Spatial and Temporal Variability in Parrotfish Assemblages on Bahamian Coral Reefs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Fish Surveys
2.3. Data and Statistical Analyses
3. Results
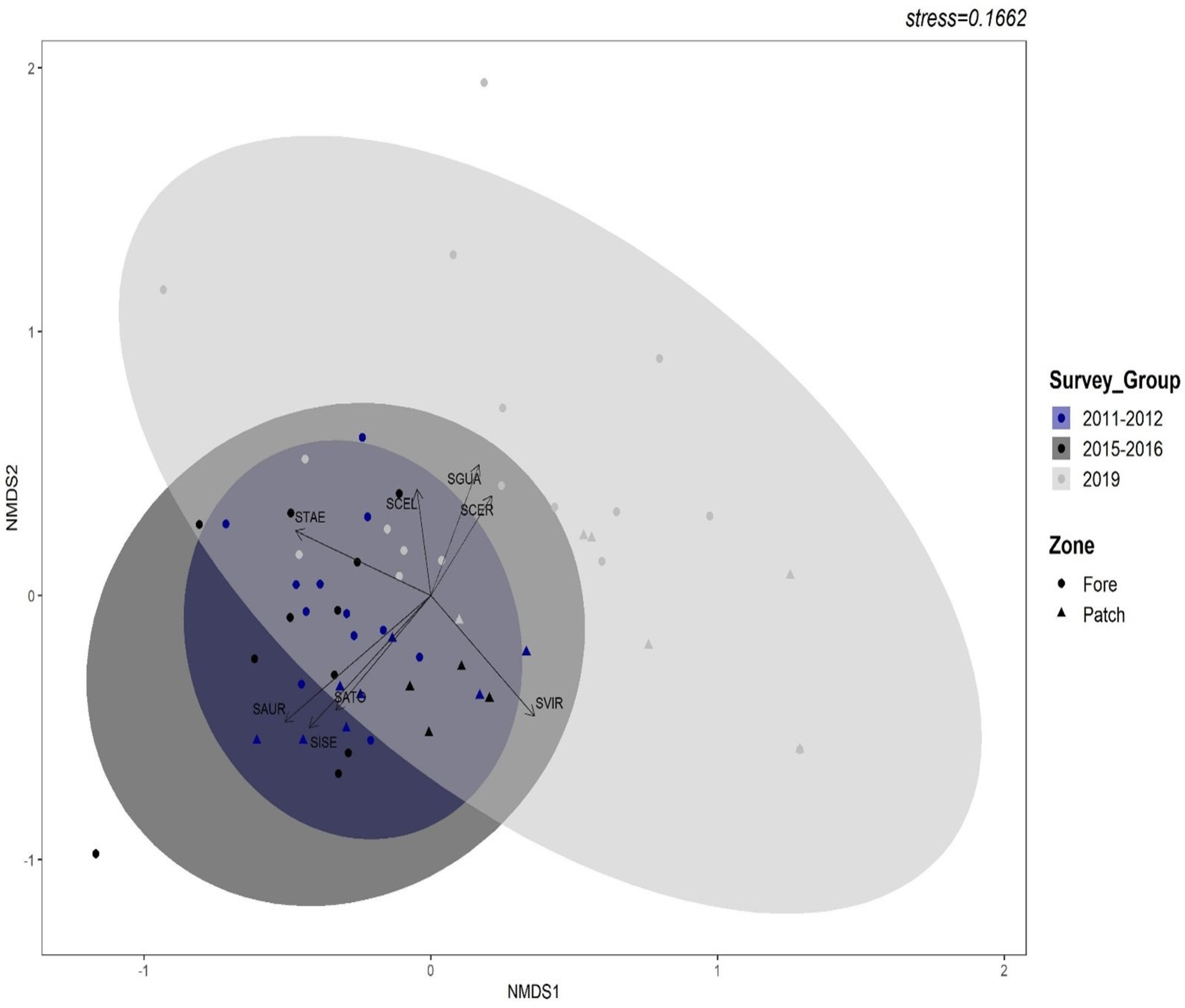
3.1. Spatial Patterns of Parrotfish Assemblages
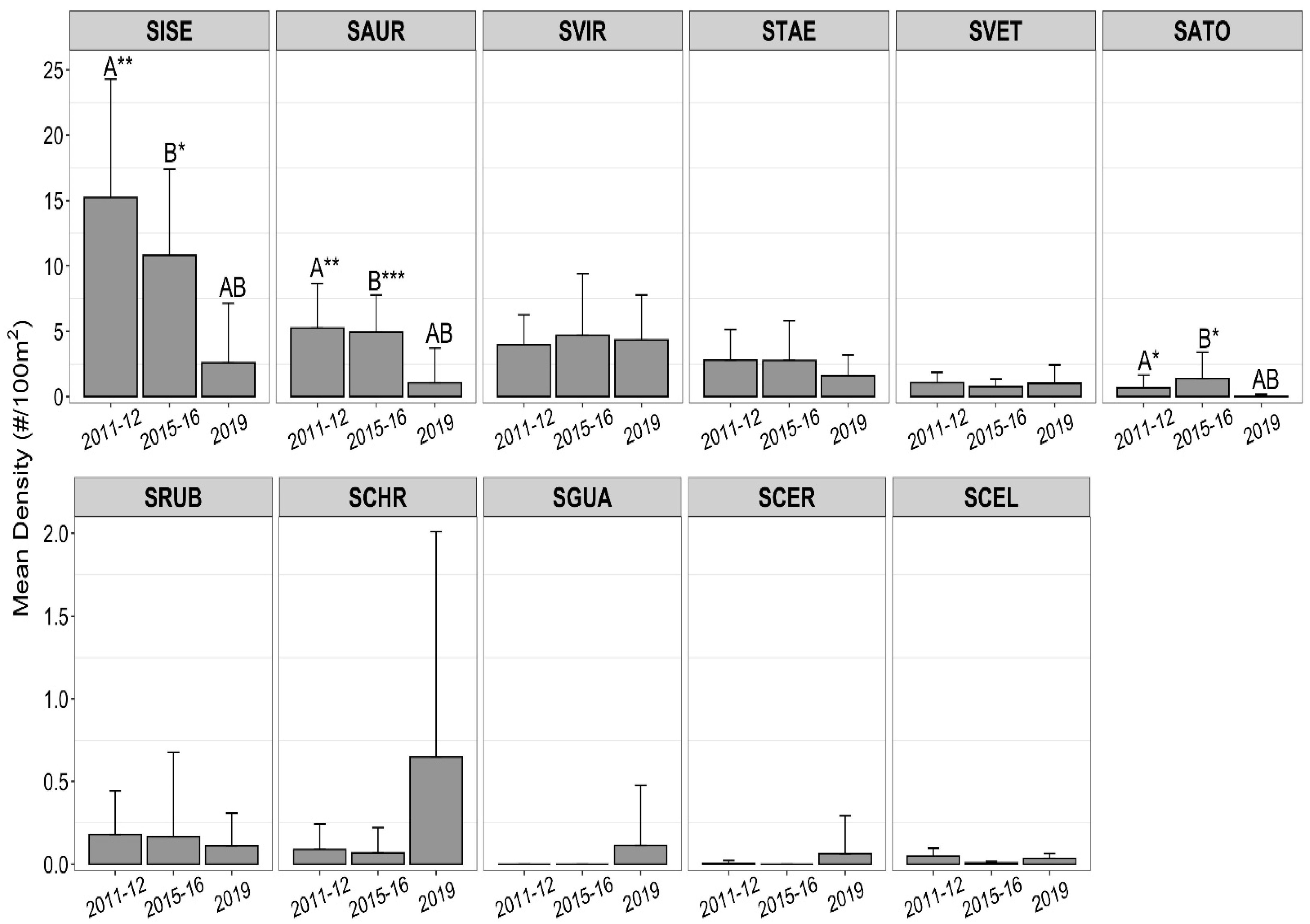
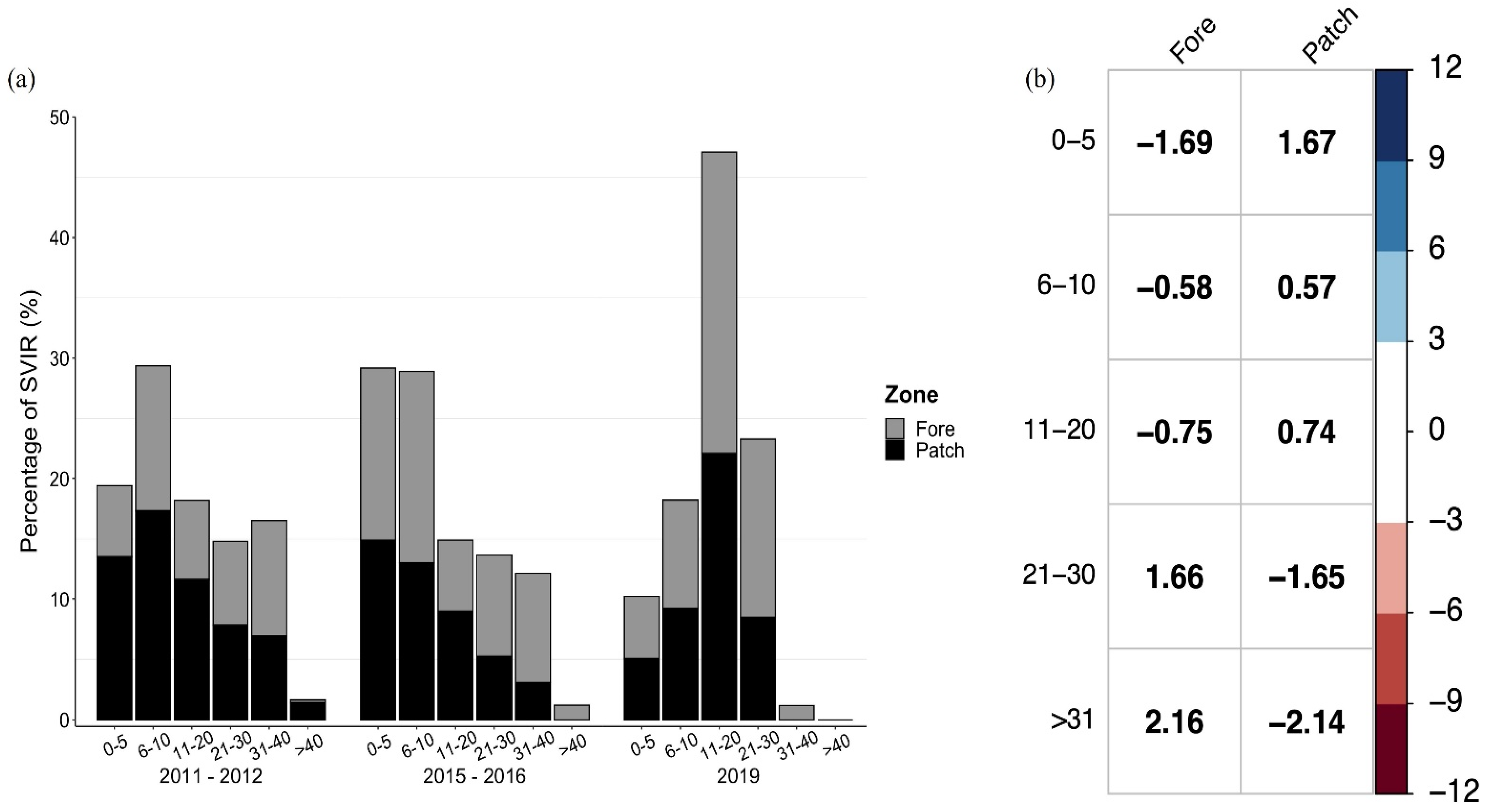
3.2. Temporal Patterns
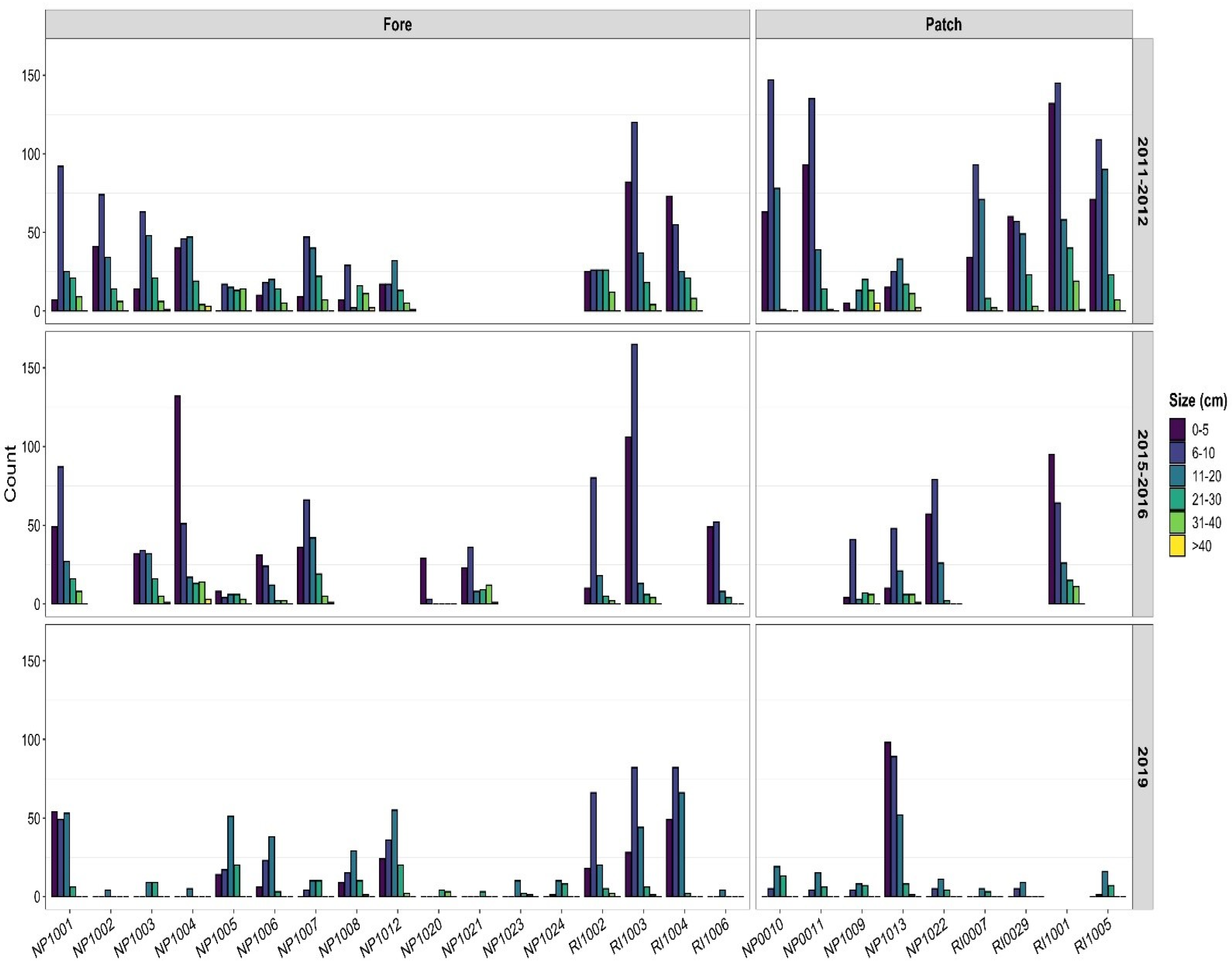
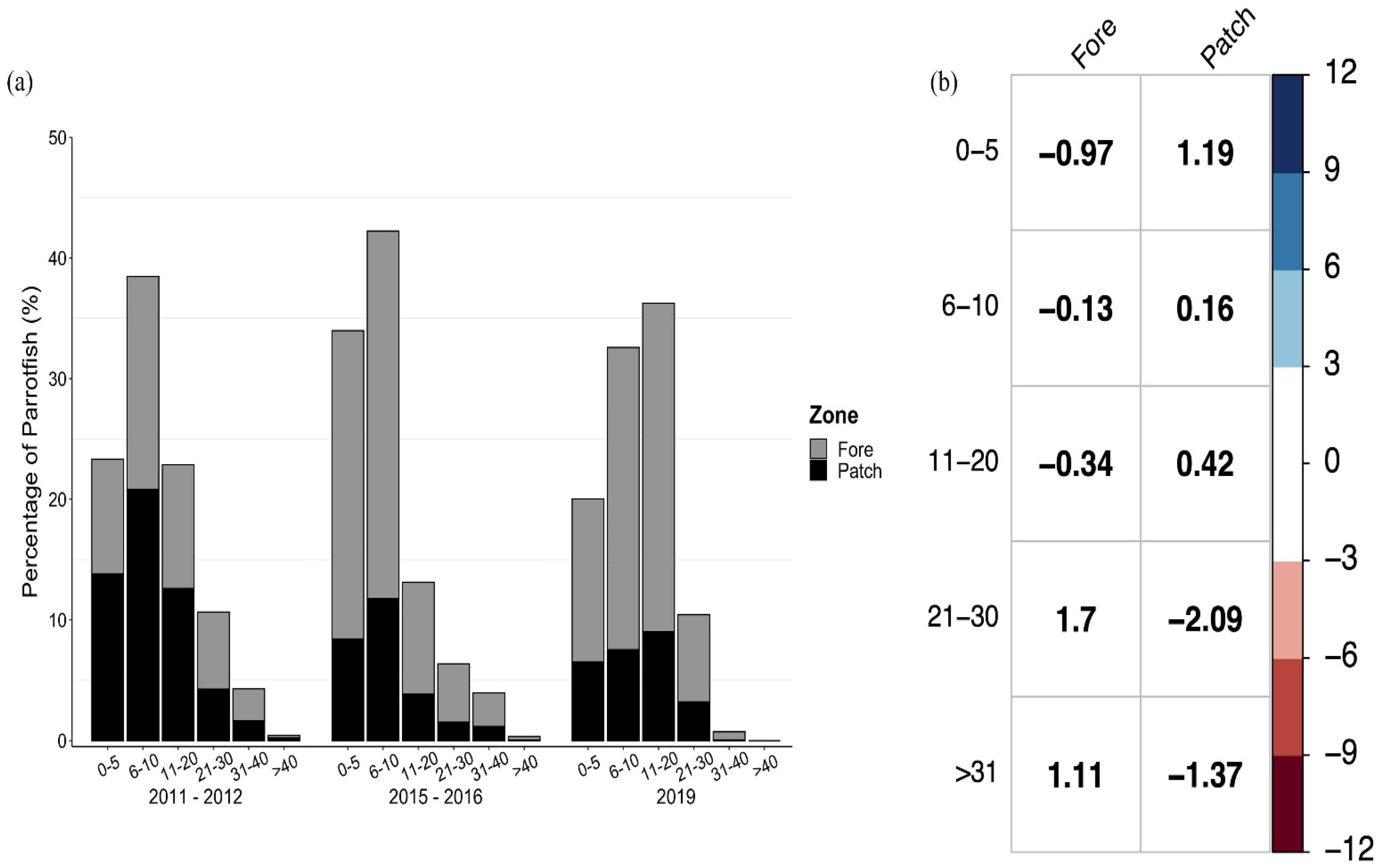
3.3. Size Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoegh-Guldberg, O. Reviving the Ocean Economy: The Case for Action—2015; WWF International: Gland, Switzerland; Geneva, Switzerland, 2015; p. 60. Available online: www.ocean.panda.org (accessed on 4 October 2021).
- Obura, D.O.; Aeby, G.; Amornthammarong, N.; Appeltans, W.; Bax, N.; Bishop, J.; Brainard, R.E.; Chan, S.; Fletcher, P.; Gordon, T.A.C.; et al. Coral Reef Monitoring, Reef Assessment Technologies, and Ecosystem-Based Management. Front. Mar. Sci. 2019, 6, 580. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.B.C.; Donovan, M.K.; Cramer, K.L.; Lam, V.V. (Eds.) Status and Trends of Caribbean Coral Reefs: 1970–2012; Global Coral Reef Monitoring Network, IUCN: Gland, Switzerland, 2014. [Google Scholar]
- Hoegh-Guldberg, O.; Poloczanska, E.S.; Skirving, W.; Dove, S. Coral reef ecosystems under climate change and ocean acidification. Front. Mar. Sci. 2017, 4, 158. [Google Scholar] [CrossRef] [Green Version]
- Dahlgren, C.; Sherman, K. Preliminary Assessment of Hurricane Dorian’s Impacts on Coral Reefs of Abaco and Grand Bahama; Report submitted to the Government of The Bahamas; Perry Institute for Marine Science: Waitsfield, VT, USA, 2020; 28p. [Google Scholar]
- Lester, S.E.; Rassweiler, A.; McCoy, S.J.; Dubel, A.K.; Donovan, M.K.; Miller, M.W.; Miller, S.D.; Ruttenberg, B.I.; Samhouri, J.F.; Hay, M.E. Caribbean reefs of the Anthropocene: Variance in ecosystem metrics indicates bright spots on coral depauperate reefs. Glob. Chang. Biol. 2020, 26, 4785–4799. [Google Scholar] [CrossRef]
- Dahlgren, C.; Pizarro, V.; Sherman, K.; Greene, W.; Oliver, J. Spatial and temporal patterns of stony coral tissue loss disease outbreaks in The Bahamas. Front. Mar. Sci. 2021, 8, 682114. [Google Scholar] [CrossRef]
- Bozec, Y.-M.; O’Farrell, S.; Bruggemann, J.H.; Luckhurst, B.E.; Mumby, P.J. Tradeoffs between fisheries harvest and the resilience of coral reefs. Proc. Natl. Acad. Sci. USA 2016, 113, 4536–4541. [Google Scholar] [CrossRef] [Green Version]
- Bellwood, D.; Hughes, T.; Folke, C.; Nyström, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Wainwright, P.C.; Fulton, C.J.; Hoey, A.S. 2006 Functional versatility supports coral reef biodiversity. Proc. R. Soc. B 2005, 273, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Mumby, P.J.; Harborne, A.R.; Williams, J.; Kappel, C.V.; Brumbaugh, D.R.; Micheli, F.; Holmes, K.E.; Dahlgren, C.P.; Paris, C.B.; Blackwell, P.G. Trophic cascade facilitates coral recruitment in a marine reserve. Proc. Natl. Acad. Sci. USA 2007, 104, 8362–8367. [Google Scholar] [CrossRef] [Green Version]
- Bonaldo, R.M.; Hoey, A.S.; Bellwood, D.R. The ecosystem roles of parrotfishes on tropical reefs. Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 81–132. [Google Scholar]
- Brandl, S.J.; Bellwood, D.R. Individual-based analyses reveal limited functional overlap in a coral reef fish community. J. Anim. Ecol. 2013, 83, 661–670. [Google Scholar] [CrossRef]
- Bozec, Y.-M.; Doropoulos, C.; Roff, G.; Mumby, P.J. Transient grazing and the dynamics of an unanticipated coral-algal phase shift. Ecosystems 2018, 22, 296–311. [Google Scholar] [CrossRef]
- Dell, C.L.A.; Longo, G.O.; Burkepile, D.E.; Manfrino, C. Few Herbivore Species Consume Dominant Macroalgae on a Caribbean Coral Reef. Front. Mar. Sci. 2020, 7, 676. [Google Scholar] [CrossRef]
- Mumby, P.J. The impact of exploiting grazers (Scaridae) on the dynamics of Caribbean coral reefs. Ecol. Appl. 2006, 16, 747–769. [Google Scholar] [CrossRef] [Green Version]
- Mumby, P.J.; Dahlgren, C.P.; Harborne, A.R.; Kappel, C.V.; Micheli, F.; Brumbaugh, D.R.; Holmes, K.E.; Mendes, J.M.; Broad, K.; Sanchirico, J.N.; et al. Fishing, trophic cascades and the process of grazing on coral reefs. Science 2006, 311, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Clements, K.D.; German, D.P.; Piché, J.; Tribollet, A.; Choat, H.A. Integrating ecological roles and trophic diversification on coral reefs: Multiple lines of evidence identify parrotfishes as microphages. Biol. J. Linn. Soc. 2016, 120, 729–751. [Google Scholar] [CrossRef]
- Ruttenberg, B.I.; Adam, T.C.; Duran, A.; Burkepile, D.E. Identity of coral reef herbivores drives variation in ecological processes over multiple spatial scales. Ecol. Appl. 2019, 29, e01893. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Clements, K.D. Resolving resource partitioning in parrotfishes (Scarini) using microhistology of feeding substrata. Coral Reefs 2020, 39, 1313–1327. [Google Scholar] [CrossRef]
- Yartlett, R.T.; Perry, C.T.; Wilson, R.W.; Harborne, A.R. Inter-habitat variability in parrotfish bioerosion rates and grazing pressure on an Indian Ocean reef platform. Diversity 2020, 12, 381. [Google Scholar] [CrossRef]
- Cox, C.E.; Jones, C.D.; Wares, J.P.; Castillo, K.D.; Bruno, J.F. Fish mislabeling in Belize: Implications for coral reef conservation. Conserv. Lett. 2013, 6, 132–140. [Google Scholar] [CrossRef]
- Vallès, H.; Oxenford, H.A. Parrotfish Size: A simple yet useful alternative indicator of fishing effects on Caribbean Reefs? PLoS ONE 2014, 9, e86291. [Google Scholar] [CrossRef] [Green Version]
- Nagelkerken, I.; Grol, M.G.G.; Mumby, P.J. Effects of marine reserves versus nursery habitat availability on structure of reef fish communities. PLoS ONE 2012, 7, e36906. [Google Scholar] [CrossRef] [Green Version]
- Eggertsen, M.; Chacin, D.H.; van Lier, J.; Eggertsen, L.; Fulton, C.J.; Wilson, S.; Halling, C.; Berkstrom, C. Seascape configuration and fine-scale habitat complexity shape parrotfish distribution and function across a coral reef lagoon. Diversity 2020, 12, 391. [Google Scholar] [CrossRef]
- Stallings, C.D. Fishery-independent data reveal negative effect of human population density on Caribbean predatory fish communities. PLoS ONE 2009, 4, e5333. [Google Scholar] [CrossRef]
- Bejarano, S.; Golbuu, Y.; Sapolu, T.; Mumby, P.J. Ecological risk and the exploitation of herbivorous reef fish across Micronesia. Mar. Ecol. Prog. Ser. 2013, 482, 197–215. [Google Scholar] [CrossRef] [Green Version]
- Sherman, K.D.; Shultz, A.D.; Dahlgren, C.P.; Thomas, C.; Brooks, E.; Brooks, A.; Brumbaugh, D.R.; Gittens, L.; Murchie, K. Contemporary and emerging fisheries in The Bahamas—Conservation and management challenges, achievements and future directions. Fish. Manag. Ecol. 2018, 25, 319–331. [Google Scholar] [CrossRef]
- Callwood, K. Examining the development of a parrotfish fishery in The Bahamas: Social considerations and management implications. Glob. Ecol. Conserv. 2021, 28, e01677. [Google Scholar] [CrossRef]
- Dahlgren, C.; Kramer, P.R.; Lang, J.; Sherman, K. New Providence and Rose Island, Bahamas 2014 Coral Reef Report Card; Perry Institute for Marine Science: Waitsfield, VT, USA, 2014. [Google Scholar]
- Dahlgren, C.; Sherman, K.; Lang, J.; Kramer, P.R.; Marks, K. Bahamas Coral Reef Report Card Volume 1: 2011–2013; Perry Institute for Marine Science: Waitsfield, VT, USA, 2016. [Google Scholar]
- Dahlgren, C.; Sherman, K.; Haines, L.; Knowles, L.; Callwood, K. Bahamas Coral Reef Report Card Volume 2: 2015–2019; Perry Institute for Marine Science: Waitsfield, VT, USA, 2020. [Google Scholar]
- Williams, I.D.; Kindinger, T.L.; Couch, C.S.; Walsh, W.J.; Minton, D.; Oliver, T.A. Can Herbivore Management Increase the Persistence of Indo-Pacific Coral Reefs? Front. Mar. Sci. 2019, 6, 557. [Google Scholar] [CrossRef]
- Bruno, J.F.; Côté, I.M.; Toth, L.T. Climate change, coral loss, and the curious case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Annu. Rev. Mar. Sci. 2019, 11, 307–334. [Google Scholar] [CrossRef] [Green Version]
- Chung, A.E.; Wedding, L.M.; Green, A.L.; Friedlander, A.M.; Goldberg, G.; Meadows, A.; Hixon, M.A. Building Coral Reef Resilience Through Spatial Herbivore Management. Front. Mar. Sci. 2019, 6, 98. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Muthiga, N.A. Change in fish and benthic communities in Belizean patch reefs in and outside of a marine reserve, across a parrotfish capture ban. Mar. Ecol. Prog. Ser. 2020, 645, 25–40. [Google Scholar] [CrossRef]
- Sullivan-Sealey, K. Large-scale ecological impacts of development on tropical island systems: Comparison of developed and undeveloped islands in the central Bahamas. Bull. Mar. Sci. 2004, 75, 295–320. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest community of southern Wisonsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Kahle, D.; Wickham, H. ggmap: Spatial Visualization with ggplot2. R J. 2013, 5, 144–161. Available online: http://journal.r-project.org/archive/2013-1/kahle-wickham.pdf (accessed on 4 October 2021). [CrossRef] [Green Version]
- Wei, T.; Simko, V. Corrplot: Visualization of a Correlation Matrix [Online]. R package version 0.92. 2021. Available online: https://cran.r-project.org/package=corrplot (accessed on 4 October 2021).
- Harms-Tuohy, C.A. Parrotfishes in the Caribbean: A Regional Review with Recommendations for Management; FAO Fisheries and Aquaculture Circular No.1240; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Brown, C.J.; Harborne, A.R.; Paris, C.B.; Mumby, P.J. Uniting paradigms of connectivity in marine ecology. Ecology 2016, 97, 2447–2457. [Google Scholar] [CrossRef] [Green Version]
- Vallès, H. Parrotfish recruitment revisited: A key role for sea surface currents. Environ. Biol. Fishes 2017, 100, 1649–1657. [Google Scholar] [CrossRef]
- Adam, T.C.; Kelley, M.; Ruttenberg, B.I.; Burkepile, D.E. Resource partitioning along multiple niche axes drives functional diversity in parrotfishes on Caribbean coral reefs. Oecologia 2015, 179, 1173–1185. [Google Scholar] [CrossRef] [Green Version]
- Graham, N.A.J.; Nash, K.L. The importance of structural complexity in coral reef ecosystems. Coral Reefs 2013, 32, 315–326. [Google Scholar] [CrossRef]
- Welsh, J.Q.; Goatley, C.H.R.; Bellwood, D.R. The ontogeny of home ranges: Evidence from coral reef fishes. Proc. R. Soc. B. 2013, 280, 20132066. [Google Scholar] [CrossRef] [Green Version]
- Green, A.L.; Maypa, A.P.; Almany, G.R.; Rhodes, K.L.; Weeks, R.; Abesamis, R.A.; Gleason, M.G.; Mumby, P.J.; White, A.T. Larval dispersal and movement patterns of coral reef fishes, and implications for marine reserve network design. Biol. Rev. 2014, 90, 1215–1247. [Google Scholar] [CrossRef]
- Roff, G.; Bejarano, S.; Priest, M.; Marshell, A.; Chollett, I.; Steneck, R.S.; Doropoulos, C.; Golbuu, Y.; Mumby, P.J. Seascapes as drivers of herbivore assemblages in coral reef ecosystems. Ecol. Monogr. 2019, 89, e01336. [Google Scholar] [CrossRef] [Green Version]
- Evensen, N.R.; Vanwonterghem, I.; Doropoulos, C.; Gouezo, M.; Botté, E.S.; Webster, N.S.; Mumby, P.J. Benthic micro- and macro-community succession and coral recruitment under overfishing and nutrient enrichment. Ecol. Accept. Author Manuscr. 2021, 102, e03536. [Google Scholar] [CrossRef]
- Holbrook, S.J.; Brooks, A.J.; Schmitt, R.J. Predictability of fish assemblages on coral patch reefs. Mar. Freshw. Res. 2002, 53, 181–188. [Google Scholar] [CrossRef]
- Hixon, M.A. Reef fishes, seaweeds, and corals. In Coral Reefs in the Anthropocene; Birkeland, C., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 195–215. [Google Scholar] [CrossRef]
- Choat, J.H. Spawning aggregations in reef fishes: Ecological and evolutionary processes. In Reef Fish Spawning Aggregations: Biology, Research and Management; Sadovy de Mitcheson, Y., Colin, P.L., Eds.; Springer: New York, NY, NY, 2012; pp. 85–116. [Google Scholar]
- Lange, I.D.; Perry, C.T.; Morgan, K.M.; Roche, R.; Benkwitt, C.E.; Graham, N.A. Site-level variation in parrotfish grazing and bioerosion as a function of species-specific feeding metrics. Diversity 2020, 12, 379. [Google Scholar] [CrossRef]
- Goetze, J.S.; Langlois, T.J.; Egli, D.P.; Harvey, E. Evidence of artisanal fishing impacts and depth refuge in assemblages of Fijian reef fish. Coral Reefs 2011, 30, 507–517. [Google Scholar] [CrossRef]
- O’Farrell, S.; Harborne, A.R.; Bozec, Y.M.; Luckhurst, B.E.; Mumby, P.J. Protection of functionally important parrotfishes increases their biomass but fails to deliver enhanced recruitment. Mar. Ecol. Prog. Ser. 2015, 522, 245–254. [Google Scholar] [CrossRef]
- Fisheries Resources (Jurisdiction and Conservation) (Amendment) Act, 2020. 21 of 2020—Fisheries Resources (Jurisdiction and Conservation) (Amendment) Act. 2020. Available online: bahamas.gov.bs (accessed on 28 January 2022).
- AGRRA. Atlantic and Gulf Rapid Reef Assessment (AGRRA): An Online Database of AGRRA Coral Reef Survey Data. 2018. Available online: http://agrra.org (accessed on 3 August 2021).
- Loera-Padilla, F.J.; Piñeros, V.J.; Baldwin, C.C.; Cox, C.E.; Simoes, N.; Ribeiro, E.; Lasso-Alcalá, O.M.; Domínguez-Domínguez, O. Phylogeography, population connectivity and demographic history of the Stoplight parrotfish, Sparisoma viride (Teleostei: Labridae), in the Greater Caribbean. Coral Reefs 2022, 41, 753–765. [Google Scholar] [CrossRef]
- Velasco-Montoya, D.A.; Millán-Márquez, A.M.; Tavera, J. Genetic connectivity in Sparisoma aurofrenatum (redband parrotfish): An unexpected journey. Hydrobiologia 2022, 849, 1727–1741. [Google Scholar] [CrossRef]




| Site Name | Site Code | Reef Zone | No. Transects | Survey Depth Range (m) | Mean Survey Depth (m) | Mean Density (# Indiv./100 m2) | Years Surveyed |
|---|---|---|---|---|---|---|---|
| Jonsa Reef | NP0010 | Patch | 14 | 6.1–7.6 | 6.9 | 38.8 (±26.7) | 2012; 2019 |
| Tao Reef | NP0011 | Patch | 14 | 4.6–7.6 | 5.8 | 36.5 (±20.0) | 2012; 2019 |
| Pumpkin Patch | NP1001 | Fore | 35 | 9.9–16.0 | 12.3 | 24.6 (±14.3) | 2011; 2016; 2019 |
| BBC | NP1002 | Fore | 12 | 13.4–13.7 | 13.6 | 24.0 (±18.3) | 2011; 2019 |
| Clifton Wall | NP1003 | Fore | 22 | 12.2–15.7 | 13.8 | 22.1 (±12.0) | 2011; 2016; 2019 |
| Nari | NP1004 | Fore | 25 | 6.1–8.4 | 7.1 | 26.3 (±18.9) | 2011; 2016; 2019 |
| Razorback | NP1005 | Fore | 20 | 12.6–20.4 | 16.0 | 15.7 (±11.5) | 2011; 2016; 2019 |
| Runway Wall | NP1006 | Fore | 23 | 9.6–14.4 | 11.8 | 15.1 (±9.0) | 2011; 2016; 2019 |
| Willaurie | NP1007 | Fore | 22 | 13.2–15.7 | 14.7 | 24.1 (±17.1) | 2011; 2016; 2019 |
| Tunnel Wall | NP1008 | Fore | 20 | 9.6–15.2 | 12.9 | 10.9 (±11.5) | 2011; 2019 |
| Elkhorn Garden | NP1009 | Patch | 17 | 2.9–6.1 | 5.0 | 13.4 (±9.2) | 2011; 2016; 2019 |
| Traveler’s Wall | NP1012 | Fore | 25 | 13.6–19.2 | 16.9 | 14.8 (±11.4) | 2012; 2019 |
| Schoolhouse Reef | NP1013 | Patch | 23 | 2.5–4.6 | 3.7 | 32.1 (±19.5) | 2012; 2016; 2019 |
| Trinity Caves | NP1020 | Fore | 10 | 8.6–13.7 | 10.2 | 6.5 (±6.9) | 2015; 2019 |
| Piece of Cake | NP1021 | Fore | 9 | 4.0–9.8 | 5.1 | 17.1 (±19.7) | 2015; 2019 |
| East End Reef | NP1022 | Patch | 9 | 3.0–6.1 | 4.5 | 34.1 (±29.6) | 2015; 2019 |
| Sand Chute | NP1023 | Fore | 4 | 12.2–13.7 | 12.6 | 5.4 (±2.1) | 2019 |
| Wreck on the Wall | NP1024 | Fore | 4 | 16.8–16.8 | 16.8 | 7.9 (±4.2) | 2019 |
| Nadia’s Reef | RI0007 | Patch | 14 | 4.0–6.1 | 4.9 | 25.7 (±22.3) | 2012; 2019 |
| Alex’s Reef | RI0029 | Patch | 16 | 4.0–4.6 | 4.3 | 21.5 (±14.7) | 2012; 2019 |
| Cannonball Reef | RI1001 | Patch | 38 | 1.2–6.1 | 4.3 | 26.6 (±25.9) | 2011; 2012; 2015; 2016; 2019 |
| Fan City | RI1002 | Fore | 29 | 7.9–9.4 | 8.6 | 19.6 (±13.7) | 2011; 2016; 2019 |
| Lindy’s Reef | RI1003 | Fore | 32 | 3.1–5.3 | 4.2 | 37.3 (±19.1) | 2011; 2015; 2016; 2019 |
| Alpha Reef | RI1004 | Fore | 21 | 4.8–11.0 | 7.5 | 30.2 (±14.2) | 2011; 2019 |
| Athol Island | RI1005 | Patch | 16 | 2.7–3.7 | 3.2 | 33.8 (±26.7) | 2012; 2019 |
| Shelley’s Reef | RI1006 | Fore | 9 | 4.4–6.1 | 5.3 | 21.7 (±20.2) | 2015; 2019 |
| TOTAL | 483 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherman, K.D.; Gomez, M.I.; Kemenes, T.; Dahlgren, C.P. Spatial and Temporal Variability in Parrotfish Assemblages on Bahamian Coral Reefs. Diversity 2022, 14, 625. https://doi.org/10.3390/d14080625
Sherman KD, Gomez MI, Kemenes T, Dahlgren CP. Spatial and Temporal Variability in Parrotfish Assemblages on Bahamian Coral Reefs. Diversity. 2022; 14(8):625. https://doi.org/10.3390/d14080625
Chicago/Turabian StyleSherman, Krista D., Maya I. Gomez, Thomas Kemenes, and Craig P. Dahlgren. 2022. "Spatial and Temporal Variability in Parrotfish Assemblages on Bahamian Coral Reefs" Diversity 14, no. 8: 625. https://doi.org/10.3390/d14080625
APA StyleSherman, K. D., Gomez, M. I., Kemenes, T., & Dahlgren, C. P. (2022). Spatial and Temporal Variability in Parrotfish Assemblages on Bahamian Coral Reefs. Diversity, 14(8), 625. https://doi.org/10.3390/d14080625






