Subtle Effects of Experimental Grassland Fragmentation on Density, Species Composition and Functional Dispersion of Gastropods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
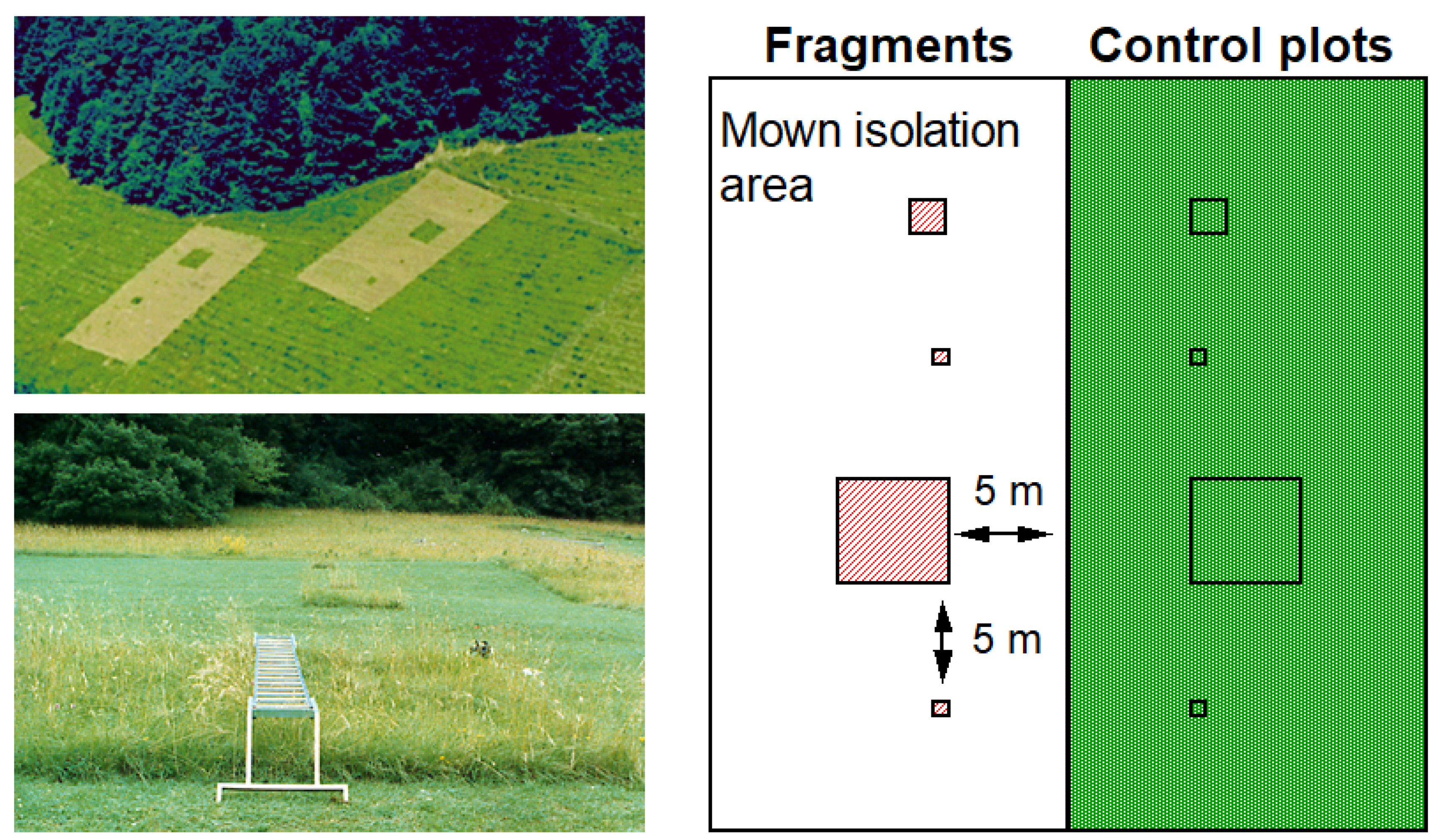
2.2. Fragmentation Experiment
2.3. Non-Invasive Gastropod Surveys
2.4. Functional Dispersion, and Morphological and Life-History Traits
2.5. Habitat Preference
2.6. Plant Biomass
2.7. Statistical Analyses
3. Results
3.1. Relative Density and Species Richness
3.2. Effect of Experimental Grassland Fragmentation
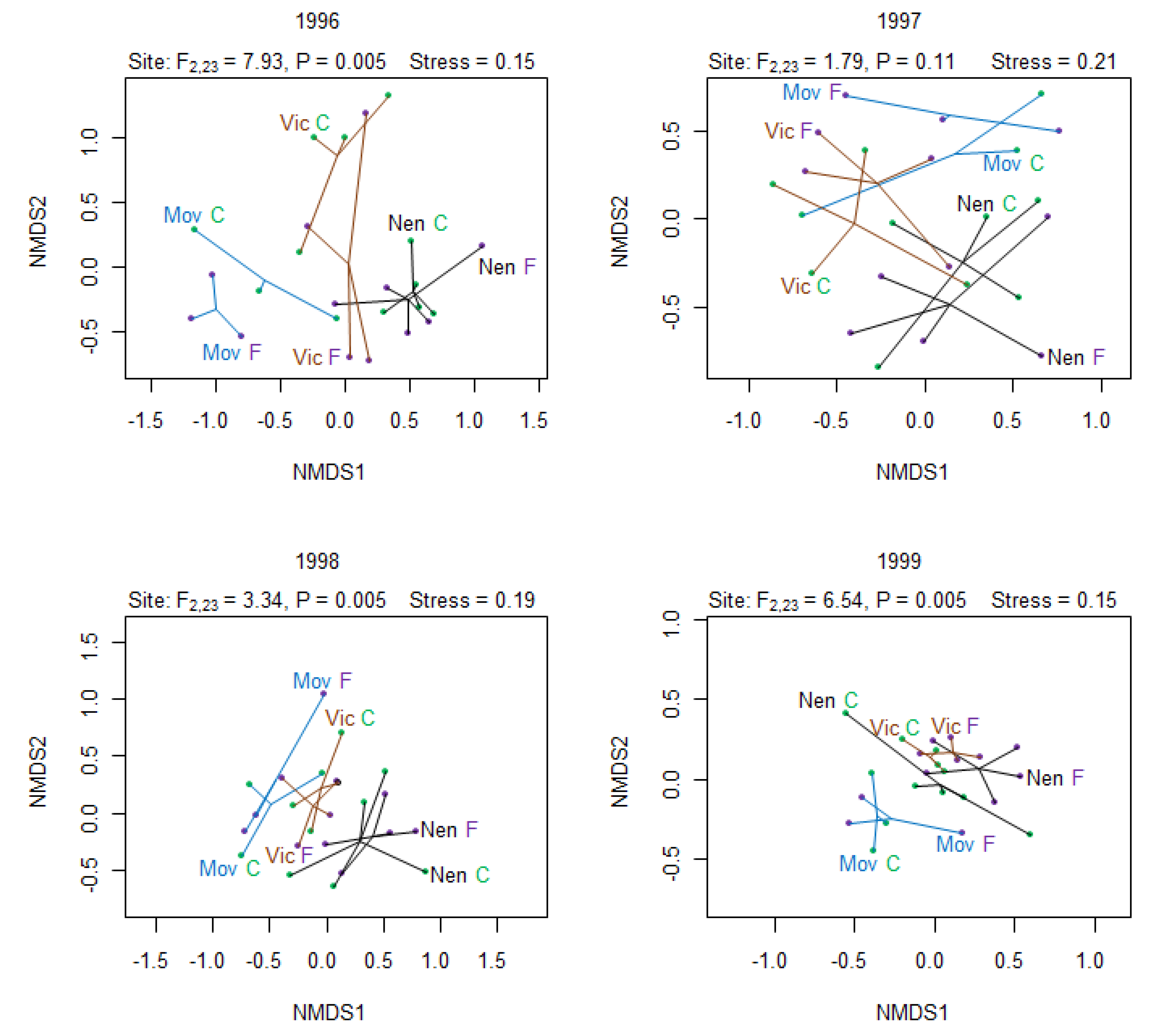
3.3. Effect on Species Composition
3.4. Functional Dispersion (FDis)
3.5. Morphological and Life-History Traits
3.6. Habitat Preferences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Biodiversity–global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. What do we really know about habitat fragmentation? Tex. J. Sci. 2000, 52, 5–22. [Google Scholar]
- Wilson, M.C.; Chen, X.-Y.; Corlett, R.T.; Didham, R.K.; Ding, P.; Holt, R.D.; Holyoak, M.; Hu, G.; Hughes, A.C.; Jiang, L.; et al. Habitat fragmentation and biodiversity conservation: Key findings and future challenges. Landsc. Ecol. 2016, 31, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Saunders, D.A.; Hobbs, R.J.; Margules, C.R. Biological consequences of ecosystem fragmentation: A review. Conserv. Biol. 1991, 5, 18–32. [Google Scholar] [CrossRef]
- Baur, B.; Erhardt, A. Habitat fragmentation and habitat alteration: Principal threats to most animal and plant species. GAIA 1995, 4, 221–226. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Braschler, B.; Rusterholz, H.-P.; Baur, B. Genetic effects of anthropogenic habitat fragmentation on remnant animal and plant populations: A meta-analysis. Ecosphere 2018, 9, e02488. [Google Scholar] [CrossRef]
- Anderson, S.J.; Kierepka, E.M.; Swihart, R.K.; Latch, E.K.; Rhodes, O.E., Jr. Assessing the permeability of the landscape features to animal movement: Using genetic structure to infer functional connectivity. PLoS ONE 2015, 10, e0117500. [Google Scholar] [CrossRef] [Green Version]
- Cooney, S.A.; Schauber, E.M.; Hellgren, E.C. Comparing permeability of matrix cover type for the Marsh Rice Rat (Oryzomys palustris). Landsc. Ecol. 2015, 30, 1307–1320. [Google Scholar] [CrossRef] [Green Version]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Doak, D.F.; Marino, P.C.; Kareiva, P.M. Spatial scale mediates the influence of habitat fragmentation on dispersal success: Implications for conservation. Theor. Popul. Biol. 1992, 41, 315–336. [Google Scholar] [CrossRef]
- Cattarino, L.; McAlpine, C.A.; Rhodes, J.R. Spatial scale and movement behaviour traits control the impacts of habitat fragmentation on individual fitness. J. Anim. Ecol. 2016, 85, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremene, C.; Groza, G.; Rakosy, L.; Schileyko, A.A.; Baur, A.; Erhardt, A.; Baur, B. Alterations of steppe-like grasslands in Eastern Europe: A threat to regional biodiversity hotspots. Conserv. Biol. 2005, 19, 1606–1618. [Google Scholar] [CrossRef]
- Baur, B.; Cremene, C.; Groza, G.; Rakoszy, L.; Schileyko, A.A.; Baur, A.; Stoll, P.; Erhardt, A. Effects of abandonment of subalpine hay meadows on plant and invertebrate diversity in Transylvania, Romania. Biol. Conserv. 2006, 132, 261–273. [Google Scholar] [CrossRef]
- Baur, B.; Joshi, J.; Schmid, B.; Hänggi, A.; Borcard, D.; Stary, J.; Pedroli-Christen, A.; Thommen, G.H.; Luka, H.; Rusterholz, H.-P.; et al. Variation in species richness of plants and diverse groups of invertebrates in three calcareous grasslands of the Swiss Jura mountains. Rev. Suisse Zool. 1996, 103, 801–833. [Google Scholar] [CrossRef]
- Zamora, J.; Verdu, J.R.; Galante, E. Species richness in Mediterranean agroecosystems: Spatial and temporal analysis for biodiversity conservation. Biol. Conserv. 2007, 134, 113–121. [Google Scholar] [CrossRef]
- Strijker, D. Marginal lands in Europe—Causes of decline. Basic Appl. Ecol. 2005, 6, 99–106. [Google Scholar] [CrossRef]
- Poschlod, P. Geschichte der Kulturlandschaft; Ulmer Verlag: Stuttgart, Germany, 2015; p. 320. [Google Scholar]
- WallisDeVries, M.F.; Poschlod, P.; Willems, J.H. Challenges for the conservation of calcareous grasslands in northwestern Europe: Integrating the requirements of flora and fauna. Biol. Conserv. 2002, 104, 265–273. [Google Scholar] [CrossRef]
- Barnett, K.L.; Facey, S.L. Grasslands, invertebrates, and precipitation: A review of the effects of climate change. Front. Plant Sci. 2016, 7, 1196. [Google Scholar] [CrossRef] [Green Version]
- Debinski, D.M.; Holt, R.D. A survey and overview of habitat fragmentation experiments. Conserv. Biol. 2000, 14, 342–355. [Google Scholar] [CrossRef]
- Zschokke, S.; Dolt, C.; Rusterholz, H.-P.; Oggier, P.; Braschler, B.; Thommen, G.H.; Lüdin, E.; Erhardt, A.; Baur, B. Short-term responses of plants and invertebrates to experimental small-scale grassland fragmentation. Oecologia 2000, 125, 559–572. [Google Scholar] [CrossRef]
- Braschler, B.; Baur, B. Diverse effects of a seven-year experimental grassland fragmentation on major invertebrate Groups. PLoS ONE 2016, 11, e0149567. [Google Scholar] [CrossRef] [PubMed]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Helm, A.; Hanski, I.; Pärtel, M. Slow response of plant species richness to habitat loss and fragmentation. Ecol. Lett. 2006, 9, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Rusterholz, H.-P.; Baur, B. Delayed response in a plant-pollinator system to experimental grassland fragmentation. Oecologia 2010, 163, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Baur, B.; Baur, A. Habitat-related dispersal in the land snail Chondrina Clienta. Ecography 1995, 18, 123–130. [Google Scholar] [CrossRef]
- Baur, A.; Baur, B. Are roads barriers to dispersal in the land snail Arianta arbustorum? Can. J. Zool. 1990, 68, 613–617. [Google Scholar] [CrossRef]
- Wirth, T.; Oggier, P.; Baur, B. Effect of road width on dispersal and genetic population structure in the land snail Helicella itala. Z. Ökol. Natursch. 1999, 8, 23–29. [Google Scholar]
- De Oliveira, T.; Hättenschwiler, S.; Handa, I.T. Snail and millipede complementarity in decomposing Mediterranean forest leaf litter mixtures. Funct. Ecol. 2010, 24, 937–946. [Google Scholar] [CrossRef]
- Meyer III, W.M.; Ostertag, R.; Cowie, R.H. Influence of terrestrial molluscs on litter decomposition and nutrient release in a Hawaiian rain forest. Biotropica 2013, 45, 719–727. [Google Scholar] [CrossRef]
- Shikov, E.V. Effects of land use changes on the land mollusc fauna in the central portion of the Russian plain. In World-Wide Snails; Solen, A., van Bruggen, A.C., Eds.; Brill & Backhuys: Leiden, The Netherlands, 1984; pp. 237–248. [Google Scholar]
- Boschi, C.; Baur, B. 2008. Past pasture management affects the land snail diversity in nutrient-poor calcareous grasslands. Basic Appl. Ecol. 2008, 9, 752–761. [Google Scholar] [CrossRef]
- Falkner, G.; Obrdlik, P.; Castella, E.; Speight, M. Shelled Gastropoda of Western Europe; Friedrich-Held-Gesellschaft: Munich, Germany, 2001; p. 267. [Google Scholar]
- Löffler, F.; Poniatowski, D.; Fartmann, T. Extinction debt across three taxa in well-connected calcareous grasslands. Biol. Conserv. 2020, 246, 108588. [Google Scholar] [CrossRef]
- Dolt, C.; Goverde, M.; Baur, B. Effects of experimental small-scale habitat fragmentation on above-ground and below-ground plant biomass in calcareous grasslands. Acta Oecol. 2005, 27, 49–56. [Google Scholar] [CrossRef]
- Braschler, B.; Zschokke, S.; Dolt, C.; Thommen, G.H.; Oggier, P.; Baur, B. Grain-dependent relationships between plant productivity and invertebrate species richness and biomass. Basic Appl. Ecol. 2004, 5, 15–24. [Google Scholar] [CrossRef]
- König, S.; Krauss, J. Get larger or grow longer wings? Impacts of habitat area and habitat amount on orthopteran assemblages and populations in semi-natural grasslands. Landsc. Ecol. 2019, 34, 175–186. [Google Scholar] [CrossRef]
- Hayder, F.; Nasri-Amman, K. Effects of habitat fragmentation on the sub-social desert terrestrial isopod Hemilepistus reaumurii. J. Arid. Environ. 2020, 178, 104173. [Google Scholar] [CrossRef]
- Tscharntke, T.; Brandl, R. Plant-insect interactions in fragmented landscapes. Annu. Rev. Entomol. 2004, 49, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: New York, NY, USA, 1992; p. 249. [Google Scholar]
- Tscharntke, T.; Steffan-Dewenter, I.; Kruess, A.; Thies, C. Characteristics of insect populations on habitat fragments: A mini review. Ecol. Res. 2002, 17, 229–239. [Google Scholar] [CrossRef]
- Zoller, H. Studien an Bromus erectus-Trockenrasengesellschaften in der Nordwestschweiz, speziell im Blauengebiet. Ber. Geobot. Inst. ETH 1947, 1946, 51–81. [Google Scholar]
- Schläpfer, M.; Zoller, H.; Körner, C. Influences of mowing and grazing on plant species composition in calcareous grassland. Bot. Helv. 1998, 108, 57–67. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- MeteoSwiss. IDAWEB 1.3.5.0. Available online: https://gate.meteoswiss.ch/idaweb/login.do (accessed on 16 May 2022).
- Oggier, P.; Zschokke, S.; Baur, B. A comparison of three methods for assessing the gastropod community in dry grasslands. Pedobiologia 1998, 42, 348–357. [Google Scholar]
- Kerney, M.P.; Cameron, R.A.D.; Jungbluth, J.H. Die Landschnecken Nord- und Mitteleuropas; Parey: Berlin/Hamburg, Germany, 1983; p. 384. [Google Scholar]
- Stoll, P.; Oggier, P.; Baur, B. Population dynamics of six land snail species in experimentally fragmented grassland. J. Anim. Ecol. 2009, 78, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, J.; Baur, B. Do pioneers have r-selected traits? Life-history patterns among colonizing terrestrial gastropods. Oecologia 1993, 94, 17–22. [Google Scholar] [CrossRef]
- Baur, B. Parental care in terrestrial gastropods. Experientia 1994, 50, 5–14. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org/ (accessed on 11 March 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 11 March 2022).
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1.0-12.1. 2014. Available online: https://cran.r-project.org/web/packages/FD/ (accessed on 11 March 2022).
- Lienert, J. Habitat fragmentation effects on fitness of plant populations: A review. J. Nat. Conserv. 2004, 12, 53–72. [Google Scholar] [CrossRef]
- Collinge, S.K. Spatial arrangement of habitat patches and corridors: Clues from ecological field experiments. Landsc. Urban Plan. 1998, 42, 157–168. [Google Scholar] [CrossRef]
- Kareiva, P. Habitat fragmentation and the stability of predator-prey interactions. Nature 1987, 326, 388–390. [Google Scholar] [CrossRef]
- Goverde, M.; Schweizer, K.; Baur, B.; Erhardt, A. Small-scale fragmentation affects pollinator behaviour: Experimental evidence from the bumblebee Bombus veteranus on calcareous grasslands. Biol. Conserv. 2002, 104, 293–299. [Google Scholar] [CrossRef]
- Braschler, B.; Lampel, G.; Baur, B. Experimental small-scale grassland fragmentation alters aphid population dynamics. Oikos 2003, 100, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Braschler, B.; Baur, B. Effects of experimental small-scale grassland fragmentation on spatial distribution, density, and persistence of ant nests. Ecol. Entomol. 2003, 28, 651–658. [Google Scholar] [CrossRef]
- Jauker, B.; Krauss, J.; Jauker, F.; Steffan-Dewenter, I. Linking life history traits to pollinator loss in fragmented calcareous grasslands. Landsc. Ecol. 2013, 28, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Biedermann, R. Body size and area-incidence relationships: Is there a general pattern? Glob. Ecol. Biogeogr. 2003, 12, 381–387. [Google Scholar] [CrossRef]
- Stevens, V.M.; Whitmee, S.; Le Galliard, J.-F.; Clobert, J.; Böhning-Gaese, K.; Bonte, D.; Brändle, M.; Dehling, D.M.; Hof, C.; Trochet, A.; et al. A comparative analysis of dispersal syndromes in terrestrial and semi-terrestrial animals. Ecol. Lett. 2014, 17, 1039–1052. [Google Scholar] [CrossRef]
- Keinath, D.A.; Doak, D.F.; Hodges, K.E.; Prugh, L.R.; Fagan, W.; Sekercioglu, C.H.; Buchart, S.H.M.; Kauffman, M. A global analysis of traits predicting species sensitivity to habitat fragmentation. Glob. Ecol. Biogeogr. 2017, 26, 115–127. [Google Scholar] [CrossRef]
- Driscoll, D.A.; Weir, T. Beetle responses to habitat fragmentation depend on ecological traits, habitat condition, and remnant size. Conserv. Biol. 2004, 19, 182–194. [Google Scholar] [CrossRef]
- Warzecha, D.; Diekötter, T.; Wolters, V.; Jauker, F. Intraspecific body size increases with habitat fragmentation in wild bee pollinators. Landsc. Ecol. 2016, 31, 1449–1455. [Google Scholar] [CrossRef]
- Riddle, W.A. High temperature tolerance in three species of land snails. J. Therm. Biol. 1990, 15, 119–124. [Google Scholar] [CrossRef]
- Nicolai, A.; Ansart, A. Conservation at a slow pace: Terrestrial gastropods facing fast-changing climate. Conserv. Physiol. 2017, 5, cox007. [Google Scholar] [CrossRef] [Green Version]
- Gibb, H.; Hochuli, D.F. Habitat fragmentation in an urban environment: Large and small fragments support different arthropod assemblages. Biol. Conserv. 2002, 106, 91–100. [Google Scholar] [CrossRef]
- Joshi, J.; Stoll, P.; Rusterholz, H.-P.; Schmid, B.; Dolt, C.; Baur, B. Small-scale experimental habitat fragmentation reduces colonization rates in species-rich grasslands. Oecologia 2006, 148, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetti, M.R.; Tscharntke, T.; Aguilar, R.; Batáry, P. Responses of insect herbivores and herbivory to habitat fragmentation: A hierarchical meta-analysis. Ecol. Lett. 2017, 20, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| (a) Abundance 1 (Relative Density) 2 | 1996 | 1997 | 1998 | 1999 | Cumulative Abundance |
| All gastropods | |||||
| Movelier 2 | 212 (0.44) | 99 (0.20) | 201 (0.41) | 498 (1.02) | 1010 (0.52) |
| Nenzlingen 2 | 252 (0.31) | 305 (0.38) | 374 (0.46) | 946 (1.17) | 1877 (0.58) |
| Vicques 2 | 101 (0.16) | 169 (0.26) | 259 (0.40) | 800 (1.23) | 1329 (0.51) |
| Snails | |||||
| Movelier 2 | 176 (0.36) | 73 (0.15) | 140 (0.29) | 252 (0.52) | 641 (0.33) |
| Nenzlingen 2 | 99 (0.12) | 64 (0.08) | 94 (0.12) | 292 (0.36) | 549 (0.17) |
| Vicques 2 | 81 (0.13) | 123 (0.19) | 136 (0.21) | 196 (0.30) | 536 (0.21) |
| (b) Species Richness (Rarefied Species Richness) 3 | 1996 | 1997 | 1998 | 1999 | Overall Species Richn |
| All gastropods | |||||
| Movelier 2 | 9 (8.1) | 10 (10.0) | 10 (10.0) | 11 (11.0) | 15 (15.0) |
| Nenzlingen 2 | 8 (6.8) | 10 (8.3) | 9 (8.3) | 12 (10.6) | 14 (14.8) |
| Vicques 2 | 7 (7.0) | 8 (7.4) | 8 (8.0) | 9 (8.2) | 10 (10.0) |
| Snails | |||||
| Movelier 2 | 7 (6.4) | 8 (7.8) | 9 (7.7) | 9 (8.5) | 12 (11.8) |
| Nenzlingen 2 | 5 (5.0) | 7 (7.0) | 5 (5.0) | 8 (7.3) | 9 (9.0) |
| Vicques 2 | 5 (5.0) | 7 (6.2) | 6 (6.0) | 7 (7.0) | 7 (7.0) |
| 1996 | 1997 | 1998 | 1999 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) All gastropods | df | F | p | df | F | p | df | F | p | df | F | p |
| Fragmentation treatment | 1,11 | 2.51 | 0.14 | 1,11 | 1.29 | 0.28 | 1,11 | 0.12 | 0.74 | 1,11 | 2.54 | 0.14 |
| (b) Snails | ||||||||||||
| Fragmentation treatment | 1,11 | 0.87 | 0.37 | 1,11 | 1.01 | 0.24 | 1,11 | 0.53 | 0.48 | 1,11 | 3.62 | 0.083 |
| 1996 | 1997 1 | 1998 1 | 1999 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) All gastropods | df | F | p | df | F | p | df | F | p | df | F | p |
| Fragmentation treatment | 1,11 | 0.08 | 0.78 | 1,11 | 0.09 | 0.77 | 1,11 | 0.49 | 0.50 | 1,11 | 1.64 | 0.22 |
| (b) Snails | ||||||||||||
| Fragmentation treatment | 1,11 | 0.01 | 0.94 | 1,10 | 0.23 | 0.64 | 1,10 | 0.56 | 0.47 | 1,11 | 5.88 | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braschler, B.; Oggier, P.; Baur, B. Subtle Effects of Experimental Grassland Fragmentation on Density, Species Composition and Functional Dispersion of Gastropods. Diversity 2022, 14, 474. https://doi.org/10.3390/d14060474
Braschler B, Oggier P, Baur B. Subtle Effects of Experimental Grassland Fragmentation on Density, Species Composition and Functional Dispersion of Gastropods. Diversity. 2022; 14(6):474. https://doi.org/10.3390/d14060474
Chicago/Turabian StyleBraschler, Brigitte, Peter Oggier, and Bruno Baur. 2022. "Subtle Effects of Experimental Grassland Fragmentation on Density, Species Composition and Functional Dispersion of Gastropods" Diversity 14, no. 6: 474. https://doi.org/10.3390/d14060474
APA StyleBraschler, B., Oggier, P., & Baur, B. (2022). Subtle Effects of Experimental Grassland Fragmentation on Density, Species Composition and Functional Dispersion of Gastropods. Diversity, 14(6), 474. https://doi.org/10.3390/d14060474






