Synthesis of Brazilian Entomobryomorpha (Collembola: Hexapoda) with Special Emphasis on the Equatorial Oceanic Islands and Redescription of the First Species of Collembola Recorded in Brazil †
Abstract
1. Introduction
2. Materials and Methods
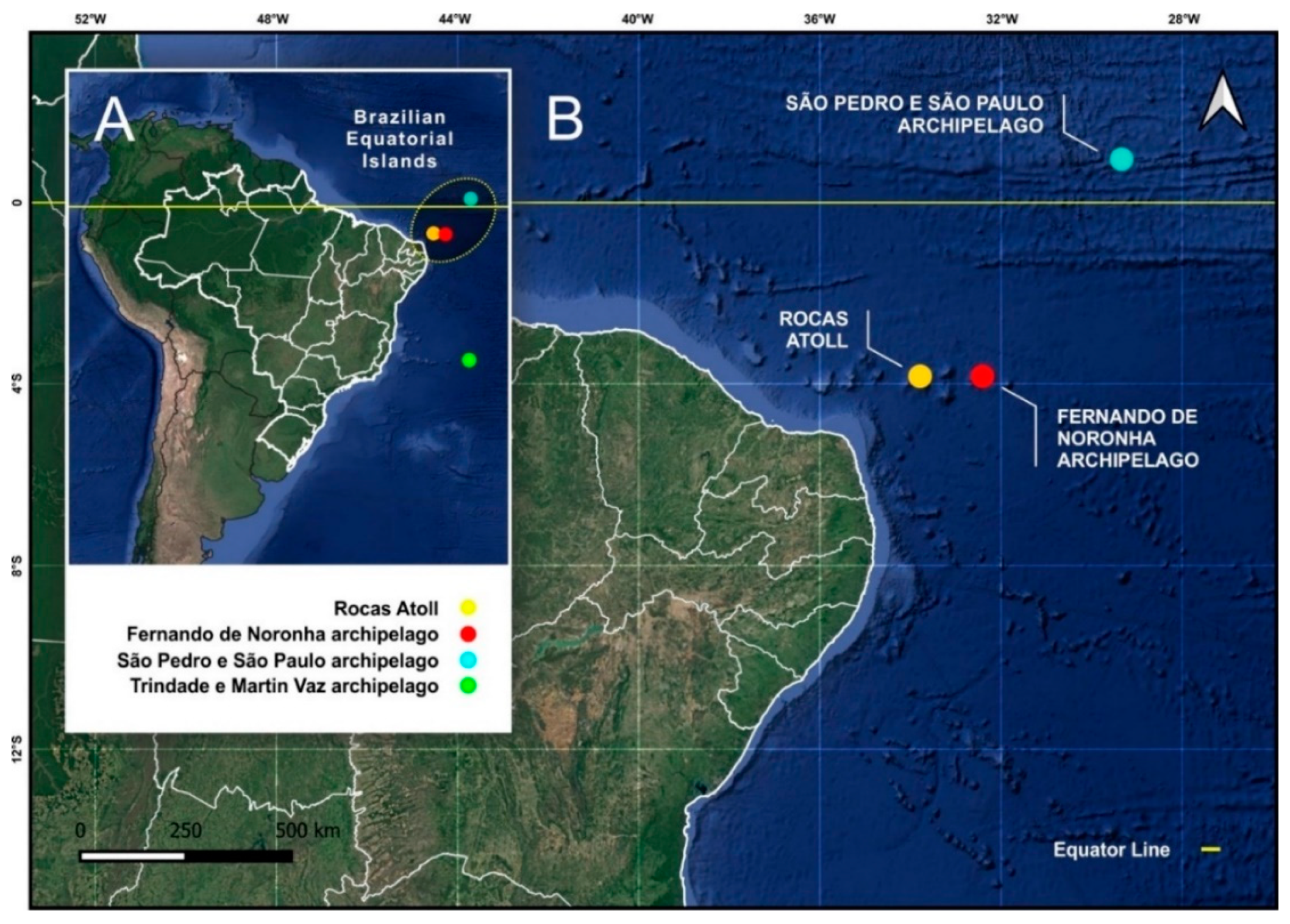
2.1. Study Area
2.2. Taxonomic Nomenclature of Seira Musarum
2.3. Sample Procedure
2.4. Species List of Brazilian Entomobryomorpha
2.5. Abbreviations
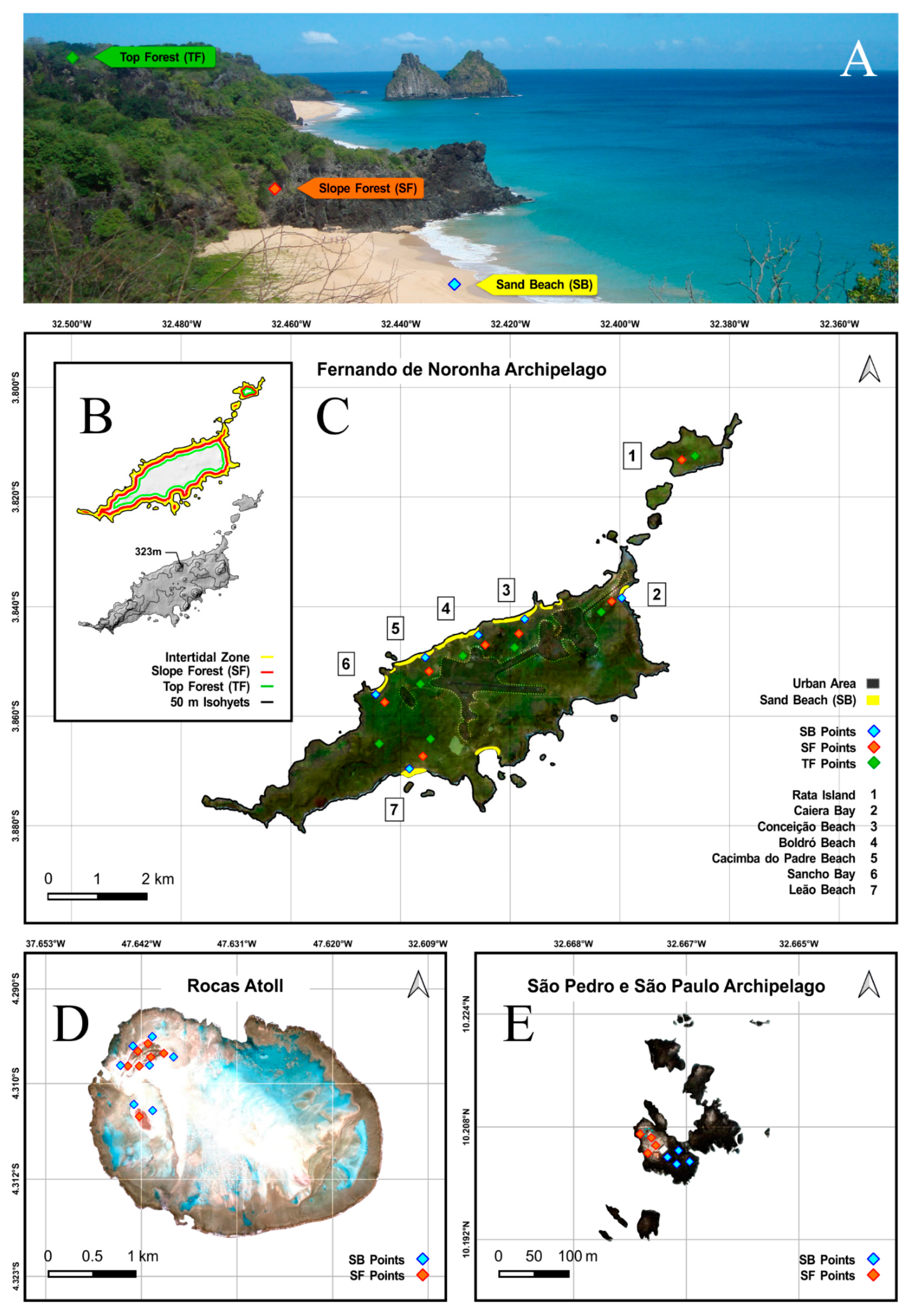
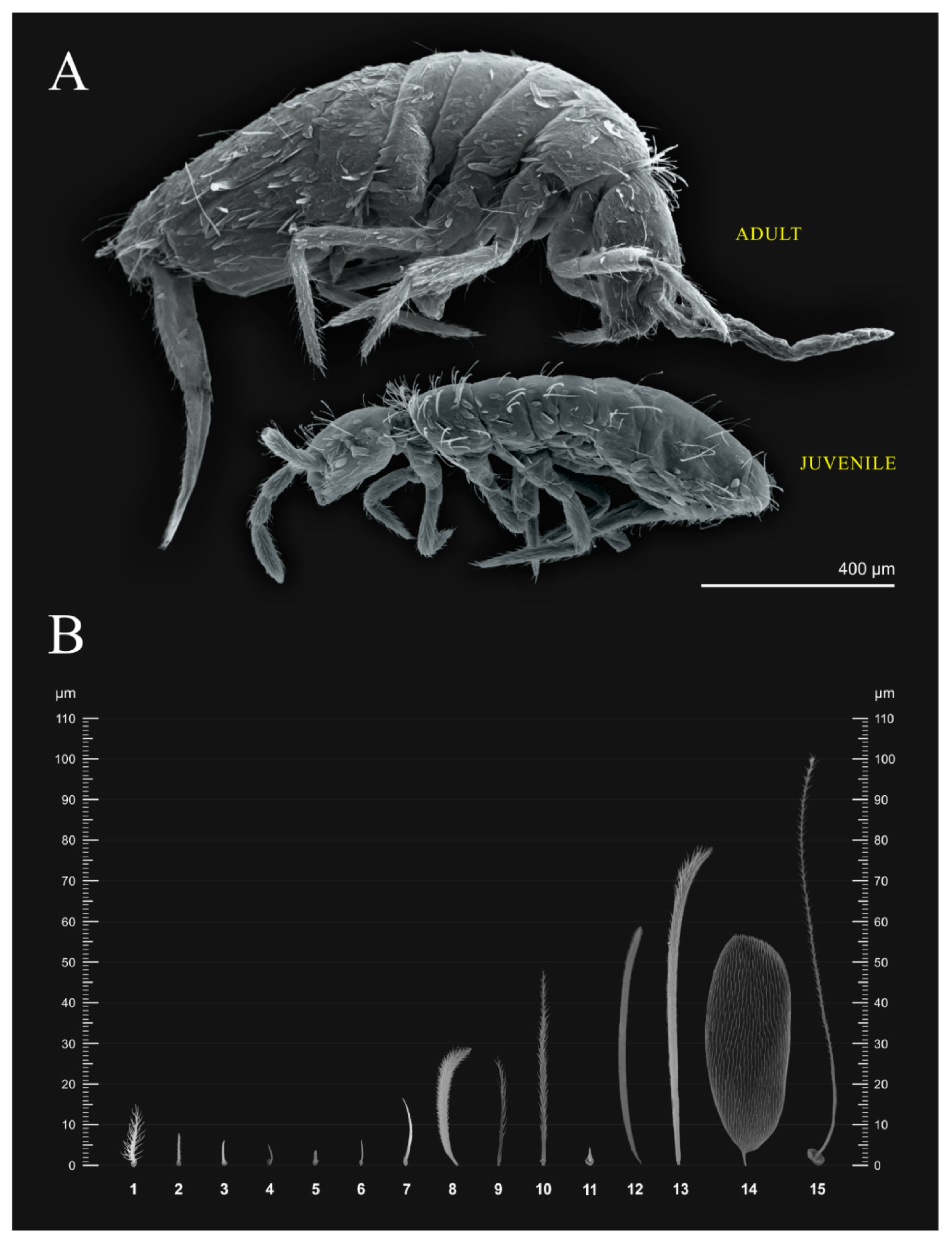
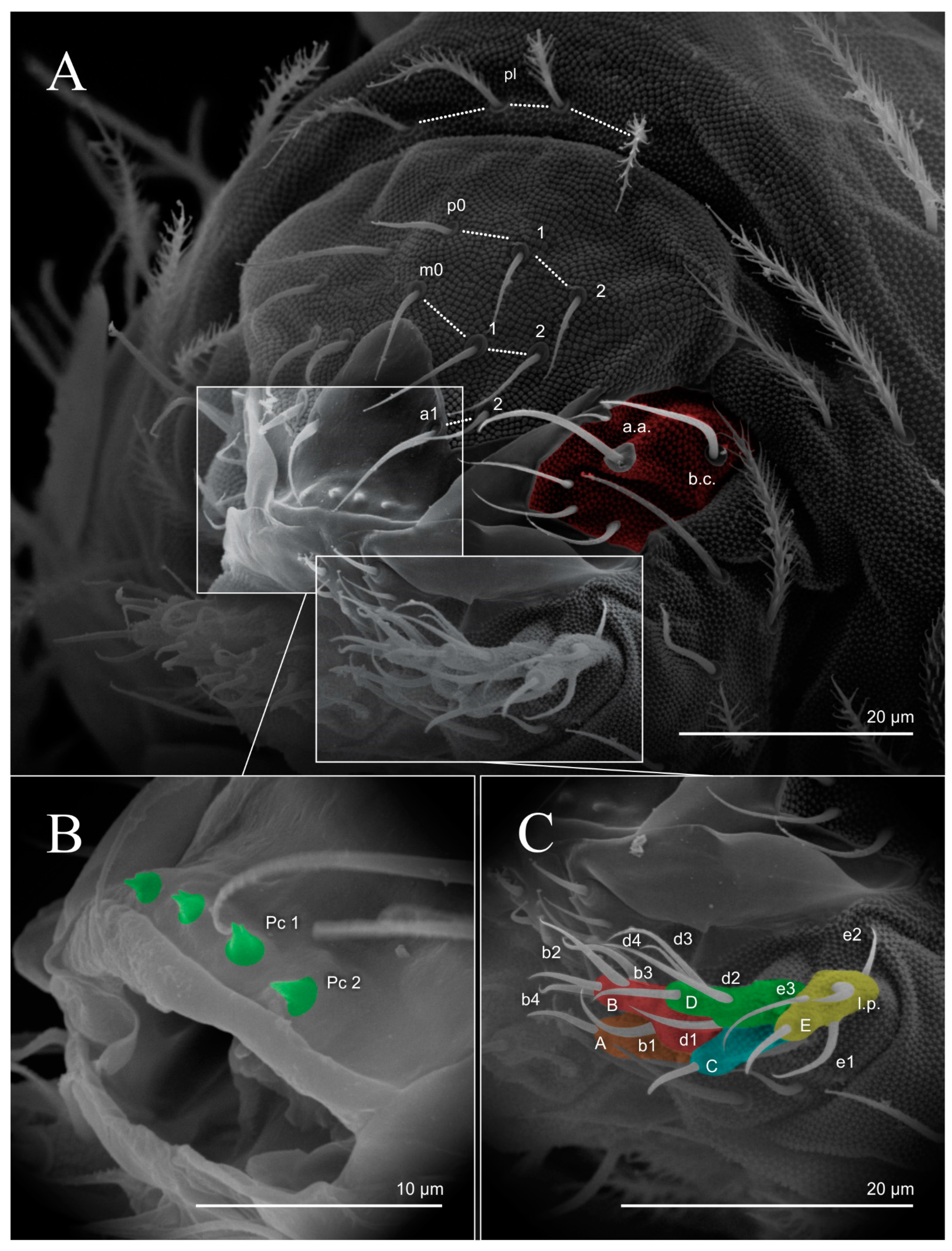
3. Results
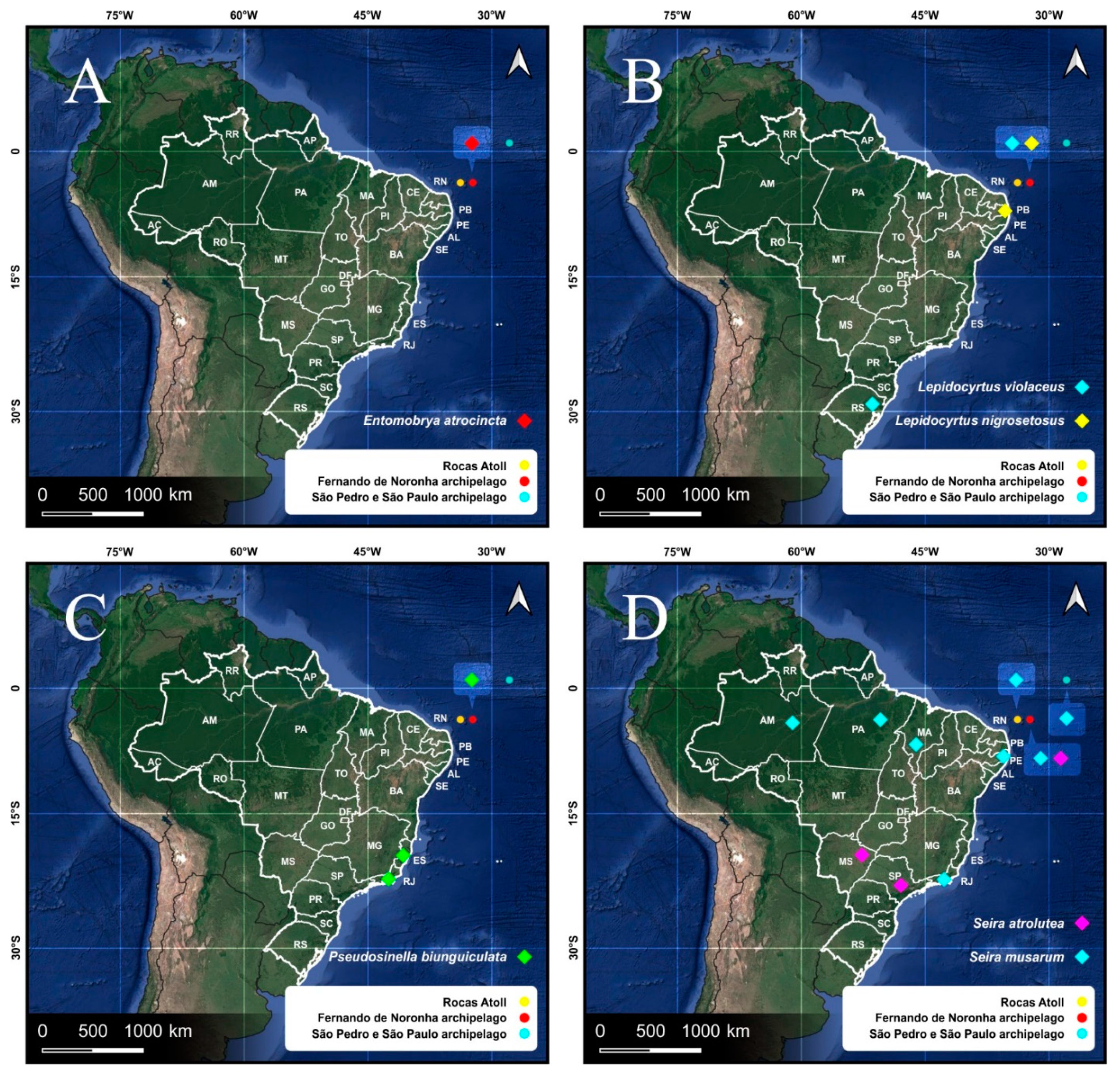
3.1. Entomobryomorpha Survey of the Brazilian Oceanic Islands
3.2. Species List of Brazilian Entomobryomorpha
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schäffer, C. Die Collembolen der Umgebung von Hamburg und benachtbaren Gebiete. Mitt. Dem Nat. Mus. 1896, 13, 147–216. [Google Scholar]
- Womersley, H. On some collembola-arthropleona from South Africa and Southern Rhodesia. Ann. S. Afr. Mus. 1934, 30, 441–475. [Google Scholar]
- Szeptycki, A. Chaetotaxy of the Entomobryidae and its Phylogenetical Significance. Morphosystematic Studies of Collembola IV. Polska Akademia Nauk, Zakład Zoologii Systematycznej i Doświadczalnej; Państwowe Wydawnictwo Naukowe: Warszawa, Kraków, 1979; p. 219. [Google Scholar]
- Soto-Adames, F.N.; Barra, J.A.; Christiansen, K.; Jordana, R. Suprageneric Classification of the entomobryomorpha Collembola. Ann. Entomol. Soc. Am. 2008, 101, 501–513. [Google Scholar] [CrossRef]
- Bellinger, P.F.; Christiansen, K.A.; Janssens, F. Checklist of the Collembola of the World. Available online: http://www.collembola.org (accessed on 7 January 2022).
- Abrantes, E.A.; Bellini, B.C.; Bernardo, A.N.; Fernandes, L.H.; Mendonça, M.C.; Oliveira, E.P.; Queiroz, G.C.; Sautter, K.D.; Silveira, T.C.; Zeppelini, D. Errata Corrigenda and update for the “Synthesis of Brazilian Collembola: An update to the species list”. Zootaxa 2012, 3168, 1–21. [Google Scholar] [CrossRef]
- Ridley, H.N. Notes on the zoology of Fernando Noronha; Thysanura and Collembola. Zool. J. Linn. Soc. 1890, 20, 556–559. [Google Scholar] [CrossRef]
- Lima, E.C.A.; Zeppelini, D. First survey of Collembola (Hexapoda: Entognatha) fauna in soil of Archipelago Fernando de Noronha, Brazil. Flor. Entomol. 2015, 98, 368–369. [Google Scholar] [CrossRef]
- Cipola, N.G.; Godeiro, N.N.; Bellini, B.C. New records of Seira dowlingi (Wray, 1953) (Collembola, Entomobryidae, Seirinae) for New World. Entomol. Commun. 2019, 1, ec01003-1. [Google Scholar] [CrossRef][Green Version]
- Rafael, J.A.; Limeira-de-Oliveira, F.; Hutchings, R.W.; Miranda, G.F.G.; Neto, A.M.S.; Somavilla, A.; Camargo, A.; Asenjo, A.; Pinto, A.P.; Bello, A.M.; et al. Insect (Hexapoda) diversity in the oceanic archipelago of Fernando de Noronha. Brazil: Updated taxonomic checklist and new records. Rev. Bras. Entomol. 2020, 64, e20200052. [Google Scholar] [CrossRef]
- Gisin, H. Espèces nouvelles et lignées évolutives de Pseudosinella endogés. Memórias Estud. Mus. Zoológico Univ. Coimbra 1967, 301, 5–25. [Google Scholar]
- Zhang, F.; Pan, Z.-X. Homology of labial chaetae in Entomobryoidea (Collembola). Zootaxa 2020, 4766, 498–500. [Google Scholar] [CrossRef]
- Fjellberg, A. The labial palp in Collembola. Zool. Anz. 1999, 237, 309–330. [Google Scholar]
- Cipola, N.G.; Morais, J.W.; Bellini, B.C. A new species of Seira (Collembola: Entomobryidae: Seirini) from Northern Brazil, with the addition of new chaetotaxic characters. Zoologia 2014, 31, 489–495. [Google Scholar] [CrossRef][Green Version]
- Mari-Mutt, J.A. A revision of the genus Dicranocentrus Schött (Insecta: Collembola: Entomobryidae). Agric. Exp. Stn. Bull. 1979, 259, 1–79. [Google Scholar]
- Szeptycki, A. Morpho-systematic studies on Collembola. III. Body chaetotaxy in the first instars of several genera of the Entomobryomorpha. Acta Zool. Cracov. 1972, 17, 341–372. [Google Scholar]
- Zhang, F.; Deharveng, L. Systematic revision of Entomobryidae (Collembola) by integrating molecular and new morphological evidence. Zool. Scrip. 2015, 44, 298–311. [Google Scholar] [CrossRef]
- Christiansen, K.; Bellinger, P. A survey of the genus Seira (Hexapoda: Collembola: Entomobryidae) in the Americas. Caribb. J. Sci. 2000, 36, 39–75. [Google Scholar]
- Jacquemart, S. Résultats de la mission Anthropologique Belge au Niger. Collemboles nouveaux du Sahara. Bull. Inst. R. Sci. Nat. Belg. 1974, 50, 1–46. [Google Scholar]
- Culik, M.P.; Zeppelini, D. Diversity and distribution of Collembola (Arthropoda: Hexapoda) of Brazil. Biodivers. Conserv. 2003, 12, 1119–1143. [Google Scholar] [CrossRef]
- Good, R. The Geography of Flowering Plants; Longman Group: London, UK, 1974; pp. 1–574. [Google Scholar]
- Christiansen, K.; Bellinger, P. The biogeography of Collembola. Bull. Entomol. Polog. 1995, 64, 279–294. [Google Scholar]
- Abrantes, E.A.; Mendonça, M.C. A new species of Psammisotoma Greenslade and Deharveng (Collembola: Isotomidae) from Brazil. Zootaxa 2009, 2295, 25–30. [Google Scholar] [CrossRef]
- International Commission on Zoological Nomenclature. International Code of Zoological Nomenclature, 4th ed.; International Trust for Zoological Nomenclature: London, UK, 1999; p. 306. [Google Scholar]
- Wray, D.L. New Collembola from Puerto Rico. J. Agric. Univ. P. R. 1953, 37, 140–150. [Google Scholar]
- Callahan, P.S. Evolution of Insects, 1st ed.; Holiday House: New York, NY, USA, 1972; p. 192. [Google Scholar]
- Palacios-Vargas, J.G. Guide of Springtails of Panama and Costa Rica (Collembola). In Insects of Panama and Mesoamerica; Quintero, D., Aiello, A., Eds.; Selected Studies; Oxford University Press: Cambridge, UK, 1992; pp. 25–36. [Google Scholar]
- Barra, J. Springtails of the genus Seira Lubbock, 1969 (Collembola: Entomobryidae) from Socotra Island. Fauna Arab. 2004, 20, 399–408. [Google Scholar]
- Bellini, B.C.; Zeppelini, D. A new species of Seira (Collembola: Entomobryidae: Seirini) from the Northeastern Brazilian coastal region. Zoologia 2011, 28, 403–406. [Google Scholar] [CrossRef][Green Version]
- Pan, Z.-X.; Shi, S.; Zhang, F. New species of Homidia (Collembola, Entomobryidae) from eastern China with description of the first instar larvae. ZooKeys 2011, 152, 21–42. [Google Scholar]
- Zhang, F.; Yu, D.-Y.; Xu, G.-L. Transformational homology of the tergal setae during postembryonic development in the Sinella-Coecobrya group (Collembola: Entomobryidae). Contrib. Zool. 2011, 80, 213–230. [Google Scholar] [CrossRef]
- Soto-Adames, F.N.; Taylor, S.J. New species and new records of Springtails (Hexapoda: Collembola) from caves in the Salem Plateau of Illinois, USA. J. Caves Karst. Stud. 2013, 75, 146–175. [Google Scholar] [CrossRef]
- Katz, A.D.; Giordano, R.; Soto-Adames, F. Taxonomic review and phylogenetic analysis of fifteen North American Entomobrya (Collembola, Entomobryidae), including four new species. ZooKeys 2015, 525, 1–75. [Google Scholar] [CrossRef]
- Soto-Adames, F.N. The dorsal chaetotaxy of first instar Trogolaphysa jataca, with description of twelve new species of Neotropical Trogolaphysa (Hexapoda: Collembola: Paronellidae). Zootaxa 2015, 4032, 1. [Google Scholar] [CrossRef][Green Version]
- Bernard, E.C.; Soto-Adames, F.N.; Wynne, J.J. Collembola of Rapa Nui (Easter Island) with description of five endemic cave-restricted species. Zootaxa 2015, 3949, 239–267. [Google Scholar] [CrossRef]
- Katz, A.D.; Taylor, S.J.; Soto-Adames, F.N.; Addison, A.; Hoese, G.B.; Sutton, M.R.; Toulkeridis, T. New records and new species of springtails (Collembola: Entomobryidae, Paronellidae) from lava tubes of the Galapagos Islands (Ecuador). Subterr. Biol. 2016, 17, 77–120. [Google Scholar]
- Soto-Adames, F.N. Chaetotaxy of first-instar Campylothorax sabanus (Wray), and description of three new Campylothorax species from Hispaniola (Collembola, Paronellidae). J. Nat. Hist. 2016, 50, 1–30. [Google Scholar] [CrossRef]
- Soto-Adames, F.N.; Anderson, E.W. Two new species and new records of springtails (Collembola: Entomobryidae, Paronellidae) from Nevis, Lesser Antilles. Fla. Entomol. 2017, 100, 32–41. [Google Scholar] [CrossRef]
- Oliveira, J.V.L.C.; Ferreira, A.S.; Zeppelini, D. Two new species of Collembola (Arthropoda: Hexapoda) from Minas Gerais, Brazil. Neotrop. Entomol. 2020, 49, 420–434. [Google Scholar] [CrossRef]
- Cipola, N.G.; Morais, J.W.; Bellini, B.C. Review of Lepidocyrtinus Börner, 1903 (Collembola, Entomobryidae, Seirinae): The African species. Zootaxa 2020, 4898, 1–110. [Google Scholar] [CrossRef] [PubMed]
- Godeiro, N.N.; Pacheco, G.; Liu, S.; Cipola, N.G.; Berbel-Filho, W.M.; Zhang, F.; Gilbert, M.T.P.; Bellini, B.C. Phylogeny of Neotropical Seirinae (Collembola, Entomobryidae) based on mitochondrial genomes. Zool. Scr. 2020, 49, 329–339. [Google Scholar] [CrossRef]
- Godeiro, N.N.; Zhang, F.; Cipola, N.G. First partial mitogenome of a new Seira Lubbock species (Collembola, Entomobryidae, Seirinae) from Cambodia reveals a possible separate lineage from the Neotropical Seirinae. Zootaxa 2020, 4890, 451–472. [Google Scholar] [CrossRef]
- Cucini, C.; Fanciulli, P.P.; Frati, F.; Convey, P.; Nardi, F.; Carapelli, A. Re-evaluating the internal phylogenetic relationships of collembola by means of mitogenome data. Genes 2021, 12, 44. [Google Scholar] [CrossRef]
- Godeiro, N.N.; Zhang, F. First record of Seira dowlingi (Wray, 1953) (Collembola, Entomobryidae, Seirinae) from China and mitogenome comparison with the New World specimens. Zootaxa 2021, 5020, 191–196. [Google Scholar] [CrossRef]
- Cipola, N.G.; Morais, J.W.; Bellini, B.C. New subgenus and four species of Lepidocyrtus Bourlet (Collembola, Entomobryidae, Lepidocyrtinae) from Amazon. Insect Syst. Evol. 2018, 50, 189–234. [Google Scholar] [CrossRef]
- Nunes, R.C.; Santos-Costa, R.C.; Bellini, B.C. The first Neotropical Capbrya Barra, 1999 (Collembola: Orchesellidae: Nothobryinae) and the reinterpretation of Nothobryinae systematics. Zool. Anz. 2020, 288, 24–42. [Google Scholar] [CrossRef]
- Brito, R.A.; Lima, E.C.A.; Zeppelini, D. Three new species of Collembola (Arthropoda: Hexapoda) from Brazil. Zootaxa 2019, 4700, 401–430. [Google Scholar] [CrossRef] [PubMed]
- Bellini, B.C.; Cipola, N.G.; Siqueira, O.J.R. A Survey of the Brazilian Dicranocentrus Schött (Collembola, Orchesellidae, Heteromurini) with the description of a new species and notes on the genus. Insect 2020, 11, 709. [Google Scholar] [CrossRef] [PubMed]
- Xisto, T.; Mendonça, M.C. New species and new records of Dicranocentrus Schött, 1893 (Collembola: Entomobryidae) from Southeastern Brazil. Insect Syst. Evol. 2017, 49, 23–58. [Google Scholar] [CrossRef]
- Bellini, B.C.; Morais, J.W.; Oliveira, F.G.L. A new species of Dicranocentrus Schött (Collembola, Entomobryidae, Orchesellinae) from Brazilian Amazon. Zootaxa 2013, 3709, 296–300. [Google Scholar] [CrossRef]
- Handschin, E. Neue myrmecophile und termitophile collembolenformen aus Süd Amerika. Neue Beiträge Syst. Insektenkunde Berl. 1924, 3, 13–28. [Google Scholar]
- Xisto, T.; Mendonça, M.C. Two new species of Dicranocentrus Schött, 1893 (Collembola: Entomobryidae) from Serra do Gandarela, Minas Gerais State, Brazil. Zootaxa 2016, 4079, 223–227. [Google Scholar] [CrossRef]
- Arlé, R.; Mendonça, C. Estudo preliminar das espécies de Dicranocentrus Schött, 1893, ocorrentes no Parque Nacional da Tijuca, Rio de Janeiro (Collembola). Rev. Bras. Biol. 1982, 42, 41–49. [Google Scholar]
- Cassagnau, P. 1963 Collemboles d’Amérique du Sud, II. Orchesellini, Paronellinae, Cyphoderinae. Biol. Amérique Australe 1963, 2, 127–148. [Google Scholar]
- Börner, C. Das System der Collembolen nebst Beschreibung neuer Collembolen des Hamburger Naturhistorischen museums. Mitt. Nat. Mus. Hambg. 1906, 23, 147–188. [Google Scholar]
- Arlé, R. Collembola Arthropleona do Brasil oriental e central. Arch. Mus. Nac. 1959, 49, 155–211. [Google Scholar]
- Bellini, B.C.; Cipola, N.G.; Godeiro, N.N. New species of Lepidocyrtus Bourlet and Entomobrya Rondani (Collembola: Entomobryoidea: Entomobryidae) from Brazil. Zootaxa 2015, 4027, 227–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santos, N.M.C.; Santos-Costa, R.C.; Siqueira, O.J.R.; Godeiro, N.N.; Bellini, B.C. Two new species of Entomobrya Rondani (Collembola, Entomobryidae) from northeastern Brazil and comments on the genus. Zootaxa 2020, 4731, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, K. Preliminary notes on the genus Entomobrya in South America with special reference to Patagonia. Biol. Amérique Australe 1963, 2, 149–168. [Google Scholar]
- Arlé, R.; Guimarães, A.E. Novas espécies de Entomobrya Rondani, 1861, do estado do Pará (Collembola, Entomobryomorpha). Bol. Mus. Para. Emilio Goeldi Nova Ser. Zool. 1978, 89, 1–18. [Google Scholar]
- Oliveira, E.P.; Deharveng, L. Response of soil Collembola (Insecta) communities to forest disturbance in central Amazonia (Brazil). In Functioning and Dynamics of Natural and Perturbed Ecosystems, Technique et Documentation; Bellan, D., Bonin, G., Emig, C., Eds.; Lavoisier, Intercept Ltd.: Mauritius, NJ, USA, 1995; pp. 361–376. [Google Scholar]
- Zeppelini, D.; Bellini, B.C.; Creão-Duarte, A.J.; Hernández, M.I.M. Collembola as bioindicators of restoration in mined sand dunes of Northeastern Brazil. Biodivers. Conserv. 2009, 18, 1161–1170. [Google Scholar] [CrossRef]
- Denis, J.R. Sur les Collemboles du Muséum de Paris (1er Partie). Ann. Soc. Entomol. Fr. 1924, 93, 211–260. [Google Scholar]
- Reuter, O.M. Notiser om finska Collembola. Medd. Af Soc. Pro Fauna Flora Fenn. Helfingfors 1892, 18, 231–232. [Google Scholar]
- Reuter, O.M. Apterygogenea Fennica Finlands Collembola och Thysanura. Acta Soc. Fauna Flora 1895, 11, 1–35. [Google Scholar]
- Kraepelin, K. Uber die durch den Schiffsverkehr in Hamburg eingeschleppten Tiere. Mitt. Aus. Dem. Nat. Mus. Hambg. 1901, 18, 183–209. [Google Scholar]
- Arlé, R. Collemboles nouveaux de Rio de Janeiro. An. Acad. Bras. Ciênc. 1939, 11, 25–32. [Google Scholar]
- Cipola, N.G.; Oliveira, F.G.L.; Morais, J.W.; Bellini, B.C. The Heteromurini Absolon & Ksenemann (Collembola, Entomobryidae): A review of the genera status and diagnoses, keys for species of Alloscopus Börnerand Heteromurtrella Mari Mutt and description of a new species. Zootaxa 2016, 4084, 151–186. [Google Scholar] [PubMed]
- Cipola, N.G.; Morais, J.W.; Bellini, B.C. The discovery of Lepidocyrtoides Schött, 1917 (Collembola, Entomobryidae, Entomobryinae) from the New World, including three new species from Brazil and one from Australia. Zootaxa 2017, 4324, 201–248. [Google Scholar] [CrossRef]
- Santos-Rocha, I.M.; Andreazze, R.; Bellini, B.C. Registros de Collembola (Arthropoda, Hexapoda) no Estado do Rio Grande do Norte, Brasil. Biota Neotrop. 2011, 11, 167–170. [Google Scholar] [CrossRef][Green Version]
- Bellini, B.C.; Zeppelini, D. First records of Collembola (Ellipura) from the state of Paraíba, Northeastern Brazil. Rev. Bras. Entomol. 2004, 48, 587–588. [Google Scholar] [CrossRef]
- Börner, C. Collembolen aus Ostafrika, Madagascar und Südamerika. In Reise in Ostafrika in den Jahren 1903–1905; Voeltzkow, A., Ed.; E. Schweizerbartsche Verlagsbuchhandlung: Stuttgart, Germany, 1907; Volume 2, pp. 147–178. [Google Scholar]
- Oliveira Filho, L.C.I.; Zeppelini, D.; Baretta, D.; Sousa, J.P.; Klauberg-Filho, O. Collembola community structure under different land management in subtropical Brazil. Ann. Appl. Biol. 2020, 177, 294–307. [Google Scholar] [CrossRef]
- Nunes, R.C.; Godeiro, N.N.; Pacheco, G.; Liu, S.; Gilbert, M.T.P.; Alvarez-Valin, F.; Zhang, F.; Bellini, B.C. The discovery of Neotropical Lepidosira (Collembola, Entomobryidae) and its systematic position. Zool. Scr. 2019, 48, 783–800. [Google Scholar] [CrossRef]
- Stach, J. Eine neue attophile collembole aus Brasilien. Zool. Anz. 1935, 110, 154–158. [Google Scholar]
- Mari Mutt, J.A. A review of the genus Mastigoceras with remarks on its systematic position (Collembola: Entomobryidae). Pan-Pac. Entomol. 1978, 54, 43–47. [Google Scholar]
- Cassagnau, P.; Oliveira, E. Sur Mastigoceras camponoti Handschin, collembole Orchesellinae d’Amazonie. Bull. Soc. Hist. Nat. 1992, 128, 27–31. [Google Scholar]
- Silveira, T.C.; Mendonça, M.C. New species of Nothobrya (Collembola: Entomobryidae) from Southeast Brazil. Zool. 2016, 33, e20160126. [Google Scholar] [CrossRef][Green Version]
- Arlé, R. Novas espécies de colêmbolas aquáticas (Nota preliminar). Atas Soc. Biol. 1961, 5, 34–37. [Google Scholar]
- Nunes, R.C.; Bellini, B.C. A new species of Nothobrya Arlé, 1961 (Collembola: Entomobryidae) from Brazil and notes on key characters for Nothobryinae taxonomy, with an identification key to the species of the subfamily. Zootaxa 2019, 4615, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Cipola, N.G.; Oliveira, J.V.L.C.; Bellini, B.C.; Ferreira, A.S.; Lima, E.C.A.; Brito, R.A.; Stievano, L.C.; Souza, P.G.C.; Zeppelini, D. Review of Eyeless Pseudosinella Schäffer (Collembola, Entomobryidae, and Lepidocyrtinae) from Brazilian Caves. Insects 2020, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.H.; Mendonça, M.C. A new species of Xennyla (Collembola, Poduromorpha, Hypogastruridae) with new records for the State of Espírito Santo, southeastern Brazil. Rev. Bras. Zool. 2010, in press. [Google Scholar]
- Zeppelini, D.; Brito, R.A.; Lima, E.C.A. Three new species of Collembola (Arthropoda: Hexapoda) from Central Brazilian shallow caves: Side effects of long term application of environmental law on conservation. Zootaxa 2018, 4500, 059–081. [Google Scholar] [CrossRef] [PubMed]
- Culik, M.P.; Martins, D.S.; Ventura, J.A. Collembola (Arthropoda: Hexapoda) communities in the soil of papaya orchards managed with conventional and integrated production in Espírito Santo, Brazil. Biota Neotrop. 2006, 6, 1–8. [Google Scholar] [CrossRef][Green Version]
- Christiansen, K. The genus Pseudosinella (Collembola, Entomobryidae) in caves of the United States. Psyche 1960, 67, 1–25. [Google Scholar] [CrossRef][Green Version]
- Nunes, R.C.; Bellini, B.C. Three new species of Entomobryoidea (Collembola: Entomobryomorpha) from Brazilian Caatinga-Cerrado transition, with identification keys to Brazilian Cyphoderus, Pseudosinella and Trogolaphysa species. Zootaxa 2018, 4420, 071–096. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.C.; Fernandes, L.H. Rhynchocyrtus gen. nov. (Collembola, Entomobryidae) from the Southeast and Northeast Brazilian regions. Zootaxa 2007, 1660, 45–51. [Google Scholar] [CrossRef]
- Bellini, B.C.; Zeppelini, D. Three new species of Seira Lubbock (Collembola, Entomobryidae) from Mataraca, Paraíba State, Brazil. Zootaxa 2008, 1773, 44–54. [Google Scholar] [CrossRef]
- Arlé, R. Collembola, anexo nº. 2, ao relatório da excursão científica do Instituto Oswaldo Cruz realizada na zona da E. F. N. O. B., em outubro de 1938. Bol. Biol. 1939, 4, 295–300. [Google Scholar]
- Godeiro, N.N.; Bellini, B.C. Two new species and two detailed chaetotaxy descriptions of Seira (Collembola: Entomobryidae) from Brazil. Zootaxa 2015, 3972, 209–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellini, B.C.; Santos, N.M.C.; Souza, P.G.C.; Weiner, W.M. Two new species of Brazilian springtails (Hexapoda: Collembola) with comments on Neotropical Brachystomella Ågren and Seira (Lepidocyrtinus) Börner. Insect Syst. Evol. 2019, 50, 297–326. [Google Scholar] [CrossRef]
- Arlé, R. Quelques collemboles de l´etat d´Espirito Santo (Brésil). Physis 1939, 17, 125–131. [Google Scholar]
- Godeiro, N.N.; Bellini, B.C. A new species of Seira (Collembola: Entomobryidae) from the state of Paraíba, Brazil. Zoologia 2013, 30, 343–345. [Google Scholar] [CrossRef][Green Version]
- Godeiro, N.N.; Bellini, B.C. Three new species of Seira Lubbock (Collembola, Entomobryidae) from Caatinga Domain, northeastern Brazil. Zootaxa 2014, 3764, 131–151. [Google Scholar] [CrossRef]
- Arlé, R.; Guimarães, A.E. Nova espécie saxícola do gênero Seira Lubbock, 1869, do Rio de Janeiro (Collembola). Rev. Bras. Entomol. 1981, 25, 1–3. [Google Scholar]
- Bellini, B.C.; Fernandes, L.H.; Zeppelini, D. Two new species of Seira (Collembola, Entomobryidae) from Brazilian coast. Zootaxa 2010, 2448, 53–60. [Google Scholar] [CrossRef]
- Nunes, R.C.; Cipola, N.G.; Bellini, B.C. Two new species of Seira Lubbock, 1870 (Collembola: Entomobryidae: Seirinae) from Brazilian Caatinga. Zootaxa 2021, 5048, 001–030. [Google Scholar] [CrossRef]
- Bellini, B.C.; Pais, A.P.; Zeppelini, D. A new species of Seira Lubbock (Collembola: Entomobryidae) from Brazil with sexually dimorphic legs. Zootaxa 2009, 2080, 38–46. [Google Scholar] [CrossRef]
- Bellini, B.C.; Godeiro, N.N. A new species of Tyrannoseira (Collembola: Entomobryidae: Seirini) from the Brazilian coastal region. Zoologia 2012, 29, 81–84. [Google Scholar] [CrossRef]
- Zeppelini, D.; Lima, E.C.A. A new species of Tyrannoseira (Collembola, Entomobryidae, Seirini) from Paraiba, Northeastern Brazil. Zootaxa 2012, 3423, 37–42. [Google Scholar] [CrossRef]
- Zeppelini, D.; Bellini, B.C. Two Seira Lubbock 1869 (Collembola, Arthropleona, Entomobryidae) new to science, with remarkable secondary sexual characters. Zootaxa 2006, 1185, 21–35. [Google Scholar] [CrossRef]
- Bellini, B.C.; Meneses, L.F. A new species of Campylothorax (Collembola: Entomobryoidea: Paronellidae) from the state of Alagoas, Brazil. Zoologia 2012, 29, 451–454. [Google Scholar] [CrossRef]
- Cipola, N.G.; Oliveira, F.G.L. A new species of Campylothorax Schött, 1893 (Collembola, Paronellidae) from Brazilian Amazon, with an identification key to the genus. Zootaxa 2016, 4109, 487–495. [Google Scholar] [CrossRef]
- Mitra, S.K.; Dallai, R. Studies of the genus Campylothorax Schött, 1893 (Collembola Entomobryidae Paronellinae) with the description of a new species from Zaire. Monit. Zool. Ital. NS Suppl. 1980, 13, 273–321. [Google Scholar] [CrossRef]
- Santos, I.P.S.; Cipola, N.G.; Morais, J.W.; Bellini, B.C. A new species of Campylothorax (Collembola: Paronellidae: Paronellinae) from Northern Brazil. Zoologia 2016, 33, 1–6. [Google Scholar] [CrossRef]
- Börner, C. Neue Cyphoderinen. Zool. Anz. 1913, 41, 274–284. [Google Scholar]
- Zeppelini, D.; Oliveira, J.V.L.C. Chaetotaxy of Neotropical Cyphoderus caetetus sp. nov. with comments on the taxonomic position of Cyphoderinae within Paronellidae (Collembola, Entomobryoidea). Zootaxa 2016, 4098, 560–570. [Google Scholar] [CrossRef]
- Oliveira, J.V.L.C.; Alves, J.L.S.; Zeppelini, D. Two new Cyphoderus (Collembola: Paronellidae) of “tridenticulati” and “bidenticulati” groups from Brazilian Amazon. Zootaxa 2017, 4350, 047–060. [Google Scholar] [CrossRef]
- Soto-Adames, F.N.; Bellini, B.C. Dorsal Chaetotaxy of Neotropical Species Supports a Basal Position for the Genus Lepidonella Among Scaled Paronellidae (Collembola, Entomobryoidea). Fla. Entomol. 2015, 98, 330–341. [Google Scholar] [CrossRef]
- Oliveira, F.G.L.; Cipola, N.G.; Almeida, E.A.B. Systematics and biogeography of Salina MacGillivray (Collembola: Entomobryoidea), with emphasis on the species groups in the New World. Insect Syst. Evol. 2018, 2018, 1–58. [Google Scholar] [CrossRef]
- Oliveira, F.G.L.; Cipola, N.G. Two new species of Salina MacGillivray (Collembola, Paronellidae) with rectangular mucro from South America. Rev. Bras. Entomol. 2016, 60, 128–136. [Google Scholar] [CrossRef]
- Bellini, B.C.; Cipola, N.G. The Neotropical genera of Paronellinae (Collembola, Entomobryoidea, Paronellidae) with description of two new species and redescription of Campylothorax mitrai. Zootaxa 2017, 4300, 151–179. [Google Scholar] [CrossRef]
- Cipola, N.G.; Morais, J.W.; Bellini, B.C. A New Epigeous Species of Troglobius (Collembola: Paronellidae: Cyphoderinae) from Brazil and Notes on the Chaetotaxy of the Genus. Fla. Entomol. 2016, 99, 658–666. [Google Scholar] [CrossRef]
- Palacios-Vargas, J.G.; Zeppelini, D. A new species of Troglobius (Collembola, Paronellidae) from Brazil. Int. J. Speleol. 1995, 23, 173–177. [Google Scholar] [CrossRef][Green Version]
- Zeppelini, D.; Silva, D.D.; Palacios-Vargas, J.G. A new species of Troglobius (Collembola, Paronellidae, Cyphoderinae) from a Brazilian iron cave. Subterr. Biol. 2014, 14, 1–14. [Google Scholar] [CrossRef][Green Version]
- Yoshii, R. Paronellid Collembola from caves of Central and South America collected by P. Strinati. Rev. Suisse Zool. 1988, 95, 449–459. [Google Scholar]
- Silva, D.D.; Bellini, B.C. Trogolaphysa formosensis sp. nov. (Collembola: Paronellidae) from Atlantic Forest, Northeast Region of Brazil. Zoologia 2015, 32, 53–58. [Google Scholar] [CrossRef]
- Arlé, R.; Guimarães, A.E. Nova espécie do gênero Paronella Schott, 1893 do Rio de Janeiro (Collembola). Rev. Bras. Entomol. 1979, 23, 213–217. [Google Scholar]
- Arlé, R. Notas sôbre a família Oncopoduridae, com descrição de duas espécies novas do Brasil (Collembola). Arch. Mus. Nac. 1960, 50, 9–23. [Google Scholar]
- Neves, A.C.; Mendonça, M.C. New Brazilian species of Archisotoma Linnaniemi, 1912 (Collembola: Isotomidae). J. Insect Biodivers. 2014, 2, 1–7. [Google Scholar] [CrossRef][Green Version]
- Strenzke, K. Axelsonia tubifera n. sp., ein neuer arthropleoner Collembole mit Geschlechtsdimorphismus aus der brasilianischen Mangrove. Acta Zool. Cracov. 1958, 2, 607–619. [Google Scholar]
- Abrantes, E.A.; Mendonça, M.C. New species and a new record of Isotomidae (Collembola) from the coast of Brazil. Zootaxa 2007, 1500, 55–60. [Google Scholar] [CrossRef]
- Thibaud, J.M.; Palacios-Vargas, J.G. Révision du genre Archisotoma Linnaniemi, 1912 (Collembola: Isotomidae). Ann. Soc. Entomol. Fr. 2001, 37, 347–356. [Google Scholar]
- Lima, E.C.A.; Zeppelini, D.; Mendonça, M.C. Brazilian Archisotoma Linnaniemi (Collembola, Isotomidae), with description of a new species from an oceanic atoll. Zootaxa 2019, 4543, 52–62. [Google Scholar] [CrossRef]
- Mendonça, M.C.; Abrantes, E.A.; Fernandes, L.H. Novas espécies de Arlea do sudeste do Brasil (Collembola, Isotomidae). Iheringia Série Zool. 2006, 96, 57–60. [Google Scholar] [CrossRef]
- Abrantes, E.A.; Mendonça, M.C. Uma nova espécie de Arlea Wormersley do sudeste do Brasil (Collembola, Isotomidae). Rev. Bras. Entomol. 2005, 22, 936939. [Google Scholar]
- Mendonça, C.; Arlé, R. Nova espécie brasileira de Arlea Womersley, 1939 (Collembola, Isotomidae). Bol. Mus. Nac. 1987, 315, 1–7. [Google Scholar]
- Arlé, R. Generalidades e importância ecológica da ordem Collembola (Apterygota). Atas Soc. Biol. 1959, 3, 4–7. [Google Scholar]
- Mendonça, C.; Reis, S.F. Geographic and interspecific variation in two Proisotoma species (Collembola, Isotomidae). Rev. Bras. Entomol. 1990, 34, 643–649. [Google Scholar]
- Mendonça, C.; Reis, S.F. Multivariate morphometric analysis of selected Proisotoma species (Collembola: Isotomidae). Zool. Anz. 1991, 227, 98–103. [Google Scholar]
- Queiroz, G.C.; Mendonça, M.C. Two new Isotomidae species (Collembola) from Espírito Santo State, Brazil. Zootaxa 2010, 2480, 37–44. [Google Scholar] [CrossRef]
- Mendonça, M.C. Contribuição para o Conhecimento de Collembola Entomobrymorpha (Insecta) do Parque Nacional da Tijuca, Rio de Janeiro, Brasil; UFRJ: Rio de Janeiro, Brasil, 1981; p. 150. [Google Scholar]
- Massoud, Z.; Rapoport, E.H. Collemboles Isotomides d’Amerique du Sud et de l’Antarctique. Biol. Amérique Australe 1968, 4, 307–337. [Google Scholar]
- Potapov, M.; Culik, M. A new species of Folsomia (Collembola: Isotomidae) from Brazil, with notes on foilsetae in the fimetaria group. Pan. Pac. Entomol. 2002, 78, 69–73. [Google Scholar]
- Mendonça, C. Contribuição ao estudo do gênero Folsomides Stach, 1922 no Brasil (Collembola, Isotomidae). Rev. Bras. Entomol. 1984, 28, 121–128. [Google Scholar]
- Thibaud, J.M.; Palacios-Vargas, J.G. Brazilian Collembola from littoral sand with description of Austrogastrura gen. n. and Isotomodes carioca sp. n. [Hypogastruridae; Isotomidae]. Rev. Fr. Entomol. 1999, 21, 25–31. [Google Scholar]
- Mendonça, M.C.; Abrantes, E.A. A new Brazilian species of Isotomiella (Collembola: Isotomidae), with notes on I. bidentata Delamare Deboutteville, 1950 and I. amazonica Oliveira & Deharveng, 1990. Zootaxa 2007, 1652, 41–48. [Google Scholar]
- Oliveira, E.; Deharveng, L. Isotomiella (Collembola, Isotomidae) d’Amazonie: Les espèces du groupe minor. Bull. Mus. Natl. Hist. Nat. 1990, 12, 75–93. [Google Scholar]
- Mendonça, M.C.; Fernandes, L.H. Três novas espécies de Isotomiella Bagnall, 1939 do sudeste do Brasil (Collembola: Isotomidae). Lundiana 2003, 4, 111–116. [Google Scholar]
- Mendonça, M.C.; Queiroz, G.C. Two new Isotomidae species (Collembola: Entomobryomorpha) from Rio de Janeiro State, Brazil. Zootaxa 2017, 4350, 385–395. [Google Scholar] [CrossRef]
- Mendonça, M.C.; Queiroz, G.C. A new Brazilian species of Isotomiella (Collembola: Isotomidae) from the state of Pará, Brazil. Zoologia 2016, 33, e20160005. [Google Scholar] [CrossRef]
- Mendonça, M.C.; Abrantes, E.; Neves, A.C.R. New species of Isotomiella Bagnall, 1939 from Southeast of Brazil (Collembola, Isotomidae). ZooKeys 2012, 233, 21–30. [Google Scholar] [CrossRef][Green Version]
- Costa, J.M. Contribuição ao Estudo dos Colembolos Isotomus Palustrias Mueller Collembola—Isotomidae. Boletim Técnico; Instituto Agronômico do Leste: Bahia, Brazil, 1961; Volume 6, pp. 43–59.
- Mendonça, C. Duas novas espécies brasileiras de Isotomurus Börner, 1903 (Collembola: Isotomidae). Rev. Bras. Biol. 1990, 50, 453–462. [Google Scholar]
- Mendonça, M.C.; Queiroz, G.C. A new species of Mucrosomia (Collembola: Isotomidae) from Brazil. Zoologia 2013, 30, 217–220. [Google Scholar] [CrossRef][Green Version]
- Mendonça, M.C.; Abrantes, E.A.; Fernandes, L.H. Two new Brazilian species of Paracerura Deharveng & Oliveira (Collembola: Isotomidae). Zootaxa 2009, 2310, 24–34. [Google Scholar]
- Abrantes, E.A.; Duarte, M. New species of Paracerura (Collembola: Isotomidae) from the state of São Paulo, Brazil. Fla. Entomol. 2013, 96, 1392–1400. [Google Scholar] [CrossRef]
- Mendonça, M.C.; Silveira, T.C. New species of Paracerura (Collembola: Isotomidae) from Minas Gerais State, Brazil. Zootaxa 2013, 3681, 73–78. [Google Scholar] [CrossRef]
- Deharveng, L.; Oliveira, E.P. Paracerura virgata n. g., n. sp. (Collembola, Isotomidae), nouveau collembole d’Amazonie centrale. Rev. Suisse Zool. 1994, 101, 441–446. [Google Scholar] [CrossRef]
- Mendonça, M.C.; Queiroz, G.C.; Silveira, T.C. Two new species of Proisotoma Börner, 1901 from Southeastern Brazil (Collembola: Isotomidae). Soil Org. 2015, 87, 51–60. [Google Scholar]
- Moniez, R. Isotoma pallida, collembole nouveau de Brésil. Rev. Biol. Nord. Fr. 1894, 6, 354. [Google Scholar]
- Deharveng, L. Un nouveau collembole Isotomidae du Brésil: Proisotoma oliveirae n. sp. Bull. Soc. Hist. Nat. 1984, 120, 123–125. [Google Scholar]
- Arlé, R. Uma nova espécie de Onychiurus (Collembola-Onychiuridae) de ocorrência periódica em Belém (Pará). Bol. Mus. Para. Emilio Goeldi Nova Ser. Zool. 1970, 72, 1–11. [Google Scholar]
- Hüther, W. Eine neue Anurophorinen-Gattung aus NordostBrasilien (Ins, Collembola). Senckenb. Biol. 1967, 48, 169–173. [Google Scholar]
- Teixeira, W.; Cordani, U.G.; Menor, E.A.; Teixeira, M.G.; Linsker, R. Arquipélago Fernando de Noronha–O Paraíso do Vulcão; Terra Virgem: São Paulo, SP, Brasil, 2003; p. 168. [Google Scholar]
- Serafine, T.Z.; França, G.B.; Andriguetto-Filho, J.M. Ilhas oceânicas brasileiras: Biodiversidade conhecida e sua relação com o histórico de uso e ocupação humana. Brazilian oceanic islands: Known biodiversity and its relation to the history of human use and occupation. Rev. Gestão Costeira Integr. J. Integr. Coast. Zone Manag. 2010, 3, 281–301. [Google Scholar] [CrossRef]
- Campos, T.F.C.; Virgens Neto, J.; Srivastava, N.K.; Petta, R.A.; Hartmann, L.A.; Moraes, J.F.S.; Mendes, L.; Silveira, S.R.M. Arquipélago de São Pedro e São Paulo—Soerguimento tectônico de rochas infracrustais no Oceano Atlântico. In Sítios Geológicos e Paleontológicos do Brasil; Winge, M., Schobbenhaus, C., Berbert-Born, M., Queiroz, E.T., Campos, D.A., Souza, C.R.G., Fernandes, A.C.S., Eds.; SIGEP: Brasilia, Brazil, 2005; Volume 2, pp. 253–263. [Google Scholar]
- Lima, E.C.A.; de Mendonça, M.C.; Queiroz, G.C.; da Silveira, T.C.; Zeppelini, D. Synthesis of the Brazilian Poduromorpha (Collembola: Hexapoda) with special emphasis on the Equatorial Oceanic Islands. Insects 2021, 12, 268. [Google Scholar] [CrossRef]
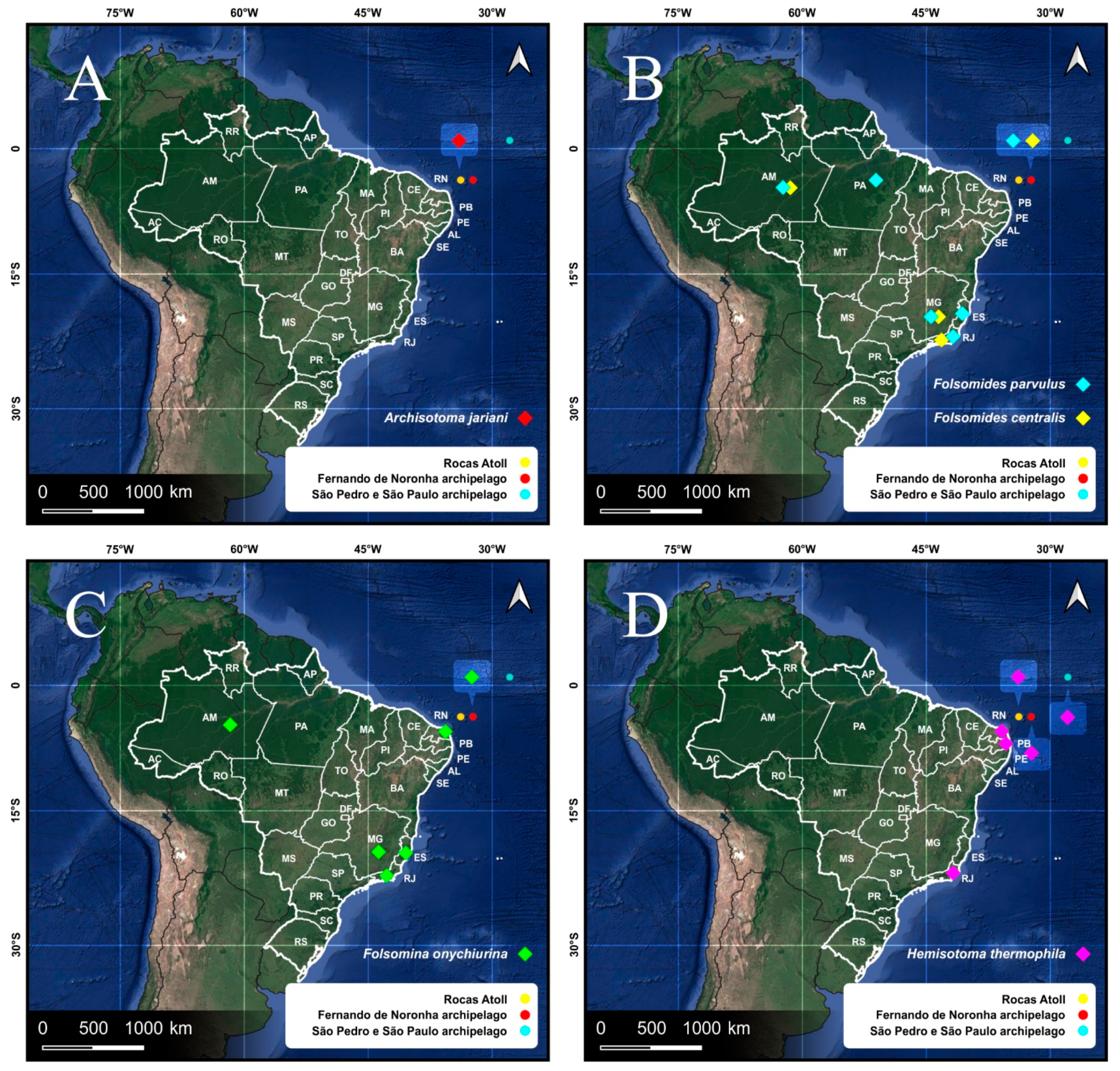
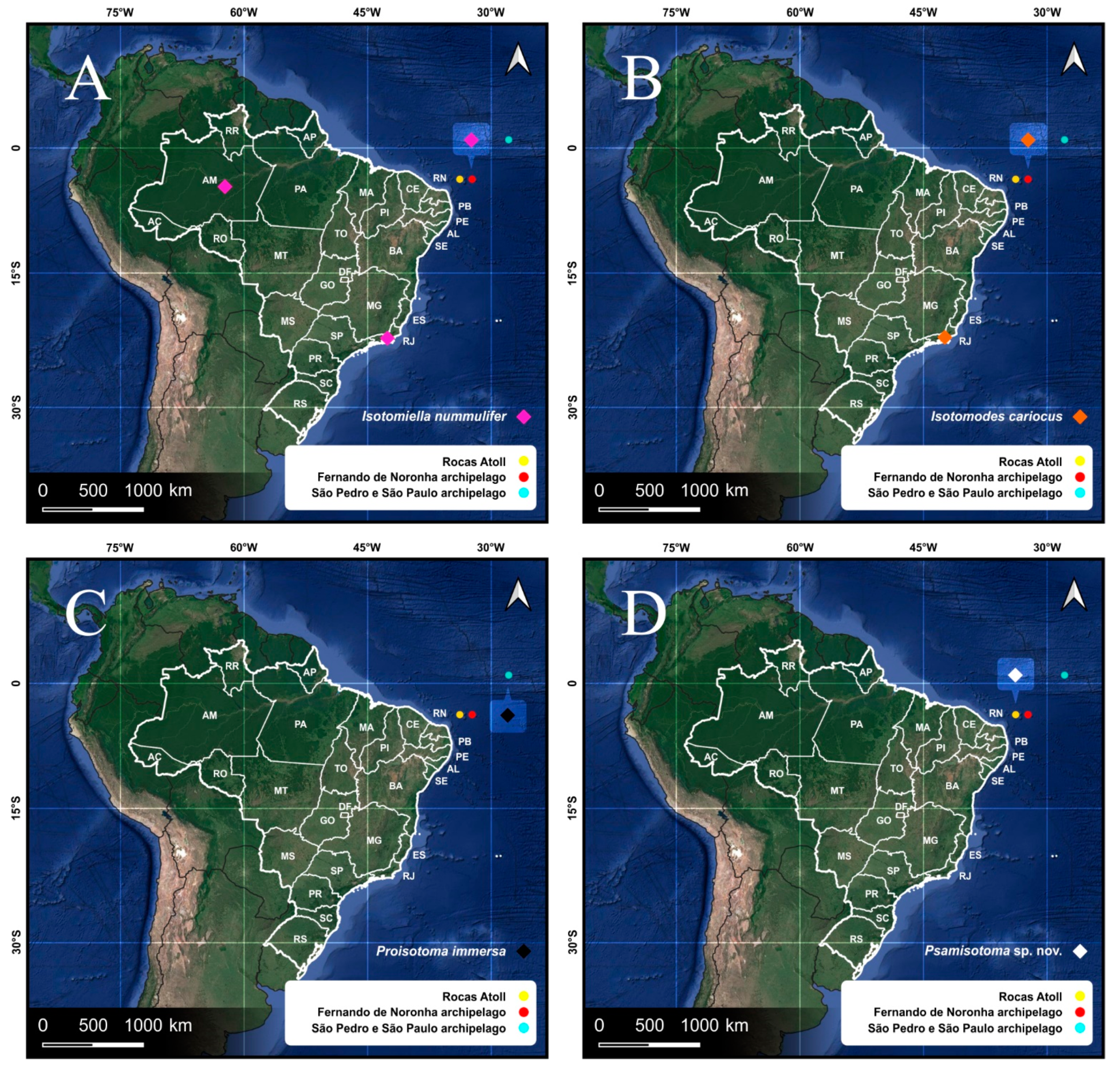
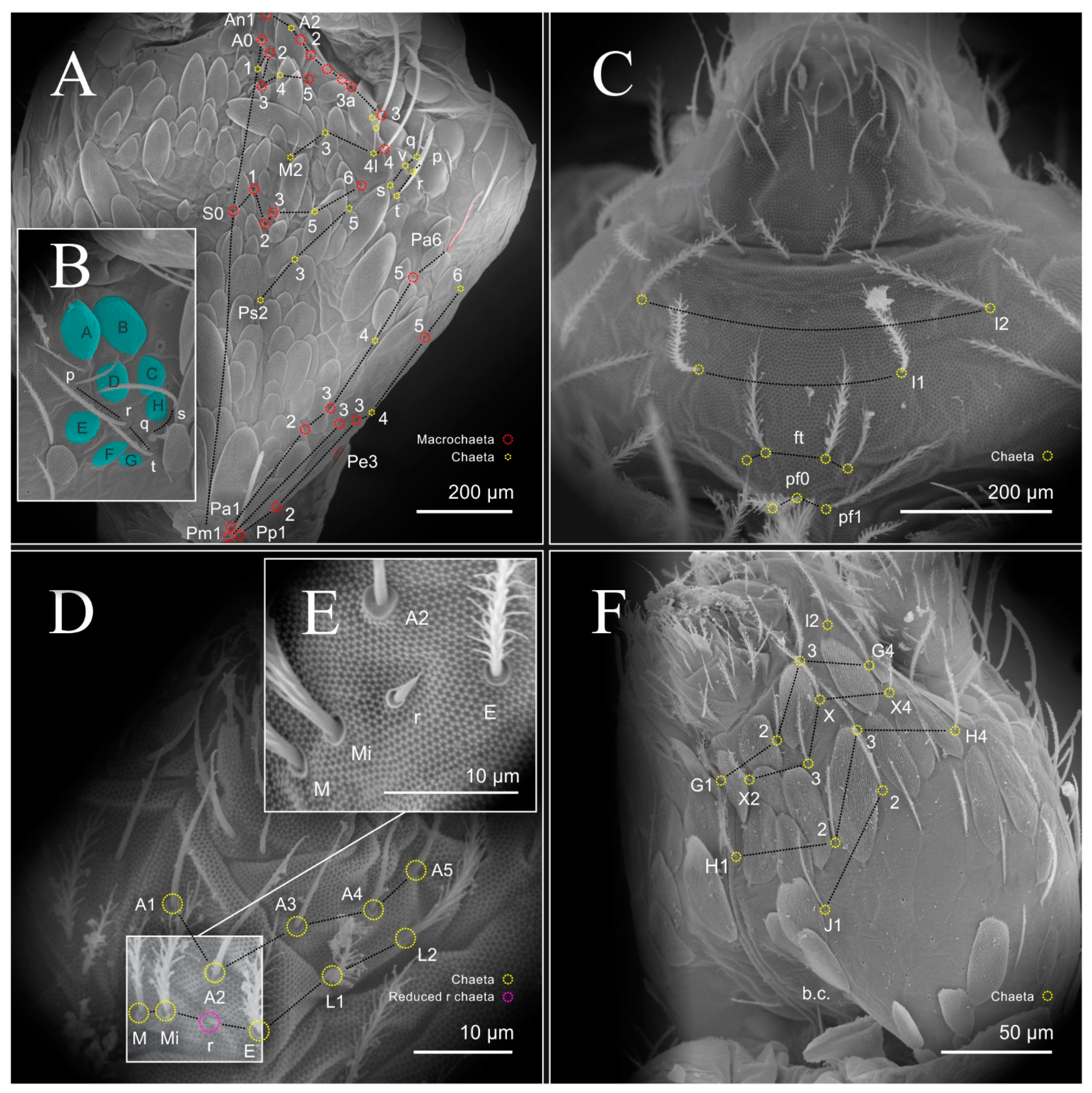
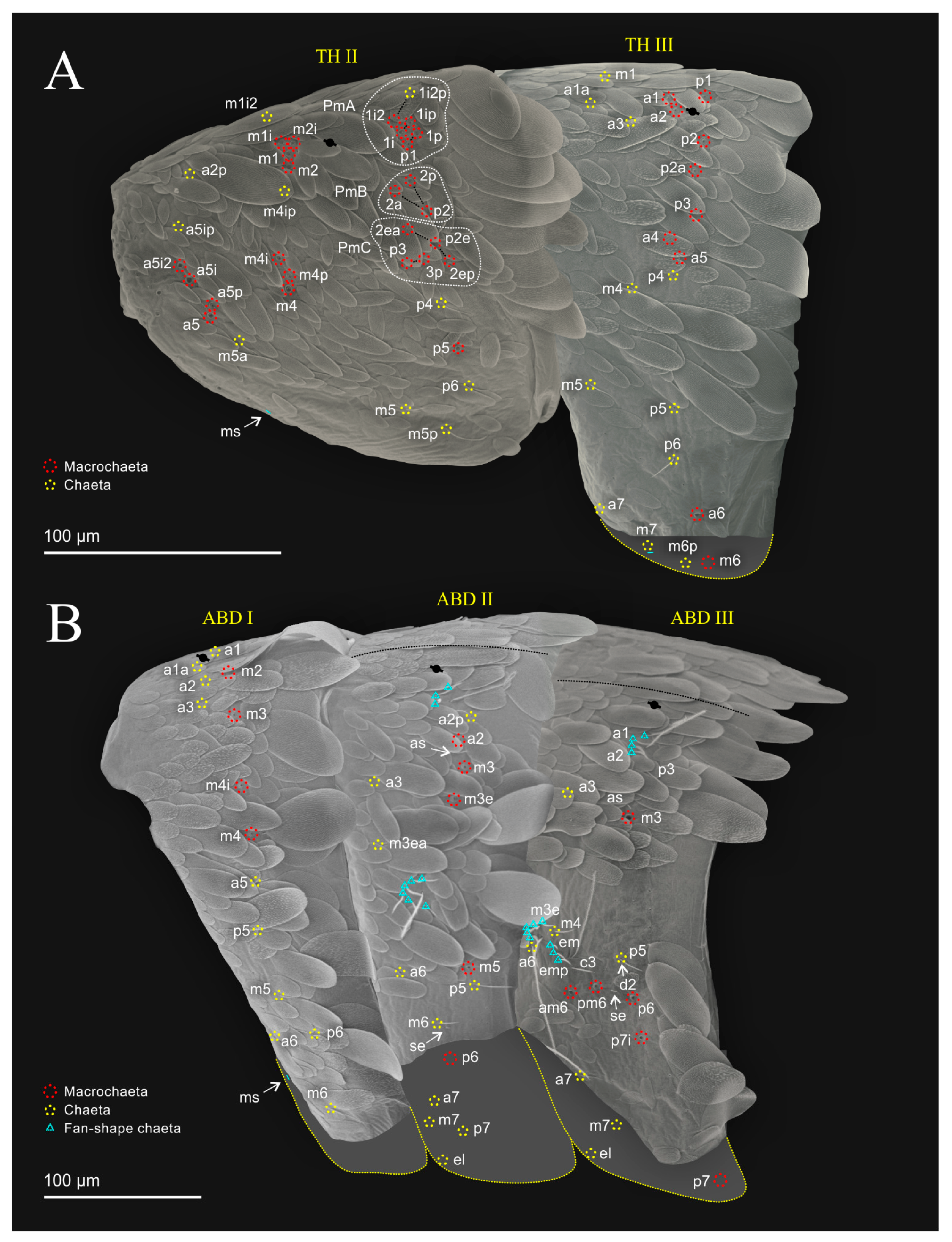
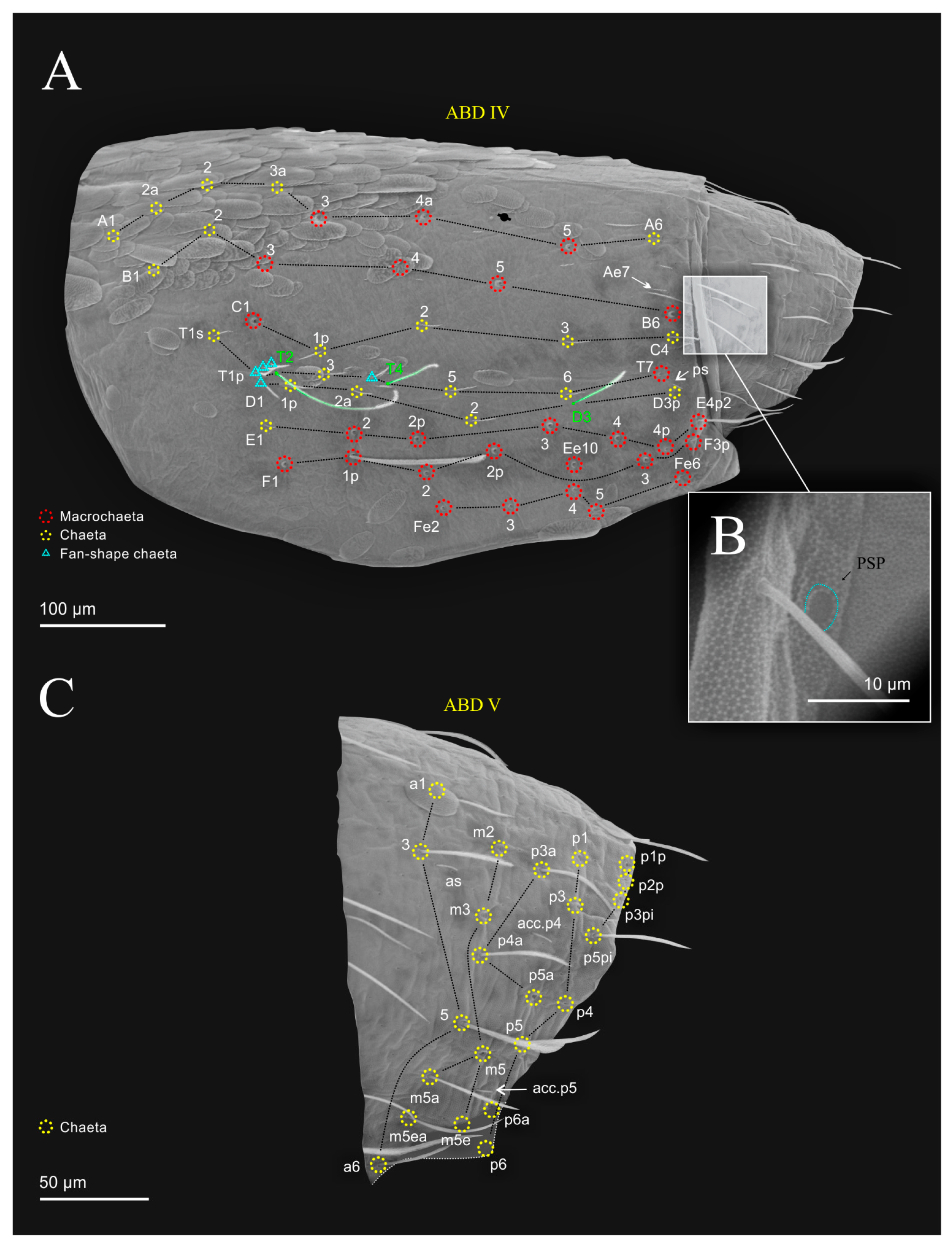
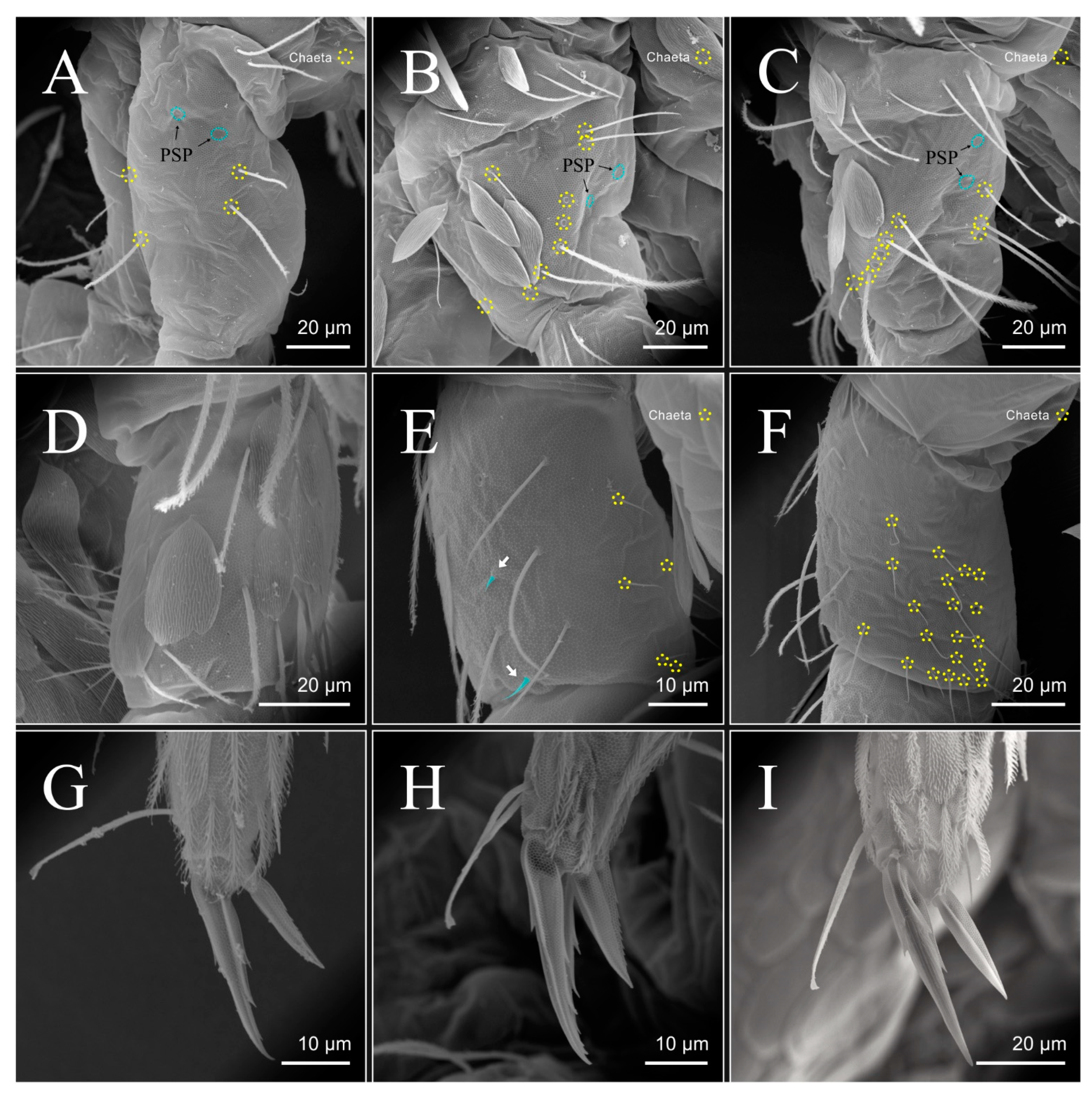
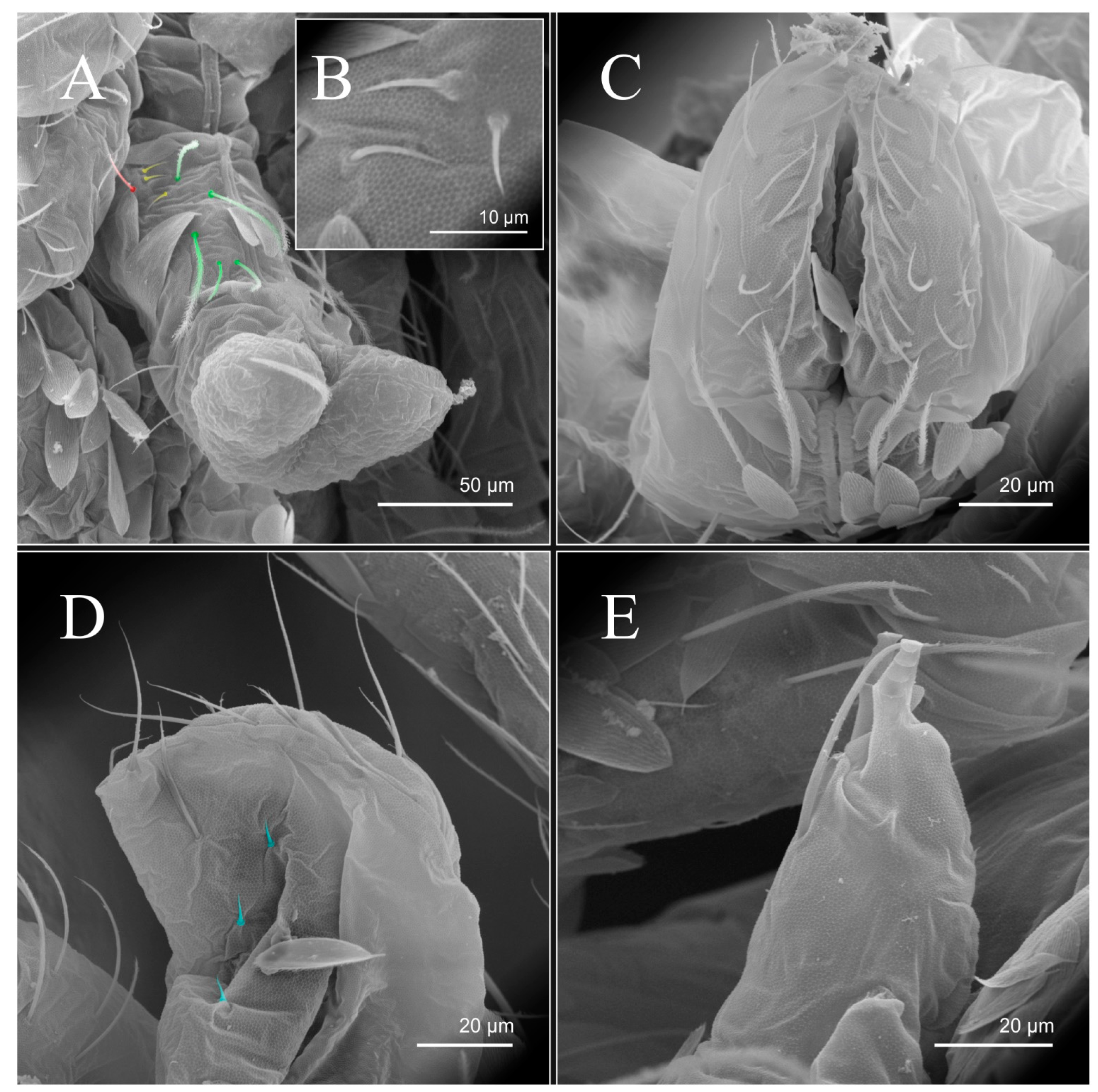
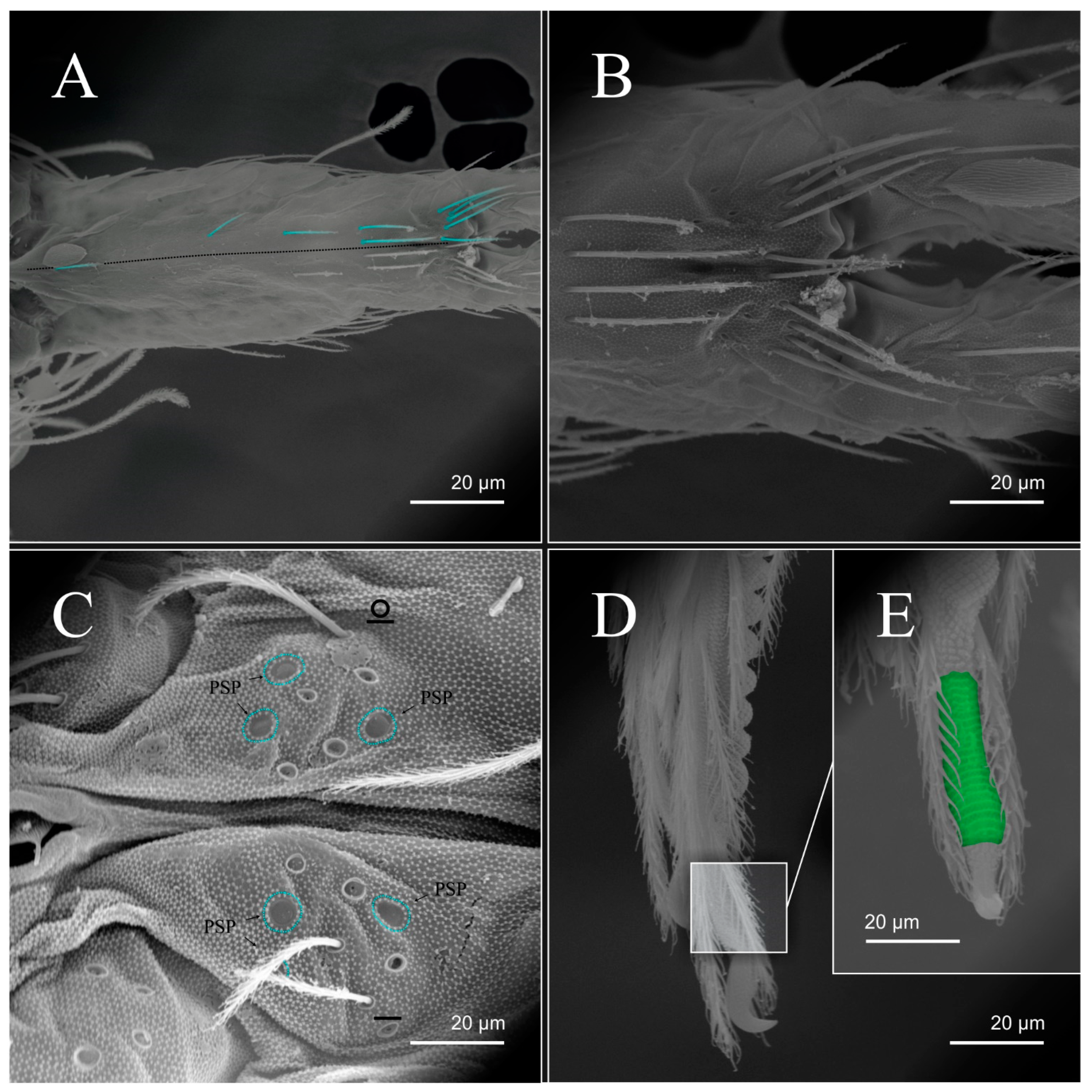
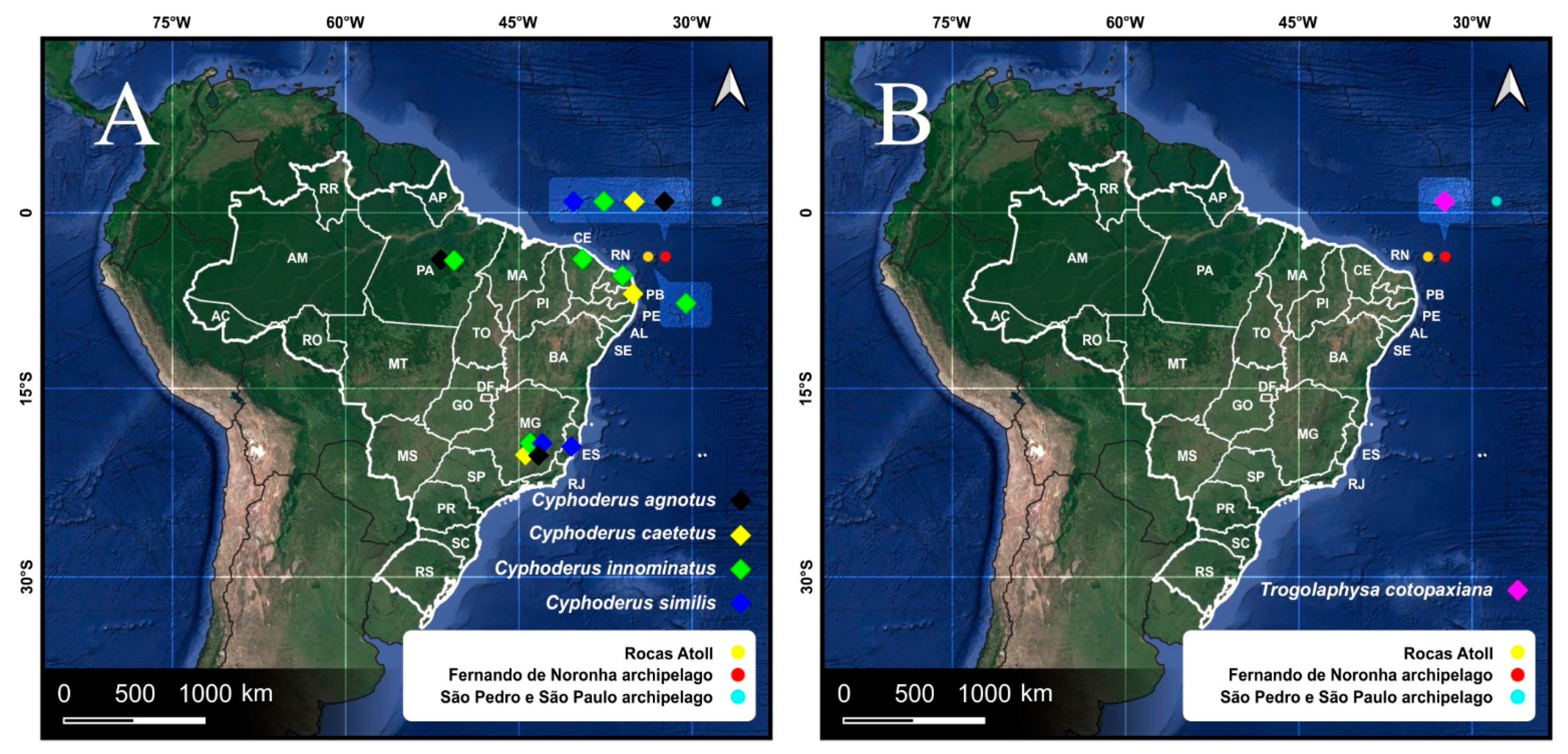
| Species | SB | SF | TF | Region | ||
|---|---|---|---|---|---|---|
| FN | Isotomidae | Folsomides centralis Denis, 1931 * | 0 | 35 | 7 | WSP |
| Folsomides parvulus Stach, 1922 * | 0 | 80 | 111 | WSP | ||
| Folsomina onychiurina Denis, 1931 * | 0 | 59 | 34 | WSP | ||
| Hemisotoma thermophila Axelson, 1900 | 0 | 223 | 153 | WSP | ||
| Isotomiella nummulifer Deharveng & Oliveira, 1990 * | 0 | 12 | 45 | WSP | ||
| Isotomodes cariocus Thibaud & Palacios-Vargas, 1999 * | 0 | 82 | 108 | Brazil | ||
| Entomobryidae | Entomobrya atrocincta Schött, 1896 | 0 | 0 | 1 | Nea | |
| Lepidocyrtus nigrosetosus Folsom, 1927 | 0 | 841 | 498 | WSP | ||
| Lepidocyrtus violaceus Fourcroy, 1785 | 0 | 0 | 1 | WSP | ||
| Pseudosinella biunguiculata Ellis, 1967 | 0 | 51 | 61 | Neo | ||
| Seira atrolutea Arlé, 1939 * | 0 | 4 | 0 | Brazil | ||
| Seira musarum Ridley, 1890 | 0 | 3 | 0 | WSP | ||
| Paronellidae | Cyphoderus agnotus Börner, 1906 | 0 | 3 | 0 | Neo | |
| Cyphoderus caetetus Zeppelini & Oliveira, 2016 | 0 | 3 | 15 | Brazil | ||
| Cyphoderus innominatus Mills, 1938 | 0 | 3 | 151 | Neo | ||
| Cyphoderus similis Folsom, 1927 | 0 | 43 | 20 | WSP | ||
| Trogolaphysa cotopaxiana Thibaud & Najt, 1988 * | 0 | 11 | 6 | Neo | ||
| SPSP | Isotomidae | Hemisotoma thermophila Axelson, 1900 * | 0 | 2 | - | WSP |
| Proisotoma immersa Folsom, 1924 * | 0 | 49 | - | Nea | ||
| Entomobryidae | Seira musarum Ridley, 1890 * | 243 | 278 | - | WSP | |
| RA | Isotomidae | Archisotoma jariani Lima, Zeppelini & Mendonça 2019 | 462 | 0 | - | RA |
| Hemisotoma thermophila Axelson, 1900 * | 0 | 2 | - | WSP | ||
| Psammisotoma sp. * | 0 | 29 | - | RA | ||
| Entomobryidae | Seira musarum Ridley, 1890 * | 0 | 6 | - | WSP | |
| Paronellidae | Cyphoderus innominatus Mills, 1938 * | 0 | 3 | - | Neo |
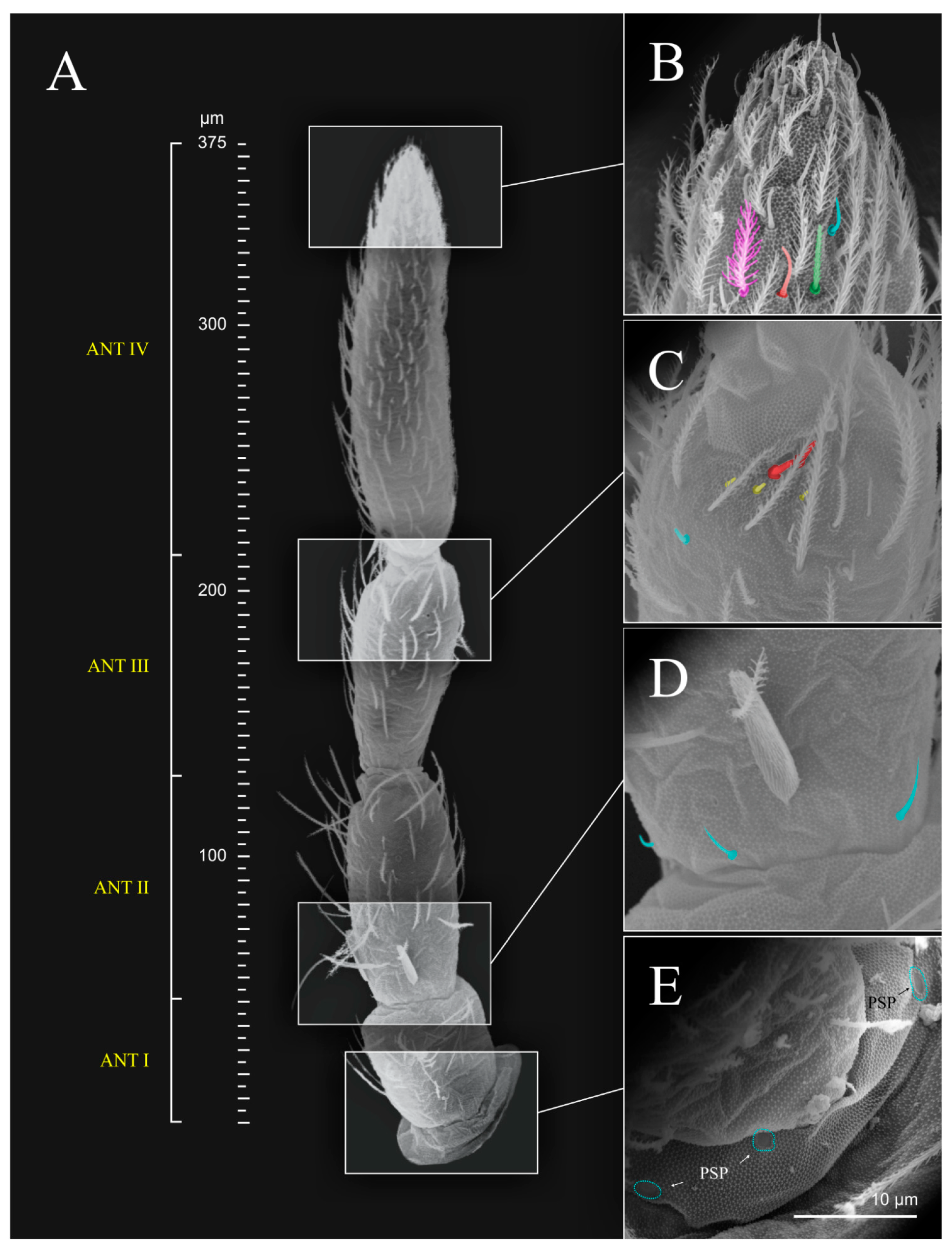
| Whole body | 1479.19 |
| Cd. | 284.42 |
| Ant. I | 82.35 |
| Ant. II | 148.13 |
| Ant. III | 211.96 |
| Ant. IV | 272.01 |
| Mucro | 11.31 |
| Dens | 407.57 |
| Manubrium | 298.90 |
| Unguis III | 45.36 |
| Unguiculus III | 26.96 |
| Tita. III | 363.50 |
| Femur III | 194.69 |
| Family/Species | Brazil Distribution | World Distribution | Habitat | Reference |
|---|---|---|---|---|
| Entomobryidae | ||||
| Amazhomidia ducke Cipola & Bellini, 2018 | AM | Amz | Soil | [45] * |
| A. thaisae Cipola & Bellini, 2018 | AM | Amz | Soil | [45] * |
| Capbrya brasiliensis Nunes, Santos-Costa & Bellini, 2020 | PI, RN | Amz | Cave | [46] * |
| Coecobrya phoenix Brito, Lima & Zeppelini, 2019 | MG, SP | NCB | Cave | [47] * |
| Dicranocentrus abestado Siqueira, Bellini & Cipola, 2020 | BA | NCB | Cave | [48] * |
| D. albicephalus Xisto & Mendonça, 2017 | ES, RJ | NCB | Atlantic forest litter | [49] * |
| D. amazonicus Bellini, Moraes & Oliveira, 2013 | AM | Amz | Forest litter | [50] * |
| D. bicolor Handschin, 1924 | SC | Pam | un | [51] |
| D. cuprum Xisto & Mendonça, 2016 | MG | NCB | Rainforest and iron ore soil | [52] * |
| D. heloisae Arlé & Mendonça, 1982 | RJ | NCB | Forest litter | [53] |
| D. magnus Xisto & Mendonça, 2017 | SP | NCB | un | [49] * |
| D. marimutti Xisto & Mendonça, 2017 | RJ | NCB | un | [49] * |
| D. melinus Xisto & Mendonça, 2016 | MG | NCB | un | [49] * |
| D. pikachu Xisto & Mendonça, 2017 | SP | NCB | un | [49] * |
| D. silvestrii Absolon, 1903 | RJ | Neo | Forest; São Francisco River | [54,55] |
| D. termitophilus Handschin, 1924 | MG | NCB | un | [51] |
| Entomobrya aipatse Arlé, 1959 | MT | NCB | Scrubland, litter | [56] |
| E. ataquensis Arlé, 1959 | MG, SP | NCB | On plants | [56] |
| E. atrocincta Schött, 1896 | FN | NCB | Forest litter | [8] * |
| E. bahiana Bellini, Cipola & Godeiro, 2015 | BA | NCB | Cave | [57] * |
| E. barbata Siqueira & Bellini, 2020 | BA | NCB | Cave | [58] * |
| E. decora Nicolet, 1847 | RJ | Neo | Duneland | [59] |
| E. egleri Arlé & Guimarães, 1978 | AM, PA | Amz | Forest litter | [60,61] |
| E. griseoolivata Packard, 1873 | PB | Bor, Neo | Forest litter, sand dune | [62] |
| E. inaequalis Arlé & Guimarães | PR | NCB | un | [63] |
| E. juneae Santos, Santos-Costa & Bellini, 2020 | RN | NCB | Cave | [58]* |
| E. nivalis Linnaeus, 1758 | PB | WSP | Forest litter, sand dune | [62] |
| E. paroara Arlé & Guimarães, 1978 | PA | Amz | Littoral | [60] |
| E. spectabilis Reuter, 1890 | AL, SE | NCB, Pal | On plants | [64,65,66] |
| E. tupiana Arlé, 1939 | RJ | NCB | Forest | [67] |
| E. uambae Arlé, 1959 | MT | Amz, NCB | Forest | [56] |
| E. wasmanni Handschin, 1924 | RJ | Neo | Forest litter | [51] |
| Heteromurtrella anae Cipola, 2016 | AM | Amz | Cave | [68] * |
| Lepidocyrtoides bicolorangelus Cipola & Bellini, 2017 | RR | Amz | Cave | [69] * |
| L. caeruleomaculatus Cipola & Bellini, 2017 | AM | Amz | Cave | [69] * |
| L. colormutatus Cipola & Bellini, 2017 | AM | Amz | Cave | [69] * |
| L. tapuia Arlé & Guimarães, 1980 | PB, RJ | NCB | Cave | [69] * |
| L. villasboasi Arlé & Guimarães, 1981 | MT | NCB | Cave | [69] * |
| Lepidocyrtus amazonicus Cipola & Bellini, 2018 | AM | Amz | Cultivation of organic guaraná | [69] * |
| L. americanus Cipola & Bellini, 2018 | AM, PA | Amz | Cultivation of conventional guaraná and rainforest | [69] * |
| L. maldonadoi Mari-Mutt, 1986 | PI, RN | NCB, Nea | Forest litter | [70] * |
| L. multisensillatus Cipola & Bellini, 2018 | AM | Amz | Rainforest | [45] * |
| L. nigrosetosus Folsom, 1927 | PB, FN | Neo | Forest litter, river sand | [8,71] * |
| L. pallidus Reuter, 1880 | RS | Bor, Neo, Pal | On plants | [66,72] |
| L. sotoi Bellini, Cipola & Godeiro, 2015 | PB | NCB | Atlantic forest and restinga woods | [57] * |
| L. violaceus Geoffroy, 1785 | SC | WSP | Soil | [8,73] * |
| Lepidosira neotropicalis Nunes & Bellini, 2019 | PI | Neo | Cave | [74]* |
| Mastigoceras camponoti Handschin, 1924 | AM, CE, MG, RJ, SP | Amz, NCB | Ant nest, forest litter, soil | [51,54,75,76,77] |
| Nothobrya arlei Silveira & Mendonça, 2016 | RJ | NCB | Urban area, litter and soil | [78] * |
| N. schubarti Arlé, 1961 | PE, PI | NCB | Lagoon | [79] |
| N. sertaneja Nunes & Bellini, 2020 | PI | Amz | Dryforest | [80] * |
| Pseudosinella acantholabrata Cipola, 2020 | MG | NCB | Cave | [81] * |
| P. alba Packard, 1873 | RJ | NCB | Forest litter | [82] |
| P. alfanjeunguiculata Bellini, Cipola & Souza, 2020 | MG | NCB | Cave | [81] * |
| P. ambigua Zeppelini, Brito & Lima, 2018 | MG | NCB | Cave | [83] * |
| P. aphelabiata Bellini, Cipola & Souza, 2020 | MG | NCB | Cave | [81] * |
| P. biunguiculata Ellis, 1967 | ES, FN | Neo | Papaya orchards, forest litter | [8,84] * |
| P. brevicornis Handschin, 1924 | RJ | NCB | Forest litter, soil and roots | [51] |
| P. brumadinhoensis Cipola, 2020 | MG | NCB | Cave | [81] * |
| P. cearensis Oliveira, Brito & Cipola, 2020 | CE | NCB | Cave | [81] * |
| P. chimerambigua Oliveira, Lima & Cipola, 2020 | MG | NCB | Cave | [81] * |
| P. diamantinensis Bellini, Cipola & Souza, 2020 | MG | NCB | Cave | [81] * |
| P. dubia Christiansen, 1960 | BA, CE, PB | NCB, Nea, Neo | Cave | [85] |
| P. guanhaensis Zeppelini, Brito & Lima, 2018 | MG | NCB | Cave | [83] * |
| P. keni Cipola, 2020 | MG | NCB | Cave | [81] * |
| P. labiociliata Cipola, 2020 | MG | NCB | Cave | [81] * |
| P. labruspinata Cipola, 2020 | MG | NCB | Cave | [81] * |
| P. macrolignicephala Oliveira, Lima & Cipola, 2020 | MG | NCB | Cave | [81] * |
| P. marianensis Bellini, Cipola & Souza, 2020 | MG | NCB | Cave | [81] * |
| P. mitodentunguilata Bellini, Cipola & Souza, 2020 | MG | NCB | Cave | [81] * |
| P. neriae Bellini, Cipola & Souza, 2020 | MG | NCB | Cave | [81] * |
| P. octopunctata Börner, 1901 | RJ | WSP | Forest soil, beach sand | [82] |
| P. paraensis Cipola, 2020 | PA | Amz | Soil and litter | [81] * |
| P. parambigua Oliveira, Lima & Cipola, 2020 | MG | NCB | Cave | [81] * |
| P. phyllunguiculata Oliveira, Lima & Cipola, 2020 | MG | NCB | Cave | [81] * |
| P. prelabruscervata Oliveira, Lima & Cipola, 2020 | MG | NCB | Cave | [81] * |
| P. pusilla Oliveira, Brito & Cipola, 2020 | PA | Amz | Cave | [81] * |
| P. serpentinensis Cipola, 2020 | MG | NCB | Cave | [81] * |
| P. spurimarianensis Bellini, Cipola & Souza, 2020 | MG | NCB | Cave | [81] * |
| P. taurina Cipola, 2020 | PA | Amz | Cave | [81] * |
| P. triocellata Nunes & Bellini, 2018 | PI | Amz | Dryforest | [86] * |
| P. unimacrochaetosa Cipola, 2020 | MG | NCB | Cave | [81] * |
| Rhynchocyrtus klausi Mendonça & Fernandes, 2007 | PB, RJ | NCB | Forest litter | [87] |
| Seira annulata Handschin, 1927 | SP | Neo | Forest | [18] |
| S. arenicola Bellini & Zeppelini, 2008 | PB | NCB | Forest litter, sand dune | [88] |
| S. atrolutea Arlé, 1939 | MS, SP, FN | NCB | Over plants, rocks and dead wood | [10,89] * |
| S. brasiliana Arlé, 1939 | MS, PB, RJ, SP | Bor, Neo | Beach sand, forest litter | [62,89] |
| S. caerucinerea Cipola, Morais & Bellini, 2014 | TO | NCB | Dryforest | [14] * |
| S. coroatensis Godeiro & Bellini, 2015 | RN | NCB | Dryforest | [90] * |
| S. dapeste Santos & Bellini, 2019 | RN | NCB | Soil | [91] * |
| S. diamantinae Godeiro & Bellini, 2015 | BA | NCB | Dryforest | [90] * |
| S. eidmanni Stach, 1935 | RJ, SP | NCB | Ant nest, tree bark | [18,75,92] |
| S. glabra Godeiro & Bellini, 2013 | PA | NCB | Dryforest | [93] * |
| S. harena Godeiro & Bellini, 2014 | PB | NCB | Soil | [94] * |
| S. jiboiensis Godeiro & Bellini, 2014 | BA | NCB | Soil | [94] * |
| S. mataraquensis Bellini & Zeppelini, 2008 | PB | NCB | Forest litter, sand dune | [88] |
| S. melloi Arlé, 1939 | ES, RJ | NCB | Lichen, moss | [92] |
| S. mendoncae Bellini & Zeppelini, 2008 | PB | NCB | Litter, soil, rocks | [88] |
| S. mirianae Arlé & Guimarães, 1981 | PB, RJ | NCB | Sand dune, forest litter, soil | [71,95] |
| S. musarum Ridley, 1890 | AM, MA, PA, PE, RJ, RN, AM, MA, PA, FN, SPSP, RA | WSP | On plants, soil, forest litter | [7,8,10] * |
| S. nigrans Arlé, 1959 | MT, PB | NCB | Scrubland, leaf litter, soil, rocks | [56,71] |
| S. nunezae Christiansen & Bellinger, 2000 | MS, SP | NCB | Litter, soil | [18] |
| S. paraibensis Bellini & Zeppelini, 2010 | PB | NCB | Forest litter, soil | [96] |
| S. paranensis Stach, 1935 | PR | NCB | un | [75] |
| S. paulae Cipola, Morais & Bellini, 2014 | PR | NCB | Atlantic forest | [14] * |
| S. picoensis Nunes, Bellini & Cipola, 2021 | PI | Amz | Dryforest | [97] * |
| S. pietata Oliveira, Ferreira & Zeppelini, 2020 | MG | NCB | Cave | [39] * |
| S. potiguara Bellini, Fernandes & Zeppelini, 2010 | RN | NCB | Sand dune | [96] * |
| S. praiana Bellini, Fernandes & Zeppelini, 2010 | RJ | NCB | Sand dune | [96] * |
| S. primaria Godeiro & Bellini, 2014 | CE | NCB | Dryforest | [94] * |
| S. prodiga Arlé, 1959 | MT, PB, PE, RJ | NCB | Forest litter, sand dune | [56,71] |
| S. pseudoannulata Bellini & Zeppelini, 2008 | PB | NCB | Forest litter, sand dune | [88] |
| S. pulcher Handschin, 1924 | SC | Bor, Pam | un | [18,51] |
| S. reichenspergeri Handschin, 1924 | SC | Pam | un | [51] |
| S. ritae Bellini & Zeppelini, 2011 | PB | Amz, NCB | Sand dune | [29] * |
| S. subannulata Denis, 1933 | ES, RJ | Neo | Lichen, moss | [92] |
| S. tinguira Cipola, Morais & Bellini, 2014 | PR | NCB | Rainforest | [12] * |
| S. trisetosa Nunes, Cipola & Bellini, 2021 | PI | Amz | Dryforest | [97] * |
| S. xinguensis Arlé, 1959 | MT; PB | NCB | Scrubland, reforestation in sand dune | [56,62] |
| Tyrannoseira bicolorcornuta Bellini, Pais & Zeppelini, 2009 | PE | NCB | Over sand, rocks | [98] * |
| T. diabolica Bellini & Godeiro, 2012 | RN | NCB | Litter, soil, rocks | [99] * |
| T. gladiata Zeppelini & Lima, 2012 | PB | NCB | Litter, soil, rocks | [100] * |
| T. raptora Zeppelini & Bellini, 2006 | PB | NCB | Litter, soil, rocks | [101] * |
| T. sex Bellini & Zeppelini, 2011 | PB | NCB | Litter soil, rocks | [29] * |
| Paronellidae | ||||
| Campylothorax cassagnau Mitra & Dallai, 1980 | RJ | Neo | Forest litter | [54] |
| C. mitrai Bellini & Meneses, 2012 | AL, BA | NCB | Forest | [102] * |
| C. plagatus Cipola & Oliveira, 2016 | AM | Amz | Rainforest | [103] * |
| C. schaefferi Börner, 1906 | AM, MG, RJ | Amz, Pam | Forest | [55,61,82,104] |
| C. viruaensis Santos, Cipola & Bellini, 2016 | RR | Amz | Rainforest | [105] * |
| Cyphoderodes xenopus Börner, 1913 | RS | NCB | Ant net | [106] |
| Cyphoderus agnotus Börner, 1906 | PE, FN | Bor?, Neo | Forest | [8,10,54] * |
| C. arlei Cassagnau, 1963 | RJ | NCB | un | [54] |
| C. caetetus Zeppelini & Oliveira, 2016 | MG, PE, FN | NCB | Rainforest | [8,10,107] * |
| C. equidenticulati Nunes & Bellini, 2018 | PI | Amz | Dryforest | [86] * |
| C. innominatus Mills, 1938 | PE, RJ, FN, RA | Neo | Sand dune, grassland | [8,10,54] * |
| C. javanus Börner, 1906 | ? | WSP | un | [86] * |
| C. mucrominimus Oliveira, Alves & Zeppelini, 2017 | PA | Amz | Cave | [108] * |
| C. mucrostrimenus Oliveira, Alves & Zeppelini, 2017 | PA | Amz | Cave | [108] * |
| C. similis Folsom, 1927 | ES, FN | Bor, Pal, Neo | Papaya orchards | [8,10,84] * |
| Lepidonella zeppelinii Soto-Adames & Bellini, 2015 | RN | NCB | Rainforest | [109] * |
| Paronellides alticolus Arlé, 1939 | RJ | NCB | Forest, leaf litter | [67] |
| Salina bellinii Oliveira & Cipola, 2018 | PA, PB, RN, RR | WSP | Rainforest | [110] * |
| S. brasiliana Oliveira & Cipola, 2018 | AC, AL, AM, MG, PE, PR, RJ, RO, RR, SC | Nea, Neo, NCB | Rainforest | [110] * |
| S. celebensis Schäffer Schäffer, 1898 | RJ | WSP | Forest | [54] |
| S. dedoris Mari-Mutt, 1987 | RR | Neo, Amz | Rainforest | [110] * |
| S. hamadae Oliveira & Cipola, 2018 | SC | NCB | Atlantic forest | [110] * |
| S. maculiflora Oliveira & Cipola, 2016 | AM | Amz | Rainforest | [111] * |
| S. maculipenis Oliveira & Cipola, 2018 | PR, RS | NCB | Atlantic forest | [110] * |
| S. serrana Oliveira & Cipola, 2018 | SP | NCB | Atlantic forest | [110] * |
| S. tocantinensis Oliveira & Cipola, 2018 | TO | NCB | Dryforest | [110] * |
| S. tristani Denis, 1931 | AM, MS, MT, PA, PR, RJ, SC, SP | Neo, Amz, NCB | Rainforest | [110] * |
| S. unisetosa Oliveira & Cipola, 2018 | AC | NCB | Rainforest | [110] * |
| S. zhangi Bellini & Cipola, 2017 | BA | NCB | Soil, litter | [112] * |
| Troglobius albertinoi Cipola & Bellini, 2016 | AP | Amz | Rainforest | [113] * |
| T. brasiliensis Palacios-Vargas & Zeppelini, 1995 | PA, SP | Amz, NCB | Cave | [114] |
| T. ferroicus Zeppelini, Silva & Palacios-Vargas, 2014 | MG | NCB | Cave | [115] * |
| Trogolaphysa aelleni Yoshii, 1988 | SP | NCB | Cave | [116] |
| T. cotopaxiana Thibaud & Najt, 1988 | FN | NCB | Forest litter | [8,10] * |
| T. ernesti Cipola & Bellini, 2017 | CE | NCB | Dryforest | [112] * |
| T. formosensis Silva & Bellini, 2015 | RN | Oriental, NCB | Atlantic forest | [117] * |
| T. hauseri Yoshii, 1988 | SP | NCB | Cave | [116] |
| T. hirtipes Handschin, 1924 | RJ, SC | Neo, Pam, NCB | Forest | [51,54] |
| T. millsi Arlé, 1939 | RJ | NCB | Forest | [67] |
| T. piracurucaensis Nunes & Bellini, 2018 | PI | Amz | Dryforest | [86] * |
| T. tijucana Arlé & Guimarães, 1979 | RJ | NCB | Forest | [118] |
| Oncopoduridae | ||||
| Oncopodura hyleana Arlé, 1961 | AP | Amz | Forest litter | [119] |
| O. itatiaiensis Arlé, 1961 | RJ | NCB | Forest litter | [119] |
| Isotomidae | ||||
| Archisotoma arariboia Neves & Mendonça, 2014 | RJ | NCB | Sand beach | [120] * |
| A. besselsii Packard Packard, 1877 | RJ | Bor, Neo | Intertidal zone | [121] |
| A. catiae Abrantes & Mendonça, 2007 | RJ | NCB | Sand dunes | [122] |
| A. gourbaultae Thibaud, 1993 | RJ | Neo | Beach | [123] |
| A. jariani Lima, Zeppelini & Mendonça, 2019 | RN, RA | NCB | Sand beach | [124] * |
| Arlea adetolai Mendonça, Abrantes & Fernandes, 2006 | RJ | NCB | Soil, litter | [125] |
| A. arenicola Abrantes & Mendonça, 2005 | RJ | NCB | Sand dune | [126] |
| A. lucifuga Arlé, 1939 | MG, RJ | NCB | Litter, soil, humus, termites’ nests | [67,127] |
| A. psammophila Mendonça, Abrantes & Fernandes, 2006 | RJ | NCB | Beach | [125] |
| A. spinisetis Mendonça & Arlé, 1987 | CE, RJ | NCB | Scrubland, beach, riparian vegetation, farming areas | [127] |
| Axelsonia littoralis Moniez, 1890 | Brazilian Coast | Bor, Aus, Neo | un | [128] |
| A. tubifera Strenzke, 1958 | SP | Neo | un | [121] |
| Ballistura fitchi Denis, 1933 | ES, RJ | Bor, Pal, Neo | Lichen, moss, soil | [92,129] |
| Clavisotoma filifera Denis, 1931 | RJ | Bor, Aus, Neo | Sand dune | [130] |
| Cryptopygus pentatomus Börner, 1906 | ? | NCB | un | [55] |
| C. separatus Denis, 1931 | RJ | Neo, Pal | Soil, litter | [82] |
| C. tingus Queiroz & Mendonça, 2010 | ES | NCB | Forest litter | [131]* |
| Desoria trispinata MacGillivray, 1896 | RJ | Bor, Pal, Neo | Soil, litter, halophyte-psammophyte vegetation | [132] |
| Folsomia candida Willem, 1902 | RJ | WSP | Litter | [133] |
| F. similis Bagnall, 1939 | RJ | Bor, Aus, Neo | Litter, under rocks | [82] |
| F. wellingdae Potapov & Culik, 2002 | ES | NCB | Soil | [134] |
| Folsomides centralis Denis, 1931 | AM, ES, RJ, FN | WSP | Soil, tree trunk, halophyte-psammophyte vegetation, foredune environments, litter, grass over rocks between sand beach, mangrove | [61,92] |
| F. parvulus Stach, 1922 | AM, ES, PA, RJ, FN | WSP | Soil, litter, humus, rotten trunk, grass over rocks between sand beach and mangrove, sand dunes, forest litter | [61,84,135] |
| F. semiparvulus Fjellberg, 1993 | RJ | Bor, Neo | Sand dune | [122] |
| Folsomina onychiurina Denis, 1931 | AM, ES, RS, FN | WSP | Beach, soil, litter, riparian vegetation, understory vegetation, sand dunes, beach, grass | [61,92,136] |
| Hemisotoma thermophila Axelson, 1900 | PB, RJ, FN, SPSP, RA | WSP | Sand dune, halophyte-psammophyte vegetation, litter | [71] * |
| Isotomiella amazonica Oliveira & Deharveng, 1990 | AM, RJ | Amz, NCB | Sand dune, litter | [137,138] |
| I. arlei Oliveira & Deharveng, 1990 | AM | Amz | Flood plain, primary and secondary forest soil, litter | [138] |
| I. barrai Deharveng & Oliveira, 1990 | AM, RJ | Amz, NCB | Primary forest soil and litter | [138,139] |
| I. barrana Mendonça & Abrantes, 2007 | RJ | NCB | Herbaceous vegetation, sand dunes, litter and soil | [137] |
| I. bidentata Delamare Deboutteville, 1950 | RJ | Neo, Pal | Litter | [137] |
| I. canina Mendonça & Fernandes, 2003 | RJ | NCB | Soil, litter | [139] |
| I. denticulata Mendonça & Queiroz, 2017 | RJ | NCB | Litter and soil in Atlantic Forest | [140] * |
| I. digitata Deharveng & Oliveira, 1990 | RO | Amz | Forest soil and litter | [138] |
| I. distincta Mendonça & Fernandes, 2003 | RJ | NCB | Soil, litter | [139] |
| I. dupliseta Deharveng & Oliveira, 1990 | AM | Amz | Soil | [138] |
| I. falcata Mendonça & Fernandes, 2003 | RJ | NCB | Soil | [139] |
| I. felina Mendonça & Fernandes, 2003 | RJ | NCB | Soil, litter | [139] |
| I. granulata Oliveira & Deharveng, 1990 | AM, RO | Amz, NCB | Scrub, forest litter | [138] |
| I. louisi Mendonça & Queiroz, 2016 | PA | Amz | Rainforest | [141] * |
| I. macedoi Mendonça, Abrantes & Neves, 2012 | RJ | NCB | Rainforest litter | [142] * |
| I. minor Schäffer, 1896 | ES | WSP | un | [92] |
| I. nummulifer Deharveng & Oliveira, 1990 | AM, RJ, FN | Pal, Neo | Soil | [138] |
| I. proxima Mendonça & Fernandes, 2003 | RJ | NCB | Soil, litter | [139] |
| I. quadriseta Deharveng & Oliveira, 1990 | AM; RJ | Amz, NCB | Litter, soil | [138,139] |
| I. sensillata Oliveira & Deharveng, 1990 | AM, RO | Amz, NCB | Litter | [138] |
| I. similis Oliveira & Deharveng, 1990 | AM | Amz | Soil | [138] |
| I. spinifer Deharveng & Oliveira, 1990 | AM | Amz | Soil | [138] |
| I. symetrimucronata Najt & Thibaud, 1987 | AM, ES, RJ | Pal, Neo | Soil, litter, sand dune | [86,138] |
| I. uai Mendonça, Abrantes & Nevez, 2012 | MG | NCB | Rainforest litter | [142] * |
| Isotomodes cariocus Thibaud & Palacios-Vargas, 1999 | RJ, FN | NCB | Beach | [136] |
| I. fernandesae Abrantes & Mendonça, 2007 | RJ | NCB | Beach | [122] |
| I. trisetosus Denis, 1923 | AM | Bor, Pal, Neo | Primary and secondary forest soil and litter | [61] |
| Isotomurus palustris Müller, 1776 | BA, ES, PE | WSP | Seedbed | [92,143] |
| I. pseudosensillatus Mendonça, 1990 | CE | NCB | Scrubland, litter | [144] |
| I. riparius Mendonça, 1990 | RJ | NCB | Duneland | [144] |
| Micranurophorus musci Bernard, 1977 | RJ | Bor, Neo | Sand dunes | [6] |
| Mucrosomia alticola Mendonça & Queiroz, 2013 | MG, RJ | Europe | Litter and soil in Atlantic Forest in 1400 m | [145] * |
| Najtia vicaria Arlé, 1960 | RJ | NCB | Soil, litter, rotten trunk, termite galleries | [56] |
| Paracerura airesi Mendonça, Abrantes & Fernandes, 2009 | TO | NCB | Soil | [146] * |
| P. bella Mendonça & Queiroz, 2017 | RJ | NCB | Soil and litter in Atlantic Forest | [140] * |
| P. cristinae Abrantes & Duarte, 2013 | SP | NCB | Soil in Atlantic Forest | [147] * |
| P. gandarela Mendonça & Silveira, 2013 | MG | NCB | Litter and soil on montane | [148] * |
| P. itatiaiensis Arlé, 1959 | RJ | NCB | Moist soil | [56] |
| P. pallida Abrantes & Duarte, 2013 | SP | NCB | Soil in Atlantic Forest | [147] * |
| P. paulista Abrantes & Duarte, 2013 | SP | NCB | Soil in Atlantic Forest | [147] * |
| P. pindorama Queiroz & Mendonça, 2010 | ES | NCB | Litter in Atlantic Forest | [131] * |
| P. serrana Mendonça, Abrantes & Fernandes, 2009 | MG | NCB | Soil on 1600 m | [146] * |
| P. virgata Deharveng & Oliveira, 1994 | AM | Amz | Forest soil | [149] |
| Proisotoma copiosa Mendonça, Queiroz & Silveira, 2015 | ES | NCB | Soil and litter in 2447 m Atlantic Forest | [150] * |
| P. douglasi Mendonça, Queiroz & Silveira, 2015 | RJ | NCB | Soil and litter in 2447 m Atlantic Forest | [150] * |
| Proisotoma immersa Folsom, 1924 | SPSP | NCB | Soil and litter | * |
| P. minima Absolon, 1901 | MG | WSP | Forest litter | [6]* |
| P. minuta Tullberg, 1871 | RJ | WSP | Soil, litter, humus | [151] |
| P. oliveirae Deharveng, 1984 | AM | Amz | Plant litter | [152] |
| P. ramosi Arlé, 1959 | MG, RJ, SP | NCB | Litter | [56,119] |
| P. subminuta Denis, 1931 | NCB | Bor, Neo | un | [127] |
| P. tenella Reuter, 1895 | ES, PR, RJ | WSP | Halophyte-psammophyte vegetation, sand dune, soil | [84,130,153] |
| Psammisotoma restingae Abrantes & Mendonça, 2009 | RJ | NCB | Halophyte-psammophyte vegetation | [23] |
| Yosiiella mira Hüther, 1967 | AM | Amz | Rainforest | [154] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima, E.C.A.; Lopes, B.C.H.; Oliveira-Neto, M.A.; de Mendonça, M.C.; Zeppelini, D. Synthesis of Brazilian Entomobryomorpha (Collembola: Hexapoda) with Special Emphasis on the Equatorial Oceanic Islands and Redescription of the First Species of Collembola Recorded in Brazil. Diversity 2022, 14, 553. https://doi.org/10.3390/d14070553
de Lima ECA, Lopes BCH, Oliveira-Neto MA, de Mendonça MC, Zeppelini D. Synthesis of Brazilian Entomobryomorpha (Collembola: Hexapoda) with Special Emphasis on the Equatorial Oceanic Islands and Redescription of the First Species of Collembola Recorded in Brazil. Diversity. 2022; 14(7):553. https://doi.org/10.3390/d14070553
Chicago/Turabian Stylede Lima, Estevam C. A., Bruna C. H. Lopes, Misael A. Oliveira-Neto, Maria Cleide de Mendonça, and Douglas Zeppelini. 2022. "Synthesis of Brazilian Entomobryomorpha (Collembola: Hexapoda) with Special Emphasis on the Equatorial Oceanic Islands and Redescription of the First Species of Collembola Recorded in Brazil" Diversity 14, no. 7: 553. https://doi.org/10.3390/d14070553
APA Stylede Lima, E. C. A., Lopes, B. C. H., Oliveira-Neto, M. A., de Mendonça, M. C., & Zeppelini, D. (2022). Synthesis of Brazilian Entomobryomorpha (Collembola: Hexapoda) with Special Emphasis on the Equatorial Oceanic Islands and Redescription of the First Species of Collembola Recorded in Brazil. Diversity, 14(7), 553. https://doi.org/10.3390/d14070553






