.09 ± 25.73 cdef | 88.08 ± 34.51 bc | 358.15 ± 46.27 abc | 885.29 ± 147.25 cd | 1156.38 ± 247.52 fg | 6162.14 ± 1543.89 ef |
| Bogdanusa | White | 1653.10 ± 1151.04 fg | 1051.29 ± 605.32 ijk | 0.00 ± 0.00 d | 19.87 ± 5.92 def | 64.99 ± 15.80 cde | 242.63 ± 78.26 defgh | 486.21 ± 180.56 fghi | 856.21 ± 399.74 ghi | 4476.81 ± 2415.95 fg |
| Cetinka | White | 3476.75 ± 277.26 de | 2245.50 ± 537.78 efgh | 0.00 ± 0.00 d | 59.74 ± 25.00 cde | 37.19 ± 21.60 e | 320.24 ± 43.37 abcde | 724.26 ± 99.56 def | 851.51 ± 89.24 ghi | 7885.48 ± 478.24 de |
| Debit | White | 858.36 ± 336.54 gh | 866.17 ± 227.03 jk | 0.00 ± 0.00 d | 37.59 ± 11.69 def | 78.0809 ± 11.82 cd | 191.75 ± 38.79 gh | 315.29 ± 51.31 hi | 602.18 ± 76.58 hi | 3069.75 ± 662.19 gh |
| Gegic | White | 263.24 ± 178.50 h | 277.53 ± 71.11 k | 0.00 ± 0.00 d | 0.00 ± 0.00 f | 78.40 ± 9.49 cd | 278.44 ± 49.90 cdefg | 276.68 ± 31.48 i | 420.18 ± 53.71 i | 1723.67 ± 308.68 h |
| Grk | White | 2326.48 ± 660.31 ef | 2741.60 ± 757.16 def | 0.00 ± 0.00 d | 68.70 ± 39.71 cd | 116.41 ± 23.17 b | 221.24 ± 23.17 fgh | 720.97 ± 101.00 def | 1166.30 ± 250.64 fg | 7538.83 ± 1793.52 de |
| M. dubrovacka | White | 1209.34 ± 465.01 fgh | 1116.76 ± 824.79 ijk | 0.00 ± 0.00 d | 11.84 ± 28.08 ef | 57.63 ± 8.23 cde | 253.30 ± 8.23 defgh | 433.70 ± 108.80 ghi | 842.20 ± 330.70 ghi | 4037.02 ± 1689.63 fgh |
| Posip | White | 1609.79 ± 739.19 fg | 2322.77 ± 1076.25 efg | 0.00 ± 0.00 d | 41.87 ± 37.06 cdef | 67.05 ± 6.45 cde | 203.98 ± 6.45 fgh | 507.88 ± 159.19 fghi | 1278.19 ± 473.46 efg | 6143.39 ± 2437.25 ef |
| Prc | White | 1809.78 ± 482.62 fg | 1394.38 ± 68.95 ghij | 0.00 ± 0.00 d | 14.27 ± 3.82 ef | 69.59 ± 28.87 cde | 197.25 ± 28.87 gh | 671.14 ± 53.84 defg | 915.02 ± 46.13 gh | 5207.20 ± 575.94 efg |
| Zilavka | White | 869.76 ± 136.38 gh | 1155.83 ± 154.08 ijk | 0.00 ± 0.00 d | 18.06 ± 9.38 def | 81.01 ± 18.65 bcd | 255.84 ± 18.65 defg | 536.72 ± 112.31 efgh | 930.76 ± 151.64 gh | 3938.97 ± 596.29 fgh |
| Pr > F | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Significant | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Variety | Color | Catechin | Epicatechin | Epigallocatechin | Epicatechin Gallate | Gallocatechin | Procyanidin B1 | Procyanidin B4 | Procyanidin B2 | Total Flavan-3-Ols |
|---|---|---|---|---|---|---|---|---|---|---|
| Babica | Red | 11.12 ± 1.92 c | 7.96 ± 0.58 de | 0.07 ± 0.01 ab | 0.30 ± 0.14 b | 0.26 ± 0.12 cd | 0.75 ± 0.09 def | 2.56 ± 0.18 cde | 3.83 ± 0.18 fg | 27.26 ± 2.03 def |
| Babic | Red | 6.63 ± 0.96 de | 7.54 ± 0.97 de | 0.00 ± 0.00 c | 0.30 ± 0.08 bc | 0.10 ± 0.01 efg | 0.65 ± 0.14 def | 1.81 ± 0.31 ef | 3.61 ± 0.34 fg | 21.08 ± 2.42 fg |
| Dobricic | Red | 10.15 ± 0.53 cd | 8.64 ± 0.75 cde | 0.01 ± 0.03 c | 0.31 ± 0.13 b | 0.14 ± 0.05 ef | 0.71 ± 0.16 def | 2.62 ± 0.21 cde | 3.89 ± 0.27 fg | 26.828 ± 1.56 def |
| Lasina | Red | 23.479 ± 5.59 a | 25.736 ± 6.43 a | 0.09.16 a | 0.775 ± 0.69 a | 0.418 ± 0.05 ab | 2.532 ± 0.29 a | 6.27 ± 1.86 a | 12.79 ± 2.77 a | 73.98 ± 17.22 a |
| Ljutun | Red | 10.08 ± 3.44 cd | 8.61 ± 0.81 cde | 0.00 ± 0.00 c | 0.32 ± 0.03 b | 0.10 ± 0.19 fg | 0.85 ± 0.18 de | 2.47 ± 0.62 cde | 4.22 ± 0.90 ef | 27.11 ± 6.01 def |
| Nincusa | Red | 1.90 ± 1.35 fg | 2.86 ± 1.67 f | 0.00 ± 0.00 c | 0.10 ± 0.02 cd | 0.16 ± 0.05 def | 0.51 ± 0.28 ef | 0.97 ± 0.45 fgh | 2.00 ± 0.96 hij | 8.82 ± 4.90 hij |
| Plavac mali | Red | 17.06 ± 0.46 b | 10.97 ± 0.21 cd | 0.11 ± 0.01 a | 0.29 ± 0.02 bc | 0.11 ± 0.00 efg | 0.83 ± 0.02 def | 3.23 ± 0.05 c | 4.64 ± 0.07 def | 37.73 ± 0.83 c |
| Plavina | Red | 25.81 ± 2.69 a | 16.15 ± 0.87 b | 0.00 ± 0.00 c | 0.68 ± 0.16 a | 0.36 ± 0.07 bc | 1.94 ± 0.11 b | 6.00 ± 0.39 a | 7.49 ± 0.46 b | 59.45 ± 3.97 b |
| Tribidrag | Red | 9.18 ± 2.51 cd | 11.17 ± 2.79 c | 0.00 ± 0.00 c | 0.08 ± 0.11 cd | 0.52 ± 0.14 a | 1.54 ± 0.35 c | 4.30 ± 0.71 b | 6.66 ± 0.99 bc | 34.50 ± 7.53 cd |
| Vranac | Red | 8.85 ± 2.69 cd | 8.35 ± 2.23 de | 0.04 ± 0.08 bc | 0.25 ± 0.12 bc | 0.46 ± 0.19 ab | 1.82 ± 0.17 bc | 4.49 ± 0.56 b | 5.85 ± 1.02 cd | 31.15 ± 6.54 cde |
| Bogdanusa | White | 3.174 ± 1.56 efg | 2.09 ± 0.70 f | 0.00 ± 0.00 c | 0.05 ± 0.02 d | 0.16 ± 0.78 def | 0.52 ± 0.08 ef | 1.02 ± 0.10 fgh | 1.75 ± 0.35 ij | 8.98 ± 2.61 hij |
| Cetinka | White | 10.64 ± 1.57 c | 7.24 ± 3.12 e | 0.00 ± 0.00 c | 0.17 ± 0.03 bcd | 0.13 ± 0.08 efg | 1.00 ± 0.30 d | 2.20 ± 0.24 de | 2.68 ± 0.79 ghi | 24.60 ± 6.20 ef |
| Debit | White | 2.38 ± 1.21 fg | 2.36 ± 0.92 f | 0.00 ± 0.00 c | 0.10 ± 0.01 cd | 0.21 ± 0.06 def | 0.52 ± 0.15 ef | 0.84 ± 0.18 gh | 1.60 ± 0.33 ij | 8.32 ± 2.83 ij |
| Gegic | White | 0.56 ± 0.42 g | 0.59 ± 0.19 f | 0.00 ± 0.00 c | 0.00 ± 0.00 d | 0.16 ± 0.02 def | 0.58 ± 0.05 ef | 0.58 ± 0.08 hi | 0.88 ± 0.15 jk | 3.62 ± 0.81 ij |
| Grk | White | 0.06 ± 0.01 g | 0.07 ± 0.01 f | 0.00 ± 0.00 c | 0.00 ± 0.00 d | 0.00 ± 0.00 g | 0.01 ± 0.00 g | 0.02 ± 0.00 i | 0.03 ± 0.00 k | 0.18 ± 0.02 j |
| M. dubrovacka | White | 8.27 ± 4.54 cd | 7.1337 ± 4.29 e | 0.00 ± 0.00 c | 0.06 ± 0.13 d | 0.36 ± 0.08 b | 1.70 ± 0.76 bc | 2.84 ± 1.13 cd | 5.51 ± 2.45 cde | 26.58 ± 12.62 ef |
| Posip | White | 4.41 ± 2.71 ef | 6.13 ± 3.43 e | 0.00± 0.00 c | 0.09 ± 0.05 cd | 0.17 ± 0.04 def | 0.52 ± 0.23 ef | 1.31 ± 0.58 fg | 3.37 ± 1.72 fgh | 16.28 ± 8.68 gh |
| Prc | White | 3.93 ± 1.61 ef | 2.94 ± 0.56 f | 0.00 ± 0.00 c | 0.03 ± 0.01 d | 0.14 ± 0.04 ef | 0.41 ± 0.09 f | 1.42 ± 0.31 fg | 1.92 ± 0.29 hij | 11.08 ± 2.87 hi |
| Zilavka | White | 2.42 ± 0.60 fg | 3.21 ± 0.71 f | 0.00 ± 0.00 c | 0.05 ± 0.03 d | 0.22 ± 0.05 de | 0.71 ± 0.13 def | 1.50 ± 0.45 fg | 2.59 ± 0.65 ghi | 10.95 ± 2.66 hi |
| Pr > F | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Significant | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andabaka, Ž.; Šikuten, I.; Tomaz, I.; Stupić, D.; Marković, Z.; Kontić, J.K.; Maletić, E.; Preiner, D. Diversity of Seed Flavan-3-Ols in Croatian Native Grapevine Cultivars (Vitis vinifera L.) Grown in Coastal Region. Diversity 2022, 14, 667. https://doi.org/10.3390/d14080667
Andabaka Ž, Šikuten I, Tomaz I, Stupić D, Marković Z, Kontić JK, Maletić E, Preiner D. Diversity of Seed Flavan-3-Ols in Croatian Native Grapevine Cultivars (Vitis vinifera L.) Grown in Coastal Region. Diversity. 2022; 14(8):667. https://doi.org/10.3390/d14080667
Chicago/Turabian StyleAndabaka, Željko, Iva Šikuten, Ivana Tomaz, Domagoj Stupić, Zvjezdana Marković, Jasminka Karoglan Kontić, Edi Maletić, and Darko Preiner. 2022. "Diversity of Seed Flavan-3-Ols in Croatian Native Grapevine Cultivars (Vitis vinifera L.) Grown in Coastal Region" Diversity 14, no. 8: 667. https://doi.org/10.3390/d14080667
APA StyleAndabaka, Ž., Šikuten, I., Tomaz, I., Stupić, D., Marković, Z., Kontić, J. K., Maletić, E., & Preiner, D. (2022). Diversity of Seed Flavan-3-Ols in Croatian Native Grapevine Cultivars (Vitis vinifera L.) Grown in Coastal Region. Diversity, 14(8), 667. https://doi.org/10.3390/d14080667









