Evidence for Genetic Hybridization between Released and Wild Game Birds: Phylogeography and Genetic Structure of Chukar Partridge, Alectoris chukar, in Turkey
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
2.2. DNA Amplification and Sequencing
2.3. Phylogeographic Analysis
3. Results
3.1. Mitochondrial Nucleotide Sequences
3.2. Microsatellite Analysis
4. Discussion
4.1. Population Genetic Structure
4.2. Heterozygosity and Inbreeding
4.3. Taxonomic and Conservation Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burri, R. Linked selection, demography and the evolution of correlated genomic landscapes in birds and beyond. Mol. Ecol. 2017, 26, 3853–3856. [Google Scholar] [CrossRef]
- Nittinger, F.; Gamauf, A.; Pinsker, W.; Wink, M.; Haring, E. Phylogeography and population structure of the saker falcon (Falco cherrug) and the influence of hybridization: Mitochondrial and microsatellite data. Mol. Ecol. 2007, 16, 1497–1517. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, T.; Gonzalez, J.; Drovetski, S.V.; Wink, M. Phylogeography and population structure of Kruper’s Nuthatch Sitta krueperi from Turkey based on microsatellites and mitochondrial DNA. J. Ornithol. 2012, 153, 405–411. [Google Scholar] [CrossRef]
- Ibiş, O.; Tez, C.; Özcan, S. Phylogenetic status of the turkish red fox (Vulpes vulpes), based on partial sequences of the mitochondrial cytochrome b gene. Vertebr. Zool. 2014, 64, 273–284. [Google Scholar]
- Schrey, A.W.; Grispo, M.; Awad, M.; Cook, M.B.; McCoy, E.D.; Mushinsky, H.R.; Albayrak, T.; Bensch, S.; Burke, T.; Butler, L.K.; et al. Broad-scale latitudinal patterns of genetic diversity among native European and introduced house sparrow (Passer domesticus) populations. Mol. Ecol. 2011, 20, 1133–1143. [Google Scholar] [CrossRef]
- Perktaş, U.; Gür, H.; Ada, E. Historical demography of the Eurasian green woodpecker: Integrating phylogeography and ecological niche modelling to test glacial refugia hypothesis. Folia Zool. 2015, 64, 284–295. [Google Scholar] [CrossRef]
- Dufresnes, C.; Strachinis, I.; Suriadna, N.; Mykytynets, G.; Cogălniceanu, D.; Székely, P.; Vukov, T.; Arntzen, J.W.; Wielstra, B.; Lymberakis, P.; et al. Phylogeography of a cryptic speciation continuum in Eurasian spadefoot toads (Pelobates). Mol. Ecol. 2019, 28, 3257–3270. [Google Scholar] [CrossRef]
- Taylan, M.S.; Şirin, D. Speciation of the genus Dolichopoda in Anatolia with reference to the role of ancient central lake system. Insect Syst. Evol. 2016, 47, 267–283. [Google Scholar] [CrossRef]
- Winkler, D.W.; Billerman, S.M.; Lovette, I.J. Pheasants, Grouse, and Allies (Phasianidae), version 1.0. In Birds of the World; Billerman, S.M., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Barilani, M.; Bernard-laurent, A.; Mucci, N.; Tabarroni, C.; Perez, A.; Randi, E.; Kark, S.; Bo, O.E. Hybridisation with introduced chukars (Alectoris chukar) threatens the gene pool integrity of native rock (A. graeca) and red-legged (A. rufa) partridge populations. Biol. Conserv. 2007, 137, 57–69. [Google Scholar] [CrossRef]
- Madge, S.; McGowan, P. Pheasants, Partridges, and Grouse: A Guide to the Pheasants, Partridges, Quails, Grouse, Guineafowl, Buttonquails, and Sandgrouse of the World; Bloomsbury Publishing: London, UK, 2002. [Google Scholar]
- Christensen, G.C. Chukar (Alectoris chukar), version 1.0. In Birds of the World; Poole, A.F., Gill, F.B., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Barbanera, F.; Guerrini, M.; Khan, A.A.; Panayides, P.; Hadjigerou, P.; Sokos, C.; Gombobaatar, S.; Samadi, S.; Khan, B.Y.; Tofanelli, S.; et al. Human-mediated introgression of exotic chukar (Alectoris chukar, Galliformes) genes from East Asia into native Mediterranean partridges. Biol. Invasions 2009, 11, 333–348. [Google Scholar] [CrossRef]
- Martínez-Fresno, M.; Henriques-Gil, N.; Arana, P. Mitochondrial DNA sequence variability in red-legged partridge, Alectoris rufa, Spanish populations and the origins of genetic contamination from A. chukar. Conserv. Genet. 2008, 9, 1223–1231. [Google Scholar] [CrossRef]
- Moulton, M.P.; Cropper, W.P.J.; Broz, A.J. Inconsistencies among secondary sources of Chukar Partridge (Alectoris chukar) introductions to the United States. PeerJ 2015, 3, e1447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Woodard, A.E. Raising Chukar Partridges; Cooperative Extension; University of California: La Jolla, CA, USA, 1982.
- Tejedor, M.T.; Monteagudo, L.V.; Mautner, S.; Hadjisterkotis, E.; Arruga, M.V. Introgression of Alectoris chukar genes into a Spanish wild Alectoris rufa population. J. Hered. 2007, 98, 179–182. [Google Scholar] [CrossRef]
- Blanco-Aguiar, J.A.; González-Jara, P.; Ferrero, M.E.; Sánchez-Barbudo, I.; Virgós, E.; Villafuerte, R.; Dávila, J.A. Assessment of game restocking contributions to anthropogenic hybridization: The case of the Iberian red-legged partridge. Anim. Conserv. 2008, 11, 535–545. [Google Scholar] [CrossRef]
- Negri, A.; Pellegrino, I.; Mucci, N.; Randi, E.; Tizzani, P.; Meneguz, P.G.; Malacarne, G. Mitochondrial DNA and microsatellite markers evidence a different pattern of hybridization in red-legged partridge (Alectoris rufa) populations from NW Italy. Eur. J. Wildl. Res. 2013, 59, 407–419. [Google Scholar] [CrossRef]
- Barilani, M.; Sfougaris, A.; Giannakopoulos, A.; Mucci, N.; Tabarroni, C.; Randi, E. Detecting introgressive hybridisation in rock partridge populations (Alectoris graeca) in Greece through Bayesian admixture analyses of multilocus genotypes. Conserv. Genet. 2007, 8, 343–354. [Google Scholar] [CrossRef]
- Barilani, M.; Deregnaucourt, S.; Gallego, S.; Galli, L.; Mucci, N.; Piombo, R.; Puigcerver, M.; Rimondi, S.; Rodríguez-Teijeiro, J.D.; Spanò, S.; et al. Detecting hybridization in wild (Coturnix c. coturnix) and domesticated (Coturnix c. japonica) quail populations. Biol. Conserv. 2005, 126, 445–455. [Google Scholar] [CrossRef]
- Söderquist, P.; Elmberg, J.; Gunnarsson, G.; Thulin, C.G.; Champagnon, J.; Guillemain, M.; Kreisinger, J.; Prins, H.H.T.; Crooijmans, R.P.M.A.; Kraus, R.H.S. Admixture between released and wild game birds: A changing genetic landscape in European mallards (Anas platyrhynchos). Eur. J. Wildl. Res. 2017, 63, 98. [Google Scholar] [CrossRef]
- Casas, F.; Mougeot, F.; Sánchez-Barbudo, I.; Dávila, J.A.; Viñuela, J. Fitness consequences of anthropogenic hybridization in wild red-legged partridge (Alectoris rufa, Phasianidae) populations. Biol. Invasions 2012, 14, 295–305. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, M.E.; González-Jara, P.; Blanco-Aguiar, J.A.; Sánchez-Barbudo, I.; Dávila, J.A. Sixteen new polymorphic microsatellite markers isolated for red-legged partridge (Alectoris rufa) and related species. Mol. Ecol. Notes 2007, 7, 1349–1351. [Google Scholar] [CrossRef]
- Ferrero, M.E.; Blanco-Aguiar, J.A.; Lougheed, S.C.; Sánchez-Barbudo, I.; De Nova, P.J.G.; Villafuerte, R.; Dávila, J.A. Phylogeography and genetic structure of the red-legged partridge (Alectoris rufa): More evidence for refugia within the Iberian glacial refugium. Mol. Ecol. 2011, 20, 2628–2642. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Dupanloup, I.; Schneider, S.; Excoffier, L. A simulated annealing approach to define the genetic structure of populations. Mol. Ecol. 2002, 11, 2571–2581. [Google Scholar] [CrossRef] [PubMed]
- Bandelt, H.-J.; Forster, P.; Rohl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Rousset, F. Genepop’007: A complete re-implementation of the GENEPOP software for Windows and Linux. Mol. Ecol. Res. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P.J. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Forcina, G.; Guerrini, M.; Khaliq, I.; Khan, A.A.; Barbanera, F. Human-modified biogeographic patterns and conservation in game birds: The dilemma of the black francolin (Francolinus francolinus, phasianidae) in Pakistan. PLoS ONE 2018, 13, e0205059. [Google Scholar] [CrossRef]
- Albayrak, T.; Aytek, A.İ. Bill Variation of Captive and Wild Chukar Partridge Populations: Shape or Size. Diversity 2022, 14, 48. [Google Scholar] [CrossRef]
- Barbanera, F.; Forcina, G.; Cappello, A.; Guerrini, M.; van Grouw, H.; Aebischer, N.J. Introductions over introductions: The genomic adulteration of an early genetically valuable alien species in the United Kingdom. Biol. Invasions 2015, 17, 409–422. [Google Scholar] [CrossRef]
- Forcina, G.; Guerrini, M.; Barbanera, F. Non-native and hybrid in a changing environment: Conservation perspectives for the last Italian red-legged partridge (Alectoris rufa) population with long natural history. Zoology 2020, 138, 125740. [Google Scholar] [CrossRef]
- Forcina, G.; Tang, Q.; Cros, E.; Guerrini, M.; Rheindt, F.E.; Barbanera, F. Genome-wide markers redeem the lost identity of a heavily managed gamebird. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210285. [Google Scholar] [CrossRef]
- Baratti, M.; Ammannati, M.; Magnelli, C.; Dessì-Fulgheri, F. Introgression of chukar genes into a reintroduced red-legged partridge (Alectoris rufa) population in central Italy. Anim. Genet. 2005, 36, 29–35. [Google Scholar] [CrossRef]
- Barbanera, F. Analysis of the genetic structure of red-legged partridge ( Alectoris rufa, Galliformes ) populations by means of mitochondrial DNA and RAPD markers: A study from central Italy. Biol. Conserv. 2005, 122, 275–287. [Google Scholar] [CrossRef]
- Barbanera, F.; Pergams, O.R.W.W.; Guerrini, M.; Forcina, G.; Panayides, P.; Dini, F. Genetic consequences of intensive management in game birds. Biol. Conserv. 2010, 143, 1259–1268. [Google Scholar] [CrossRef]
- Barbanera, F.; Marchi, C.; Guerrini, M.; Panayides, P.; Sokos, C.; Hadjigerou, P. Genetic structure of mediterranean chukar (Alectoris chukar, Galliformes) populations: Conservation and management implications. Naturwissenschaften 2009, 96, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Panayides, P.; Guerrini, M.; Barbanera, F. Conservation genetics and management of the Chukar Partridge (Alectoris chukar) in Cyprus and the Middle East. Sandgrouse 2011, 33, 34–43. [Google Scholar]
- Casas, F.; Mougeot, F.; Ferrero, M.E.; Sánchez-Barbudo, I.; Dávila, J.A.; Viñuela, J. Phenotypic differences in body size, body condition and circulating carotenoids between hybrid and ‘“pure”’ red-legged partridges (Alectoris rufa) in the wild. J. Ornithol. 2013, 154, 803–811. [Google Scholar] [CrossRef]
- Perktaş, U.; Gür, H.; Saʇlam, I.K.; Quintero, E. Climate-driven range shifts and demographic events over the history of Kruper’s Nuthatch Sitta krueperi. Bird Study 2015, 62, 14–28. [Google Scholar] [CrossRef]
- Şenkul, Ç.; Doǧan, U. Vegetation and climate of Anatolia and adjacent regions during the Last Glacial period. Quat. Int. 2013, 302, 110–122. [Google Scholar] [CrossRef]
- Gür, H. The effects of the Late Quaternary glacial-interglacial cycles on Anatolian ground squirrels: Range expansion during the glacial periods? Biol. J. Linn. Soc. 2013, 109, 19–32. [Google Scholar] [CrossRef]
- Özüdoğru, B.; Özgişi, K.; Perktaş, U.; Gür, H. The Quaternary range dynamics of Noccaea iberidea (Brassicaceae), a typical representative of subalpine/alpine steppe communities of Anatolian mountains. Biol. J. Linn. Soc. 2020, 131, 986–1001. [Google Scholar] [CrossRef]
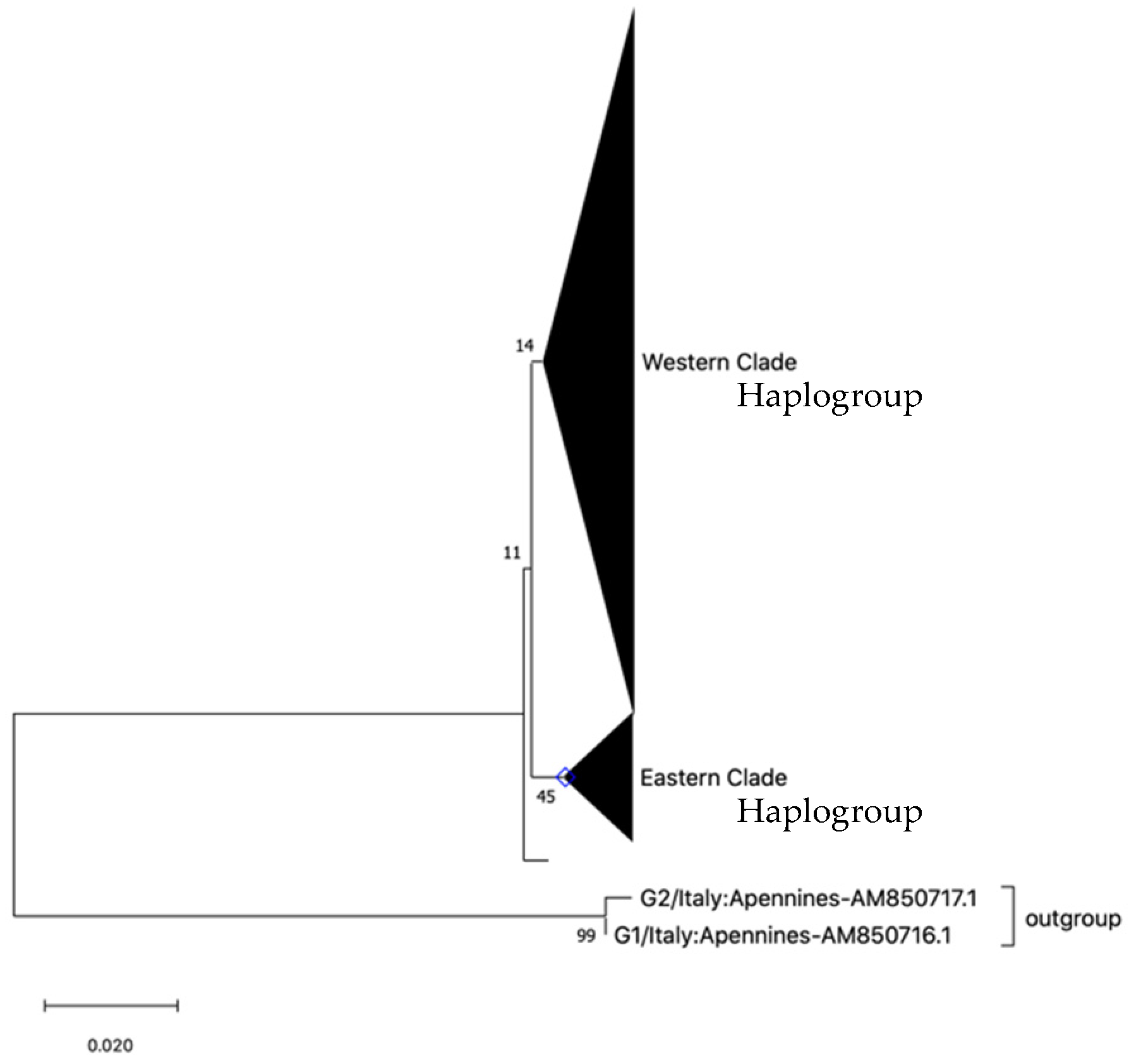
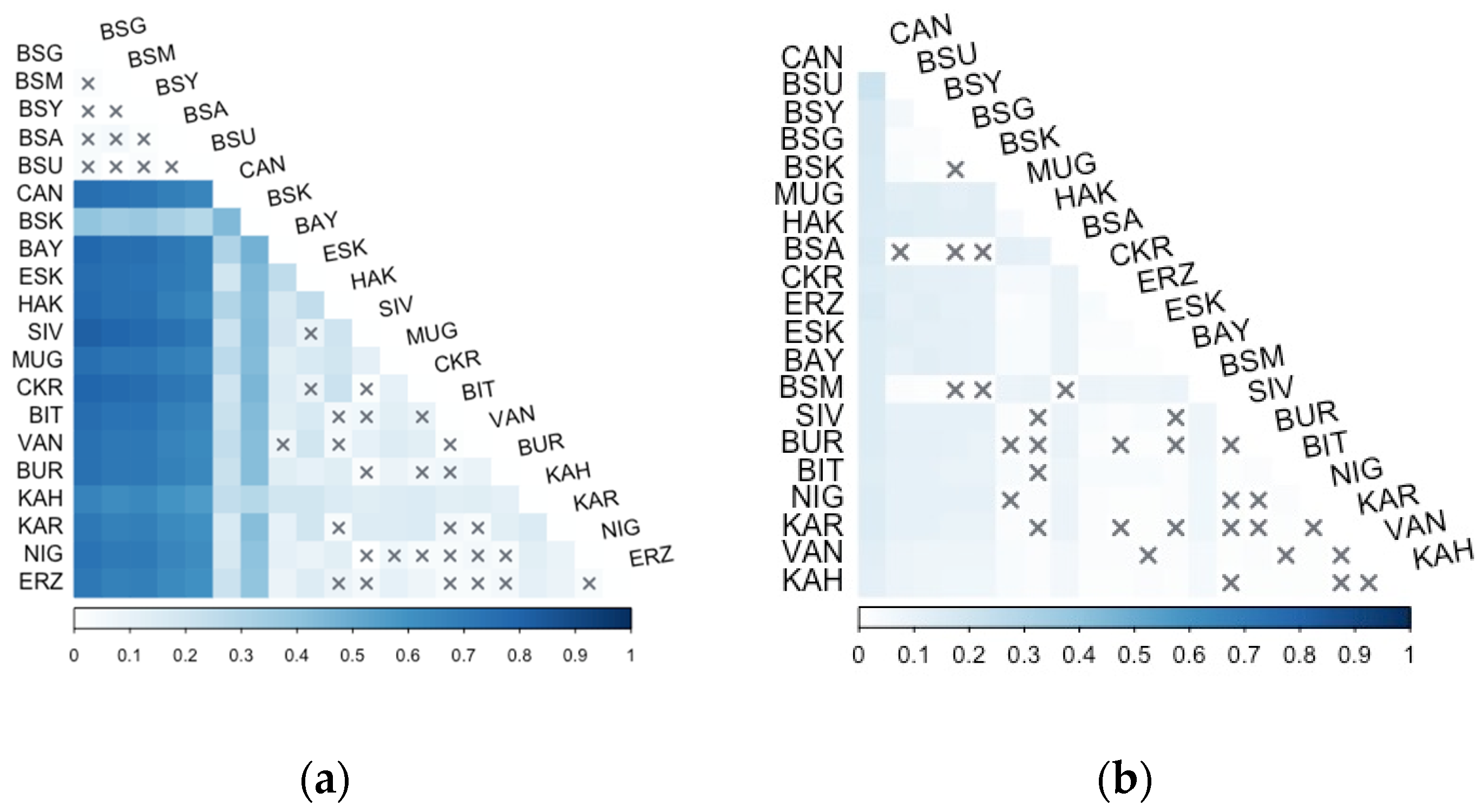



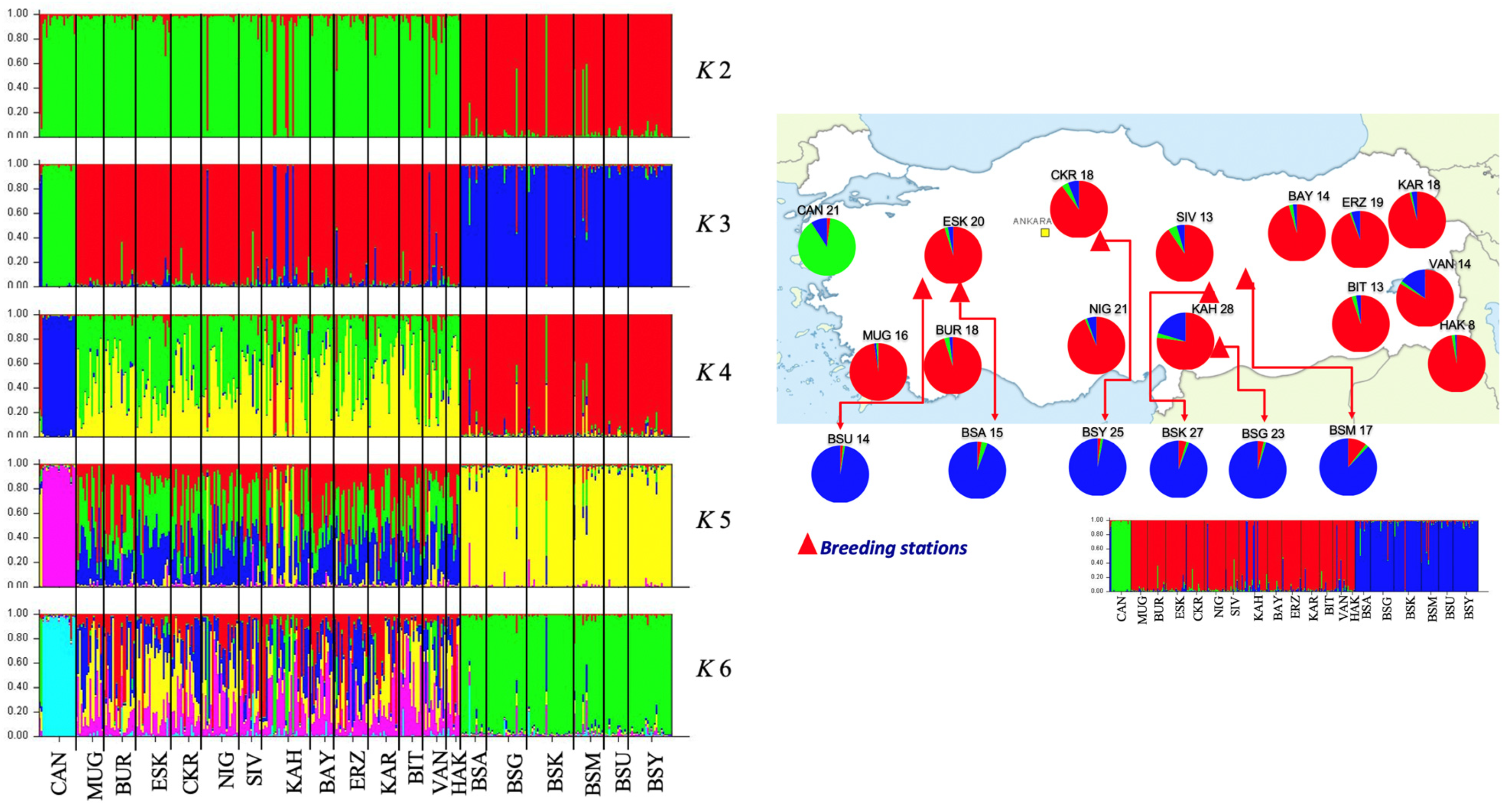
| No. | Source | Locality | Mt DNA (n) | Microsatellites (n) | Reference |
|---|---|---|---|---|---|
| 1 | Wild population | Çanakkale (CAN) | 24 | 21 | This study |
| 2 | Muğla (MUG) | 19 | 16 | This study | |
| 3 | Burdur (BUR) | 19 | 18 | This study | |
| 4 | Eskişehir (ESK) | 21 | 20 | This study | |
| 5 | Çankırı (CKR) | 18 | 18 | This study | |
| 6 | Niğde (NIG) | 22 | 21 | This study | |
| 7 | Sivas (SIV) | 20 | 13 | This study | |
| 8 | Kayramanmaraş (KAH) | 29 | 28 | This study | |
| 9 | Bayburt (BAY) | 14 | 14 | This study | |
| 10 | Erzurum (ERZ) | 19 | 19 | This study | |
| 11 | Kars (KAR) | 18 | 18 | This study | |
| 12 | Bitlis BIT) | 13 | 13 | This study | |
| 13 | Van (VAN) | 14 | 14 | This study | |
| 14 | Hakkari (HAK) | 8 | 8 | This study | |
| BS1 | Breeding station | Afyon (BSA) | 19 | 15 | This study |
| BS2 | Gaziantep (BSG) | 24 | 23 | This study | |
| BS3 | Kahramanmaraş (BSK) | 28 | 27 | This study | |
| BS4 | Malatya (BSM) | 18 | 17 | This study | |
| BS5 | Uşak (BSU) | 14 | 14 | This study | |
| BS6 | Yozgat (BSY) | 26 | 25 | This study | |
| GenBank | 12 Countries | 86 | - | Barbanera et al. [13] | |
| Alectoris greaca | 2 | - | Barbanera et al. [13] | ||
| Total | 475 | 362 |
| SAMOVA Groups | Population | N | eN (%) | S | Haplotypes | Hd | π (×10−3) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| h | eh (%) | uh | ||||||||
| 1 | CAN | 22 | 1 (4.5) | 25 | 13 | 1 (7.7) | 8 | 0.91 ± 0.05 | 1.63 ± 0.46 | |
| 2 | KAH | 29 | 3 (10.3) | 36 | 28 | 3 (10.7) | 21 | 0.99 ± 0.02 | 2.98 ± 0.30 | |
| 3 | BAY | 14 | no | 23 | 12 | no | 8 | 0.98 ± 0.04 | 1.91 ± 0.31 | |
| 4 | ESK | 20 | no | 10 | 8 | no | 4 | 0.87 ± 0.05 | 1.69 ± 0.13 | |
| 5 | Center | BUR | 18 | no | 28 | 15 | no | 11 | 0.98 ± 0.02 | 2.45 ± 0.36 |
| 5 | MUG | 18 | no | 22 | 15 | no | 13 | 0.98 ± 0.02 | 2.04 ± 0.28 | |
| 5 | CKR | 16 | no | 16 | 13 | no | 7 | 0.98 ± 0.03 | 1.62 ± 0.24 | |
| 5 | NIG | 19 | 1 (5.3) | 28 | 16 | 1 (6.3) | 9 | 0.98 ± 0.02 | 2.07 ± 0.43 | |
| 5 | SIV | 12 | no | 11 | 10 | no | 3 | 0.97 ± 0.04 | 1.25 ± 0.19 | |
| 5 | ERZ | 19 | no | 34 | 15 | no | 11 | 0.97 ± 0.02 | 2.84 ± 0.23 | |
| 5-total center | 102 | 1 (1) | 74 | 72 | 1 (1.4) | 54 | 0.99 ± 0.00 | 2.17 ± 0.15 | ||
| 6 | East | KAR | 18 | no | 23 | 16 | no | 10 | 0.99 ± 0.02 | 2.69 ± 0.24 |
| 6 | BIT | 12 | no | 21 | 10 | no | 7 | 0.97 ± 0.04 | 2.32 ± 0.29 | |
| 6 | VAN | 13 | no | 20 | 10 | no | 5 | 0.95 ± 0.05 | 2.47 ± 0.37 | |
| 6 | HAK | 8 | no | 12 | 7 | no | 1 | 0.96 ± 0.08 | 2.35 ± 0.25 | |
| 6-total east | 51 | no | 34 | 35 | no | 23 | 0.98 ± 0.01 | 2.49 ± 0.13 | ||
| Total wild | 238 | 5 (2.1) | 103 | 146 | 5 (3.4) | 139 | 0.99 ± 0.00 | 2.47 ± 0.10 | ||
| 1 * | BSA | 14 | 12 (85.7) | 14 | 4 | 3 (75.0) | no | 0.71 ± 0.10 | 1.77 ± 0.75 | |
| 2 * | BSG | 22 | 21 (95.5) | 18 | 5 | 4 (80.0) | no | 0.52 ± 0.11 | 1.02 ± 0.56 | |
| 3 * | BSK | 26 | 17 (65.4) | 22 | 18 | 12 (66.7) | 4 | 0.97 ± 0.02 | 3.26 ± 0.32 | |
| 4 * | BSM | 15 | 14 (93.3) | 11 | 6 | 5 (83.3) | 1 | 0.76 ± 0.08 | 1.08 ± 0.35 | |
| 4 * | BSU | 14 | 12 (85.7) | 20 | 5 | 3 (60.0) | no | 0.72 ± 0.09 | 1.97 ± 0.85 | |
| 4 * | BSY | 25 | 23 (92.0) | 16 | 8 | 6 (75.0) | no | 0.66 ± 0.09 | 1.29 ± 0.51 | |
| Total captive | 116 | 99 (85.3) | 26 | 30 | 20 (66.7) | 7 | 0.79 ± 0.03 | 2.23 ± 0.27 | ||
| TOTAL | 354 | 104 (29.4) | 105 | 169 | 21 (12.4) | 148 | 0.97 ± 0.00 | 3.81 ± 0.08 | ||
| Mt DNA | Microsatellite | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | 5 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 4 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| FCT | 0.56* | 0.10* | 0.10* | 0.10* | 0.11* | 0.11* | 0.12* | 0.12* | 0.12* | 0.11* | 0.08* | 0.07* | 0.06* | 0.05* | 0.05* | 0.05* | |
| Group composition | Group composition | ||||||||||||||||
| Wild populations | BUR | 1 | 3 | 4 | 5 | 6 | 7 | 7 | 7 | 4 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| CAN | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| MUG | 1 | 3 | 4 | 5 | 6 | 5 | 5 | 5 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| ESK | 1 | 3 | 4 | 5 | 4 | 4 | 4 | 4 | 4 | 3 | 4 | 5 | 6 | 7 | 8 | 2 | |
| CKR | 1 | 3 | 4 | 5 | 6 | 7 | 7 | 7 | 4 | 3 | 4 | 5 | 6 | 6 | 6 | 6 | |
| SIV | 1 | 3 | 4 | 5 | 6 | 7 | 7 | 7 | 4 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| NIG | 1 | 3 | 4 | 5 | 6 | 7 | 7 | 7 | 4 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| KAH | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 3 | 4 | 5 | 6 | 7 | 8 | 8 | |
| BAY | 1 | 3 | 4 | 3 | 3 | 3 | 3 | 3 | 4 | 3 | 4 | 5 | 6 | 7 | 5 | 5 | |
| ERZ | 1 | 3 | 4 | 5 | 6 | 7 | 6 | 6 | 4 | 3 | 4 | 5 | 6 | 7 | 7 | 7 | |
| KAR | 1 | 3 | 3 | 4 | 5 | 6 | 8 | 9 | 4 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| BIT | 1 | 3 | 4 | 4 | 5 | 6 | 6 | 8 | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | |
| VAN | 1 | 3 | 4 | 4 | 5 | 6 | 6 | 8 | 4 | 3 | 4 | 5 | 5 | 5 | 8 | 4 | |
| HAK | 1 | 3 | 3 | 4 | 5 | 6 | 8 | 9 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | 9 | |
| Breeding stations | BSA | 2 | 2 | ||||||||||||||
| BSG | 3 | 2 | |||||||||||||||
| BSK | 4 | 2 | |||||||||||||||
| BSU | 5 | 3 | |||||||||||||||
| BSY | 5 | 2 | |||||||||||||||
| BSM | 5 | 2 | |||||||||||||||
| Location | N | A | Ho | He | p | FIS |
|---|---|---|---|---|---|---|
| CAN | 21 | 7.5 ± 3.7 | 0.63 ± 0.23 | 0.69 ± 0.22 | 0.0002 | 0.0991 |
| MUG | 16 | 9.0 ± 3.7 | 0.62 ± 0.13 | 0.82 ± 0.11 | 0.0000 | 0.2523 |
| BUR | 18 | 10.4 ± 4.1 | 0.66 ± 0.18 | 0.86 ± 0.06 | 0.0000 | 0.2325 |
| ESK | 20 | 10.0 ± 3.4 | 0.74 ± 0.14 | 0.84 ± 0.06 | 0.0000 | 0.1286 |
| CKR | 18 | 10.1 ± 4.9 | 0.69 ± 0.18 | 0.81 ± 0.15 | 0.0000 | 0.1404 |
| SIV | 13 | 9.7 ± 3.5 | 0.74 ± 0.16 | 0.86 ± 0.08 | 0.0000 | 0.1482 |
| NIG | 21 | 11.4 ± 4.4 | 0.74 ± 0.13 | 0.85 ± 0.09 | 0.0000 | 0.1344 |
| KAH | 28 | 13.2 ± 5.7 | 0.71 ± 0.12 | 0.86 ± 0.08 | 0.0000 | 0.1749 |
| BAY | 14 | 9.5 ± 3.4 | 0.76 ± 0.18 | 0.84 ± 0.06 | 0.0000 | 0.1125 |
| ERZ | 19 | 10.2 ± 3.6 | 0.79 ± 0.09 | 0.84 ± 0.08 | 0.0155 | 0.0506 |
| KAR | 18 | 11.3 ± 4.4 | 0.78 ± 0.15 | 0.87 ± 0.06 | 0.0000 | 0.0995 |
| BIT | 13 | 10.1 ± 3.2 | 0.79 ± 0.17 | 0.86 ± 0.08 | 0.0000 | 0.0790 |
| VAN | 14 | 9.3 ± 3.5 | 0.77 ± 0.18 | 0.86 ± 0.07 | 0.0000 | 0.1080 |
| HAK | 8 | 6.6 ± 2.4 | 0.71 ± 0.26 | 0.84 ± 0.08 | 0.0004 | 0.1644 |
| BSA | 15 | 7.0 ± 2.8 | 0.72 ± 0.14 | 0.75 ± 0.15 | 0.1004 | 0.0473 |
| BSG | 23 | 8.2 ± 3.3 | 0.73 ± 0.15 | 0.73 ± 0.18 | 0.1194 | −0.0062 |
| BSK | 27 | 7.8 ± 3.3 | 0.66 ± 0.18 | 0.74 ± 0.16 | 0.0000 | 0.0974 |
| BSY | 25 | 8.3 ± 3.8 | 0.70 ± 0.17 | 0.72 ± 0.18 | 0.0152 | 0.0368 |
| BSM | 17 | 8.2 ± 3.1 | 0.71 ± 0.17 | 0.79 ± 0.12 | 0.0000 | 0.1109 |
| BSU | 14 | 6.9 ± 3.6 | 0.64 ± 0.16 | 0.71 ± 0.19 | 0.0329 | 0.0913 |
| Location | Aru1B3 | Aru1E7 | Aru1E97 | Aru1G4 | Aru1G49 | Aru2D020 | Aru1A | Aru1E93 | AruF114 | AruF25 |
|---|---|---|---|---|---|---|---|---|---|---|
| T | 24 (9–16) | 10 (5–10) | 43 (7–25) | 13 (4–9) | 23 (6–15) | 20 (4–13) | 13 (4–9) | 9 (2–9) | 13 (6–10) | 35 (6–20) |
| Np | 12.6/1.9 | 6.4/1.3 | 14.7/4.6 | 6.9/1.3 | 11.5/2.1 | 8.5/2.7 | 7.1/1.4 | 4.4/1.8 | 8.7/1.2 | 11.7/4.0 |
| CAN | -/HWE | -/HWE | -/HWE | -/HWE | -/HD | -/HD | -/HD | -/HWE | -/HWE | -/HWE |
| MUG | -/HD | -/HWE | 1/HWE | -/HD | -/HD | -/HD | -/HWE | -/HWE | -/HD | -/HD |
| BUR | -/HD | -/HD | 1/HD | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HD | -/HWE |
| ESK | -/HD | -/HWE | 2/HWE | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HD | -/HWE |
| CKR | -/HD | -/HWE | -/HWE | -/HWE | -/HWE | -/HWE | -/HWE | -/HWE | -/HD | -/HWE |
| SIV | -/HWE | -/HWE | -/HWE | 1/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HWE | 1/HWE |
| NIG | -/HWE | 1/HD | 2/HWE | -/HWE | -/HD | -/HE | -/HWE | -/HWE | -/HD | -/HWE |
| KAH | -/HD | -/HWE | -/HD | 1/HWE | 1/HD | 1/HD | -/HWE | 2/HWE | -/HD | -/HWE |
| BAY | -/HD | -/HWE | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HWE | -/HWE | -/HWE |
| ERZ | -/HD | -/HWE | -/HWE | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HD | 1/HWE |
| KAR | 1/HWE | -/HWE | -/HWE | 1/HWE | 1/HD | -/HD | -/HWE | -/HWE | -/HWE | -/HWE |
| BIT | -/HWE | -/HWE | -/HWE | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HWE | 2/HWE |
| VAN | -/HD | -/HWE | 1/HWE | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HWE | -/HWE |
| HAK | 1/HD | -/HWE | -/HD | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HD | 2/HWE |
| BSA | -/HWE | -/HWE | -/HWE | -/HWE | -/HWE | -/HWE | -/HWE | -/HWE | -/HD | -/HWE |
| BSG | -/HWE | -/HWE | -/HWE | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HD | -/HWE |
| BSK | -/HWE | -/HWE | -/HWE | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HD | -/HWE |
| BSY | -/HWE | -/HWE | -/HWE | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HD | -/HWE |
| BSM | -/HWE | -/HWE | -/HWE | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HD | -/HWE |
| BSU | -/HWE | -/HWE | 1/HWE | -/HWE | -/HD | -/HWE | -/HWE | -/HWE | -/HD | -/HWE |
| Location | n | Wild (%) | Captive (%) | Main Admixture (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Cluster I | Cluster II | Cluster III | Cluster I and II | Cluster I and III | Cluster II and III | |||
| Wild | CAN | 21 | 0 | 90 | 10 | 0 | 0 | 0 |
| MUG | 16 | 100 | 0 | 0 | 0 | 0 | 0 | |
| BUR | 18 | 94 | 0 | 0 | 6 | 0 | 0 | |
| ESK | 20 | 95 | 0 | 0 | 0 | 5 | 0 | |
| CKR | 18 | 89 | 0 | 6 | 6 | 0 | 0 | |
| NIG | 21 | 95 | 0 | 5 | 0 | 0 | 0 | |
| SIV | 13 | 85 | 0 | 0 | 8 | 8 | 0 | |
| KAH | 28 | 75 | 0 | 18 | 4 | 4 | 0 | |
| BAY | 14 | 100 | 0 | 0 | 0 | 0 | 0 | |
| ERZ | 19 | 95 | 0 | 0 | 0 | 5 | 0 | |
| KAR | 18 | 94 | 0 | 0 | 0 | 6 | 0 | |
| BIT | 13 | 92 | 0 | 0 | 8 | 0 | 0 | |
| VAN | 14 | 86 | 0 | 7 | 0 | 7 | 0 | |
| HAK | 8 | 100 | 0 | 0 | 0 | 0 | 0 | |
| Total | 241 | 83 | 8 | 4 | 2 | 2 | 0 | |
| Breeding stations | BSA | 15 | 0 | 0 | 93 | 0 | 0 | 7 |
| BSG | 23 | 0 | 0 | 96 | 0 | 4 | 0 | |
| BSK | 27 | 4 | 0 | 93 | 0 | 0 | 4 | |
| BSM | 17 | 0 | 0 | 82 | 0 | 18 | 0 | |
| BSU | 14 | 0 | 0 | 100 | 0 | 0 | 0 | |
| BSY | 25 | 0 | 0 | 100 | 0 | 0 | 0 | |
| Total | 121 | 1 | 0 | 94 | 0 | 3 | 2 | |
| Total | 362 | 56 | 5 | 34 | 1 | 3 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albayrak, T.; García, J.A.D.; Özmen, Ö.; Karadas, F.; Ateş, D.; Wink, M. Evidence for Genetic Hybridization between Released and Wild Game Birds: Phylogeography and Genetic Structure of Chukar Partridge, Alectoris chukar, in Turkey. Diversity 2022, 14, 571. https://doi.org/10.3390/d14070571
Albayrak T, García JAD, Özmen Ö, Karadas F, Ateş D, Wink M. Evidence for Genetic Hybridization between Released and Wild Game Birds: Phylogeography and Genetic Structure of Chukar Partridge, Alectoris chukar, in Turkey. Diversity. 2022; 14(7):571. https://doi.org/10.3390/d14070571
Chicago/Turabian StyleAlbayrak, Tamer, José Antonio Dávila García, Özlem Özmen, Filiz Karadas, Duygu Ateş, and Michael Wink. 2022. "Evidence for Genetic Hybridization between Released and Wild Game Birds: Phylogeography and Genetic Structure of Chukar Partridge, Alectoris chukar, in Turkey" Diversity 14, no. 7: 571. https://doi.org/10.3390/d14070571
APA StyleAlbayrak, T., García, J. A. D., Özmen, Ö., Karadas, F., Ateş, D., & Wink, M. (2022). Evidence for Genetic Hybridization between Released and Wild Game Birds: Phylogeography and Genetic Structure of Chukar Partridge, Alectoris chukar, in Turkey. Diversity, 14(7), 571. https://doi.org/10.3390/d14070571







