Abstract
We quantified functional traits (escape strategy, sprint speed and predatory performance) and population density across 10 lizard species representing morphotype stages in the acquisition of burrowing snake-like morphotypes (BSLM), from Brazil. We used phylogenetic mixed models to test if: (a) morphotype and substrate affects flight strategy and speed, (b) BSLM species more effectively access different potential prey types than lacertoid species, when in syntopy, and (c) morphotype is correlated with population abundance and habitat use in a way expected from the output of the previous experiments. BSLM rigidly relied on burrowing as flight strategy, while syntopic lacertoid species changed their strategy according to the substrate. In addition, sand had opposing effects on sprint speed depending on morphotype, making lacertoids run more slowly and BSLM faster. Even though BSLM were overall slower than lacertoids, they were equally effective hunters of challengingly fast prey, and better hunters of underground prey. In their shared habitats, prey is most abundant in the superficial layer of leaf litter, although a large fraction is found beneath this layer, under bushes. Experimental results support the observed higher importance of sand for BSLM’s density and the higher importance of vegetation for lacertoids’ density. Finally, although BSLM species reached the highest population densities among the studied species, a systematic effect of morphological evolution on the abundance of limbless lizards remains elusive.
1. Introduction
For more than half a century, researchers have been striving to understand how organismal function relate to the abundance and habitat use of populations [1,2]. Classical morphofunctional theory (AKA, the organismal performance paradigm [3,4]) states that evolutionary changes in individuals’ morphology, behavior, or physiology interact with the environment to influence their performance (i.e., speed) during tasks morphotype, such as escaping predators, capturing prey, etc. Within this paradigm, natural selection filters individuals depending on their performance during important tasks for survival (ex. prey speed during predatory attacks.) The literature shows many examples of selection favoring higher sprint speed [5] and how different morphologies induce different levels of sprint speed, or correlate with individuals’ microhabitat preferences (ex. in lizards [6,7,8,9].)
Nonetheless, slow morphotypes have often evolved in nature. For example, the Slow worm (Anguis spp.) and sloths (Ex. Choloepus spp.) have most likely derived from faster ancestors, although we cannot identify them today. This raises the question of how this process relates to changes in morphology, behavior and population abundance. Likely, evolving a slower morphotype requires ways of compensating for low speed during tasks such as escaping or hunting. That compensation might come from protective morphological traits (ex. a tortoises’ armor) or compensatory escaping or hunting strategies, as shown by many vertebrates, such as fish [10] or birds [11]. For instance, fast lizard species have been observed avoiding “slower” microhabitats (ex. thin branches), where fast locomotion is difficult [7]. Still, how behavior compensates for the evolution of slower morphotypes is largely unexplored.
Additionally, understanding how the evolution of phenotypes relates to population level traits (ex. abundance), remains a key gap for linking patterns across different levels of biological organization [2]. Existing evidence remains heavily fragmented across different animal groups. For example, antipredator behavior has been shown to explain habitat use [12], but population density has been more related to access to food [13,14]. Additionally, a match between species’ phenotypes and microhabitat use is often observed [15]. Thus, as phenotypes evolve, subsequent changes in individuals’ performance during predatory interactions might lead to ecological patterns at the population level.
Even individuals’ performance during experimental tasks may not be necessarily relevant for the traits of their populations in the wild. For example, while changes in morphology have been experimentally shown to affect prey handling in limb-reduced lizards [16], a subsequent study did not find the expected restrictions of evolving limb-reduced morphotype on diet [17]. Therefore, to better understand relationships between morphology, performance, and population density, it is recommended to combine experimental observations with field observations (ex. predatory performance with natural prey availability [18]). Yet, such integrative studies are scarce, possibly due in part to the large time and effort required to combine experimental trials with field work at remote sites.
The evolution of burrowing snake-like morphotypes (BSLM) in squamates provides an interesting example to test hypothesized links between functional evolution and ecological traits at the population level. Across the world, lacertoid species (i.e., that have four fully developed limbs with at least four digits) have often evolved into BSLM, developing elongated bodies, reduced or absent limbs and digits, and a burrowing lifestyle [19,20,21,22]. This morphology may make BSLM relatively slow on the surface [23] but able to burrow faster and deeper than many lacertoids [24,25]. The evolution of their predatory performance is not well understood, with some studies considering them as diet specialists [15,26] and others not [16,27]. Many BSLM lineages have evolved in open sandy environments [28,29,30] to which BSLM’s distribution seems to be constrained [21,27]. Still, some studies suggest they might be locally abundant [27,31]. Taken together, these studies suggest that morphofunctional evolution in BSLM could be linked to their population level traits, such as population density. However, these links have never been evaluated.
Herein, we report how the evolution of BSLM may influence their performance in different situations, and related their performance with their variation in population density. For that, we tested the following hypotheses: (a) morphotype and substrate can change individuals’ speed and flight strategy, (b) different lizard morphotypes have different predatory performance over prey of different types and in different situations (i.e., fast vs. slow crickets, and buried vs. surface-active termites). (c) Either the availability of sand or vegetation can affect the population density of each morphotype. (d) BSLM species have denser populations than lacertoid species at sampling plots where they are syntopic.
2. Materials and Methods
2.1. Species Accounts
Our study species comprehend two monophyletic sister groups of Gymnopthalmidae lizards [32] (Figure 1a). One is composed by BSLMs (Calyptommatus leiolepis, Calyptommatus nicterus, Calyptommatus sinebrachiatus, Nothobachia ablephara, and Scriptosaura catimbau), whose forelimbs are either absent or vestigial. The other is compound of surface active lacertoid ecomorphotypes (Vanzosaura multiscutata, Micrablepharus maximiliani, Procellosaurinus tetradactylus, P. eryhtrocercus, and Psilops paeminosus; [27,33]). These species are also capable of burrowing but more superficially [27]. They represent the sole known acquisition of BSLM within the lizard tribe Gymnophthalmini, and a lacertoid ancestral state is assumed since no snake-like species is found close to the basal node shown in Figure 1a [34]. As it is observed in other BSLM lineages [29], our study lizards of both morphotypes may live syntopically; In this case, at a few sandy spots within the open biome of the Brazilian Caatingas. All of the studied species are endemic to sandy areas in such region [28], except for Vanzosaura multiscutata, which occurs on other soil types, and Micrablepharus maximilliani, which ranges widely across Brazil. We acknowledge that a single evolutionary acquisition of such morphotypes cannot demonstrate causation, and thus we simply aim to show how different functional traits correlate with morphotype in these species [3]. Additionally, the natural “common garden” in which these endemic species have diverged makes our study system especially suitable for documenting the inter actions between phenotypic evolution, environment and population level traits.
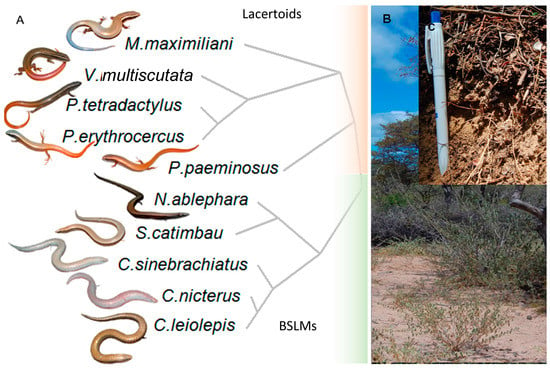
Figure 1.
(A). Variation in body shape and phylogenetic relationships among Lacertoid and BSLM Gymnopthalmini lizards from the Caatinga (Brazil). (B). typical vegetation (semi-desertic shrubs). (C) typical soil structure inhabited by those species (leaf litter layer over sandy soil).
Lizards were obtained at sites which represent most of sandy locations where BSLM and the studied lacertoid Gymnopthalmini species (Figure 1) are syntopic in Brazil: Alagoado (9°29′19.65″ S, 41°22′34.06″ W, municipal district of Casanova, Bahia State, 400 m a.s.l. in February 2010), Pedra Vermelha (−11°7′37.05″ S, −42°44′28.78″ W Santo Inácio, Bahia state, 450 m a.s.l. in September 2010), Gameleira de Assuruá (11°18′6.78″ S, 42°39′28.11″ W, municipal district of Gentio d’Ouro, Bahia state 750 m a.s.l. in September 2010), Vacaria, (10°40′38.22″ S, 42°37′46.30″ W, municipal district of Xique-Xique, Bahia state, 400 m a.s.l.) and Catimbau (8°35′29.23″ S, 37°14′44.32″ W, municipal district of Buique, Pernambuco state, 750 m a.s.l.). Lizard collection and experimental methods were approved by an official ethics committee and registered under the IBAMA license number (17086-2).
During field trips, specimens were kept individually or in small groups, when found under the same bush, in transparent plastic bags (1.5 L) with substrate from the exact place where they were collected. An incandescent lamp positioned close to these bags maintained thermal and light gradients within them. Animals were fed with termites and sprayed with water every two or three days. In the lab, animals were kept in 15 × 60 × 40 cm plastic terraria, separated by species but maintaining the groups found together in nature under single bushes during fieldwork. All terraria received UV (L12:D12) and heating (L10:D14) light, allowing lizards to thermoregulate normally. Termites were provided and water was sprayed in the terraria three times a week. Experimental and collection procedures were approved by the official ethics committee at the Instituto de Biociências da Universidade de São Paulo. Due to permit limitations, all experimental procedures were carried out over different, partially overlapped subsamples of 15 individuals, always including animals of both sexes and juveniles. Any specimen noticeably unhealthy (emaciated or slow) was excerpted from the experiments. Specimens run for each species and tests are shown at the corresponding results table.
2.2. Effects of Morphotype on Flight Strategy
Flight strategy of all specimens was observed within 48 h of their respective collection and at air temperatures between 30 and 35 °C (within preferred ranges of all species, see Camacho et al. 2014). One of us made lizards escape within an opaque plastic box (measures 13 × 35 × 29 cm). The box had its bottom covered by 3 cm of sand, and a half of the surface was additionally covered by a thin layer of leaf litter from the collection sites. Within that box, each lizard was deposited, in random order, over each of the two box’s halves: bare sand or sand covered by a leaf litter layer. The immediate following action of the animal, right after depositing it, was coded as “burrowing”, if the animal burrowed completely under the sand; as “hiding”, if the animal left a part of the trunk exposed or just sheltered under the leaf litter layer; or as ”running”, if the animal kept running after the first 2 or 3 steps/body undulations.
Later, we used phylogenetic logistic mixed models to test for the relative effects of two categorical fixed factors: morphotype (BSLM vs. Lacertoid) and environment (Sand vs. Leaf litter) on the odds of using a given flight strategy against another (hiding vs. running, hiding vs. burrowing, burrowing vs. running). Since our response variable, Flight strategy, is a categorical variable with three options, we fitted three separate logisticmodels comparing each of the three flight options with the others, one pair at a time. In this way, we tested if changes in our fixed factors altered the odds of running vs. hiding, running vs. burrowing and hiding vs. burrowing. For that, we used the function “pglmm”, implemented in the package “phyr” [34], in Rstudio [35]. We used the phylogenetic relationships proposed for these species [32]. Individuals entered as the grouping variable, to account for the two measurements made for each individual.
2.3. Effects of Morphotype on Sprint Speed over Different Substrates
We estimated the maximum sprint speed of each species in two substrates (i.e., sand paper and loose soil). The sand paper substrate was constructed with dune sand glued to a wood card whereas the loose soil context was generated by adding a 2–3 mm layer of the same sand to the sand paper. That depth provided enough lateral support for body undulations while preventing lizards from burrowing under it. Lizards were maintained for a week in terraria prior to initiate speed measurements. Species’ different thermal optima and loss of motivation were accounted for by making each lizard run in a random sequence of five different room temperatures (22 °C, 27 °C, 32 °C, 37 °C, and 42 °C). Daily, each lizard ran twice per substrate at one temperature, during its typical activity period, and after a period of at least 40 min to equalize body temperature with room temperature. Each trial was recorded using a high-speed video camera (Redlake MotionXtra HG-SE, 250 frames per second) and illuminated with fluorescent lamps. This procedure generated 2054 videos over which we calculated individuals’ linear speeds using the program Tracker 3.0 (Cabrillo edu®). We considered linear speed as the total distance covered along 3 steps/body undulations of acceptably straight advance, divided by the time to do it. For each temperature, the fastest run of each lizard at each substrate was selected to represent its speed.
We again used phylogenetic mixed models. This time we used a gaussian link function to test if speed (response variable) was affected by two categorical fixed factors, each one with two levels: morphotype (BSLM vs. lacertoid) or substrate (loose vs. compacted soil). We also controlled for phylogenetic relationships and used individual identity as grouping factor to control for repeated measurements of each individual across the different temperatures. Modeling the non-linear effects of temperature would unnecessarily increase the complexity of the model and these are outside of the scope of this study. Thus, we did not test for such effects.
2.4. Effects of Morphotype on Predatory Performance over Different Prey Types
We evaluated the predatory performance of all lizard species for four prey types: slow vs. fast prey, and surface-active prey vs. buried prey. In all experiments, we stimulated lizard’s appetite by only providing water for 4 days before trials. We used captive raised cricket hatchlings which often have their jumping legs injured and thus allowed the comparison of similar prey with different speeds. In this case, we added 5 crickets with no jumping legs and 5 uninjured crickets at the same time to 1 L plastic boxes, each one containing 2 cm layer of sand and a lizard. We kept each lizard and its prey for 24 h in the plastic box and then counted the existing injured and uninjured crickets.
To see if one of the two morphotypes was less effective when preying on superficial or buried prey, we performed feeding experiments on individual lizards placed in plastic boxes. These boxes were only provided with two centimeters of clean sand from their habitats and prey. The selected sand depth is accessible by all the studied species (Camacho et al. 2014). Before placing the lizards, one of us introduced 5 termites, either on the surface or buried at the bottom of each box. An overnight observation had previously confirmed that termites were unable to neither resurge from this deep nor dig into the sand or leave the box. After placing ten termites in the bottom of each box, we waited for 24 h and counted how many termites each lizard ate. On a different day, we repeated this procedure but with 5 termites left above the sand. This experiment was carried out in two steps to enable us to see if lizards would move a buried termite to the surface, by their own movements, and keep the lizards hungry during the experiment.
For each species, predatory performance was represented by the mean number of prey items ingested, by prey type and individual lizard. We tested for the effects of morphotype and prey type on the number of prey items ingested across species. We used phylogenetic mixed models, separately for each predation experiment (i. e. comparing slow vs. fast prey, and surface active vs. buried prey). This time, we used a Poisson’s link function, suitable for count data [36]. In each of the two fitted models, the number of prey items ingested is predicted by morphotype and prey type, and the interaction between them. Normality could be assumed thanks to the large number of trials (176)
2.5. Estimating the Availability of Feeding Resources
In order to compare the predatory performance of the lizards with the availability of prey in their natural habitats, we measured the abundance and diversity of potential prey across four of our lizard sampling sites. Concretely, we collected 1 substrate sample of 1 L volume at each combination of microhabitat (Surface vs. down to 10 cm underground) and habitat (open vs. under bushes or trees). In this way, we obtained soil from 33 sampling points, totaling 132 samples. Each sample of leaf litter was quickly collected in an area of less than half a squared meter over the ground, and then another liter of soil from up to a depth of 10 cm (shovel length) within one meter of the leaf litter sample, was collected.
Each soil sample was examined in a white dish within 4 to 6 h of collection. Since we observed failed predation attempts in the laboratory of pupae of 5 mm in minimum diameter, we only counted complete invertebrates whose minimum diameter was shorter than that length. This criterion encompassed 21 prey categories (mostly identified to taxonomic orders and a larvae category). We then preserved the sample material in alcohol. Using these datasets of prey diversity and abundance, we tested for the effects of vegetation and microhabitat on both traits using a mixed model with a Poisson link function, in which sampling point entered as grouping variable.
2.6. Ecological Distribution and Density of Syntopic Lacertoid and BSLM Species
We estimated each species’ population density in ellipsoidal sampling units, located across all our five fieldwork localities. Sampling units consisted of natural, easily identifiable, vegetation patches (i.e., bushes, small trees) and similarly sized and shaped open patches that naturally occurred between vegetation patches, which we first raked and then measured their longest diameters. Following McCoy [27], the first author and one assistant in each case thoroughly raked the entire unit, reaching the leaf litter and underlying sand layers of each sampling unit, down to the first 14 cm of sand. Then, we extracted, identified to species level, and counted all the lizards found. Then we characterized each unit as “shaded” or “exposed”, depending on the presence of woody vegetation cover or just bare sand. We also categorized units as “no sand”, if there was no sand between the leaf litter and the compacted soil, or “sand”, if the sand layer was a least 5 cm deep under the leaf litter. To estimate each unit’s area, we measured the two major perpendicular axes of each patch, and used those measures to solve the ellipsoid area equation. Using this procedure we sampled 277 units, collecting data for all species of this study.
We fitted a phylogenetic mixed model with gaussian link function, to test whether the density (abundance divided by plot size) of each lizard morphotype was predicted by the habitat and/or sand availability in the plot. Habitat and sand availability were both binary fixed factors (levels: open vs. vegetated, and no sand vs. sandy soil, respectively). Sampling site entered as grouping variable.
Finally, to test for overall effects of morphotype evolution on the average density of each species, we fitted a phylogenetic mixed model (gaussian link function), using morphotype as fixed factor, and sampling site as random factor. For this test, we omitted plots devoid of lizards before testing.
3. Results
3.1. Effects of Morphotype and Context on Flight Strategy
Across the three phylogenetic logistic mixed models, BSLMs escaped by burrowing significantly more frequently than species of the lacertoid sister clade (PMM: trials = 306, individuals = 198, species = 10, range of z-scores = 6.3–10.6, p values always <0.001, Table 1). Changing flight context, from being at bare sand to leaf litter had detectable effects for two of the three models. (PMM: trials = 306, individuals = 198, species = 10, range of z-scores = (−4.56) to (−7.57), p values always <0.001, Table 1). Only the comparison of hiding vs. running was not altered by the flight context (See Table S1b). (PMM: trials = 306, individuals = 198, species = 10, z-value = 0.4, p value = 0.4, Table 1). Thus, BSLM maintain a rigid escape strategy while lacertoid species of gymnopthalmini lizards swiftly adjust their escape strategy to the environmental context (Figure 2). Our non-phylogenetic mixed model analysis detected similar effects and readers can re-run them using our supporting R scripts and data tables).

Table 1.
Morphotype interacts with substrate type, driving the flight strategy of lacertoid and snake-like lizards. Estimates represent changes in the odds of choosing to burrow or hide with respect to choose running as flight strategy. Burrowing snake-like morphotype (BSLM) exhibit greater odds of burrowing during escape, either on clean sand or in sand covered with leaf litter cover. In turn, clean sand decreases the odds of burrowing or hiding because lacertoids mostly chose those strategies in presence of leaf litter. B = Burrow, H = Hide.
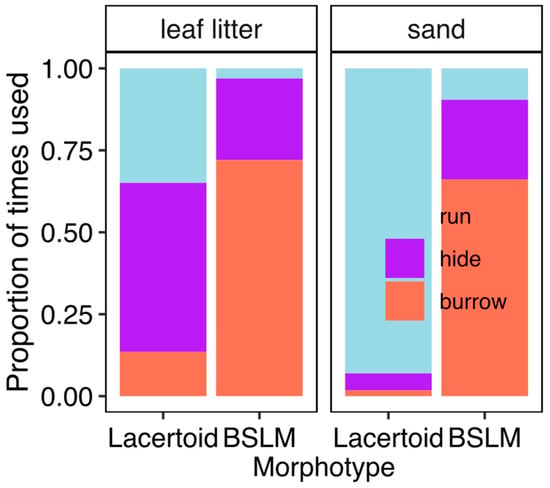
Figure 2.
Variation in the use of escape strategy used by ten species of lizards of two morphotypes that were made escape over bare sand layer and sand covered by a thin leaf litter layer.
3.2. Effects of Morphotype on Sprint Speed in Different Substrates
There was a significant interaction of morphotype and substrate (compacted soil vs. loose sand) (PMM gaussian: trials = 972, species = 10, slope = −17.54, SD = 2.50, z-score = −6.85, p < 0.001). BSLMs were always slower than lacertoids (PMM gaussian: trials = 972, species = 10, slope = −32.54, SD = 2.27, z-score = −14.29, p < 0.001), although differently from them, sprinted faster on sand than on compacted soil, Figure 3).
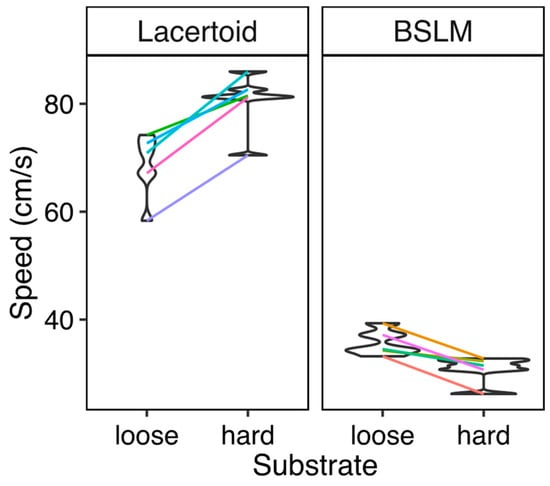
Figure 3.
Sprint speed of five lacertoid and five BSLM lizard species in different substrates. Each line connects the mean of maximum speeds of species measured across each substrate, in five different temperatures. The width of violins represents the frequency of species’ values across the response variable. Colored lines connect the mean values of the same species in each treatment.
3.3. Predatory Performance and Distribution of Prey in Nature
Our cricket hunting experiment showed clear effects of prey speed on hunting performance, indicating that uninjured crickets were a difficult prey for species of both morphotypes (PMM poisson: trials = 176, species = 10, slope = 1.19, SD = 0.27, z-score = 4.8, p < 0.001). However, BSLM species consumed uninjured crickets as effectively as lacertoid species (PMM poisson: trials = 176, species = 10, slope = 0.53, SD = 0.37, z-score = 1.4, p = 0.15). For this experiment, there was no statistically detectable interaction between morphotype and prey type (PMM poisson: trials = 176, species = 10, slope = −0.22, SD = 0.37, z score = −0.59, p = 0.55). See Figure 4 (above) and Table S3 (Supplementary material) for details.
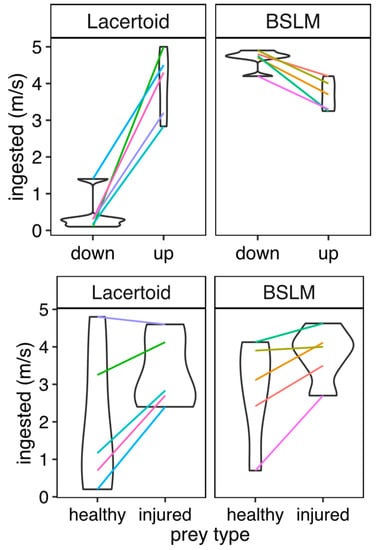
Figure 4.
(Upper row). Comparison of predatory performance between 5 BSLM and 5 lacertoid species eating uninjured and leg injured crickets during a 24 h experiment. (Lower row). Results of an analogous experiment (134 observations) on the same species but comparing lizards’ capability of hunting on surface prey vs. prey located under sand (termites). Colored lines connect the mean values of the same species in each treatment.
The termite hunting experiment also showed a significant interaction of morphotype and prey position (PMM poisson: trials = 176, species = 10, slope = −4.54, SD = 0.33, z-score = −13.06 p < 0.001), showing that buried termites were hard to access for lacertoids, while BSLM ate epigeal and buried prey equally well. See Figure 4 (lower row) and Table S4 for details.
More prey categories were found at soil surface, particularly if under vegetation. That was shown by a significant interaction of vegetation cover and microhabitat. (GMM poisson: samples = 132, sampling points = 33, Slope = 0.39, SD = 0.002, z-score = 149, p < 0.001). Prey abundance was also lower on bare ground (GMM poisson: samples = 132, sampling points = 33, Slope = −1.28, SD = 0.39, z-score = −3.27, p = 0.001) and higher under vegetation (GMM poisson: samples = 132, sampling points = 33, Slope = 2.07, SD = 0.19, z-score = 10.68, p < 0.001). However, this time, the interaction among these terms was not statistically significant (p = 0.384). See Figure 5 (lower row), Table 2 and Table S5 for details.
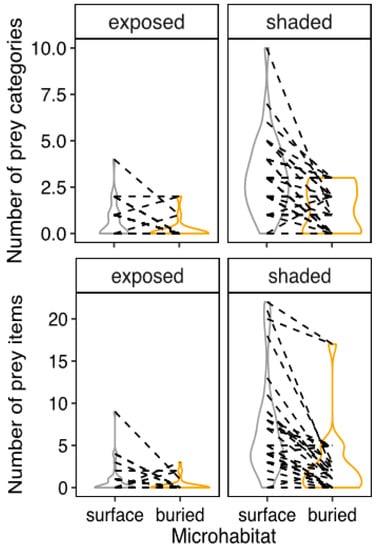
Figure 5.
Distribution of the number of prey types (upper row) and prey items (lower row) in different habitats (exposed, shaded) and microhabitats (soil surface, underground) in each of 33 sampling points, collected across 4 sites of Northeastern Brazil. Lines connect samples collected at the very same microsite.

Table 2.
Effects of habitat (open, under vegetation) and microhabitat (soil surface, underground) over the abundance and diversity of prey in 125 samples distributed across 4 localities of the Caatinga, where BSLM lizard species have evolved.
3.4. Effects of Vegetation Cover and Soil Type on Each Morph’s Density
The density of BSLM species was affected by the presence of vegetation (PMM gaussian: sampling points = 277, groups = 5, Slope = 0.01, SD = 0.002, z-score = 8.322, p < 0.001) and of sand, exhibiting a significant interaction with vegetation cover (PMM gaussian: sampling points = 277, groups = 5, Slope = 0.006, SD = 0.002, z-score = 2.306, p = 0.02). In contrast, lacertoid species were only, and much more strongly, affected by vegetation cover (PMM gaussian: sampling points = 277, groups = 5, Slope = 0.01, SD = 0.001, z-score = 13.77, p < 0.001). See Figure 6 A and Table 3 for results, and Table S6 for more details.
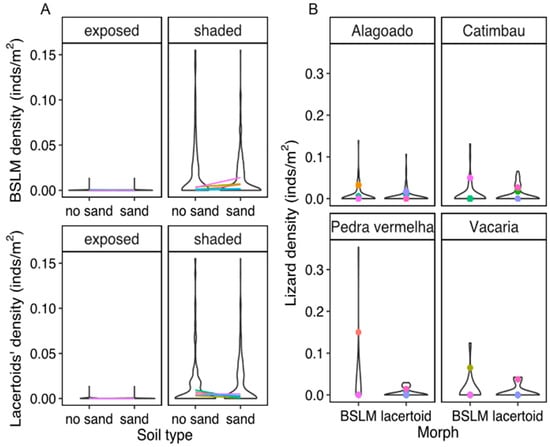
Figure 6.
(A). Variation in the density of lizards with different morphotype across 284 samples obtained at 6 localities of Northeastern Brazil. (B). Comparison of the density of lizards with different morphotype. We only used sampling plots where both morphotypes were found. Colors indicate or connect mean values for each species.

Table 3.
Effects of sand availability, presence of vegetation, and sampling unit size over 284 samples of lizards of two different morphotypes, made in 5 sandy sites in Northeastern Brazil. Z-values show soil type and microhabitat affected the abundance of BSLM lizards more than that of lacertoid lizards. Additionally, BSLM numbers increased more steeply with the size of the sampling unit.
4. Discussion
Many studies have focused on the variation of particular performance traits (e.g., the speed of ingesting prey, burrowing, sprinting [15,23,24] or in the variation in microhabitat use among different species [8,28,33]. Following Irschick and Losos [7], we integrated these different approaches to understand the evolution of limbless lizards more comprehensively.
Our results suggest that, at least among gymnophthalmini lizards, the evolution of BSLM implies a loss of speed and flexibility in escape strategy, concomitant with their specialization as burrowers. This contrasts with previous evidence, and the widespread view that a faster sprint speed confers higher individuals’ fitness in lizards [5,6]. This raises the question of how these a priori impairments in performance could have evolved. Risks of visually oriented predation and overheating are common at the open and sandy habitats where BSLM have most often evolved [24,25,29]. As seen herein, sand and leaf litter typically decrease the sprint speed for lacertoid species [37]. At these sandy habitats, the loss of traction and speed can lead to at least two opposite strategies, the evolution of morphological modifications that increase speed [38] and the acquisition of a slower, burrowing morphotype [28,29]). Species with this morphotype are also more prone to burrow, and do it faster and deeper, either when escaping predators, as shown by Australian lineages [24] or when avoiding thermal extremes, as found for Brazilian lineages [25].
When it comes to foraging, gymnophthalmini BSLM species do it mostly during the night, locomoting under the sand’s surface [33]. During captive care, it was possible to observe that they arise close to surface-active prey to eat it by surprise. Our results further show they can successfully attack challengingly fast prey in the surface, and that they can reach prey underground much more effectively than syntopic lacertoid relatives. Thus, in an environment where sprinting is impeded, the evolution of a slower morphotype seems to be promoted when the environment offers opportunities to behaviorally compensate for a slower speed (ex. a soft substrate that allows sheltering and ambushing). In our study system, this might be the case, as sand not only allows BSLM’s fixed flight strategy and access to a stock of underground prey, but also provides more options for thermoregulation [25].
The population density of our studied species correlated with the availability of sand and vegetation cover in ways expected from existing theory and our behavioral experiments. First, the density of both morphotypes was affected by the presence of vegetation. A major factor explaining lizards’ density is access to food [12], and we found that, across sandy habitats of the Brazilian Caatinga, prey is systematically concentrated in the leaf litter layers that exist under the vegetation. BSLM population density was arguably more dependent on sand availability than that of lacertoids, as we observed only for them a significant interaction of sand availability (Table 3). Furthermore, vegetation impacted lacertoids’ density more strongly (z-score of 13 for lacertoids vs. 8 for BSLM, Table 3), which is expected from their higher dependence of it to avoid thermal extremes [25].
BSLM’s enhanced access to prey and thermal refuges suggest that the evolution of these morphotypes may cause increases in population density. However, our analyses provided mixed support for that hypothesis. While we did not find a systematic effect of morphotype on population density, BSLM had the highest population densities in all but one of our sampling sites. This happened at Gameleira, where sandy habitats are rarer. These results suggest that an evolutionary process such as the acquisition of BSLM, despite involving a decrease in sprint performance and a more fixed escape strategy may help the new morphotypes to reach particularly high densities in sandy habitats, but that further factors may still restrict their population to levels which are similar to their sister taxa. This capacity to reach high population density within sandy habitats, supported by observations of other radiations [27,31], and the high functional dependence of BLSM on sand might explain why this morphotype has evolved so many times within Squamates [21], and its high propensity to vicariance following the fragmentation of sandy patches [39]. To test these hypotheses, integrative studies involving multiple radiations seem necessary.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d14060482/s1.
Author Contributions
A.C.: idea, design and general development, collection of field and experimental data, data analysis, graphs and manuscript writing. A.T.Y.: feeding experiments. C.A.N.: advice and revisions, M.T.R.: advice and revisions. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the people who helped with fieldwork (R. Brandt; R. Recoder), and with reviewing previous versions of this manuscript (R. Telemeco, T. Kholsdorf). We thank FAPESP grants 2008/06143-0 and 2011/50146-6.
Institutional Review Board Statement
Experimental and collection procedures were approved by the official ethics committee (protocol 079/2008) at the Instituto de Biociências da Universidade de São Paulo. Due to permit limitations (IBAMA number 17086-2).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated in this study were provided as suplementary material.
Acknowledgments
The authors are grateful to the people who kindly helped with fieldwork (R. Brandt; R. Recoder), and with reviewing previous versions of this manuscript (R. Telemeco, T. Kholsdorf).
Conflicts of Interest
The authors declare no conflict of interest.
References
- McNab, B. Bioenergetics and the determination of home range size. Am. Nat. 1963, 97, 133–140. [Google Scholar] [CrossRef]
- Violle, C.; Reich, P.B.; Pacala, S.W.; Enquist, B.J.; Kattge, J. The emergence and promise of functional biogeography. Proc. Natl. Acad. Sci. USA 2014, 111, 13690–13696. [Google Scholar] [CrossRef] [PubMed]
- Garland, T.; Adolph, S. Why not to do two-species comparative studies: Limitations on inferring adaptation. Physiol. Zool. 1994, 67, 797–828. [Google Scholar] [CrossRef]
- Garland, T., Jr.; Carter, P.A. Evolutionary physiology. Annu. Rev. Physiol. 1994, 56, 579–621. [Google Scholar] [CrossRef]
- Garland, T., Jr.; Losos, J.B. Ecological morphology of locomotor performance in squamate reptiles. In Ecological Morphology: Integrative Organismal Biology; Wainwright, P.C., Reilly, S.M., Eds.; University of Chicago Press: Chicago, IL, USA, 1994; pp. 240–302. [Google Scholar]
- Miles, D.B. The race goes to the swift: Fitness consequences of variation in sprint performance in juvenile lizards. Evol. Ecol. Res. 2004, 6, 63–75. [Google Scholar]
- Losos, J.B.; Sinervo, B. The effects of morphology and perch diameter on sprint performance of Anolis lizards. J. Exp. Biol. 1989, 145, 23–30. [Google Scholar] [CrossRef]
- Irschick, D.J.; Losos, J.B. Do lizards avoid habitats in which performance is submaximal? the relationship between sprinting capabilities and structural habitat use in caribbean anoles. Am. Nat. 1999, 154, 293. [Google Scholar] [CrossRef]
- Goodman, B. Nowhere to run: The role of habitat openness and refuge use in defining patterns of morphological and performance evolution in tropical lizards. J. Evol. Biol. 2009, 22, 1535–1544. [Google Scholar] [CrossRef]
- Domenici, P. Context-dependent variability in the components of fish escape response: Integrating locomotor performance and behavior. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2010, 313A, 59–79. [Google Scholar] [CrossRef]
- Hedenstrom, A. Predator vs. prey: On aerial hunting and escape strategies in birds. Behav. Ecol. 2001, 12, 150–156. [Google Scholar] [CrossRef]
- Heithaus, M.; Wirsing, A.; Burkholder, D.; Thomson, J.; Dill, L. Towards a predictive framework for predator risk effects: The interaction of landscape features and prey escape tactics. J. Anim. Ecol. 2009, 78, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Girard, I.; Brown, T. Energetics of free-ranging mammals, reptiles, and birds. Annu. Rev. Nutr. 1999, 19, 247–277. [Google Scholar] [CrossRef] [PubMed]
- Sabo, J.L.; Power, M.E. River–watershed exchange: Effects of riverine subsidies on riparian lizards and their terrestrial prey. Ecology 2002, 83, 1860–1869. [Google Scholar]
- Jacob, S.; Bestion, E.; Legrand, D.; Clobert, J.; Cote, J. Habitat matching and spatial heterogeneity of phenotypes: Implications for metapopulation and metacommunity functioning. Evol. Ecol. 2015, 29, 851–871. [Google Scholar] [CrossRef]
- Andrews, R.; Pough, F.; Collazo, A.; de Queiroz, A. The ecological cost of morphological specialization: Feeding by a fossorial lizard. Oecologia 1987, 73, 139–145. [Google Scholar] [CrossRef]
- Barros, F.; Herrel, A.; Kohlsdorf, T. Head shape evolution in Gymnophthalmidae: Does habitat use constrain the evolution of cranial design in fossorial lizards? J. Evol. Biol. 2011, 24, 2423–2433. [Google Scholar] [CrossRef]
- Pough, F.; Preest, M.; Fusari, M. Prey-handling and the evolutionary ecology of sand-swimming lizards (Lerista: Scincidae). Oecologia 1997, 112, 351–361. [Google Scholar] [CrossRef]
- Gans, C. Tetrapod limblessness: Evolution and functional corollaries. Am. Zool. 1975, 15, 455–467. [Google Scholar] [CrossRef]
- Greer, A. Limb reduction in squamates: Identification of the lineages and discussion of the trends. J. Herpetol. 1991, 25, 166. [Google Scholar] [CrossRef]
- Wiens, J.; Slingluff, J. How lizards turn into snakes: A phylogenetic analysis of body-form evolution in anguid lizards. Evolution 2001, 55, 2303–2318. [Google Scholar] [CrossRef]
- Wiens, J.; Brandley, M.; Reeder, T. Why does a trait evolve multiple times within a clade? repeated evolution of snakelike body form in squamate reptiles. Evolution 2006, 60, 123. [Google Scholar] [CrossRef] [PubMed]
- Shine, R.; Wall, M. Interactions between locomotion, feeding, and bodily elongation during the evolution of snakes. Biol. J. Linn. Soc. 2008, 95, 293–304. [Google Scholar] [CrossRef][Green Version]
- Bergmann, P.; Irschick, D. Alternate pathways of body shape evolution translate into common patterns of locomotor evolution in two clades of lizards. Evolution 2009, 64, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Pavão, R.; Moreira, C.; Pinto, A.; Navas, C.; Rodrigues, M. Interaction of morphology, thermal physiology and burrowing performance during the evolution of fossoriality in Gymnophthalmini lizards. Funct. Ecol. 2014, 29, 515–521. [Google Scholar] [CrossRef]
- Shine, R. evolutionary advantages of limblessness: Evidence from the pygopodid lizards. Copeia 1986, 1986, 525. [Google Scholar] [CrossRef]
- McCoy, E.; Sutton, P.; Mushinsky, H. The role of guesswork in conserving the threatened sand skink. Conserv. Biol. 1999, 13, 190–194. [Google Scholar] [CrossRef]
- Rodrigues, M. Lizards, snakes, and amphisbaenians from the quaternary sand dunes of the middle Rio São Francisco, Bahia, Brazil. J. Herpetol. 1996, 30, 513. [Google Scholar] [CrossRef]
- Pianka, E.; Vitt, L. Lizards; University of California Press: Berkeley, CA, USA, 2006. [Google Scholar]
- Grizante, M.; Brandt, R.; Kohlsdorf, T. Evolution of body elongation in gymnophthalmid lizards: Relationships with climate. PLoS ONE 2012, 7, e49772. [Google Scholar] [CrossRef]
- Enge, K.; Marion, W. Effects of clearcutting and site preparation on herpetofauna of a North Florida flatwoods. Forest Ecol. Manag. 1986, 14, 177–192. [Google Scholar] [CrossRef]
- Goicoechea, N.; Frost, D.R.; De la Riva, I.; Pellegrino, K.C.; Sites, J., Jr.; Rodrigues, M.T.; Padial, J.M. Molecular systematics of teioid lizards (Teioidea/Gymnophthalmoidea: Squamata) based on the analysis of 48 loci under tree-alignment and similarity-alignment. Cladistics 2016, 32, 624–671. [Google Scholar] [CrossRef]
- Rocha, P.L.; Rodrigues, M.T. Electivities and resource use by an assemblage of lizards endemic to the dunes of the São Francisco River, northeastern Brazil. Papéis Avulsos De Zool. 2005, 45, 261–284. [Google Scholar]
- Li, D.; Dinnage, R.; Nell, L.A.; Helmus, M.R.; Ives, A.R. phyr: An R package for phylogenetic species-distribution modelling in ecological communities. Methods Ecol. Evol. 2020, 11, 1455–1463. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R. Boston, MA, USA, 2015. Ghost Orchid. Release (fc9e2179, 2022-01-04). Available online: http://www.rstudio.com (accessed on 20 September 2021).
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Brandt, R.; Galvani, F.; Kohlsdorf, T. Sprint performance of a generalist lizard running on different substrates: Grip matters. J. Zool. 2015, 297, 15–21. [Google Scholar] [CrossRef]
- Carothers, J.H. An experimental confirmation of morphological adaptation: Toe fringes in the sand-dwelling lizard Uma scoparia. Evolution 1986, 40, 871–874. [Google Scholar]
- Siedchlag, A.C.; Benozzati, M.L.; Passoni, J.C.; Rodrigues, M.T. Genetic structure, phylogeny, and biogeography of Brazilian eyelid-less lizards of genera Calyptommatus and Nothobachia (Squamata, Gymnophthalmidae) as inferred from mitochondrial DNA sequences. Mol. Phylogenetics Evol. 2010, 56, 622–630. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).