Abstract
The temporal partitioning hypothesis refers to the promotion of stable species’ coexistence by reducing the likelihood of competitive exclusion, such as when species are active at different months of the year. However, the studies confirming the mechanisms of species’ coexistence focus on spatial scale, and temporal partitioning hypothesis for species’ coexistence remains underexplored. Fungal sporophores that are sensitive to seasonality change are ideal candidates for studying the role of temporal differentiation hypothesis in species’ coexistence. In this study, a field survey of fungal sporocarps was conducted from May to October, and the entity and abundance of different species of fungal sporocarps in a 5-hectare forest dynamic plot in a temperate, deciduous broad-leaved forest were recorded. The results showed that the emergence of fungal sporocarps based on month was highly specialized and uneven. The torus-translation test showed that 56 species exhibited ecological habitat preferences for different months (47/100, 47%). The distribution of soil fungal sporocarps (35/75, 46.67%) based on months showed higher specialization compared with that of rotten-wood fungal sporocarps (9/22, 40.90%). The findings suggest the importance of temporal partitioning in maintaining local diversity in the fungal community.
1. Introduction
Macrofungi constitute an important part of the ecological system and form a large share of species’ diversity [1,2]. Fungal sporocarps usually grow on the substrate and play an important role in the material cycles of forest ecosystems. They can decompose dead plants and play an important role in the ecological balance of nature. The assembly mechanism of fungal sporophores is poorly understood and difficult to study because of their hidden nature and frequently sporadic and short-lived characteristics [3,4]. In particular, the mechanism underlying the community dynamics of fungal sporophores on a time scale has been rarely studied.
Species’ coexistence of macrofungal sporocarps is achieved through the availability of different substrates, both spatially and temporally [5,6]. Therefore, different species’ combinations should form at different community levels in different spaces or at different times [7,8]. If the environmental conditions of the availability of substrates have a spatial structure, it should be reflected in the distribution of species through their correlation with different habitats or time [3]. Therefore, different habitats or times should form different species’ combinations at the community level [9]. Many species possess distinct, specific temporal preferences, highlighting the importance of temporal characteristics for defining species’ assemblages [8,10,11,12]. If the availability of substrates at the temporal scale is important for maintaining the species’ richness of fungal communities, then the distribution of fungal sporophores will be strongly influenced by time [8]. However, the temporal partitioning that drives fungal community assembly is not as well-known as those in animals [13,14,15] and plants [16,17,18].
Temporal partitioning refers to the promotion of the coexistence of stable species by reducing the possibility of competitive exclusion, such as the changes in species’ composition in the different months of a year’s growth active period [19,20]. On the time scale, temperature and rainfall are the major determinants for the variation in fungal sporophore composition [21,22,23,24]. Remarkable differences in precipitation and temperature in different months of a year were observed in temperate, deciduous broad-leaved forests [25]. In the temperate zone, the growth season of fungal sporocarps is from May to October [2]. In these ecosystems, fungal sporophores are species rich and their diversity varies substantially among different months [26]. Therefore, temporal partitioning may be an important reason for the fungal sporophore diversity in temperate, deciduous broad-leaved forests.
Environmental factors are geographically distinct, and the growth of fungal sporocarps is closely related to temperature, light, and precipitation [1]. Therefore, the growth habits and characteristics of fungal sporocarps growing in different substrates are different [27]. Fungal sporophores can inhabit different substrates, such as rotten wood, soil, and living wood; thus, fungal sporocarps can also be divided into rotten-wood fungal sporocarps, soil fungal sporocarps, living-wood fungal sporocarps, and so on [1]. Previous studies showed that the mechanism of species’ coexistence differs among fungal sporophores in different substrates [27]. However, whether community assembly differs on a time scale for different functional guilds of fungal sporophores (e.g., soil and rotten-wood fungi) is not well understood. Funiu Mountain is located in the transition region from the warm temperate zone to the north subtropical zone and has the climatic characteristics of both the warm temperate zone and the north subtropical zone. Baiyun Mountain National Forest Park is located in the heart of the Funiu Mountains. However, the diversity and composition of fungal sporocarps in Baiyun Mountain National Forest Park have not been systematically investigated. Previous studies of the relationship between macrofungi and environmental factors in the area have shown differences in fruiting–environmental relationships between microhabitats defined by topography, understory light availability, and plant communities. However, the temporal partitioning of fungal sporocarps in Baiyun Mountain National Forest Park has not been investigated systematically.
In the present study, we hypothesized that temporal partitioning is important for structuring epigeous fungal communities in temperate, deciduous broad-leaved forests and, hence, would be important for the maintenance of their local species’ diversity. These hypotheses were tested by investigating fungal sporophores monthly from May to October in a 5-hectare forest dynamic plot in a temperate forest in China. The temporal preferences of fungal species were examined by network analysis and torus-translation testing.
2. Materials and Methods
2.1. Study Site and Sampling
Field work was carried out in a temperate forest in the Baiyunshan National Nature Reserve, Henan Province (111°48′–112°16′ E, 33°33′–33°56′ N, China). The Baiyun Mountain belongs to the warm temperate zone and is influenced by the monsoon. The study area has a forest coverage of 81.2% and a deciduous broad-leaved forest as the typical zonal vegetation. Quercus aliena var. acuteserrata, Toxicodendron vernicifluum, and Sorbus alnifolia are some dominant tree species in this forest [27].
According to the construction standards of the Smithsonian Institution’s Center for Tropical Forestry Research [28], a 5-hectare plot (250 m × 200 m, horizontal distance) was established in the Baiyunshan National Nature Reserve. The 5-hectare plot was divided into 125 quadrats (400 m2 each). The average annual temperature in the study area is 13.5 °C, and the average annual precipitation is 1200 mm. The topography of the plot is rugged, the elevation ranges from 1538 m to 1600 m, and the slopes range from 4.3° to 55.5° [27].
2.2. Collection and Identification of Fungal Sporocarps
Macrofungi growth is closely related to climatic conditions, such as temperature and precipitation; therefore, fungal sporocarps should be monitored over time. The rainy season in the Baiyun Mountain begins in May and ends in October, the time when fungal sporocarps gather spawn. Therefore, we chose the end of each month from May to October to investigate the fungal sporocarps.
Fungal sporocarps in different habitats, such as herb layer, leaf layer, dead branches, and tree trunk, were observed with each 20 m × 20 m quadrat as the basic unit. All species present in the plots were marked and photographed during each survey, including those that had not disappeared since the previous survey. The fungi were not removed from the plot to avoid the impact of sample collection on the next investigation. The species’ number, habitat type (rock, dead wood, ground, etc.), and habitat distribution (single occurrence, overlapping, cluster, scatter, etc.) of the fungal sporocarps were recorded.
Three to five photos of each individual fungal sporocarp were captured from different angles, and each photo was numbered. The features of the fungal sporocarps, including the front, side, and different parts (including the external shape, color, velum, prosthecae, and mediotrastum), as well as grade profile, were recorded [2].
The Chinese names of the fungal sporocarps were determined according to the classification system of Hawksworth [29]. Based on the substrate and mycorrhizal formation, the fungal sporocarps were divided into four ecological types: living tree fungal sporocarps, litterfall fungal sporocarps, soil fungal sporocarps, and rotten-wood fungal sporocarps (Figure S1).
2.3. Data Analysis
Kruskal–Wallis test is often used to compare three or more populations. This method was used to test for differences in the richness and abundance of fungal sporocarps’ species during the 6 months. Species’ richness refers to the number of species, and species’ abundance is the number of individuals of all species in the 400 m2 quadrats in the plot.
The impact of the month on the beta diversity of the fungal sporocarps’ species was assessed using the betadisper function. Permutational multivariate ANOVA (PERMANOVA) was performed to test whether these distances differed among months. PERMANOVA was applied to explore the significant differences based on 999 permutations [30]. The betadisper test was conducted using the “betadisper” command in the “vegan” package.
A fungal sporocarps–month network graph was used to visualize the strong potential relationships between fungal sporophores and months. The structure of the sporophore–month network was evaluated using the H2′ metric of specialization and connectance index [31]. The architecture of the sporophore–month network was visualized using the “bipartite” package of R software [32].
The associations of fungal sporophores with months were determined by torus-translation tests [10,11]. In the 5-hectare plot, environmental conditions, such as light, temperature, and precipitation, vary greatly between each month. Torus-translation tests compare the true relative densities of focal species in a month with expected relative densities under a null model, in which the species are distributed randomly among months [11]. The method considers the temporal autocorrelation of species’ distribution among months. Further details on torus-translation tests are provided in the study by Harms et al. [10]. In our study plot, 750 unique torus-translation habitat maps were initially possible (125 quadrats in the plot, 6 months). Then, three original maps, namely, 180° rotation, mirror image, and 180° rotation of the mirror images, could be generated to continue the torus-translation [10,11]. In total, these procedures provided one real and 2999 translated maps. If the true relative density of the fungal sporophores in the focal month was greater or smaller than at least 97.5% of the expected relative densities, then it was statistically associated with that month (positively or negatively associated, α = 0.05 level of significance for a two-tailed test). Month associations were only tested for fungal sporophores with more than five individuals in the 5-hectare plot. Nearly 46.08% of the fungal sporophores (100/217) recorded had greater than five individuals in the 5-hectare plot [11].
All data analyses in the study were conducted in R version 3.4.0 [33] (R Development Core Team, 2017).
3. Results
3.1. Species’ Diversity Differences among Months
A total of 2433 fungal sporophores from 217 species in 33 families were recorded (Table S2). A total of 32, 16, 73, 104, 42, and 55 fungal sporocarps’ species were recorded from May to October, respectively. Soil and rotten-wood fungal sporocarps were the main components of the fungal communities (Figure S1). The abundance and richness of the fungal sporophores differed among the different months (Figure 1). The abundance and richness of the fungal sporocarps reached the maximum in August (Figure 1). PERMANOVA tests showed significant differences in the community dispersion of fungal sporocarps during the 6 months (F = 7.5066, p = 0.001; Figure 2). The results indicated that the species’ diversity of the fungal sporocarps was seasonal and the abundance of the fungal sporocarps varied remarkably.
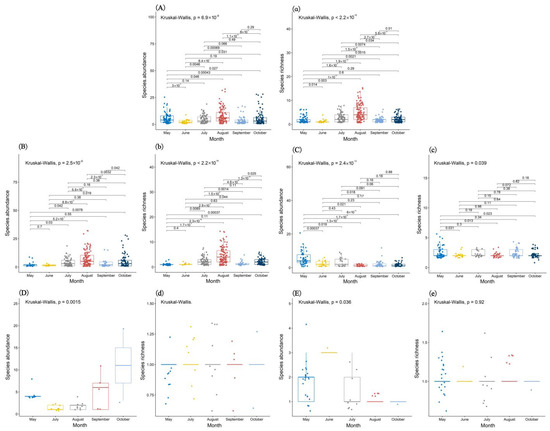
Figure 1.
Species’ richness and abundance of all fungal sporocarps (A,a), soil fungal sporocarps (B,b), rotten-wood fungal sporocarps (C,c), litterfall fungal sporocarps (D,d), and living-tree fungal sporocarps (E,e) in 6 months, as demonstrated by a boxplot diagram. The different colored boxplots represent the species’ richness or abundance of the fungal sporophores in 6 months.
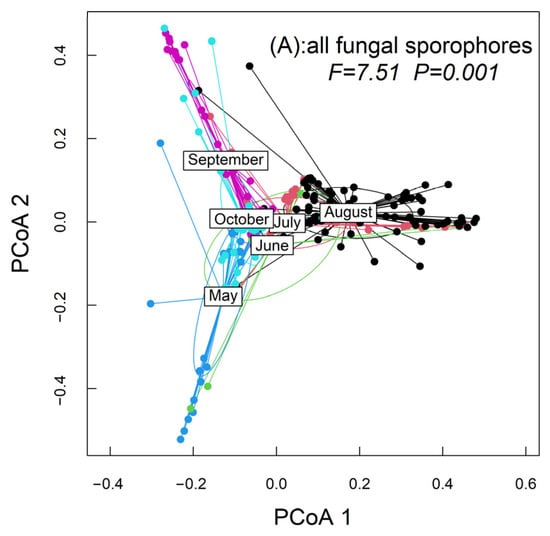
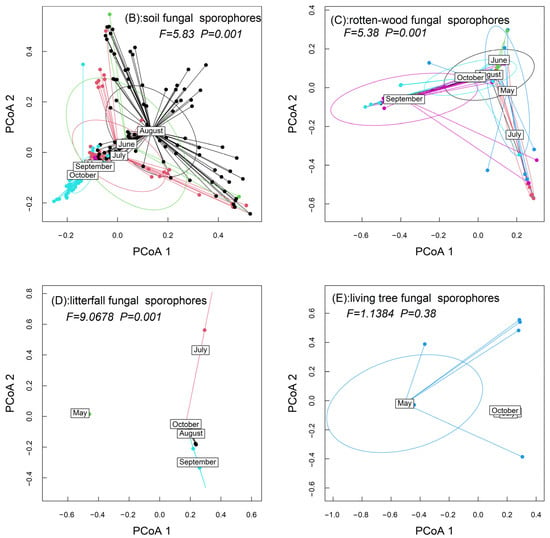
Figure 2.
Effect of months on beta diversity of the fungal sporocarps by PCoA. PERMANOVA was applied to test how these distances differed among months. Capital “F” refers to F values, and capital “P” refers to p values. PCoA1 and PCoA2 are the first and second sort axes in the “PERMANOVA” analysis, respectively.
3.2. Associations between Sporophores and Months
The architecture of the fungal sporocarps–month network showed that 10, 6, 35, 57, 15, and 22 fungal sporocarps’ species were recorded from May to October, respectively (Figure 3). The connectance index, which shows the potential associations between fungal sporophores and the months, was 24.73%. The H2′ estimate of interaction specialization was 74.87%.
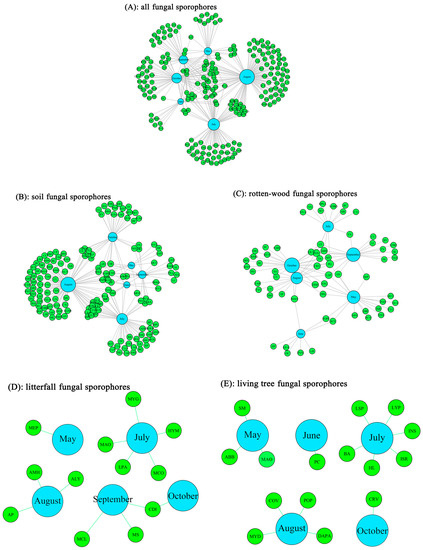
Figure 3.
Network diagram of the fungal sporocarps–month network in a temperate forest in China. The network includes 217 species of fungal sporocarps, including 150 soil fungal sporocarps, 64 rotten-wood fungal sporocarps, 12 litterfall fungal sporocarps, and 15 living-tree fungal sporocarps. The thickness of lines and the size of rectangles represent the number of observed associations.
Based on the torus-translation tests, the 47 species examined were associated with the months (47/100, 47%). A total of 4, 5, 9, 8, 15, and 7 species showed positive associations (p < 0.05) to May, June, July, August, September, and October, respectively (Table S1). Two of the species (Pycnoporus coccineus and Volvariella volvacea) examined were associated positively with two months. Moreover, only Phellinus gilvus showed a negative association with May and August. The detailed associations of the fungal sporocarps’ species with months are shown in Table S1.
3.3. Association Difference between Fungal Sporocarps
The variations in abundance and richness were higher in the soil fungal sporocarps than in the rotten-wood fungal sporocarps (Figure 1). PERMANOVA tests showed significant differences in the community dispersion of the soil fungal sporocarps (F = 5.8294, p = 0.001) and rotten-wood fungal sporocarps (F = 5.3819, p = 0.001) during the 6 months (Figure 2).
Based on the connectance index, 25.26% and 22.89% of the possible interactions among fungal sporophores and months actually occurred in the soil and rotten-wood fungal sporocarps, respectively. The H2′ estimates of interaction specialization were 76.75% and 68.05% in the soil and rotten-wood fungal sporocarps, respectively (Figure 3).
Based on the torus-translation tests, 35 (35/75, 46.67%) positive associations with months were detected in the soil fungal sporocarps, whereas only 9 (9/22, 40.90%) were found in the rotten-wood fungal sporocarps (Table S1).
4. Discussion
In this study, the abundance and richness of species over their fruiting periods were approximately symmetrical in distribution from May to October. This finding matched previous reports on intra-annual fungal fruiting in other regions [21,23]. The H2′ estimate of interaction specialization for the sporophore–month network was 0.749, which was as high as those previously reported in fungal sporocarps–month networks (0.265) [34], plant–seed disperser networks (0.354) [35], and plant–pollinator networks (0.533) [9]. The distribution of fungal sporophores in different months showed specialization and unevenness. Baiyun Mountain belongs to a deciduous broad-leaved forest; environmental factors, such as temperature, precipitation, and light availability, remarkably differ among different months of the year in temperate, deciduous broad-leaved forests [21,22,23,24]. These differences also provide diverse habitats for the growth of diverse fungal sporophores’ species on a time scale. The growth of fungal sporocarps depends not only on temperature and precipitation but also on the response of species to environmental factors. The results showed that the growth and diffusion of fungal sporocarps could be facilitated by low light and good ventilation [2,25,27]. The finding suggested that the distribution of macrofungal sporophores among different months is not random and has specialization.
The distribution of fungal sporocarps in the microhabitats of different months is not random but specialized. Torus-translation tests showed that nearly half of the fungal species exhibited ecological habitat preferences for different months. Temperature and precipitation are the two major determinants of fungal sporophore production on a time scale [21,22,23,24]. In the forest ecosystem, the species composition of fungal sporophore changed obviously every month, and the dead leaves of the deciduous woody plants in autumn provided a good substrate for the growth and reproduction of fungal sporophore in the deciduous broad-leaved forest [14]. Temperature and precipitation show remarkable differences among different months of the year in temperate deciduous broad-leaved forests [25]. This phenomenon may be an important reason why fungal sporophores exhibited ecological specialization. Our study demonstrated the importance of temporal partitioning in maintaining local diversity in the fungal sporophore community.
The patterns of fruiting timing are strongly correlated with temperature in August [21,23]. However, in the present study, a lower number of species–month associations were detected (9) to August and compared with July and September (13 and 16 positive associations, respectively), although the species’ richness and abundance of sporophores were the highest in August. The higher temperatures and stronger light intensity in August than in July and September are more conducive to evaporation [25]. The environmental changes of a temperate, deciduous broad-leaved forest over a period of five months are difficult to express simply. There are many indicators of habitat factors, such as wind and grass movement; as long as it is related to soil, the factors that can cause soil change can influence the diversity of fungal sporophores in soil more or less. In previous studies, environmental conditions, such as high temperatures, strong light, and low humidity, are not conducive to sporophore growth [26,27]. The temperature drops in September and October, and the fruiting bodies of macrofungi enter the decay period [36,37,38]. The conditions and resources for fungal sporophore growth vary; therefore, a temporal differentiation in distribution and preference may exist, and some fungal sporophore species may not prefer August.
Fungal sporophore species were categorized into two main groups, namely, soil and rotten-wood fungal sporophores, to determine their associations with months. Compared with the distribution of rotten-wood fungal sporophores, the distribution of soil fungal sporophores based on months showed higher specialization. In addition, more soil macrofungal sporophores than rotten-wood fungal sporophores exhibited distinct temporal preferences. This finding could be caused by community assembly differences between soil and rotten-wood fungal sporophores. The light environment, humidity, humus, and other factors in the plant community have differences that will influence fungal sporophores [27]. In the temperate, deciduous broad-leaved forest, the precipitation and temperature in different months of the year also have remarkable differences [14]. Plants first absorb CO2 through photosynthesis to produce organic matter stored in the body, which is the process by which forests absorb carbon. Through the respiration of plants themselves, some carbon is released and stored in the soil in the form of litter, root debris, etc.; some of the carbon in the soil is released into the atmosphere by microorganisms and other heterotrophs through decomposition and respiration. In this process, the formation of fungal sporophores, which grow in litter, soil, and rotten-wood, is affected by many factors. Fungal sporocarps have many species and their resource needs are diverse [27]. The effect of temporal partitioning on the diversity of fungal sporocarps is reflected by temporal preference. Previous studies observed light as the major driver for soil fungal sporophores, and unknown spatial processes are the major drivers for rotten-wood fungal sporophores [27]. Compared with the soil microhabitat, the distribution of fallen dead wood is a discontinuous microhabitat in space. Dispersal can be a limiting factor for the occurrence of rotten-wood fungal sporophores [27]. The number and distribution of fallen dead woods limit the growth space for rotten-wood fungal sporophores. However, light availability differences were distinct among the different months in the temperate deciduous broad-leaved forest [25]. Therefore, more soil fungal sporophores’ species exhibited distinct temporal preferences than rotten-wood fungal sporophores.
5. Conclusions
In conclusion, our study found that the distribution of fungal sporocarps in different months showed high specialization and unevenness. We further found that the effects of temporal partitioning were stronger on soil fungal sporophores than on rotten-wood fungal sporophores. Moreover, the environmental heterogeneity caused by temporal partitioning makes the growth of fungal sporophores complex and diverse, and the time distribution preference of fungal sporophores is the result of human disturbance, plant community, and other environmental factors. Overall, these findings highlighted the importance of temporal partitioning in maintaining local diversity in the fungal community. Therefore, the growing preference for months of fungal sporophores, especially soil fungal sporophores, should be considered in fungal sporophores’ diversity conservation in temperate, deciduous broad-leaved forests.
In addition, the monitoring time is too short to study the effects of different seasons and climates on soil fungal sporophores from a spatial perspective. Our study did not address all fungal communities but was limited to sporocarps. Therefore, many factors and mechanisms affecting the species’ diversity of fungal sporophores in soils need to be further studied; the below-ground fungal community is also the focus of our future research.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d14060483/s1. Table S1: Significant positive associations of fungal sporophores’ species with months (p = 0.05 level of significance for torus-translation test). Table S2: Fungal sporophores’ species’ composition, abundance. Figure S1: The number of fungal sporophores’ species in different substrates. In the fungal sporocarps–month network, fungal sporophores’ species (green) interact with months (blue). The size of nodes represents the relative abundance of fungal sporophores’ species. Figure S2: Monthly mean temperature and humidity changes.
Author Contributions
Z.Z. and Y.C. conceived the study; S.W. and X.W. conducted the experiment and analyzed the samples; Z.Z. and M.X. performed the data analysis; Z.Y., W.L. and E.G. discussed the results, commented, and edited the manuscript; and Z.Z. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Youth Talent Promotion Project by Henan Province (2020HYTP037); Young Talents project funded by Henan Agricultural University (111/30500744); Youth Foundation of Natural Science Foundation of Henan Province (212300410153); Basic research expenses of Henan academy of forestry (2021JB02014) and the Youth Innovation Promotion Association CAS (grant 2018084).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the Field Scientific Observation and Research Station of Forest Ecosystem in the North-South Transition Zone of Funiu Mountain, Zhengzhou, China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Senn-Irlet, B.; Heilmann-Clausen, J.; Genney, D.; Dahlberg, A. Guidance for Conservation of Macrofungi in Europe; European Council for Conservation of Fungi (ECCF): Strasbourg, France, 2007. [Google Scholar]
- Chen, Y.; Yuan, Z.; Bi, S.; Wang, X.; Ye, Y.; Svenning, J.C. Macrofungal species distributions depend on habitat partitioning of topography, light, and vegetation in a temperate mountain forest. Sci. Rep. 2018, 8, 13589. [Google Scholar] [CrossRef] [PubMed]
- Balmford, A.; Lyon, A.J.E.; Lang, R.M. Testing the higher-taxon approach to conservation planning in a megadiverse group: The macrofungi. Biol. Conserv. 2000, 93, 209–217. [Google Scholar] [CrossRef]
- Andrew, E.E.; Kinge, T.R.; Tabi, E.M.; Thiobal, N.; Mih, A.M. Diversity and distribution of macrofungi (mushrooms) in the Mount Cameroon Region. J. Ecol. Nat. Environ. 2013, 5, 318–334. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, Z.; Li, P.; Cao, R.; Jia, H.; Ye, Y. Effects of environment and space on species turnover of woody plants across multiple forest dynamic plots in East Asia. Front. Plant Sci. 2016, 7, 1533. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, B.; Mallik, A.U.; Huang, F.; Xiang, W.; Ding, T.; Li, X. Topographic species–habitat associations of tree species in a heterogeneous tropical karst seasonal rain forest, China. J. Plant Ecol. 2017, 10, 450460. [Google Scholar] [CrossRef]
- Svenning, J.C. Microhabitat specialization in a species-rich palm community in Amazonian Ecuador. J. Ecol. 1999, 87, 55–65. [Google Scholar] [CrossRef]
- Ignasi, B.; Montserrat, V.; Luís, S. Contrasting effects of invasive plants in plant-pollinator networks. Oecologia 2008, 155, 761–770. [Google Scholar] [CrossRef]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Plant Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Lai, J.; Mi, X.; Ren, H.; Ma, K. Species-habitat associations change in a subtropical forest of China. J. Veg. Sci. 2009, 20, 415–423. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, Y.; Xi, J.; Yuan, Z.; Ye, Y.; Wang, T. Community Preferences of Woody Plant Species in a Heterogeneous Temperate Forest, China. Front. Ecol. Evol. 2020, 8, 165. [Google Scholar] [CrossRef]
- Ziv, Y.; Abramsky, Z.; Kotler, B.P.; Subach, A. Interference competition and temporal and habitat partitioning in two gerbil species. Oikos 1993, 66, 237–246. [Google Scholar] [CrossRef]
- Hayward, M.W.; Slotow, R. Temporal partitioning of activity in large African carnivores: Tests of multiple hypotheses. S. Afr. J. Wildl. Res. 2009, 39, 109–125. Available online: https://hdl.handle.net/10520/EJC117325 (accessed on 13 March 2022). [CrossRef]
- Bourgis, F.; Kilaru, A.; Cao, X.; Ngando-Ebongue, G.F.; Drira, N.; Ohlrogge, J.B.; Arondel, V. Comparative transcriptome and metabolite analysis of oil palm and date palm mesocarp that differ dramatically in carbon partitioning. Proc. Natl. Acad. Sci. USA 2011, 108, 12527–12532. [Google Scholar] [CrossRef] [PubMed]
- Anten, N.P.; Hirose, T. Interspecific differences in above-ground growth patterns result in spatial and temporal partitioning of light among species in a tall-grass meadow. J. Ecol. 1999, 87, 583–597. [Google Scholar] [CrossRef]
- Hansen, R.A. Effects of Habitat Complexity and Composition on a Diverse Litter Microarthropod Assemblage. Ecology 2000, 81, 1120–1132. [Google Scholar] [CrossRef]
- Pronk, T.E.; During, H.J.; Schieving, F. Coexistence by temporal partitioning of the available light in plants with different height and leaf investments. Ecol. Model. 2007, 204, 349–358. [Google Scholar] [CrossRef]
- Hustad, V.P.; Meiners, S.J.; Methven, A.S. Terrestrial Macrofungi of Illinois Old-Growth Prairie Groves. Am. Midl. Nat. 2011, 166, 13–28. [Google Scholar] [CrossRef][Green Version]
- Koide, R.T.; Shumway, D.L.; Xu, B.; Sharda, J.N. On temporal partitioning of a community of ectomycorrhizal fungi. New Phytol. 2007, 174, 420–429. [Google Scholar] [CrossRef]
- Büntgen, U.; Kauserud, H.; Egli, S. Linking climate variability to mushroom productivity and phenology. Front. Ecol. Environ. 2012, 10, 14–19. [Google Scholar] [CrossRef]
- Vacher, C.; Vile, D.; Helion, E.; Piou, D.; Desprez-Loustau, M.L. Distribution of parasitic fungal species richness: Influence of climate versus host species diversity. Divers. Distrib. 2008, 14, 786–798. [Google Scholar] [CrossRef]
- Hawksworth, D. Species richness, abundance, and phenology of fungal fruit bodies over 21 years in a Swiss forest plot. Mycol. Res. 2001, 105, 515–523. [Google Scholar] [CrossRef]
- Ágreda, T.; Águeda, B.; Fernández-Toirán, M.; Vicente-Serrano, S.M.; Òlano, J.M. Long-term monitoring reveals a highly structured interspecific variability in climatic control of sporophores production. Agric. For. Meteorol. 2016, 223, 39–47. [Google Scholar] [CrossRef]
- Hou, H.Y.; Hou, X.Y. Vegetation of China with reference to its geographical distribution. Ann. Mo. Bot. Gard. 1983, 70, 509–549. [Google Scholar] [CrossRef]
- Miles, P.G.; Chang, S.T. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–431. [Google Scholar] [CrossRef]
- Chen, Y.; Svenning, J.C.; Wang, X.; Cao, R.; Yuan, Z.; Ye, Y. Drivers of Macrofungi Community Structure Differ between Soil and Rotten-Wood Substrates in a Temperate Mountain Forest in China. Front. Microbiol. 2018, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Condit, R. Research in large long-term tropical forest plots. Trends Ecol. Evol. 1995, 10, 18–22. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Sutton, B.C.; Ainsworth, G.C. Ainsworth and Bisby’s Dictionary of the Fungi Seventh Edition; Commonwealth Agricultural Bureaux: Slough, UK, 1983. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. Vegan: Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 13 December 2021).
- Blüthgen, N.; Menzel, F.; Hovestadt, T.; Fiala, B.; Blüthgen, N. Specialization, constraints, and conflicting interests in mutualistic networks. Curr. Biol. 2007, 17, 341–346. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Open Access Indices, Graphs and Null Models: Analyzing Bipartite Ecological Networks. Open Ecol. J. 2013, 2, 7–24. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 15 June 2019).
- Toju, H.; Guimaraes, P.R.; Olesen, J.M.; Thompson, J.N. Assembly of complex plant–fungus networks. Nat. Commun. 2014, 5, 5273. [Google Scholar] [CrossRef]
- Dicks, L.V.; Corbet, S.A.; Pywell, R.F. Compartmentalization in Plant–Insect flower visitor webs. J. Anim. Ecol. 2002, 71, 32–43. [Google Scholar] [CrossRef]
- Marc, B.; Jean, P.M.; Bernd, Z.; Andrianarisoa, S.; Ranger, J.; Courtecuisse, R.; Marçais, B.; Tacon, F.L. Influence of tree species on richness and diversity of epigeous fungal communities in a French temperate forest stand. Fungal Ecol. 2011, 4, 22–31. [Google Scholar] [CrossRef]
- Tedersoo, L.; Jairus, T.; Horton, B.M.; Abarenkov, K.; Suvi, T.; Saar, I. Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol. 2008, 180, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Alday, J.G.; De Aragón, J.M.; de-Miguel, S.; Bonet, J.A. Mushroom biomass and diversity are driven by different spatio-temporal scales along Mediterranean elevation gradients. Sci. Rep. 2017, 7, 45824. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).