The Bioconcentration and the Translocation of Heavy Metals in Recently Consumed Salicornia ramosissima J. Woods in Highly Contaminated Estuary Marshes and Its Food Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species Studied
2.2. Soils Sampling and Analysis
2.3. Plants Sampling and Analysis
2.4. Translocation and Bioconcentration Factors
2.5. Assessment of Food Risk to Human Health
2.6. Statistical Analyses
3. Results
3.1. pH and Soil Conductivity Found in Populations and Habitats
3.2. Heavy Metal Concentrations in Soil
3.3. Heavy Metal Concentrations in Plants
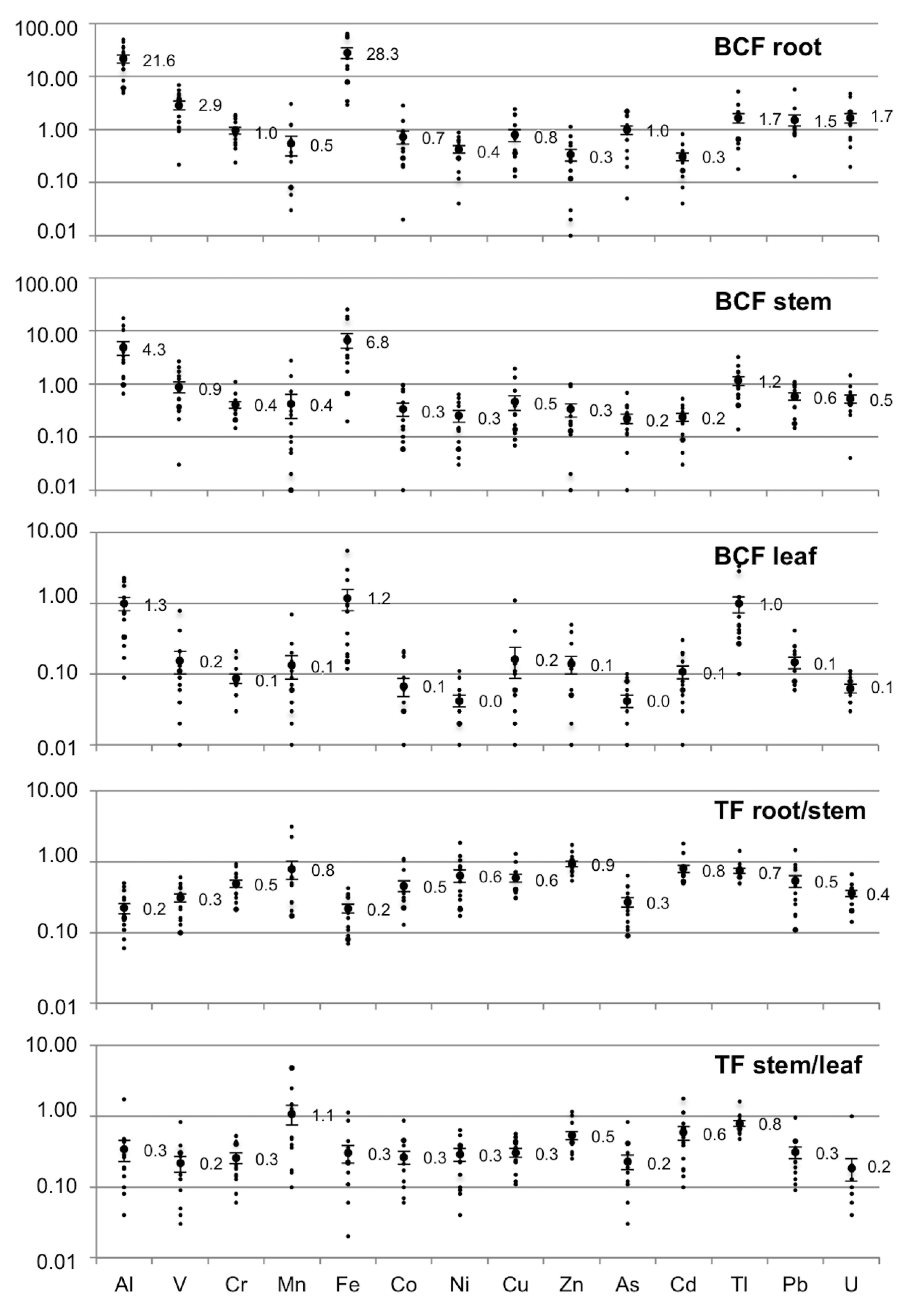
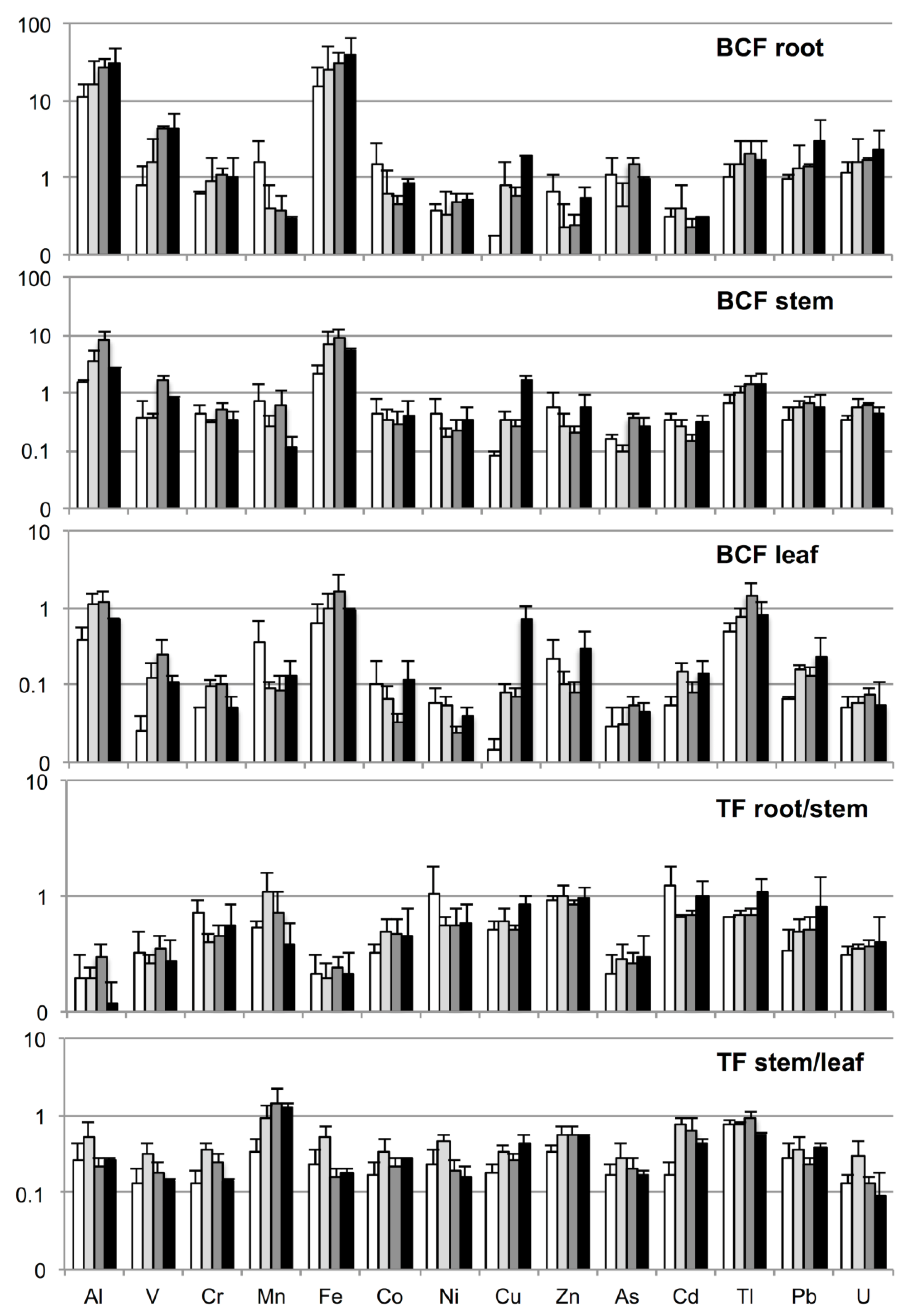
3.4. Translocation and Bioconcentration Factors
3.5. Assessment of Food Risk to Human Health
| Metal | Limit Content in Food | PTWI | TUIL | RfD | EDI Min. | EDI Max. | HRI Min. | HRI Max. |
|---|---|---|---|---|---|---|---|---|
| Al | - | 2 (1) | - | 1(14) | 0.001352 | 0.034180 | 0.001352 | 0.034180 |
| V | - | - | 1.8 (2,3) | 0.005(14) | 0.000008 | 0.000082 | 0.001600 | 0.016400 |
| Cr | 0.5 for vegetables (15) | - | - | 0.003(14) | 0.000014 | 0.000064 | 0.004640 | 0.021213 |
| Mn | - | - | 11 (2) | 0.14(14) | 0.001104 | 0.022785 | 0.007884 | 0.162747 |
| Fe | - | 0.8 (4) | 45 (2) | 0.7(14) | 0.003697 | 0.038215 | 0.005281 | 0.054593 |
| Co | - | - | - | 0.0003(14) | 0.000012 | 0.000274 | 0.040000 | 0.913333 |
| Ni | - | - | 1 (2); 0.15–0.90 (5) | 0.02(14) | 0.000028 | 0.000139 | 0.001392 | 0.006960 |
| Cu | - | - | 10 (2); 2–3 (4); 5 (6) | 0.04(14) | 0.000708 | 0.003248 | 0.017699 | 0.081189 |
| Zn | - | 1(4) | 40 (2); 25 (6) | 0.3(14) | 0.002917 | 0.009319 | 0.009725 | 0.031064 |
| As | 0.5 for vegetables (15) | 0.015 (7) | 0.003–0.008 (8) | 0.0003(12) | 0.000014 | 0.000263 | 0.046403 | 0.875028 |
| Cd | 0.2 (7); 0.1 (9); 0.2 (15) for leaf vegetables | 0.025 (4); 0.0025 (10) | - | 0.0001(14) | 0.000002 | 0.000046 | 0.019890 | 0.457400 |
| Tl | - | - | - | 0.00001(14) | 0.000012 | 0.000372 | 1.200000 | 37.200000 |
| Pb | 2 for food (7); 0.3 (11); 0.3 (15) for leaf vegetables | 0.025 (16) | - | 0.004(13) | 0.000039 | 0.000181 | 0.008452 | 0.045243 |
| U | - | - | - | 0.0002(14) | 0.000000 | 0.000008 | 0.000000 | 0.040000 |
4. Discussion
4.1. pH and Soil Conductivity
4.2. Heavy Metal Concentrations Found in Soil
4.3. Heavy Metal Concentrations in Plants
4.4. Translocation and Bioconcentration Factors
4.5. Assessment of Food Risk to Human Health
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greger, M. Metal availability and bioconcentration in plants. In Heavy Metal Stress in Plants; Springer: Berlin, Heidelberg, 1999; pp. 1–27. [Google Scholar]
- Yu, M.H. Environmental Toxicology-Biological and Health Effects of Pollutants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Nel, M.A.; Rubidge, G.; Adams, J.B.; Human, L.R. Rhizosediments of Salicornia tegetaria indicate metal contamination in the intertidal estuary zone. Front. Environ. Sci. 2020, 8, 175. [Google Scholar] [CrossRef]
- Agrawal, S.B.; Singh, A.; Sharma, R.K.; Agrawal, M. Bioaccumulation of heavy metals in vegetables: A threat to human health. Terr. Aquat. Environ. Toxicol. 2007, 1, 13–23. [Google Scholar]
- Pandey, R.; Dwivedi, M.K.; Singh, P.K.; Patel, B.; Pandey, S.; Patel, B.; Singh, B. Effluences of heavy metals, way of exposure and bio-toxic impacts: An update. J. Chem. Sci. 2016, 66, 2319–7625. [Google Scholar]
- Singh, A.K.; Sathya, M.; Verma, S.; Jayakumar, S. Health risk assessment of heavy metals in crop grains grown on open soils of Kanwar wetland, India. EMJE 2018, 3, 29. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- European Union Council Directive (EUCD) 1986/278 of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. 1986, 181, 6–12.
- Spanish Government Real Decreto (SGRD) 1310/1990, de 29 de octubre, por el que se regula la utilización de los lodos de depuración en el sector agrario. Boletín Of. Estado 1990, 262, 32339–32340.
- Consejería de Medio Ambiente. Los Criterios y Estándares para Declarar un Suelo Contaminado en Andalucía y la Metodología y Técnicas de Toma de Muestra y Análisis para su Investigación; Junta de Andalucía: Sevilla, Spain, 1999; Volume 246, pp. 61–64. [Google Scholar]
- Khan, M.U.; Malik, R.N.; Muhammad, S. Human health risk from heavy metal via food crops consumption with wastewater irrigation practices in Pakistan. Chemosphere 2013, 93, 2230–2238. [Google Scholar] [CrossRef]
- Yang, J.; Lv, F.; Zhou, J.; Song, Y.; Li, F. Health risk assessment of vegetables grown on the contaminated soils in Daye City of Hubei Province, China. Sustainability 2017, 9, 2141. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Kananke, T.; Wansapala, J.; Gunaratne, A. Detection of Ni, Cd, and Cu in green leafy vegetables collected from different cultivation areas in and around Colombo District, Sri Lanka. Environ. Monit. Assess. 2016, 188, 187. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, S.; Shi, Y.; Wang, C.; Li, B.; Li, Y.; Wu, S. Heavy metals in food crops, soil, and water in the Lihe River Watershed of the Taihu Region and their potential health risks when ingested. Sci. Total Environ. 2018, 615, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chabchoubi, I.B.; Bouguerra, S.; Ksibi, M.; Hentati, O. Health risk assessment of heavy metals exposure via consumption of crops grown in phosphogypsum-contaminated soils. Environ. Geochem. Health 2021, 43, 1953–1981. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.I.; Wang, Y.B.; Xin, G.U.; Su, Y.B.; Gang, W.A.N.G. Risk assessment of heavy metals in soils and vegetables around non-ferrous metals mining and smelting sites, Baiyin, China. Res. J. Environ. Sci. 2006, 18, 1124–1134. [Google Scholar] [CrossRef]
- Ramesh, H.L.; Yogananda Moorthy, V.N. Assessment of heavy metal contamination in green leafy vegetables grown in Bangalore urban district of Karnataka. Adv. Life Sci. 2012, 6, 40–51. [Google Scholar]
- Chang, C.Y.; Yu, H.Y.; Chen, J.J.; Li, F.B.; Zhang, H.H.; Liu, C.P. Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environ. Monit. Assess. 2014, 186, 1547–1560. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO); World Health Organization (WHO); Expert Committee on Food Additives. Codex Alimentarius. General Standard for Contaminants and Toxins in Food and Feed. Adopted in 1995, Revised in 1997, 2006, 2008, 2009 and Amended in 2010, 2012, 2013, 2014, 2015, 2016, 2017, 2018. 2019. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 25 May 2022).
- European Union Commission Regulation (EUCR) 2021/1323 of 10 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in certain foodstuffs (Text with EEA relevance). Off. J. 2021, 288, 13–18.
- European Union Commission Regulation (EUCR) 2021/1317 of 9 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs (Text with EEA relevance). Off. J. 2021, 286, 1–4.
- Food and Agriculture Organization (FAO); World Health Organization (WHO); Expert Committee on Food Additives. Compendium of Food Additive Specifications; FAO: Rome, Itaiy, 2011; Available online: https://www.fao.org/3/i2358e/i2358e.pdf (accessed on 25 May 2022).
- United States Institute of Medicine (USIM), Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222310 (accessed on 25 May 2022).
- European Food Safety Authority (EFSA). Tolerable Upper Intake Levels for Vitamins and Minerals; EFSA: Parma, Italy, 2006. [Google Scholar]
- European Food Safety Authority (EFSA). Overview on Tolerable Upper Intake Levels Asderived by the Scientific Committee on Food (SCF) and the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). 2018. Available online: https://www.efsa.europa.eu/sites/default/files/assets/UL_Summary_tables.pdf (accessed on 25 May 2022).
- European Food Safety Authority (EFSA). Scientific Report on the Chronic Dietary Exposure to Inorganic Arsenic. 2021. Available online: https://doi.org/10.2903/j.efsa.2021.6380 (accessed on 25 May 2022).
- Agencia Española de Seguridad Alimentaria y Nutrición (AESAN). Metales y Metaloides. Available online: https://www.aesan.gob.es/AECOSAN/web/home/aecosan_inicio.htm (accessed on 1 December 2021).
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; Licence: CC BY-NC-SA 3.0 IGO; WHO: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/rest/bitstreams/1080656/retrieve (accessed on 25 May 2022).
- United States Environmental Protection Agency (USEPA). Handbook for Non-Cancer Health Effects Evaluation; Environmental Protection Agency Science and Policy Council: Washington, DC, USA, 2000.
- United States Environmental Protection Agency (USEPA). Regional Screening Level (RSL) Subchronic Toxicity Supporting Table November 2021. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 25 May 2022).
- United States Environmental Protection Agency (USEPA). Integrated Risk Information System. Available online: https://www.epa.gov/iris (accessed on 25 January 2022).
- Huang, Z.; Pan, X.D.; Wu, P.G.; Han, J.L.; Chen, Q. Heavy metals in vegetables and the health risk to population in Zhejiang, China. Food Control 2014, 36, 248–252. [Google Scholar] [CrossRef]
- Antoine, J.M.; Fung LA, H.; Grant, C.N. Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 2017, 4, 181–187. [Google Scholar] [CrossRef]
- Woldetsadik, D.; Drechsel, P.; Keraita, B.; Itanna, F.; Gebrekidan, H. Heavy metal accumulation and health risk assessment in wastewater-irrigated urban vegetable farming sites of Addis Ababa, Ethiopia. Int. J. Food Contam. 2017, 4, 9. [Google Scholar] [CrossRef]
- Pal, J.; Bishnoi, M.; Kaur, M. Heavy metals in soil and vegetables and their effect on health. Int. J. Eng. Sci. Technol. 2017, 2, 17–27. [Google Scholar] [CrossRef]
- Rahmdel, S.; Rezaei, M.; Ekhlasi, J.; Zarei, S.H.; Akhlaghi, M.; Abdollahzadeh, S.M.; Sefidkar, R.; Mazloomi, S.M. Heavy metals (Pb, Cd, Cu, Zn, Ni, Co) in leafy vegetables collected from production sites: Their potential health risk to the general population in Shiraz, Iran. Environ. Monit. Assess. 2018, 190, 650. [Google Scholar] [CrossRef] [PubMed]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Sharma, R.; Wungrampha, S.; Singh, V.; Pareek, A.; Sharma, M.K. Halophytes as bioenergy crops. Front. Plant Sci. 2016, 7, 1372. [Google Scholar] [CrossRef]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From wild salt marsh species to sustainable edible crops. Agriculture 2019, 9, 14. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An overview of the emerging trends of the Salicornia L. genus as a sustainable crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Parnian, A.; Spasiano, D.; Race, M.; Ashraf, M. Haloculture: A system to mitigate the negative impacts of pandemics on the environment, society and economy, emphasizing COVID-19. Environ. Res. 2021, 198, 111228. [Google Scholar] [CrossRef]
- Abdelly, C.; Öztürk, M.; Ashraf, M.; Grignon, C. (Eds.) Biosaline Agriculture and High Salinity Tolerance; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Manousaki, E.; Kalogerakis, N. Halophytes—an emerging trend in phytoremediation. Int. J. Phytoremediation 2011, 13, 959–969. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Maggio, A. Functional biology of halophytes in the phytoremediation of heavy metal contaminated soils. Environ. Exp. Bot. 2015, 111, 135–146. [Google Scholar] [CrossRef]
- Mujeeb, A.; Aziz, I.; Ahmed, M.Z.; Alvi, S.K.; Shafiq, S. Comparative assessment of heavy metal accumulation and bio-indication in coastal dune halophytes. Ecotoxicol. Environ. Saf. 2020, 195, 110486. [Google Scholar] [CrossRef] [PubMed]
- Caparrós, P.G.; Ozturk, M.; Gul, A.; Batool, T.S.; Pirasteh-Anosheh, H.; Unal, B.T.; Toderich, K.N. Halophytes have potential as heavy metal phytoremediators: A comprehensive review. Environ. Exp. Bot. 2022, 193, 104666. [Google Scholar] [CrossRef]
- European Union Commission Recommendation (EUCR) 2018/464 of 19 March 2018 on the monitoring of metals and iodine in seaweed, halophytes and products based on seaweed (Text with EEA relevance). Off. J. 2018, 78, 16–18.
- Rose, M.; Lewis, J.; Langford, N.; Baxter, M.; Origgi, S.; Barber, M.; MacBain, H.; Thomas, K. Arsenic in seaweed-forms, concentration and dietary exposure. Food Chem. Toxicol. 2007, 45, 1263–1267. [Google Scholar] [CrossRef]
- Valdés, B.; Salicornia, L.S. Flora Vascular de Andalucía Occidental 1; Valdés, B., Talavera, S., Fernández-Galiano, E., Eds.; Ketres Editora: Sevilla, Spain, 1987; pp. 184–185. [Google Scholar]
- Kadereit, G.; Ball, P.; Beer, S.; Mucina, L.; Sokoloff, D.; Teege, P.; Yaprak, A.E.; Freitag, H. A taxonomic nightmare comes true: Phylogeny and biogeography of glassworts (Salicornia L., Chenopodiaceae). Taxon 2007, 56, 1143–1170. [Google Scholar] [CrossRef]
- Davy, A.J.; Bishop, G.F.; Costa, C.S.B. Salicornia L. (Salicornia pusilla J. Woods, S. ramosissima J. Woods, S. europaea L., S. obscura P. W. Ball & Tutin, S. nitens P. W. Ball & Tutin, S. fragilis P. W. Ball & Tutin and S. dolichostachya Moss). J. Ecol. 2001, 89, 681–707. [Google Scholar] [CrossRef]
- Rubio-Casal, A.E.; Castillo, J.M.; Luque, C.J.; Figueroa, M.E. Influence of salinity on germination and seeds viability of two primary colonizers of Mediterranean salt pans. J. Arid Environ. 2003, 53, 145–154. [Google Scholar] [CrossRef]
- Muñoz-Rodríguez, A.F.; Sanjosé, I.; Márquez-García, B.; Infante-Izquierdo, M.D.; Polo-Ávila, A.; Nieva FJ, J.; Castillo, J.M. Germination syndromes in response to salinity of Chenopodiaceae halophytes along the intertidal gradient. Aquat. Bot. 2017, 139, 48–56. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech. 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Lefèvre, G.; Rivière, C. Amaranthaceae halophytes from the French Flanders coast of the North Sea: A review of their phytochemistry and biological activities. Phytochem. Rev. 2020, 19, 1263–1302. [Google Scholar] [CrossRef]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of cultivation salinity in the nutritional composition, antioxidant capacity and microbial quality of Salicornia ramosissima commercially produced in soilless systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef]
- Santos, E.S.; Salazar, M.; Mendes, S.; Lopes, M.; Pacheco, J.; Marques, D. Rehabilitation of abandoned areas from a Mediterranean nature reserve by Salicornia crop: Influence of the salinity and shading. Arid. Land Res. Manag. 2017, 31, 29–45. [Google Scholar] [CrossRef]
- Shpigel, M.; Ben-Ezra, D.; Shauli, L.; Sagi, M.; Ventura, Y.; Samocha, T.; Lee, J.J. Constructed wetland with Salicornia as a biofilter for mariculture effluents. Aquaculture 2013, 412, 52–63. [Google Scholar] [CrossRef]
- Smillie, C. Salicornia spp. as a biomonitor of Cu and Zn in salt marsh sediments. Ecol. Indic. 2015, 56, 70–78. [Google Scholar] [CrossRef]
- Lin, Z.-Q.; Schemenauer, R.S.; Cervinka, V.; Zayed, A.; Lee, A.; Terry, N. Selenium Volatilization from a Soil—Plant System for the Remediation of Contaminated Water and Soil in the San Joaquin Valley. J. Environ. Qual. 2000, 29, 1048–1056. [Google Scholar] [CrossRef]
- Kaviani, E.; Niazi, A.; Heydarian, Z.; Moghadam, A.; Ghasemi-Fasaei, R.; Abdollahzadeh, T. Phytoremediation of Pb-Contaminated Soil by Salicornia iranica: Key Physiological and Molecular Mechanisms Involved in Pb Detoxification. CLEAN–Soil Air Water 2017, 45, 1500964. [Google Scholar] [CrossRef]
- Kaviani, E.; Niazi, A.; Moghadam, A.; Taherishirazi, M.; Heydarian, Z. Phytoremediation of Ni-contaminated soil by Salicornia iranica. Environ. Technol. 2019, 40, 270–281. [Google Scholar] [CrossRef]
- Pedro, C.A.; Santos, M.S.; Ferreira, S.M.; Gonçalves, S.C. The influence of cadmium contamination and salinity on the survival, growth and phytoremediation capacity of the saltmarsh plant Salicornia ramosissima. Mar. Environ. Res. 2013, 92, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Marín, J.; Pérez-Romero, J.A.; Redondo-Gómez, S.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Mateos-Naranjo, E. Impact of plant growth promoting bacteria on Salicornia ramosissima ecophysiology and heavy metal phytoremediation capacity in estuarine soils. Front. Microbiol. 2020, 2148. [Google Scholar] [CrossRef] [PubMed]
- Gavhane, S.K.; Sapkale, J.B.; Susware, N.K.; Sapkale, S.J. Impact of Heavy Metals in Riverine and Estuarine Environment: A review. Res. J. Chem. Environ. 2021, 25, 226–233. [Google Scholar]
- Nelson, C.H.; Lamothe, P.J. Heavy metal anomalies in the Tinto and Odiel river and estuary system, Spain. Estuaries 1993, 16, 496–511. [Google Scholar] [CrossRef]
- Sainz, A.; Grande, J.A.; De la Torre, M.L. Characterisation of heavy metal discharge into the Ria of Huelva. Environ. Int. 2004, 30, 557–566. [Google Scholar] [CrossRef]
- Torre, B.M.; Borrero-Santiago, A.R.; Fabbri, E.; Guerra, R. Trace metal levels and toxicity in the Huelva Estuary (Spain): A case study with comparisons to historical levels from the past decades. Environ. Toxicol. Chem. 2019, 1, 12–18. [Google Scholar] [CrossRef]
- GB 2762-2017. National Standard for Food Safety—Limits of Contaminant in Food; ChFDA (China Food and Drug Administration): Beijing, China, 2017. [Google Scholar]
- Fernández-Illescas, F.; Nieva, F.J.J.; Silva, I.; Tormo, R.; Muñoz, A.F. Pollen production of Chenopodiaceae species at habitat and landscape scale in Mediterranean salt marshes: An ecological and phenological study. Rev. Palaeobot. Palynol. 2010, 161, 127–136. [Google Scholar] [CrossRef]
- Long, S.P.; Mason, C.F. Saltmarsh Ecology; Blackie, Tertiary Level Biology Series; Chapman & Hall: New York, NY, USA, 1983. [Google Scholar]
- Contreras-Cruzado, I.; Infante-Izquierdo, M.D.; Márquez-García, B.; Hermoso-López, V.; Polo, A.; Nieva, F.J.J.; Cartes-Barroso, J.B.; Castillo, J.M.; Muñoz-Rodríguez, A.F. Relationships between spatio-temporal changes in the sedimentary environment and halophytes zonation in salt marshes. Geoderma 2017, 305, 173–187. [Google Scholar] [CrossRef]
- Polo-Ávila, A.; Fragoso, A.; Infante-Izquierdo, M.D.; Nieva, F.J.; Muñoz-Rodríguez, A.F.; Castillo, J.M. Seed bank dynamics of the annual halophyte Salicornia ramosissima: Towards a sustainable exploitation of its wild populations. Plant Ecol. 2021, 222, 647–657. [Google Scholar] [CrossRef]
- Alan, M.; Kara, D. Comparison of a new sequential extraction method and the BCR sequential extraction method for mobility assessment of elements around boron mines in Turkey. Talanta 2019, 194, 189–198. [Google Scholar] [CrossRef]
- Stenner, R.D.; Nickless, G. Heavy metals in organisms of the Atlantic coast of S.W. Spain and Portugal. Mar. Pollut. Bull. 1975, 6, 89–92. [Google Scholar] [CrossRef]
- Achterberg, E.P.; Herzl, V.M.; Braungardt, C.B.; Millward, G.E. Metal behaviour in an estuary polluted by acid mine drainage: The role of particulate matter. Environ. Pollut. 2003, 121, 283–292. [Google Scholar] [CrossRef]
- Borrego, J.; Morales, J.; De la Torre, M.; Grande, J. Geochemical characteristics of heavy metal pollution in surface sediments of the Tinto and Odiel river estuary (Southwestern Spain). Environ. Geol. 2002, 41, 785–796. [Google Scholar] [CrossRef]
- Braungardt, C.B.; Achterberg, E.P.; Elbaz-Poulichet, F.; Morley, N.H. Metal geochemistry in a mine-polluted estuarine system in Spain. J. Appl. Geochem. 2003, 18, 1757–1771. [Google Scholar] [CrossRef]
- Elbaz-Poulichet, F.; Morley, N.H.; Cruzado, A.; Velasquez, Z.; Achterberg, E.P.; Braungardt, C.B. Trace metal and nutrient distribution in an extremely low pH (2.5) river–estuarine system, the Ria of Huelva (South–West Spain). Sci. Total Environ. 1999, 227, 73–83. [Google Scholar] [CrossRef]
- Elbaz-Poulichet, F.; Braungardt, C.; Achterberg, E.; Morley, N.; Cossa, D.; Beckers, J.M.; Nomèrange, P.; Cruzado, A.; Leblanc, M. Metal biogeochemistry in the Tinto–Odiel rivers (Southern Spain) and in the Gulf of Cadiz: A synthesis of the results of TOROS project. Cont. Shelf Res. 2001, 21, 1961–1973. [Google Scholar] [CrossRef]
- Fernández-Caliani, J.; Ruiz-Muñoz, F.; Galán, E. Clay mineral and heavy metal distributions in the lower estuary of Huelva and adjacent Atlantic shelf, SW Spain. Sci. Total Environ. 1997, 198, 181–200. [Google Scholar] [CrossRef]
- Bermejo, J.S.; Beltrán, R.; Ariza, J.G. Spatial variations of heavy metals contamination in sediments from Odiel river (Southwest Spain). Environ. Int. 2003, 29, 69–77. [Google Scholar] [CrossRef]
- Galán, E.; Gómez-Ariza, J.L.; González, I.; Fernández-Caliani, J.C.; Morales, E.; Giráldez, I. Heavy metal partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt. J. Appl. Geochem. 2003, 18, 409–421. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; De Andrés, J.R.; Clemente, L.; Martín, J.A.; González-Vila, F.J. Organic carbon and environmental quality of riverine and off-shore sediments from the Gulf of Cádiz, Spain. Environ. Chem. Lett. 2008, 6, 41–46. [Google Scholar] [CrossRef][Green Version]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.H.; Hoffman, R.S. Heavy metal chelation in neurotoxic exposures. Neurol. Clin. 2011, 29, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, D.A.; Selim, E.M.M.; El-Sherbiny, M.M. Ecological assessment of heavy metals in the grey mangrove (Avicennia marina) and associated sediments along the Red Sea coast of Saudi Arabia. Oceanologia 2018, 60, 513–526. [Google Scholar] [CrossRef]
- Staven, L.H.; Napier, B.A.; Rhoads, K.; Strenge, D.L. A Compendium of Transfer Factors for Agricultural and Animal Products (No. PNNL-13421); Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2003. [Google Scholar] [CrossRef][Green Version]
- Agudo, A.; Amiano, P.; Barcos, A.; Barricarte, A.; Beguiristain, J.M.; Chirlaque, M.D.; Dorronsoro, M.; González, C.A.; Lasheras, C.; Martínez, C.; et al. Dietary intake of vegetables and fruits among adults in five regions of Spain. Eur. J. Clin. Nutr. 1999, 53, 174–180. [Google Scholar] [CrossRef]
- Walpole, S.C.; Prieto-Merino, D.; Edwards, P.; Cleland, J.; Stevens, G.; Roberts, I. The weight of nations: An estimation of adult human biomass. BMC Public Health 2012, 12, 439. [Google Scholar] [CrossRef]
- European Union Commission Recommendation (EUCR) 2014/193 of 4 April 2014 on the reduction of the presence of cadmium in foodstuffs Text with EEA relevance. Off. J. 2014, 104, 80–81.
- European Food Safety Authority (EFSA): Panel on Contaminants in the Food Chain. Scientific Opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- Blaser, P.; Zimmermann, S.; Luster, J.; Shotyk, W. Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb, and Zn in Swiss forest soils. Sci. Total Environ. 2000, 249, 257–280. [Google Scholar] [CrossRef]
- Richards, B.K.; Steenhuis, T.S.; Peverly, J.H.; McBride, M.B. Effect of sludge-processing mode, soil texture and soil pH on metal mobility in undisturbed soil columns under accelerated loading. Environ. Pollut. 2000, 109, 327–346. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Adelaide, Australia, 2011; ISBN 9780123849069. [Google Scholar]
- Mesa, J.; Mateos-Naranjo, E.; Pajuelo, E.; Caviedes, M.Á.; Rodríguez-Llorente, I.D. Heavy metal pollution structures soil bacterial community dynamics in SW spain polluted salt marshes. Water Air Soil Pollut. 2016, 227, 466. [Google Scholar] [CrossRef]
- United States Dept. of Agriculture; Soil Survey Division; United States Division of Soil Survey. Soil Survey Manual (No. 18); US Department of Agriculture: Washington, DC, USA, 1993.
- Ghassemi, F.; Jakeman, A.J.; Nix, H.A. Salinisation of land and water resources: Human causes, extent, management and case studies; CAB International: Wallingford, UK, 1995. [Google Scholar]
- López-González, N.; Borrego, J.; Ruiz, F.; Carro, B.; Lozano-Soria, O.; Abad, M. Geochemical variations in estuarine sediments: Provenance and environmental changes (Southern Spain). Estuar. Coast. Shelf Sci. 2006, 67, 313–320. [Google Scholar] [CrossRef]
- Otte, M.L.; Haarsma, M.S.; Broekman, R.A.; Rozema, J. Relation between heavy metal concentrations in salt marsh plants and soil. Environ. Pollut. 1993, 82, 13–22. [Google Scholar] [CrossRef]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.D.P. El muelle minero de Tharsis, en Huelva: Historia de un maltrato. Energía Minas 2010, 8, 54–57. [Google Scholar]
- Luque, C.J.; Castellanos, E.M.; Castillo, J.M.; González, M.; González-Vilches, M.C.; Figueroa, M.E. Metals in halophytes of a contaminated Estuary (Odiel saltmarshes, SW Spain). Mar. Pollut. Bull. 1999, 38, 49–51. [Google Scholar] [CrossRef]
- Sánchez-Gavilán, I.; Rufo, L.; Rodriguez, N.; de la Fuente, V. On the elemental composition of the Mediterranean euhalophyte Salicornia patula Duval-Jouve (Chenopodiaceae) from saline habitats in Spain (Huelva, Toledo and Zamora). Environ. Sci. Pollut. Res. 2021, 28, 2719–2727. [Google Scholar] [CrossRef]
- Williams, T.P.; Bubb, J.M.; Lester, J.N. The occurrence and distribution of trace metals in halophytes. Chemosphere 1994, 28, 1189–1199. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Fitzgerald, E.J.; Caffrey, J.M.; Nesaratnam, S.T.; McLoughlin, P. Copper and lead concentrations in salt marsh plants on the Suir Estuary, Ireland. Environ. Pollut. 2003, 123, 67–74. [Google Scholar] [CrossRef]
- Khodaverdiloo, H.; Hamzenejad Taghlidabad, R. Phytoavailability and potential transfer of Pb from a salt-affected soil to Atriplex verucifera, Salicornia europaea and Chenopodium album. J. Chem. Ecol. 2014, 30, 216–226. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Suprasanna, P. Coping with metal toxicity–cues from halophytes. Front. Plant Sci. 2018, 9, 777. [Google Scholar] [CrossRef]
- Tani, F.H.; Barrington, S. Zinc and copper uptake by plants under two transpiration rates. Part I. Wheat (Triticum aestivum L.). Environ. Pollut. 2005, 138, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yang, J.; Zhang, D.; Mu, S. Lead complexation behaviour of root exudates of salt marsh plant Salicornia europaea L. Chem. Speciat. Bioavailab. 2012, 24, 60–63. [Google Scholar] [CrossRef]
- Khalilzadeh, R.; Pirzad, A.; Sepehr, E.; Khan, S.; Anwar, S. The Salicornia europaea potential for phytoremediation of heavy metals in the soils under different times of wastewater irrigation in northwestern Iran. Environ. Sci. Pollut. Res. 2021, 28, 47605–47618. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.P.; Zhao, F.J. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef]
- Van der Ent, A.; Baker, A.J.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil. 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Arnot, J.A.; Gobas, F.A. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Baker, A.J. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Fuente, V.; Rufo, L.; Rodríguez, N.; Amils, R.; Zuluaga, J. Metal accumulation screening of the Río Tinto flora (Huelva, Spain). Biol. Trace Elem. Res. 2010, 134, 318–341. [Google Scholar] [CrossRef]
- Sánchez-Martínez, M.A.; Riosmena-Rodríguez, R.; Marmolejo-Rodríguez, A.J.; Sánchez-González, A. Trace elements in two wetland plants (Maytenus phyllanthoides and Salicornia subterminalis) and sediment in a semiarid area influenced by gold mining. Reg. Stud. 2017, 10, 65–74. [Google Scholar] [CrossRef]
- Pérez-Romero, J.A.; Redondo-Gómez, S.; Mateos-Naranjo, E. Growth and photosynthetic limitation analysis of the Cd-accumulator Salicornia ramosissima under excessive cadmium concentrations and optimum salinity conditions. Plant Physiol. Biochem. 2016, 109, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Dear, S.E.; Moore, N.G.; Dobos, S.K.; Watling, K.M.; Ahern, C.R. Soil management guidelines. In Queensland Acid Sul-Fate Soil Technical Manual; Queensland Department of Natural, Mines and Energy: Indooroopilly, QLD, Australia, 2002; pp. 50–64. Available online: http://www.nrm.qld.gov.au/land/ass/pdfs/soilmgmtguidelinesv38.pdf (accessed on 25 May 2022).
- Murányi, A.; Seeling, B.; Ladewig, E.; Jungk, A. Acidification in the rhizosphere of rape seedlings and in bulk soil by nitrification and ammonium uptake. Z. Für Pflanzenernahr. Bodenkd. 1994, 157, 61–65. [Google Scholar] [CrossRef]
- Römheld, V. The role of phytosiderophores in acquisition of iron and other micronutrients in graminaceous species: An ecological approach. In Iron Nutrition and Interactions in Plants; Springer: Dordrecht, The Netherlands, 1991; pp. 159–166. [Google Scholar] [CrossRef]
- Liu, W.X.; Li, H.H.; Li, S.R.; Wang, Y.W. Heavy metal accumulation of edible vegetables cultivated in agricultural soil in the suburb of Zhengzhou City, People’s Republic of China. Bull. Environ. Contam. Toxicol. 2006, 76, 163–170. [Google Scholar] [CrossRef]
- Baker, A.J.M.; McGrath, S.P.; Sidoli, C.M.D.; Reeves, R.D. The possibility of in situ heavy metal decontamination of polluted soils using crops of metal-accumulating plants. Resour. Conserv. Recycl. 1994, 11, 41–49. [Google Scholar] [CrossRef]
- Watanabe, M.E. Phytoremediation on the brink of commercialization. Environ. Sci. Technol. 1997, 31, A182–A186. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Ghaly, A.E.; Mahmoud, N.; Côté, R. Phytoaccumulation of heavy metals by aquatic plants. Environ. Int. 2004, 29, 1029–1039. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Soil–plant transfer of trace elements—an environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Monteiro, M.; Santos, C.; Mann, R.M.; Soares, A.M.; Lopes, T. Evaluation of cadmium genotoxicity in Lactuca sativa L. using nuclear microsatellites. Environ. Exp. Bot. 2007, 60, 421–427. [Google Scholar] [CrossRef]
- Intawongse, M.; Dean, J.R. Uptake of heavy metals by vegetable plants grown on contaminated soil and their bioavailability in the human gastrointestinal tract. Food Addit. Contam. 2006, 23, 36–48. [Google Scholar] [CrossRef]
- Rusyniak, D.E. Clinical Neurotoxicology E-Book: Syndromes, Substances, Environments. Dobbs, M.R., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009; pp. 277–281. [Google Scholar]


| Sampling Point | Longitude | Latitude | Habitat | Soil pH | Soil Conductivity |
|---|---|---|---|---|---|
| 1 | 37°16′18.75″ N | 6°58′59.81″ W | Saltpan | 6.14 ± 0.01 | 38.05 ± 0.25 |
| 2 | 37°15′40.85″ N | 6°58′34.57″ W | Saltwork | 5.66 ± 0.06 | 24.95 ± 0.15 |
| 3 | 37°15′39.13″ N | 6°58′37.06″ W | Saltwork | 5.92 ± 0.01 | 32.45 ± 0.05 |
| 4 | 37°15′12.12″ N | 6°57′59.79″ W | Low marsh | 6.20 ± 0.00 | 31.60 ± 0.20 |
| 5 | 37°14′31.80″ N | 6°57′56.65″ W | Saltpan | 5.92 ± 0.00 | 32.45 ± 0.05 |
| 6 | 37°14′31.54″ N | 6°57′54.44″ W | Saltpan | 5.03 ± 0.01 | 23.30 ± 0.00 |
| 7 | 37°13′32.80″ N | 6°57′52.52″ W | Medium marsh | 5.11 ± 0.02 | 34.40 ± 0.20 |
| 8 | 37°13′17.47″ N | 6°57′41.09″ W | Saltpan | 6.33 ± 0.02 | 15.60 ± 0.00 |
| 9 | 37°13′16.31″ N | 6°57′43.75″ W | Medium marsh | 6.48 ± 0.00 | 23.70 ± 0.40 |
| 10 | 37°13′04.99″ N | 6°57′48.85″ W | Medium marsh | 5.73 ± 0.01 | 36.05 ± 0.05 |
| 11 | 37°12′29.71″ N | 6°57′32.60″ W | Low marsh | 5.69 ± 0.01 | 36.50 ± 0.30 |
| 12 | 37°12′26.06″ N | 6°57′33.75″ W | Saltpan | 5.55 ± 0.25 | 28.75 ± 0.15 |
| 13 | 37°12′20.09″ N | 6°57′04.41″ W | Medium marsh | 5.96 ± 0.03 | 21.25 ± 0.15 |
| 14 | 37°11′02.84″ N | 6°56′29.89″ W | Medium marsh | 6.21 ± 0.01 | 32.00 ± 0.10 |
| Metal | Material | Low Marsh | Medium Marsh | Saltpan | Saltwork | Kruskal Wallis Test | |
|---|---|---|---|---|---|---|---|
| Al | SOIL | 102.96 ± 27.32 | 74.83 ± 7.20 | 147.22 ± 47.14 | 49.98 ± 2.80 | H(3, N=42) = 2.78 | p = 0.4273 |
| ROOT | 795.79 ± 40.70 | 1468.69 ± 319.61 | 1994.83 ± 178.26 | 1677.93 ± 474.13 | H(3, N=42) = 6.01 | p = 0.1109 | |
| STEM | 165.21 a ± 48.65 | 178.08 a ± 35.05 | 574.17 b ± 92.46 | 133.67 a ± 10.26 | H(3, N=42) = 21.29 | p = 0.0001 | |
| LEAF | 26.24 a ± 1.19 | 46.57 a ± 12.94 | 101.24 b ± 15.28 | 36.67 ab ± 2.16 | H(3, N=42) = 16.64 | p = 0.0008 | |
| V | SOIL | 5.43 a ± 1.96 | 2.05 a ± 0.17 | 0.85 b ± 0.11 | 0.62 b ± 0.03 | H(3, N=42) = 25.57 | p = 0.0000 |
| ROOT | 1.82 a ± 0.15 | 29.19 b ± 17.38 | 3.73 b ± 0.48 | 2.63 ab ± 0.67 | H(3, N=42) = 8.17 | p = 0.0425 | |
| STEM | 0.54 ± 0.09 | 0.78 ± 0.09 | 1.52 ± 0.39 | 0.49 ± 0.01 | H(3, N=42) = 8.85 | p = 0.0314 | |
| LEAF | 0.06 a ± 0.01 | 0.17 b ± 0.03 | 0.16 b ± 0.03 | 0.07 ab ± 0.00 | H(3, N=42) = 15.08 | p = 0.0018 | |
| Cr | SOIL | 1.67 ± 0.15 | 1.68 ± 0.09 | 1.70 ± 0.13 | 1.98 ± 0.52 | H(3, N=42) = 0.91 | p = 0.8228 |
| ROOT | 1.02 ± 0.04 | 8.85 ± 4.99 | 1.88 ± 0.13 | 1.46 ± 0.36 | H(3, N=42) = 6.92 | p = 0.0744 | |
| STEM | 0.71 ± 0.07 | 0.53 ± 0.03 | 0.86 ± 0.13 | 0.57 ± 0.01 | H(3, N=42) = 5.84 | p = 0.1198 | |
| LEAF | 0.09 a ± 0.01 | 0.17 b ± 0.02 | 0.17 b ± 0.02 | 0.08 a ± 0.00 | H(3, N=42) = 20.71 | p = 0.0001 | |
| Mn | SOIL | 410.51 ± 180.09 | 1190.85 ± 336.31 | 799.61 ± 240.70 | 203.83 ± 67.45 | H(3, N=42) = 2.53 | p = 0.4704 |
| ROOT | 42.54 ± 8.27 | 93.23 ± 27.75 | 88.53 ± 16.86 | 53.45 ± 16.39 | H(3, N=42) = 2.11 | p = 0.5498 | |
| STEM | 24.01 ab ± 5.75 | 68.42 a ± 15.99 | 44.65 ab ± 9.37 | 13.70 b ± 1.90 | H(3, N=42) = 10.96 | p = 0.0120 | |
| LEAF | 5.90 a ± 0.17 | 43.92 b ± 10.74 | 28.92 b ± 7.14 | 18.66 ab ± 3.52 | H(3, N=42) = 13.19 | p = 0.0043 | |
| Fe | SOIL | 119.55 ± 31.78 | 118.98 ± 23.01 | 144.75 ± 41.20 | 29.55 ± 1.85 | H(3, N=42) = 4.68 | p = 0.1971 |
| ROOT | 989.57 ± 152.21 | 1305.59 ± 228.56 | 2167.75 ± 277.07 | 1197.09 ± 360.23 | H(3, N=42) = 8.52 | p = 0.0365 | |
| STEM | 177.32 a ± 10.97 | 203.62 a ± 27.24 | 504.88 b ± 98.36 | 152.74 a ± 12.89 | H(3, N=42) = 21.68 | p = 0.0001 | |
| LEAF | 39.57 ± 7.95 | 66.28 ± 17.17 | 58.82 ± 10.55 | 27.93 ± 1.15 | H(3, N=42) = 3.81 | p = 0.2832 | |
| Co | SOIL | 4.93 ab ± 1.92 | 14.21 a ± 2.94 | 9.01 ab ± 1.41 | 2.44 b ± 0.94 | H(3, N=42) = 10.10 | p = 0.0177 |
| ROOT | 1.87 ± 0.04 | 5.01 ± 1.84 | 3.31 ± 0.46 | 1.96 ± 0.74 | H(3, N=42) = 2.76 | p = 0.4300 | |
| STEM | 0.63 ab ± 0.05 | 1.06 a ± 0.14 | 1.24 a ± 0.13 | 0.34 b ± 0.05 | H(3, N=42) = 16.10 | p = 0.0011 | |
| LEAF | 0.10 ± 0.01 | 0.38 ± 0.13 | 0.24 ± 0.04 | 0.09 ± 0.01 | H(3, N=42) = 6.81 | p = 0.0782 | |
| Ni | SOIL | 4.91 a ± 0.15 | 12.93 b ± 2.70 | 10.34 b ± 1.13 | 6.41 ab ± 0.33 | H(3, N=42) = 14.00 | p = 0.0029 |
| ROOT | 1.78 ± 0.15 | 6.35 ± 2.70 | 4.62 ± 0.79 | 3.42 ± 0.49 | H(3, N=42) = 6.99 | p = 0.0723 | |
| STEM | 2.06 ± 0.74 | 1.05 ± 0.15 | 1.94 ± 0.62 | 2.59 ± 0.99 | H(3, N=42) = 2.92 | p = 0.4035 | |
| LEAF | 0.28 ab ± 0.05 | 0.45 a ± 0.04 | 0.24 b ± 0.02 | 0.26 ab ± 0.04 | H(3, N=42) = 16.98 | p = 0.0007 | |
| Cu | SOIL | 246.83 a ± 20.72 | 188.17 a ± 31.15 | 172.81 a ± 22.11 | 13.23 b ± 3.86 | H(3, N=42) = 16.60 | p = 0.0009 |
| ROOT | 43.09 ab ± 2.74 | 71.31 a ± 9.56 | 70.61 a ± 3.77 | 24.91 b ± 7.02 | H(3, N=42) = 15.89 | p = 0.0012 | |
| STEM | 22.91 ab ± 3.72 | 30.58 ab ± 1.22 | 34.74 a ± 2.28 | 18.70 b ± 4.29 | H(3, N=42) = 10.67 | p = 0.0137 | |
| LEAF | 3.57 a ± 0.02 | 8.76 b ± 1.21 | 8.91 b ± 0.93 | 6.96 ab ± 0.82 | H(3, N=42) = 16.01 | p = 0.0011 | |
| Zn | SOIL | 166.23 ab ± 54.54 | 1706.96 a ± 500.86 | 647.84 a ± 194.64 | 88.14 b ± 22.28 | H(3, N=42) = 13.18 | p = 0.0046 |
| ROOT | 50.00 ab ± 0.69 | 67.58 a ± 6.95 | 59.25 a ± 1.78 | 39.09 b ± 4.42 | H(3, N=42) = 11.94 | p = 0.0076 | |
| STEM | 47.12 ab ± 1.32 | 57.82 a ± 2.98 | 49.62 a ± 2.49 | 34.38 b ± 0.44 | H(3, N=42) = 19.78 | p = 0.0002 | |
| LEAF | 16.11 a ± 0.78 | 27.86 b ± 2.69 | 26.88 b ± 2.82 | 17.76 ab ± 0.77 | H(3, N=42) = 14.56 | p = 0.0022 | |
| As | SOIL | 8.64 ab ± 1.07 | 24.28 a ± 4.31 | 6.23 b ± 0.43 | 7.74 ab ± 2.68 | H(3, N=42) = 22.75 | p = 0.0000 |
| ROOT | 7.79 ± 1.55 | 13.84 ± 4.21 | 9.71 ± 1.28 | 7.78 ± 2.80 | H(3, N=42) = 1.19 | p = 0.7562 | |
| STEM | 1.29 ± 0.04 | 1.49 ± 0.15 | 2.26 ± 0.35 | 1.28 ± 0.27 | H(3, N=42) = 6.07 | p = 0.1084 | |
| LEAF | 0.22 ± 0.03 | 0.42 ± 0.12 | 0.36 ± 0.05 | 0.24 ± 0.06 | H(3, N=42) = 2.49 | p = 0.4776 | |
| Cd | SOIL | 0.49 ab ± 0.09 | 1.53 ab ± 0.57 | 1.05 a ± 0.09 | 0.39 b ± 0.02 | H(3, N=42) = 13.58 | p = 0.0035 |
| ROOT | 0.14 ab ± 0.01 | 3.69 a ± 2.36 | 0.20 ab ± 0.03 | 0.12 b ± 0.00 | H(3, N=42) = 14.82 | p = 0.0020 | |
| STEM | 0.19 ± 0.05 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.02 | H(3, N=42) = 2.33 | p = 0.5062 | |
| LEAF | 0.03 ± 0.00 | 0.10 ± 0.01 | 0.08 ± 0.02 | 0.06 ± 0.01 | H(3, N=42) = 6.93 | p = 0.0740 | |
| Tl | SOIL | 0.19 a ± 0.01 | 0.44 ab ± 0.09 | 0.37 b ± 0.04 | 0.21 ab ± 0.02 | H(3, N=42) = 12.83 | p = 0.0050 |
| ROOT | 0.18 ± 0.03 | 0.49 ± 0.06 | 0.98 ± 0.28 | 0.42 ± 0.16 | H(3, N=42) = 5.31 | p = 0.1502 | |
| STEM | 0.12 ± 0.02 | 0.29 ± 0.06 | 0.65 ± 0.18 | 0.34 ± 0.11 | H(3, N=42) = 4.39 | p = 0.2221 | |
| LEAF | 0.09 ± 0.01 | 0.22 ± 0.04 | 0.70 ± 0.20 | 0.19 ± 0.06 | H(3, N=42) = 5.82 | p = 0.1205 | |
| Pb | SOIL | 4.74 ± 0.38 | 4.71 ± 0.53 | 3.55 ± 0.24 | 4.52 ± 1.37 | H(3, N=42) = 3.99 | p = 0.2625 |
| ROOT | 4.57 ± 0.22 | 5.62 ± 0.94 | 5.03 ± 0.56 | 4.66 ± 1.66 | H(3, N=42) = 0.75 | p = 0.8604 | |
| STEM | 1.52 ± 0.30 | 1.96 ± 0.25 | 2.36 ± 0.36 | 1.38 ± 0.04 | H(3, N=42) = 2.45 | p = 0.4843 | |
| LEAF | 0.31 ± 0.03 | 0.57 ± 0.08 | 0.43 ± 0.05 | 0.55 ± 0.03 | H(3, N=42) = 7.37 | p = 0.0611 | |
| U | SOIL | 0.27 ab ± 0.05 | 0.23 ab ± 0.01 | 0.28 a ± 0.03 | 0.16 b ± 0.01 | H(3, N=42) = 10.69 | p = 0.0135 |
| ROOT | 0.25 ± 0.01 | 0.38 ± 0.10 | 0.50 ± 0.08 | 0.43 ± 0.16 | H(3, N=42) = 6.86 | p = 0.0764 | |
| STEM | 0.08 ab ± 0.01 | 0.13 ab ± 0.03 | 0.16 a ± 0.01 | 0.07 b ± 0.02 | H(3, N=42) = 11.95 | p = 0.0075 | |
| LEAF | 0.01 a ± 0.00 | 0.01 ab ± 0.00 | 0.02 b ± 0.00 | 0.01 ab ± 0.00 | H(3, N=42) = 11.79 | p = 0.0081 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanjosé, I.; Navarro-Roldán, F.; Montero, Y.; Ramírez-Acosta, S.; Jiménez-Nieva, F.J.; Infante-Izquierdo, M.D.; Polo-Ávila, A.; Muñoz-Rodríguez, A.F. The Bioconcentration and the Translocation of Heavy Metals in Recently Consumed Salicornia ramosissima J. Woods in Highly Contaminated Estuary Marshes and Its Food Risk. Diversity 2022, 14, 452. https://doi.org/10.3390/d14060452
Sanjosé I, Navarro-Roldán F, Montero Y, Ramírez-Acosta S, Jiménez-Nieva FJ, Infante-Izquierdo MD, Polo-Ávila A, Muñoz-Rodríguez AF. The Bioconcentration and the Translocation of Heavy Metals in Recently Consumed Salicornia ramosissima J. Woods in Highly Contaminated Estuary Marshes and Its Food Risk. Diversity. 2022; 14(6):452. https://doi.org/10.3390/d14060452
Chicago/Turabian StyleSanjosé, Israel, Francisco Navarro-Roldán, Yina Montero, Sara Ramírez-Acosta, Francisco Javier Jiménez-Nieva, María Dolores Infante-Izquierdo, Alejandro Polo-Ávila, and Adolfo Francisco Muñoz-Rodríguez. 2022. "The Bioconcentration and the Translocation of Heavy Metals in Recently Consumed Salicornia ramosissima J. Woods in Highly Contaminated Estuary Marshes and Its Food Risk" Diversity 14, no. 6: 452. https://doi.org/10.3390/d14060452
APA StyleSanjosé, I., Navarro-Roldán, F., Montero, Y., Ramírez-Acosta, S., Jiménez-Nieva, F. J., Infante-Izquierdo, M. D., Polo-Ávila, A., & Muñoz-Rodríguez, A. F. (2022). The Bioconcentration and the Translocation of Heavy Metals in Recently Consumed Salicornia ramosissima J. Woods in Highly Contaminated Estuary Marshes and Its Food Risk. Diversity, 14(6), 452. https://doi.org/10.3390/d14060452






