A Decade-Long Change in the Elevational Distribution of Non-Volant Small Mammals on Mount Meru, Tanzania
Abstract
:1. Introduction
2. Materials and Methods
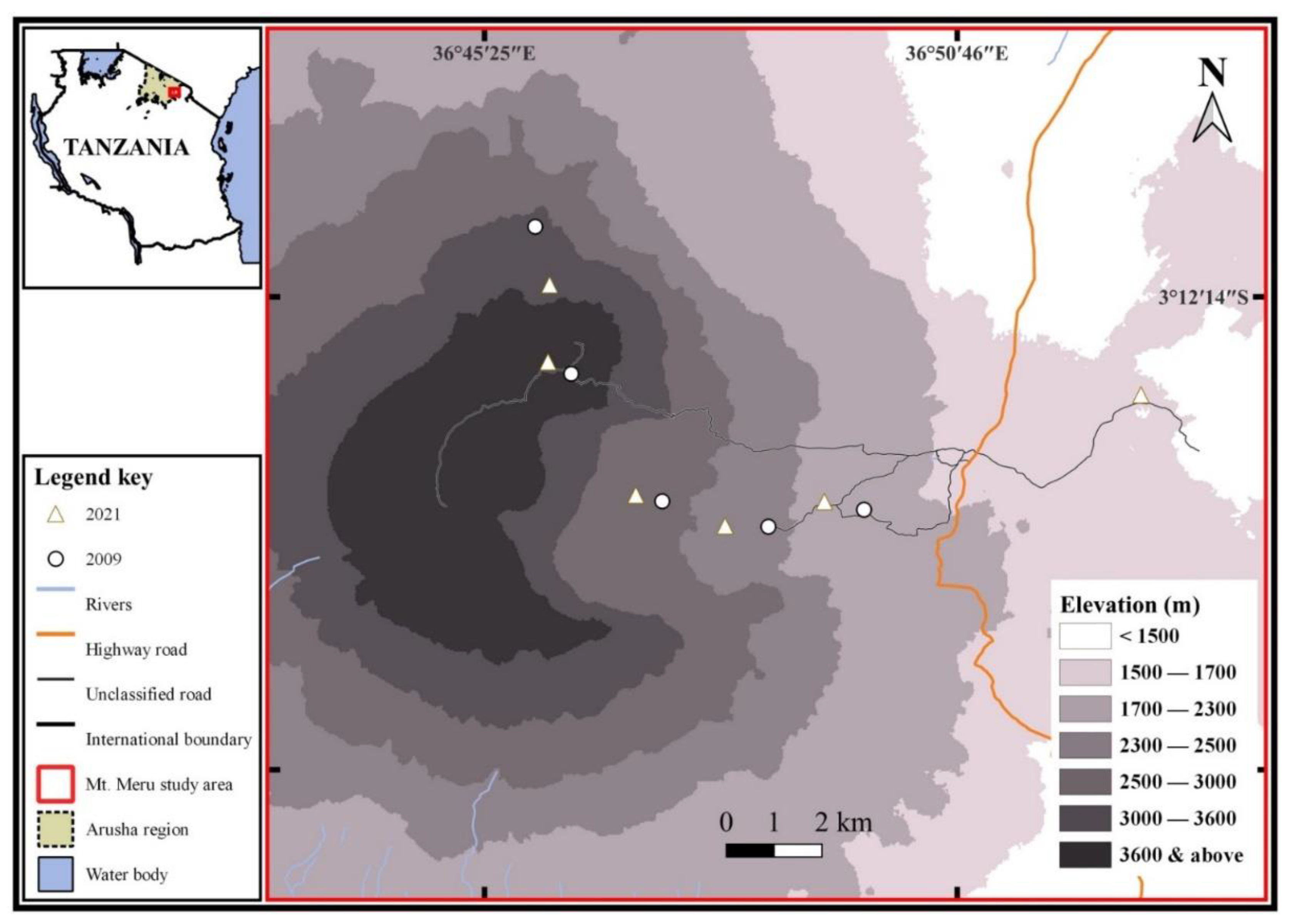
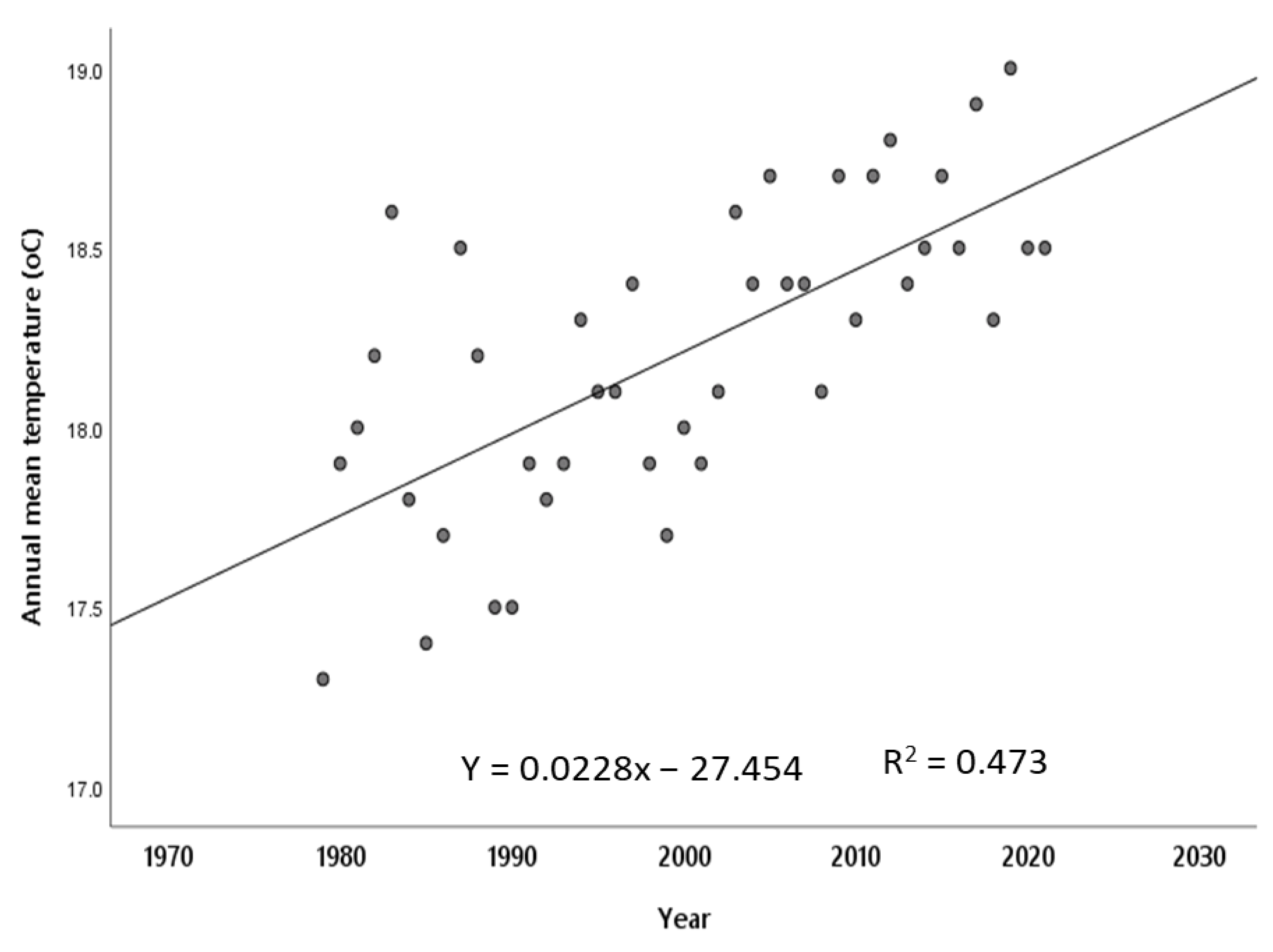
2.1. Study Area
2.2. Sampling Sites and Vegetation Types

2.3. Trapping Techniques and Identification
2.4. Data Analysis
2.5. Analysing and Comparing the 2009 and 2021 Surveys
3. Results
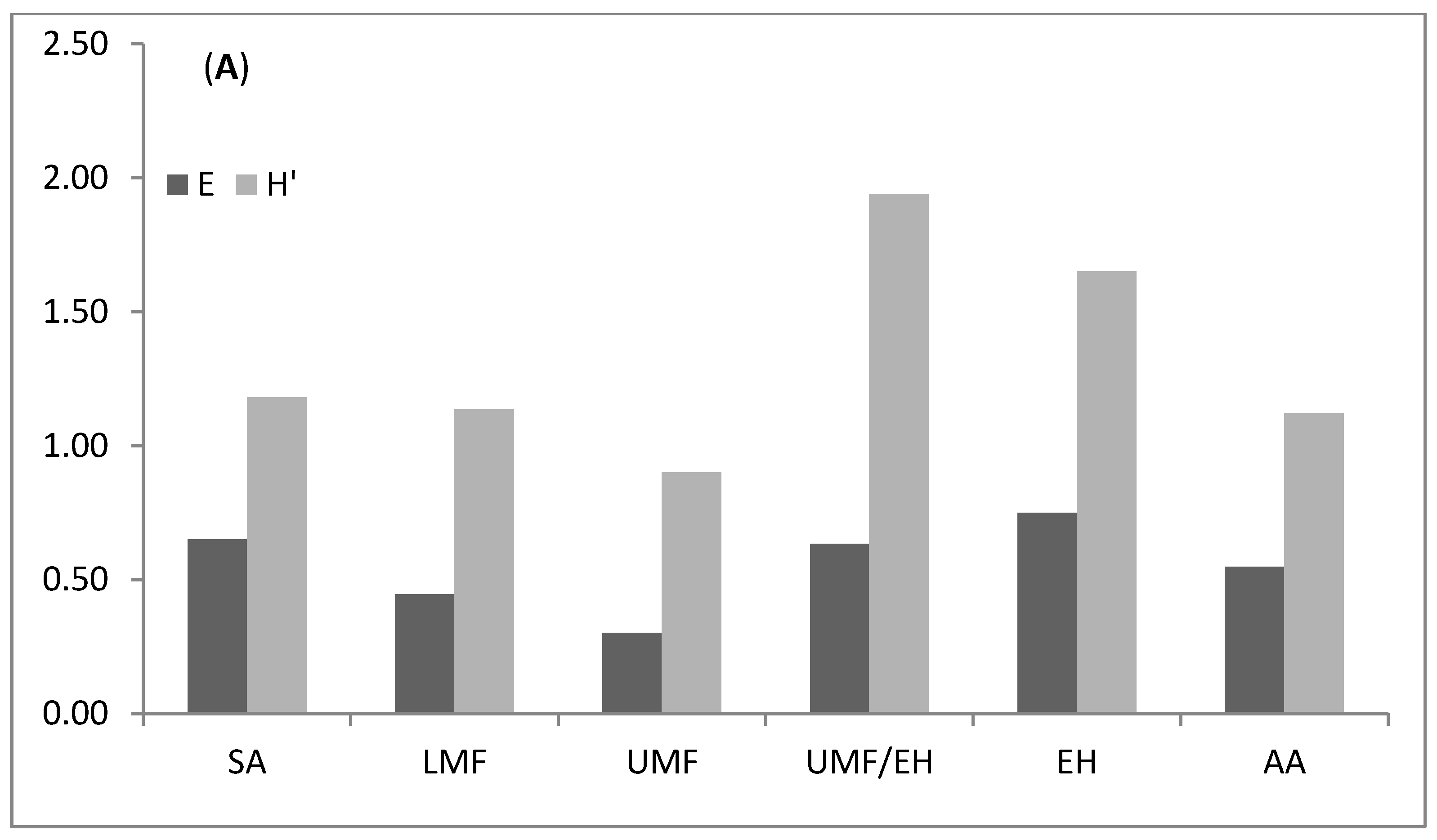
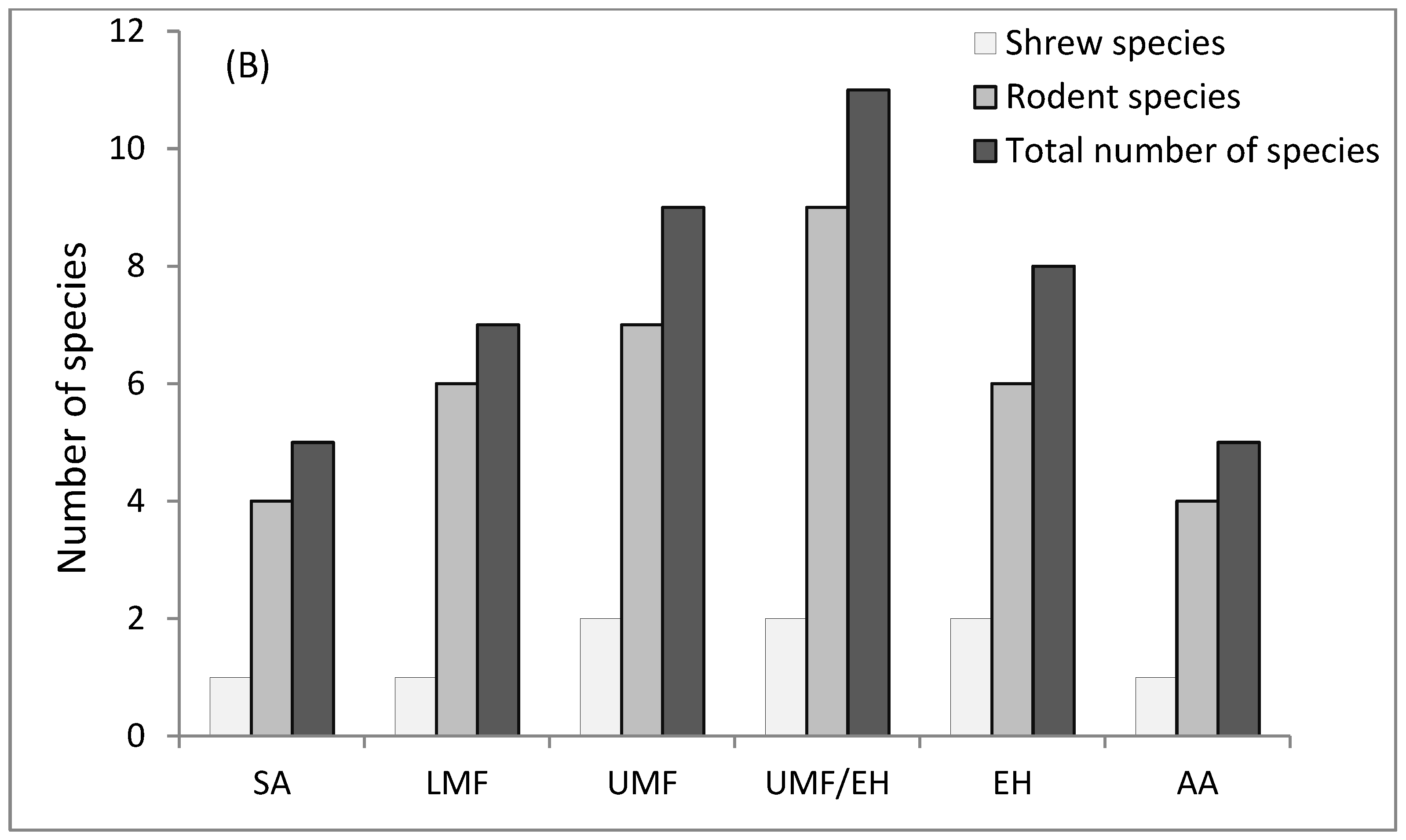
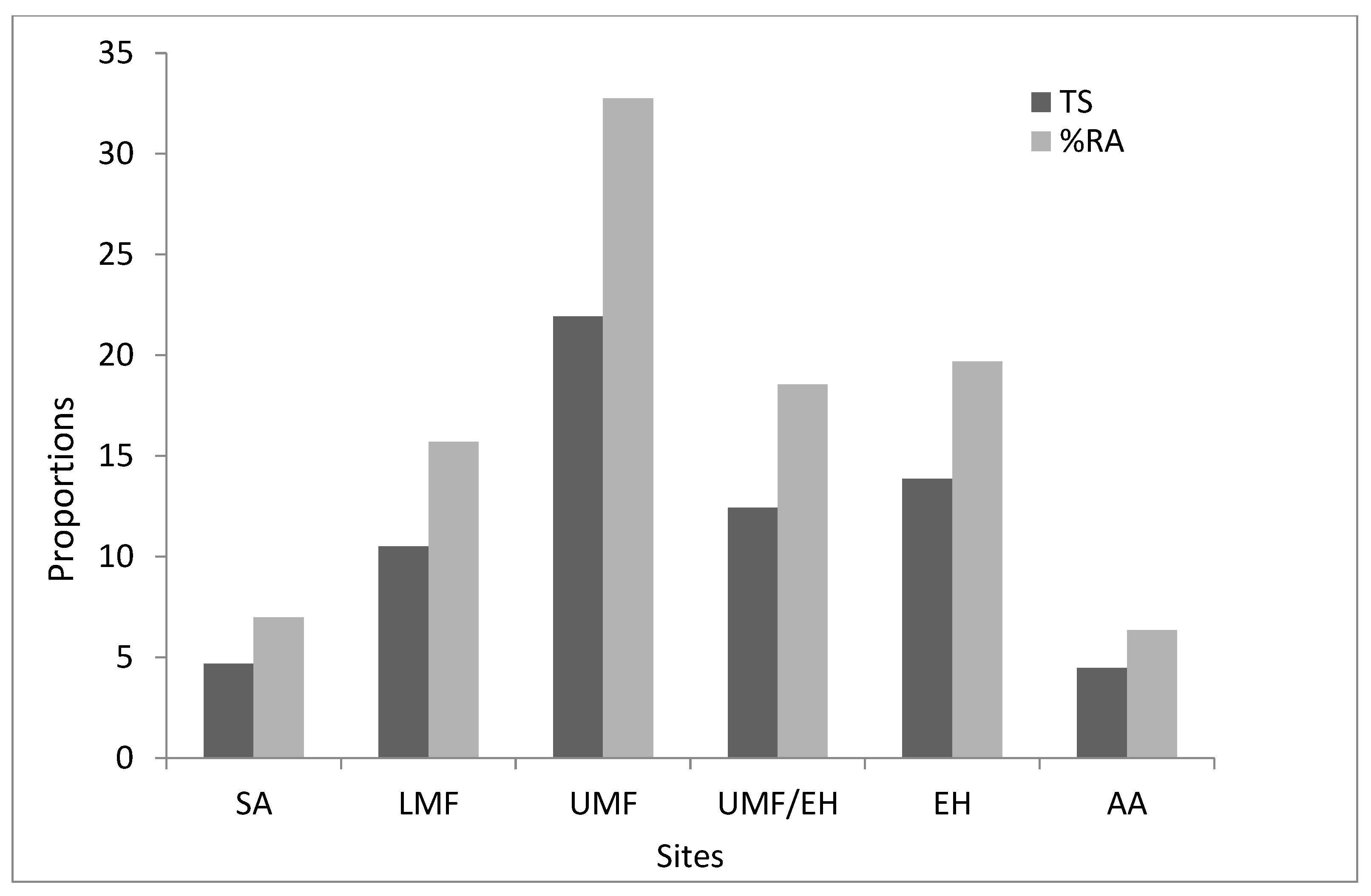
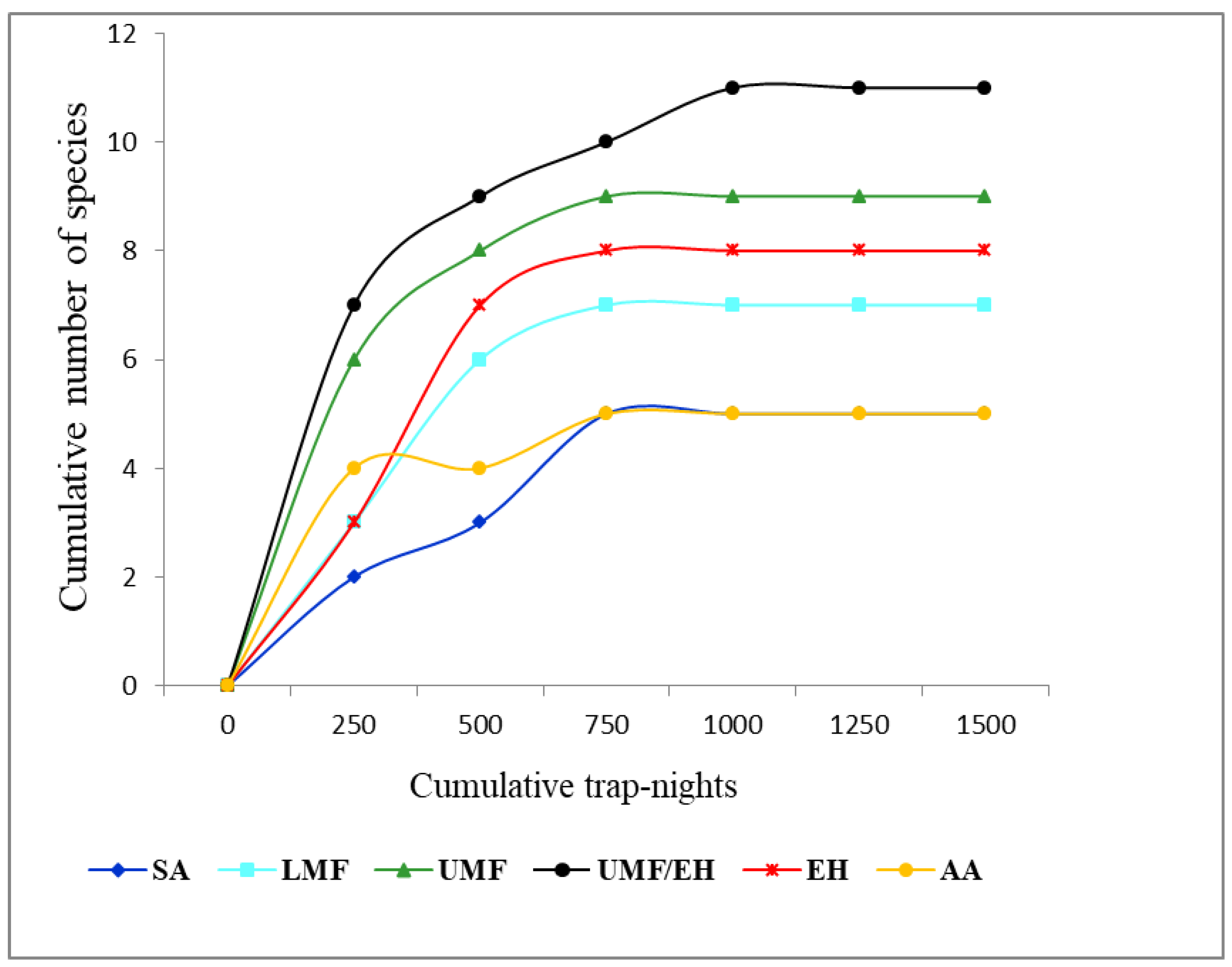
3.1. Abundance and Species Richness across Elevation Graident
| Sites | |||||||
|---|---|---|---|---|---|---|---|
| SA | LMF | UMF | UMF/EH | EH | AA | Totals | |
| Rodents | |||||||
| Rhabdomys dilectus | 3.6(2) | 0.8(1) | 1.1(3) | 17.4(26) | 12.7(20) | 70.6(36) | 11.0(88) |
| Praomys taitae | – | 57.1(72) | 73.0(192) | 26.2(39) | 11.4(18) | – | 40.0(321) |
| Montemys delectorum @ | – | – | 0.8(2) | 0.7(1) | – | – | 0.4(3) |
| Mus triton | – | – | – | – | – | 7.8(4) | 0.5(4) |
| Mastomys natalensis | 51.8(29) | – | – | – | – | – | 3.6(29) |
| Lophuromys verhageni | – | – | – | 8.1(12) | 23.4(37) | 9.8(5) | 6.7(54) |
| Lemniscomys striatus | – | – | 1.1(3) | 0.7(1) | – | – | 0.5(4) |
| Graphiurus murinus | – | – | – | 14.1(21) | 3.2(5) | – | 3.2(26) |
| Grammomys dolichurus | 10.7(6) | 2.4(3) | 1.9(5) | 2.7(4) | 1.9(3) | – | 2.6(21) |
| Dendromus insignis | – | – | – | – | 2.5(4) | 5.9(3) | 0.9(7) |
| Dasymys incomtus | – | 2.4(3) | 1.1(3) | 0.7(1) | – | – | 0.9(7) |
| Cricetomys ansorgei | – | 3.2(4) | 1.1(3) | 1.3(2) | ¥ | ¥ | 1.1(9) |
| Arvicanthis niloticus | 30.4(17) | 4(5) | – | – | – | – | 2.7(22) |
| Shrews | |||||||
| Crocidura newmarki | 3.6(2) | 30.2(38) | 7.2(19) | 15.4(23) | 26.6(42) | 5.9(3) | 15.8(127) |
| Crocidura allex | – | – | 12.5(33) | 12.8(19) | 29(18.4) | – | 10.1(81) |
| Rodent abundance | 96.4(54) | 69.8(88) | 80.2(211) | 71.80(107) | 55.1(87) | 94.1(48) | 74.1(595) |
| Shrew abundance | 3.6(2) | 30.2(38) | 19.8(52) | 28.2(42) | 44.9(71) | 5.9(3) | 25.9(208) |
| Rodent species | 4 | 6 | 7 | 9 | 6 | 4 | 13 |
| Shrew species | 1 | 1 | 2 | 2 | 2 | 1 | 2 |
| Trap success (%) | 4.7 | 10.5 | 21.9 | 12.4 | 13.9 | 4.5 | 11.3 |
| Trap effort | 1200 | 1200 | 1200 | 1200 | 1140 | 1140 | 7080 |
| SA | LMF | UMF | UMF/EH | EH | AA | |
|---|---|---|---|---|---|---|
| X2 | 27.00 | 22.87 | 15.00 | 6.27 | 19.20 | 27.00 |
| df | 5 | 7 | 9 | 10 | 8 | 5 |
| p–value | 0.000 | 0.001 | 0.035 | 0.134 | 0.003 | 0.00 |
3.2. Changes in Community Composition in the Last Decade, between 2009 and 2021
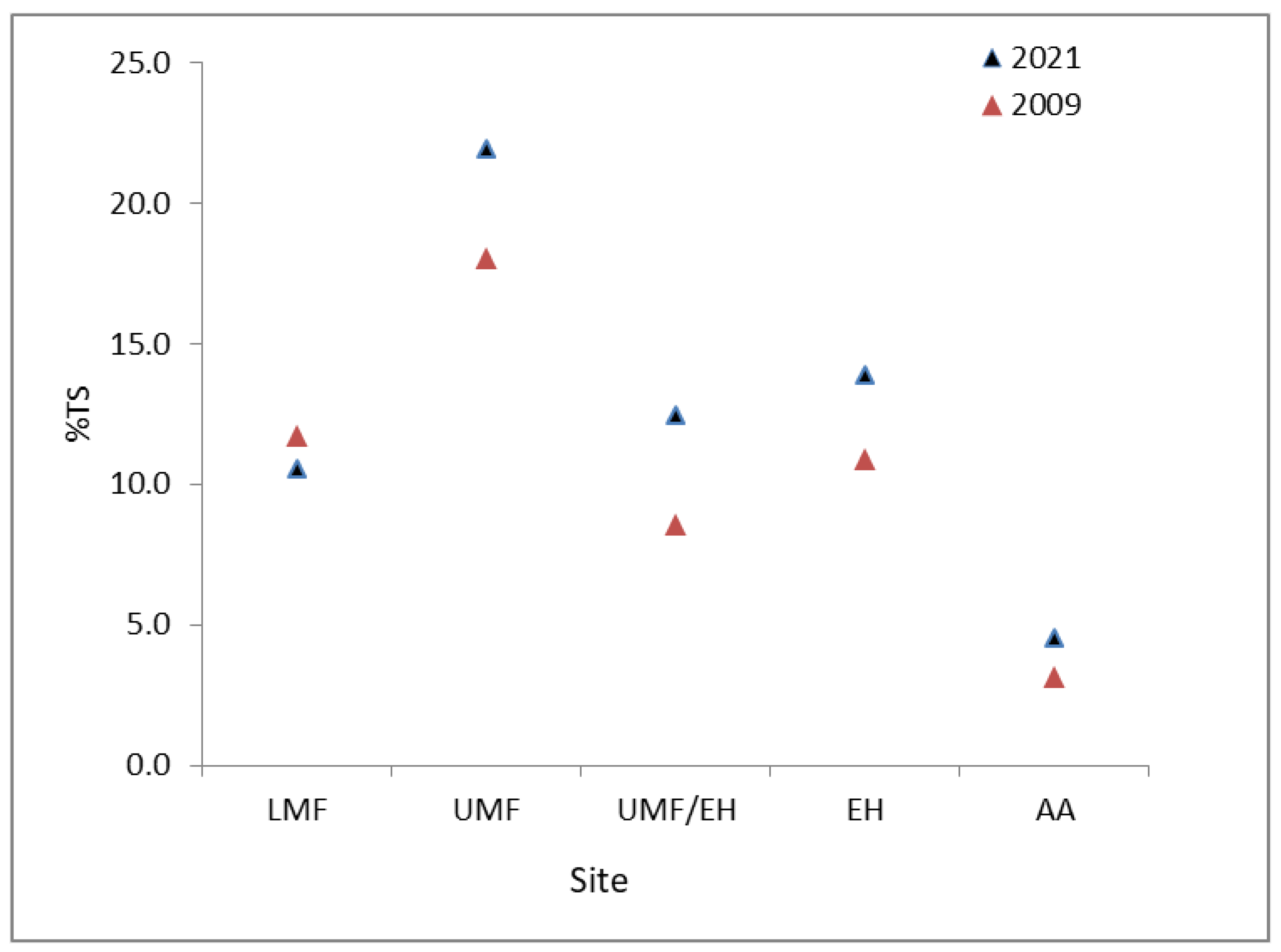
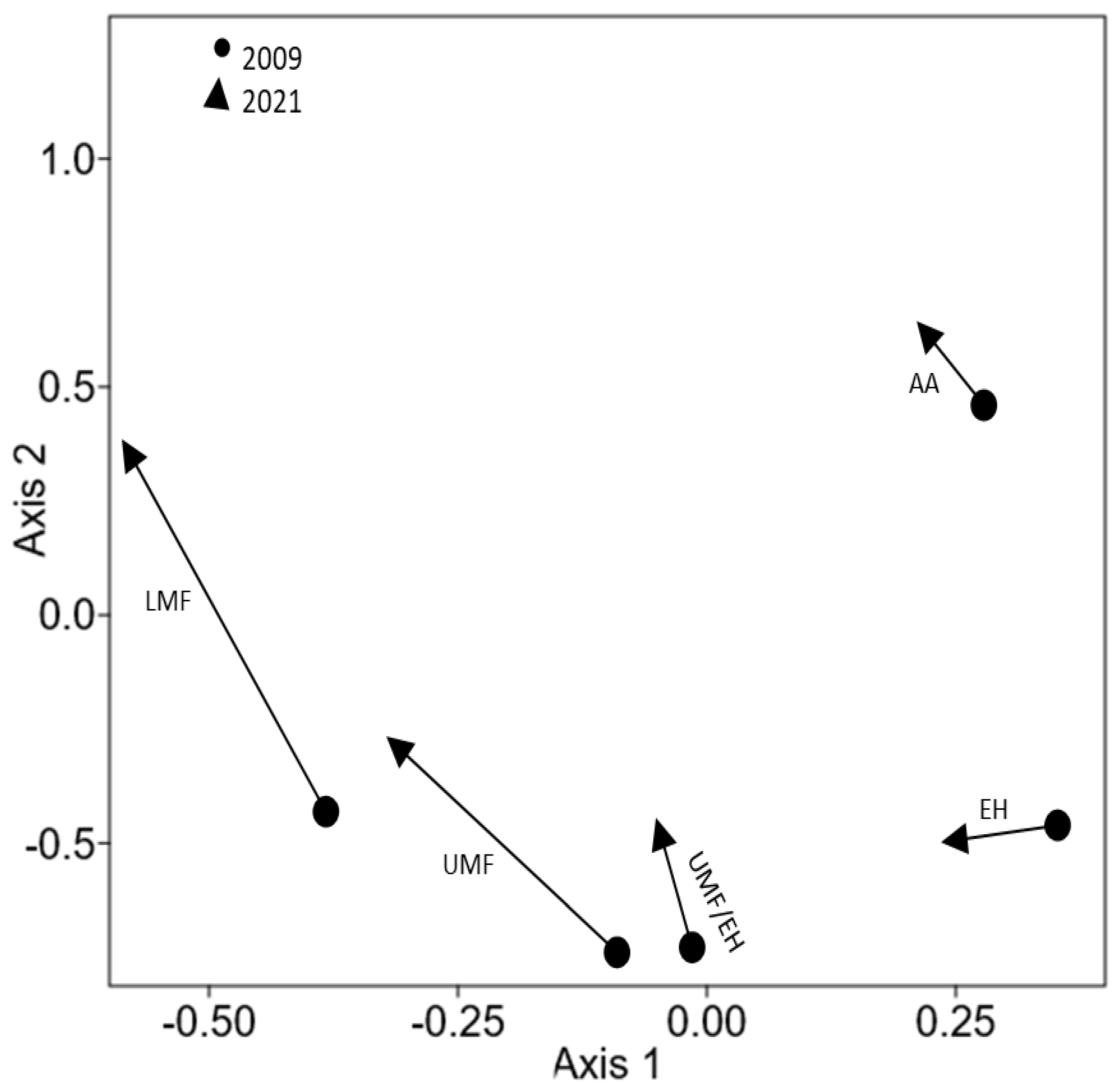
4. Discussion
4.1. Diversity and Distribution Patterns
4.2. Possible Changes in Species Composition between 2009 and 2021
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Small Mammal Species | SA ns 2021 | LMF | UMF | UMF/EH | EH | AA | TOTAL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2021 | 2009 | 2021 | 2009 | 2021 | 2009 | 2021 | 2009 | 2021 | 2009 | 2021 | ||
| Rhabdomys dilectus | 2 | 0 | 1 | 0 | 3 | 24 | 26 | 7 | 20 | 24 | 36 | 55 | 88 |
| Otomys tropicali | 0 | 0 | 72 | 0 | 192 | 2 | 39 | 1 | 18 | 1 | 0 | 4 | 321 |
| Praomys taitae | 0 | 79 | 0 | 185 | 0 | 38 | 0 | 4 | 0 | 0 | 0 | 306 | 0 |
| Montemys delectorum @ | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| Mus triton | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 1 | 4 |
| Mastomys natalensis | 29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 29 |
| Lophuromys verhageni | 0 | 0 | 0 | 18 | 0 | 9 | 12 | 30 | 37 | 2 | 5 | 59 | 54 |
| Lemniscomys striatus | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| Graphiurus murinus | 0 | 5 | 0 | 1 | 0 | 6 | 21 | 15 | 5 | 0 | 0 | 27 | 26 |
| Grammomys dolichurus | 6 | 3 | 3 | 4 | 5 | 2 | 4 | 4 | 3 | 0 | 0 | 13 | 21 |
| Dendromus insignis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 7 | 4 | 2 | 3 | 10 | 7 |
| Dasymys incomtus | 0 | 0 | 3 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 7 |
| Cricetomys ansorgei | 0 | 0 | 4 | 0 | 3 | 0 | 2 | 0 | – | 0 | – | 0 | 9 |
| Arvicanthis niloticus | 17 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 |
| Crocidura newmarki | 2 | 49 | 38 | 18 | 19 | 22 | 23 | 51 | 42 | 4 | 3 | 144 | 127 |
| Crocidura allex | 0 | 31 | 0 | 31 | 33 | 18 | 19 | 36 | 29 | 16 | 0 | 132 | 81 |
| Species richness | 5 | 5 | 7 | 6 | 9 | 9 | 11 | 10 | 8 | 6 | 5 | 10 | 15 |
| Total number of captures | 56 | 167 | 126 | 257 | 263 | 122 | 149 | 155 | 158 | 50 | 51 | 751 | 803 |
| Total trap-effort | 1200 | 1426 | 1200 | 1426 | 1200 | 1426 | 1200 | 1426 | 1140 | 1407 | 1140 | 7111 | 7080 |
| Trap success | 4.7 | 11.7 | 10.5 | 18 | 21.9 | 8.6 | 12.4 | 10.9 | 13.8 | 3.5 | 4.5 | 10.5 | 11.3 |
| Elevation Zone | Daily Rainfall (mm) | Daily Minimum Temperature (°C) | Daily Maximum Temperature (°C) |
|---|---|---|---|
| SA | |||
| Mean ± SD | 0.1 ± 0.3 | 20.6 ± 5.8 | 20.9 ± 5.8 |
| R | 0–0.7 | 12.5–28.1 | 12.6–28.3 |
| N | 6(1) | 6 | 6 |
| LMF | |||
| Mean ± SD | 0 | 14.5 ± 1.5 | 15.6 ± 1.8 |
| R | 0 | 11.6–15.7 | 12.3–17.5 |
| N | 6(0) | 6 | 6 |
| UMF | |||
| Mean ± SD | 2.5 ± 1.4 | 14.7 ± 2.2 | 15.4 ± 2.8 |
| R | 0–4.1 | 13.3–18.9 | 13.6 ± 19.1 |
| N | 6(2) | 6 | 6 |
| UMF/EH | |||
| Mean ± SD | 2.2 ± 1.7 | 10 ± 0.34 | 12.5 ± 2.0 |
| R | 0–3.8 | 9.5–10.5 | 10.4–15.9 |
| N | 5(4) | 5 | 5 |
| EH | |||
| Mean ± SD | 3.3 ± 1.6 | 10.3 ± 0.2 | 12.8 ± 2.2 |
| R | 0–4.2 | 10.1–10.5 | 10.6–16.4 |
| N | 6(2) | 6 | 6 |
| AA | |||
| Mean ± SD | 1.7 ± 1.9 | 5.5 ± 2.4 | 8 ± 1.3 |
| R | 0–3.8 | 2.3 ± 8.3 | 6.9–10.3 |
| N | 5(3) | 5 | 5 |
References
- Elsen, P.R.; Monahan, W.B.; Merenlender, A.M. Global Patterns of Protection of Elevational Gradients in Mountain Ranges. Proc. Natl. Acad. Sci. USA 2018, 115, 6004–6009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta–Holme, N.; Nogues–Bravo, D.; Whittaker, R.J.; Fjeldsa, J. Humboldt’s Enigma: What Causes Global Patterns of Mountain Biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef]
- Neate–Clegg, M.H.C.; Jones, S.E.I.; Burdekin, O.; Jocque, M.; Şekercioğlu, Ç.H. Elevational Changes in the Avian Community of a Mesoamerican Cloud Forest Park. Biotropica 2018, 50, 805–815. [Google Scholar] [CrossRef]
- Rogora, M.; Frate, L.; Carranza, M.L.; Freppaz, M.; Stanisci, A.; Bertani, I.; Bottarin, R.; Brambilla, A.; Canullo, R.; Carbognani, M.; et al. Assessment of Climate Change Effects on Mountain Ecosystems through a Cross–Site Analysis in the Alps and Apennines. Sci. Total Environ. 2018, 624, 1429–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willig, M.R.; Presley, S.J. Biodiversity and Metacommunity Structure of Animals along Altitudinal Gradients in Tropical Montane Forests. J. Trop. Ecol. 2015, 32, 421–436. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Song, W.; Li, Q.; Onditi, K.; Khanal, L.; Jiang, X. Small Mammal Species Richness and Turnover along Elevational Gradient in Yulong Mountain, Yunnan, Southwest China. Ecol. Evol. 2020, 10, 2545–2558. [Google Scholar] [CrossRef]
- Pacifici, M.; Rondinini, C.; Rhodes, J.R.; Burbidge, A.A.; Cristiano, A.; Watson, J.E.M.; Woinarski, J.C.Z.; Di Marco, M. Global Correlates of Range Contractions and Expansions in Terrestrial Mammals. Nat. Commun. 2020, 11, 2840. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Sanders, N.J.; Wardle, D.A. Community and Ecosystem Responses to Elevational Gradients: Processes, Mechanisms, and Insights for Global Change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 261–280. [Google Scholar] [CrossRef] [Green Version]
- Craig, E.W.; Stanley, W.T.; Kerbis Peterhans, J.C.; Bryja, J.; Meheretu, Y. Small Terrestrial Mammal Distributions in Simien Mountains National Park, Ethiopia: A Reassessment after 88 Years. J. Mammal. 2020, 101, 634–647. [Google Scholar] [CrossRef]
- Mena, J.L.; Pacheco, V. Mountains and Traits: Environmental Heterogeneity and Mammal Assemblages along an Elevational Gradient in the Northern Andes. Stud. Neotrop. Fauna Environ. 2020, 13, 1–3. [Google Scholar] [CrossRef]
- Heaney, L.R.; Heideman, P.D.; Rickart, E.A.; Utzurrum, R.B.; Klompen, J.S.H. Elevational Zonation of Mammals in the Central Philippines. J. Trop. Ecol. 1989, 5, 259–280. [Google Scholar] [CrossRef]
- Rickart, E.A.; Heaney, L.R.; Utzurrum, R.C.B. Distribution and Ecology of Small Mammals along an Elevational Transect in Southeastern Luzon, Philippines. J. Mammal. 1991, 72, 458–469. [Google Scholar] [CrossRef]
- Kamenišťák, J.; Baláž, I.; Tulis, F.; Jakab, I.; Ševčík, M.; Poláčiková, Z.; Klimant, P.; Ambros, M.; Rychlik, L. Changes of Small Mammal Communities with the Altitude Gradient. Biologia 2019, 75, 713–722. [Google Scholar] [CrossRef]
- Benedek, A.M.; Sîrbu, I. Dynamics of Small–Mammal Communities along an Elevational Gradient. Can. J. Zool. 2019, 97, 312–318. [Google Scholar] [CrossRef]
- Moritz, C.; Patton, J.L.; Conroy, C.J.; Parra, J.L.; White, G.C.; Beissinger, S.R. Impact of a Century of Climate Change on Small–Mammal Communities in Yosemite National Park, USA. Science 2008, 322, 261–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulungu, L.S.; Makundi, R.H.; Massawe, A.W.; Machang’u, R.S.; Mbije, N.E. Diversity and Distribution of Rodent and Shrew Species Associated with Variations in Altitude on Mount Kilimanjaro, Tanzania. Mammalia 2008, 72, 178–185. [Google Scholar] [CrossRef]
- Stanley, W.T.; Rogers, M.A.; Kihaule, P.M.; Munissi, M.J. Elevational Distribution and Ecology of Small Mammals on Africa’s Highest Mountain. PLoS ONE 2014, 9, e109904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clausnitzer, V.; Kityo, R. Altitudinal Distribution of Rodents (Muridae and Gliridae) on Mt Elgon, Uganda. Trop. Zool. 2001, 14, 95–118. [Google Scholar] [CrossRef]
- Clausnitzer, V.; Denys, C.; Granjon, L.; Poulet, A. Rodents of the afro–alpine zone of Mt. Elgon. Afr. Small Mamm. 2001, 427–443. [Google Scholar]
- Musila, S.; Chen, Z.Z.; Li, Q.; Yego, R.; Zhang, B.; Onditi, K.; Jiang, X.L. Diversity and distribution patterns of Non–Volant small mammals along different elevation gradients on Mt. Kenya, Kenya. Zool. Res. 2019, 40, 53–60. [Google Scholar] [CrossRef]
- Stanley, W.T.; Hutterer, R. Differences in Abundance and Species Richness between Shrews and Rodents along an Elevational Gradient in the Udzungwa Mountains, Tanzania. Acta Theriol. 2007, 52, 261–275. [Google Scholar] [CrossRef]
- Pratt, D.M.; Anderson, V.H. Population, Distribution, and Behaviour of Giraffe in the Arusha National Park, Tanzania. J. Nat. Hist. 1982, 16, 481–489. [Google Scholar] [CrossRef]
- Maleko, D.D.; Mbassa, G.N.; Maanga, W.F.; Sisya, E.S. Impacts of wildlife–livestock interactions in and around Arusha National Park, Tanzania. Curr. Res. J. Biol. Sci. 2012, 4, 471–476. [Google Scholar]
- Morand, S.; Krasnov, B.R.; Poulin, R. Micromammals and Macroparasites: From Evolutionary Ecology to Management; Morand, S., Krasnov, B.R., Poulin, R., Eds.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Demeter, A.; Hutterer, R. Small mammals from Mt. Meru and its environs (Northern Tanzania). Cimbebasia 1986, 8, 199–207. [Google Scholar]
- Stanley, W.T.; Kihaule, P.M. Elevational Distribution and Ecology of Small Mammals on Tanzania’s Second Highest Mountain. PLoS ONE 2016, 11, e0162009. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.G.; Brecher, H.H.; Mosley–Thompson, E.; Hardy, D.R.; Mark, B.G. Glacier Loss on Kilimanjaro Continues Unabated. Proc. Natl. Acad. Sci. USA 2009, 106, 19770–19775. [Google Scholar] [CrossRef] [Green Version]
- Rowe, R.J.; Finarelli, J.A.; Rickart, E.A. Range Dynamics of Small Mammals along an Elevational Gradient over an 80–Year Interval. Glob. Change Biol. 2009, 16, 2930–2943. [Google Scholar] [CrossRef]
- Bussmann, R.W. Vegetation zonation and nomenclature of African Mountains–an overview. Lyonia 2006, 11, 41–66. [Google Scholar]
- Common Plants of Arusha National Park lowlands. Available online: https://fieldguides.fieldmuseum.org/sites/default/files/rapid–color–guides–pdfs/330_Arusha_Park_Lowlands_d1_1.pdf (accessed on 13 March 2021).
- Sikes, R.S.; Gannon, W.L. Guidelines of the American Society of Mammalogists for the Use of Wild Mammals in Research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- Happold, D.C.D.; Kingdon, J. Rodents, Hares and Rabbits. In Mammals of Africa; Happold, D.C.D., Ed.; Bloomsbury: London, UK, 2013; Volume III. [Google Scholar]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9.1.0. User’s Guide and Application. 2013. Available online: http://purl.oclc.org/estimates (accessed on 15 November 2021).
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Climate Change Arusha National Park. Available online: https://www.meteoblue.com/en/climate–change/arusha–national–park_tanzania_161323 (accessed on 24 December 2021).
- Buytaert, W.; Cuesta–Camacho, F.; Tobon, C. Potential Impacts of Climate Change on the Environmental Services of Humid Tropical Alpine Regions. Glob. Ecol. Biogeogr. 2010, 20, 19–33. [Google Scholar] [CrossRef]
- Ademola, O.J.; Vanden Broecke, B.; Leirs, H.; Mulungu, L.S.; Massawe, A.W.; Makundi, R.H. Effects of Forest Disturbance on the Fitness of an Endemic Rodent in a Biodiversity Hotspot. Ecol. Evol. 2021, 11, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Ademola, O.J.; Massawe, A.W.; Mulungu, L.S.; Hieronimo, P.; Makonda, F.B.S.; Makundi, R.H. Habitat Type Impacts Small Mammal Diversity in the Ukaguru Mountains, Tanzania. Mammalia 2021, 86, 123–133. [Google Scholar] [CrossRef]
- Taylor, P.J. Regional patterns of small mammal abundance and community composition in protected areas in KwaZulu–Natal. Durb. Mus. Novit. 1998, 23, 42–51. [Google Scholar]
- Happold, D.C.D.; Happold, M. An Ecological Study of Small Rodents in the Thicket-Clump Savanna of Lengwe National Park, Malawi. J. Zool. 1991, 223, 527–547. [Google Scholar] [CrossRef]
- Makundi, R.H.; Massawe, A.W.; Mulungu, L.S. Reproduction and Population Dynamics of Mastomys Natalensis Smith, 1834 in an Agricultural Landscape in the Western Usambara Mountains, Tanzania. Integr. Zool. 2007, 2, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.T.; Kihaule, P.M.; Howell, K.M.; Hutterer, R. Small Mammals of the Eastern Arc Mountains, Tanzania. J. East Afr. Nat. Hist. 1998, 87, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Stanley, W.T.; Kihaule, P.M. Correction: Elevational Distribution and Ecology of Small Mammals on Tanzania’s Second Highest Mountain. PLoS ONE 2019, 14, e0225985. [Google Scholar] [CrossRef]
- Kasangaki, A.; Kityo, R.; Kerbis, J. Diversity of Rodents and Shrews along an Elevational Gradient in Bwindi Impenetrable National Park, South–Western Uganda. Afr. J. Ecol. 2003, 41, 115–123. [Google Scholar] [CrossRef]
- Andrade, A.; Monjeau, A. Patterns in Community Assemblage and Species Richness of Small Mammals across an Altitudinal Gradient in Semi–Arid Patagonia, Argentina. J. Arid Environ. 2014, 106, 18–26. [Google Scholar] [CrossRef]
- Brown, J.H. Mammals on Mountainsides: Elevational Patterns of Diversity. Glob. Ecol. Biogeogr. 2001, 10, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Zu, K.; Wang, Z.; Zhu, X.; Lenoir, J.; Shrestha, N.; Lyu, T.; Luo, A.; Li, Y.; Ji, C.; Peng, S.; et al. Upward Shift and Elevational Range Contractions of Subtropical Mountain Plants in Response to Climate Change. Sci. Total Environ. 2021, 783, 146896. [Google Scholar] [CrossRef] [PubMed]
- Elsen, P.R.; Monahan, W.B.; Merenlender, A.M. Topography and Human Pressure in Mountain Ranges Alter Expected Species Responses to Climate Change. Nat. Commun. 2020, 11, 1974. [Google Scholar] [CrossRef] [PubMed]
- Neate–Clegg, M.H.C.; Jones, S.E.I.; Tobias, J.A.; Newmark, W.D.; Şekercioǧlu, Ç.H. Ecological Correlates of Elevational Range Shifts in Tropical Birds. Front. Ecol. Evol. 2021, 9, 215. [Google Scholar] [CrossRef]
- Colwell, R.K.; Brehm, G.; Cardelus, C.L.; Gilman, A.C.; Longino, J.T. Global Warming, Elevational Range Shifts, and Lowland Biotic Attrition in the Wet Tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, B.G.; Scholer, M.N.; Ruiz–Gutierrez, V.; Fitzpatrick, J.W. Climate Change Causes Upslope Shifts and Mountaintop Extirpations in a Tropical Bird Community. Proc. Natl. Acad. Sci. USA 2018, 115, 11982–11987. [Google Scholar] [CrossRef] [PubMed]
- Herzog, S.K.; For, I.; Council, I. Climate Change and Biodiversity in the Tropical Andes; Inter–American Institute for Global Change Research: São Paulo, Brazil, 2011. [Google Scholar]
- La Sorte, F.A.; Jetz, W. Projected Range Contractions of Montane Biodiversity under Global Warming. Proc. R. Soc. B 2010, 277, 3401–3410. [Google Scholar] [CrossRef] [Green Version]
- Mt. Meru in Tanzania on Fire. Available online: https://youtu.be/PjThKgOTKEA (accessed on 15 October 2021).
- Shore, R.F.; Garbett, S.D. Notes on the Small Mammals of the Shira Plateau, Mt. Kilimanjaro. Mammalia 1991, 55, 601–608. [Google Scholar] [CrossRef]
| Small Mammal Species | SA | LMF | UMF | UMF/EH | EH | AA | Total (R2) |
|---|---|---|---|---|---|---|---|
| Rhabdomys dilectus | 2.3 | 1.1 | 3.4 | 29.5 | 22.7 | 40.9 | 11.0(88) †† |
| Praomys taitae | 0.0 | 22.4 | 59.8 | 12.1 | 5.6 | 0.0 | 40.0(321) |
| Montemys delectorum | 0.0 | 0.0 | 66.7 | 33.3 | 0.0 | 0.0 | 0.4(3) |
| Mus triton | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 0.5(4) |
| Mastomys natalensis | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.6(29) |
| Lophuromys verhageni | 0.0 | 0.0 | 0.0 | 22.2 | 68.5 | 9.3 | 6.7(54) |
| Lemniscomys striatus | 0.0 | 0.0 | 75.0 | 25.0 | 0.0 | 0.0 | 0.5(4) |
| Graphiurus murinus | 0.0 | 0.0 | 0.0 | 80.8 | 19.2 | 0.0 | 3.2(26) |
| Grammomys dolichurus | 28.6 | 14.3 | 23.8 | 19.0 | 14.3 | 0.0 | 2.6(21) |
| Dendromus insignis | 0.0 | 0.0 | 0.0 | 0.0 | 57.1 | 42.9 | 0.9(7) †† |
| Dasymys incomtus | 0.0 | 42.9 | 42.9 | 14.3 | 0.0 | 0.0 | 0.9(7) |
| Cricetomys ansorgei | 0.0 | 44.4 | 33.3 | 22.2 | ¥ | ¥ | 1.1(9) ¥ |
| Arvicanthis niloticus | 77.3 | 22.7 | 0.0 | 0.0 | 0.0 | 0.0 | 2.7(22) †† |
| Crocidura newmarki | 1.6 | 29.9 | 15.0 | 18.1 | 33.1 | 2.4 | 15.8(127) |
| Crocidura allex | 0.0 | 0.0 | 40.7 | 23.5 | 35.8 | 0.0 | 10.1(81) |
| Species | SA ns | LMF | UMF | UMF/EH | EH | AA |
|---|---|---|---|---|---|---|
| Rhabdomys dilectus | –/3.6 | –/0.8 | –/1.1 | 19.7/17.4 | 4.5/12.7 | 48/70.6 |
| Otomys tropicali | –/0.0 | –/– | –/– | 1.6/– | 0.6/– | 2.0/– |
| Praomys taitae D | –/0.0 | 47.3/57.1 | 72.0/73.0 | 31.1/26.2 | 2.6/11.4 | –/– |
| Montemys delectorum | –/0.0 | –/– | –/0.8 | –/0.7 | –/– | –/– |
| Mus triton | –/0.0 | –/– | –/– | –/– | 0.6/– | –/7.8 |
| Mastomys natalensis d | –/51.8 | –/– | –/– | –/– | –/– | –/– |
| Lophuromys verhageni | –/0.0 | –/– | 7.0/– | 7.4/8.1 | 19.4/23.4 | 4.1/9.8 |
| Lemniscomys striatus D | –/0.0 | –/– | –/1.1 | –/0.7 | –/– | –/– |
| Graphiurus murinus D | –/0.0 | 3.0/– | 0.4/– | 4.9/14.1 | 9.7/3.2 | –/– |
| Grammomys dolichurus D | –/10.7 | 1.8/2.4 | 1.6/1.9 | 1.6//2.7 | 2.6/1.9 | –/– |
| Dendromus insignis | –/0.0 | –/– | –/– | 0.8/– | 4.5/2.5 | 4/5.9 |
| Dasymys incomtus | –/0.0 | –/2.4 | –/1.1 | –/0.7 | –/– | –/– |
| Cricetomys ansorgei | –/0.0 | –/3.2 | –/1.1 | –/1.3 | –/– | –/– |
| Arvicanthis niloticus | –/30.4 | –/4.0 | –/– | –/– | –/– | –/– |
| Crocidura newmarki | –/3.6 | 29.3/30.2 | 7.0/7.2 | 18.0/15.4 | 32.9/26.6 | 8.0/5.9 |
| Crocidura allex D | –/0.0 | 18.6/– | 12.1/12.5 | 14.8/12.8 | 23.2/18.4 | 32.0/– |
| Percentage of contribution per site | –/7.0 | 22.2/15.7 | 34.2/32.8 | 16.2/18.6 | 20.6/19.7 | 6.7/6.4 |
| Total number of species | –/5 | 5/7 | 6/9 | 9/11 | 10/8 | 6/5 |
| Total number of captures | –/56 | 167/126 | 257/263 | 122/149 | 156/158 | 49/51 |
| Trap effort | –/1200 | 1426/1200 | 1426/1200 | 1426/1200 | 1426/1140 | 1407/1140 |
| Trap success (%) | –/4.7 | 11.7/10.5 | 18.0/21.9 | 8.6/12.4 | 10.9/13.9 | 3.5/4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebrezgiher, G.B.; Makundi, R.H.; Meheretu, Y.; Mulungu, L.S.; Katakweba, A.A.S. A Decade-Long Change in the Elevational Distribution of Non-Volant Small Mammals on Mount Meru, Tanzania. Diversity 2022, 14, 454. https://doi.org/10.3390/d14060454
Gebrezgiher GB, Makundi RH, Meheretu Y, Mulungu LS, Katakweba AAS. A Decade-Long Change in the Elevational Distribution of Non-Volant Small Mammals on Mount Meru, Tanzania. Diversity. 2022; 14(6):454. https://doi.org/10.3390/d14060454
Chicago/Turabian StyleGebrezgiher, Genet Berhe, Rhodes H. Makundi, Yonas Meheretu, Loth S. Mulungu, and Abdul A. S. Katakweba. 2022. "A Decade-Long Change in the Elevational Distribution of Non-Volant Small Mammals on Mount Meru, Tanzania" Diversity 14, no. 6: 454. https://doi.org/10.3390/d14060454
APA StyleGebrezgiher, G. B., Makundi, R. H., Meheretu, Y., Mulungu, L. S., & Katakweba, A. A. S. (2022). A Decade-Long Change in the Elevational Distribution of Non-Volant Small Mammals on Mount Meru, Tanzania. Diversity, 14(6), 454. https://doi.org/10.3390/d14060454







