The Bacterial Composition and Diversity in a Eucalyptus pellita Plantation in South Sumatra, Indonesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. Soil Chemical Analysis
2.3. Soil DNA Extraction
2.4. PCR Amplification and NGS Sequencing
2.5. Data Analysis
3. Results
3.1. Soil Chemical Properties
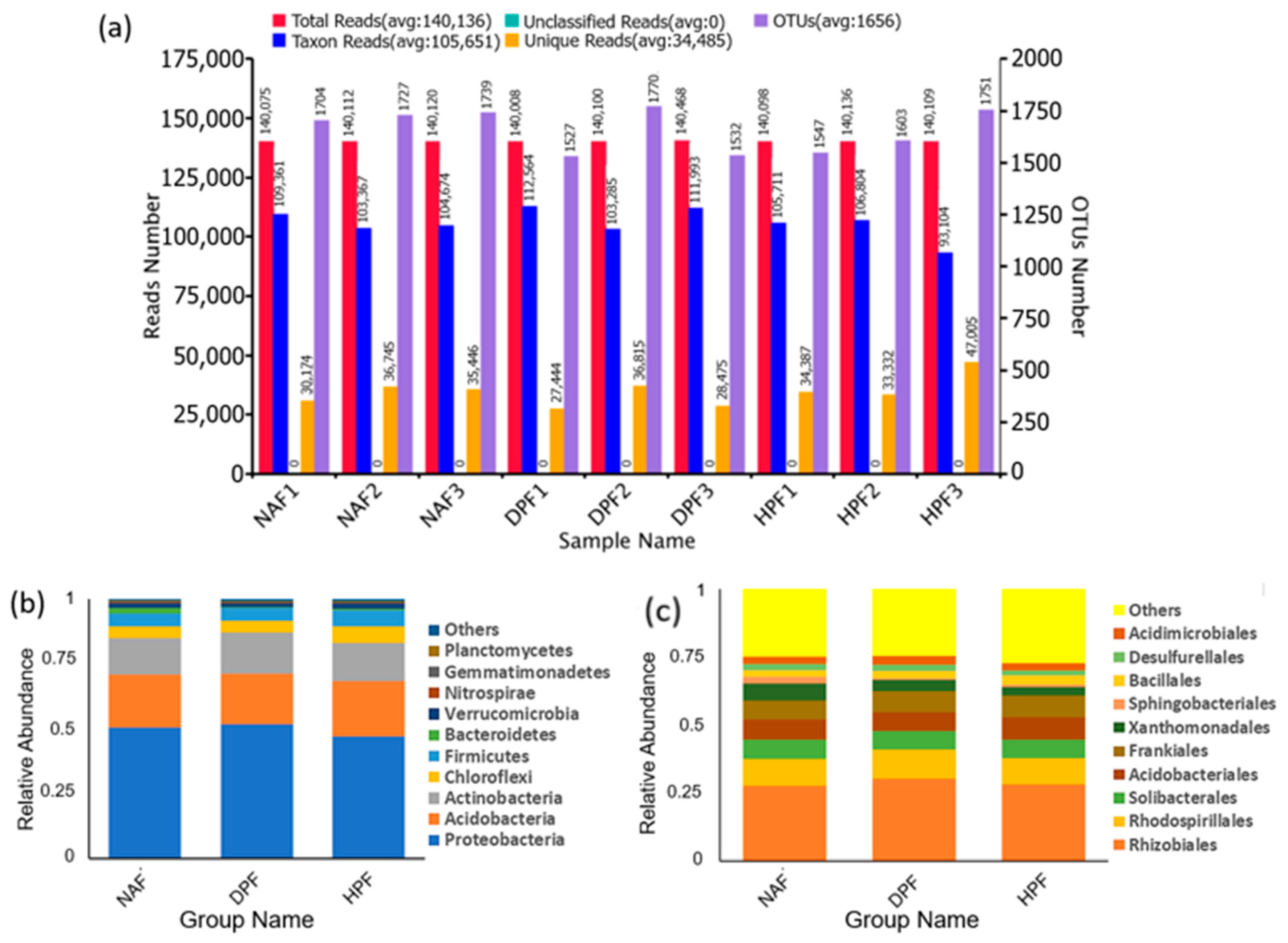
3.2. Bacterial Abundance
3.3. Bacterial Diversity
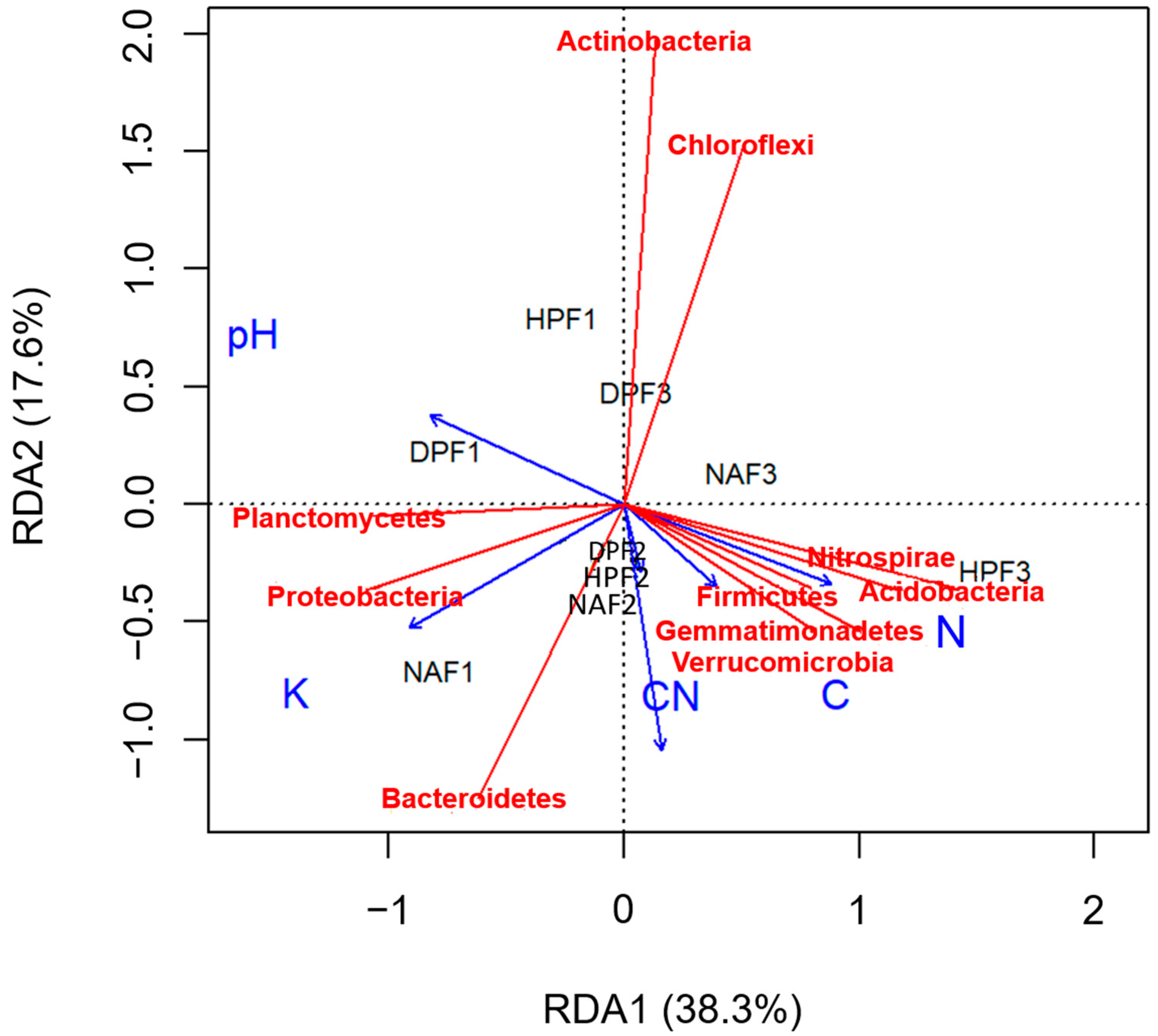
3.4. Relationship between Soil Chemical Properties and Microbiome
4. Discussion
4.1. The Difference between Bacterial Diversity in Natural Forests and Plantation Forests
4.2. Relationship between Soil Physicochemical Properties and Bacterial Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BPS-Statistics Indonesia. Statistics of Timber Culture Establishment 2020; BPS-Statistics Indonesia: Jakarta, Indonesia, 2021; pp. 16–22. [Google Scholar]
- Setyawati. Indonesia-Investments. Available online: https://www.indonesia-investments.com/news/todays-headlines/pulp-andpaper-industry-indonesia-challenges-and-opportunities/item7738 (accessed on 17 February 2021).
- Nambiar, E.K.S.; Harwood, C.E.; Mendham, D.S. Paths to sustainable wood supply to the pulp and paper industry in Indonesia after diseases have forced a change of species from acacia to eucalypts. Aust. For. 2018, 81, 148–161. [Google Scholar] [CrossRef]
- Meng, S.; Peng, T.; Liu, X.; Wang, H.; Huang, T.; Gu, J.; Hu, Z. Ecological role of bacteria involved in the biogeochemical cycles of mangroves based on functional genes detected through GeoChip 5.0. ASM 2022, 7, e00936-21. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Gu, J.D. Ecological distribution and potential roles of Woesearchaeota in anaerobic biogeochemical cycling unveiled by genomic analysis. Comput. Struct. Biotechnol. J. 2021, 19, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Bever, J.D.; Westover, K.M.; Antonovics, J. Incorporating the soil community into plant population dynamics: The utility of the feedback approach. J. Ecol. 1997, 85, 561–573. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Bessler, H.; Engels, C.; Gleixner, G.; Habekost, M.; Milcu, A.; Partsch, S.; Sabais, A.C.W.; Scherber, C.; Steinbeiss, S.; et al. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology 2010, 91, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93, fix050. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Dicke, M. Plant interactions with microbes and insects: From molecular mechanisms to ecology. Trends Plant Sci. 2007, 12, 564–569. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, K.H. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Li, J.; Xiao, Y.; Gu, Y.; Liu, H.; Liang, Y.; Liu, X.; Hu, J.; Meng, D.; Yin, H. An integrated insight into the relationship between soil microbial community and tobacco bacterial wilt disease. Front. Microbiol. 2017, 8, 2179. [Google Scholar] [CrossRef] [Green Version]
- Jansson, J.K.; Hofmockel, K.S. The soil microbiome-from metagenomics to metaphenomics. Curr. Opin. Micrbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Duy, M.V.; Hoi, N.T.; Ve, N.B.; Thuc, L.V.; Trang, N.Q. Influence of Cellulomonas flavigena, Azospirillum sp. and Pseudomonas sp. on rice growth and yield grown in submerged soil amended with rice straw. In Recent Trends in PGPR Research for Sustainable Crop Productivity; Sayyed, R.Z., Reddy, M.S., Al-Turki, A.I., Eds.; Scientific Publishers: Hanoi Vietnam, India, 2016; pp. 238–242. [Google Scholar]
- Siegel-Hertz, K.; Edel-Hermann, V.; Chapelle, E.; Terrat, S.; Raaijmakers, J.M.; Steinberg, C. Comparative microbiome analysis of a Fusarium wilt suppressive soil and a Fusarium wilt conducive soil from the Châteaurenard region. Front. Microbiol. 2018, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, X.; Yuan, F.; Deng, B.; Yu, X. Biocatalysis of heterogenously-expressed chitosanase for the preparation of desirable chitosan oligosaccharides applied againts phytopathogenic fungi. ACS Sustain. Chem. Eng. 2020, 8, 4781–4791. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, X.; Meng, D.; Tao, J.; Gu, Y.; Yin, H.; Lia, J. The role of soil bacterial community during winter follow period in the incidence of tobacco bacterial wilt disease. Appl. Microbial. Biotechnol. 2018, 102, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Siong, A.; Li, Z.; Wang, E.; Xu, D.; Wang, S.; Bi, J.; Wang, H.; Jeyakumar, P.; Li, Z.; Fan, F. Supplying silicon alters community and reduces soil cadmium bioavailability to promote health wheat growth and yield. Sci. Total Environ. 2021, 796, 148797. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A.; Thomas, G.V. Bespoke microbiome therapy to manage plant diseases. Front. Microbiol. 2013, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Forde, B.M.; O’Toole, P.W. Next-generation sequencing technologies and their impact on microbial genomics. Brief Funct. Genom. 2013, 12, 440–453. [Google Scholar] [CrossRef] [Green Version]
- Rinke, C.; Schwientek, P.; Sczyrba, A.; Ivanova, N.N.; Anderson, I.J.; Cheng, J.F.; Darling, A.; Malfatti, S.; Swan, B.K.; Gies, E.A.; et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature 2013, 499, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Balai Penelitian Tanah. Petunjuk Teknis Edisi 2. Analisis Kimia Tanah, Tanaman, Air, dan Pupuk; Balai Penelitian Tanah: Bogor, Indonesia, 2009. [Google Scholar]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.M.; Edwards, D.P.; Mendes, L.W.; Kim, M.; Dong, K.; Kim, H.; Adams, J. The impact of tropical forest logging and oil palm agriculture on the soil microbiome. Mol. Ecol. 2016, 25, 2244–2257. [Google Scholar] [CrossRef]
- Schneider, D.; Engelhaupt, M.; Allen, K.; Kurniawan, S.; Krashevska, V.; Heinemann, M.; Nacke, H.; Wijayanti, M.; Meryandini, A.; Corre, M.D.; et al. Impact of lowland rainforest transformation on diversity and composition of soil prokaryotic communities in Sumatra (Indonesia). Front. Microbiol. 2015, 6, 1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkelmann, B.; Schneider, D.; Engelhaupt, M.; Heinemann, M.; Christel, S.; Wijayanti, M.; Meryandini, A.; Daniel, R. How rainforest conversion to agricultural systems in Sumatra (Indonesia) affects active soil bacterial communities. Front. Microbiol. 2018, 9, 2381. [Google Scholar] [CrossRef]
- Berkelmann, B.; Schneider, D.; Meryandini, A.; Daniel, R. Unravelling the effects of tropical land use conversion on the soil microbiome. Environ. Microbiomes 2020, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Hansel, C.M.; Fendorf, S.; Jardine, P.M.; Francis, C.A. Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl. Environ. Microbiol. 2008, 74, 1620–1633. [Google Scholar] [CrossRef] [Green Version]
- Leff, J.W.; Nemergut, D.R.; Grandy, A.S.; O’Neill, S.P.; Wickings, K.; Townsend, A.R.; Cleveland, C.C. The effects of soil bacterial community structure on decomposition in a tropical rain forest. Ecosystems 2012, 15, 284–298. [Google Scholar] [CrossRef]
- Erlarcer, A.; Cernava, T.; Cardinal, M.; Soh, J.; Sensen, C.W.; Grube, M.; Berg, G. Rhizobiales as functional and endosymbiontic members in the lichen symbiosis of Lobaria pulmonaria L. Front. Microbiol. 2015, 6, 53. [Google Scholar] [CrossRef]
- Barabote, R.D.; Xie, G.; Leu, D.H.; Normand, P.; Necsulea, A.; Daubin, V.; Médigue, C.; Adney, W.S.; Xu, X.C.; Lapidus, A.; et al. Complete genome of the cellulolytic thermophile Acidothermus cellulolyticus 11B provides insights into its ecophysiological and evolutionary adaptations. Genome Res. 2009, 19, 1033–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, N.L.; Challacombe, J.F.; Janssen, P.H.; Henrissat, B.; Coutinho, P.M.; Wu, M.; Xie, G.; Haft, D.H.; Sait, M.; Badger, J.; et al. Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl. Environ. Microbiol. 2009, 75, 2046–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, S.A.; Gilbert, J.A.; Leff, J.W.; Fierer, N.; D’Angelo, H.; Bateman, C.; Gedallovich, S.M.; Gillikin, C.M.; Gradoville, M.R.; Mansor, P.; et al. Consequences of tropical forest conversion to oil palm on soil bacterial community and network structure. Soil Biol. Biochem. 2017, 112, 258–268. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant microbiome–an account of the factors that shape community composition and diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Amoo, A.E.; Babalola, O.O. Impact of Land Use on Bacterial Diversity and Community Structure in Temperate Pine and Indigenous Forest Soils. Diversity 2019, 11, 217. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, N.; Schneider, D.; Sahner, J.; Ballauff, J.; Edy, N.; Barus, H.; Irawan, B.; Budi, S.W.; Qaim, M.; Daniel, R.; et al. Intensive tropical land use massively shifts soil fungal communities. Sci. Rep. 2019, 9, 3403. [Google Scholar] [CrossRef] [Green Version]
- Rodrigus, J.L.M.; Pellizari, V.H.; Mueller, R.; Baek, K.; Jesus, E.C.; Paula, F.S.; Mirza, B.; Hamaoui, G.S., Jr.; Tsai, S.M.; Feigl, B.; et al. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2013, 110, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Lan, G.; Wu, Z.; Yang, C.; Sun, R.; Chen, B.; Zhang, X. Forest conversion alters the structure and functional processes of tropical forest soil microbial communities. Land Degrad. Dev. 2020, 32, 613–627. [Google Scholar] [CrossRef]
- Song, H.; Singh, D.; Tomlinson, K.W.; Yang, X.; Ogwu, M.C.; Slik, J.W.F.; Adams, J.M. Tropical forest conversion to rubber plantation in southwest China results in lower fungal beta diversity and reduced network complexity. FEMS Microbiol. Ecol. 2019, 95, fiz092. [Google Scholar] [CrossRef] [Green Version]
- Meng, M.; Lin, J.; Guo, X.; Liu, X.; Wu, J.; Zhao, Y.; Zhang, J. Impacts of forest conversion on soil bacterial community composition and diversity in subtropical forests. Catena 2019, 175, 167–173. [Google Scholar] [CrossRef]
- McHugh, T.A.; Schwartz, E. Changes in plant community composition and reduced precipitation have limited effects on the structure of soil bacterial and fungal communities present in a semiarid grassland. Plant Soil 2015, 388, 175–186. [Google Scholar] [CrossRef]
- Lima-Perim, J.E.; Romagnoli, E.M.; DiniAndreote, F.; Durrer, A.; Dias, A.C.F.; Andreote, F.D. Linking the composition of bacterial and archaeal communities to characteristics of soil and flora composition in the Atlantic rainforest. PLoS ONE 2016, 11, e0146566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitali, F.; Mastromei, G.; Senatore, G.; Caroppo, C.; Casalone, E. Long lasting effects of the conversion from natural forest to poplar plantation on soil microbial communities. Microbiol. Res. 2016, 182, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Grayston, S.J.; Griffith, G.S.; Mawdsley, J.L.; Campbell, C.D.; Bardgett, R.D. Accounting for variability in soil microbial communities of temperate upland grassland ecosystems. Soil Biol. Biochem. 2001, 33, 533–551. [Google Scholar] [CrossRef]
- Prober, S.M.; Leff, J.W.; Bates, S.T.; Borer, E.T.; Firn, J.; Harpole, W.S.; Lind, E.M.; Seabloom, E.W.; Adler, P.B.; Bakker, J.D.; et al. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 2015, 18, 85–95. [Google Scholar] [CrossRef]
- He, S.; Guo, L.; Niu, M.; Miao, F.; Jiao, S.; Hu, T.; Long, M. Ecological diversity and co-occurrence patterns of bacterial community through soil profile in response to long-term switchgrass cultivation. Sci. Rep. 2017, 7, 3608. [Google Scholar] [CrossRef]
- Aponte, C.; García, L.V.; Marañón, T. Tree species effect on litter decomposition and nutrient release in Mediterranean oak forests changes over time. Ecosystems 2012, 15, 1204–1218. [Google Scholar] [CrossRef] [Green Version]
- Dawud, S.M.; Raulund-Rasmussen, K.; Domisch, T.; Finer, L.; Jaroszewicz, W.; Vesterdal, L. Is tree species diversity or species identity the more important driver of soil carbon stocks, C/N ratio, and pH? Ecosystems 2016, 19, 645–660. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.F.; Jiang, P.K.; Wu, J.S.; Zhou, G.M.; Shen, R.F.; Fuhrmann, J.J. Bamboo invasion of native broadleaf forest modified soil microbial communities and diversity. Biol. Invasions 2015, 17, 433–444. [Google Scholar] [CrossRef]
- Wolińska, A.; Kuźniar, A.; Zielenkiewicz, U.; Szafranek-Nakonieczna, A.; Banach, A.; Błaszczyk, M. Bacteroidetes as a sensitive biological indicator of agricultural soil usage revealed by a culture-independent approach. Appl. Soil Ecol. 2017, 119, 128–137. [Google Scholar] [CrossRef]
- Haas, J.C.; Street, N.R.; Sjödin, A.; Lee, N.M.; Högberg, M.N.; Näsholm, T.; Hurry, V. Microbial community response to growing season and plant nutrient optimisation in a boreal Norway spruce forest. Soil Biol. Biochem. 2018, 125, 197–209. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Lauber, C.L.; Knight, R.; Bradford, M.A.; Fierer, N. Consistent effects of nitrogen fertilization on soil bacterial communities in contrasting systems. Ecology 2010, 91, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community composition at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, A.; Becker, R. Soil parent material is a key determinant of the bacterial community structure in arable soils. FEMS Microbial. Ecol. 2006, 56, 430–443. [Google Scholar] [CrossRef] [Green Version]
- Purnamayani, R.; Asni, N. Teknologi Pemupukan Karet Unggul dan Lokal Spesifik Lokasi; Balai Pengkajian Teknologi Pertanian Jambi: Jambi, Indonesia, 2013. [Google Scholar]
- Kiswanto; Purwanta, J.H.; Wijayanto, B. Teknologi Budidaya Kelapa Sawit. Balai Besar Pengkajian dan Pengembangan Teknologi Pertanian; Badan Penelitian dan Pengembangan Pertanian: Bogor, Indonesia, 2008; pp. 12–13. [Google Scholar]
- Dai, Z.; Su, W.; Chen, H.; Barberan, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Longterm nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Turlapati, S.A.; Minocha, R.; Bhiravarasa, P.S.; Tisa, L.S.; Thomas, W.K.; Minocha, S.C. Chronic N-amended soils exhibit an altered bacterial community structure in Harvard Forest, MA, USA. FEMS Microbiol. Ecol. 2013, 83, 478–493. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.; Carnol, M.; Dharmarajah, A.; Brunner, I.; Schleppi, P. Only minor changes in the soil microbiome of a sub-alpine forest after 20 years of moderately increased nitrogen loads. Front. For. Glob. Chang. 2020, 3, 77. [Google Scholar] [CrossRef]
- Pierre, W.H. Nitrogenous fertilizers and soil acidity: Effect of various nitrogenous fertilizers on soil reaction. Agron. J. 1928, 20, 2–16. [Google Scholar] [CrossRef] [Green Version]
- Kissel, D.E.; Bock, B.R.; Ogles, C.Z. Thoughts on acidification of soils by nitrogen and sulfur fertilizers. Agrosyst. Geosci. Environ. 2020, 3, e20060. [Google Scholar] [CrossRef]
- Hartman, W.H.; Richardson, C.J.; Vilgalys, R.; Bruland, G.R. Environmental and anthropogenic controls over bacterial communities in wetland soils. Proc. Natl. Acad. Sci. USA 2008, 105, 17842–17847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bárcenas-Moreno, G.; Bååth, E.; Rousk, J. Functional implications of the pH-trait distribution of the microbial community in a re-inoculation experiment across a pH gradient. Soil Biol. Biochem. 2016, 93, 69–78. [Google Scholar] [CrossRef]
- Cho, S.J.; Kim, M.H.; Lee, Y.O. Effect of pH on soil bacterial diversity. J. Ecol. Environ. Sci. 2016, 40, 10. [Google Scholar] [CrossRef] [Green Version]
- Truog, E. Soil reaction influence on availability of plant nutrients. Soil Sci. Soc. Am. Proc. 1946, 11, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef]




| Code | Type of Forest | Unit Location | Latitude & Longitude | Silviculture Practices | Dominant Species |
|---|---|---|---|---|---|
| NAF1 | Natural | Sodong | −3.64444, 103.96361 | - | Mallotus paniculatus (Lamk.) Muell.Arg, Eugenia cerina M.R. Hend., Trema orientale (L) Blume, Macaranga sp., Jasminum elongatum (P.J.Bergius) Willd, Macaranga gigantea (Rchb.f. & Zoll.) Müll.Arg., Psychotria viridiflora Reinw. ex Blume, Cratoxylum formosum (Jacq.) Benth. & Hook.f. ex Dyer, Schima noronhae Reinw. ex Blume Eucalyptus pellita F.Muell., Acacia mangium Willd. |
| NAF2 | Natural | Sodong | −3.63917, 103.95500 | - | Mallotus paniculatus (Lamk.) Muell.Arg, Eugenia cerina M.R. Hend., Trema orientale (L) Blume, Macaranga sp., Jasminum elongatum (P.J.Bergius) Willd, Macaranga gigantea (Rchb.f. & Zoll.) Müll.Arg, Psychotria viridiflora Reinw. ex Blume, Cratoxylum formosum (Jacq.) Benth. & Hook.f. ex Dyer, Schima noronhae Reinw. ex Blume Eucalyptus pellita F.Muell., Acacia mangium Willd. |
| NAF3 | Natural | Sodong | −3.66611, 104.02694 | - | Endiandra rubescens (Blume) Miq., Psychotria sp., Schima wallichii Choisy, Macaranga sp., Microcos tomentosa Sm., Aporosa octandra var. malesiana Schot, Mallotus paniculatus (Lamk.) Muell.Arg, Adinandra dumosa Jack, Eugenia sp., Planchonia valida (Blume) Blume, Buchanania arborescens (Blume) Blume |
| HPF1 | Plantation | Sodong | −3.72111, 104.17528 | Planting: Jun 2016 Fertilizer: 45 g/tree TSP at planting Herbicide * | Eucalyptus pellita F.Muell. |
| HPF2 | Plantation | Sodong | −3.76861, 104.02667 | Planting: Mar 2016 Fertilizer: 45 g/tree TSP at planting Herbicide * | Eucalyptus pellita F.Muell. |
| HPF3 | Plantation | Caban | −3.62194, 103.93611 | Planting: Feb 2016 Fertilizer: 45 g/tree TSP at planting Herbicide * | Eucalyptus pellita F.Muell. |
| DPF1 | Plantation | Subanjeriji | −3.93694, 104.07750 | Planting: Jan 2015 Fertilizer: 45 g/tree at planting Herbicide * | Eucalyptus pellita F.Muell. |
| DPF2 | Plantation | Subanjeriji | −3.89083, 104.11083 | Planting: Apr 2015 Fertilizer: 45 g/tree TSP at planting Herbicide * | Eucalyptus pellita F.Muell. |
| DPF3 | Plantation | Subanjeriji | −3.77167, 104.17917 | Planting: Jan 2015 Fertilizer: 45 g/tree TSP at planting Herbicide * | Eucalyptus pellita F.Muell. |
| Variable | NAF | DPF | HPF |
|---|---|---|---|
| pH (H2O) | 4.33 ± 0.06 | 4.67 ± 0.06 | 4.33 ± 0.06 |
| C-organic (%) | 2.65 ± 0.51 | 2.43 ± 0.16 | 2.96 ± 0.13 |
| N total (%) | 0.19 ± 0.02 | 0.18 ± 0.01 | 0.20 ± 0.01 |
| C/N ratio | 14.00 ± 1.73 | 13.33 ± 0.58 | 15.00 ± 0.00 |
| P total (ppm) | 4.00 ± 1.73 | 3.00 ± 1.00 | 4.33 ± 2.08 |
| K (cmol/kg) | 31.33 ± 15.01 | 26.00 ± 7.55 | 20.00 ± 3.60 |
| Clay | 26.33 ± 4.62 | 28.00 ± 1.73 | 28.00 ± 4.36 |
| Silt | 30.33 ± 2.31 | 26.33 ± 2.52 | 25.00 ± 1.73 |
| Sand | 43.33 ± 2.31 | 45.67 ± 1.15 | 47.00 ± 2.65 |
| Texture | Silty Clay | Silty Clay | Silty Clay |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lelana, N.E.; Utami, S.; Widyati, E.; Puspitaningtyas, D.M.; Yulianti; Supriadi, B.; Oktarina, S.; Priatna, D. The Bacterial Composition and Diversity in a Eucalyptus pellita Plantation in South Sumatra, Indonesia. Diversity 2022, 14, 442. https://doi.org/10.3390/d14060442
Lelana NE, Utami S, Widyati E, Puspitaningtyas DM, Yulianti, Supriadi B, Oktarina S, Priatna D. The Bacterial Composition and Diversity in a Eucalyptus pellita Plantation in South Sumatra, Indonesia. Diversity. 2022; 14(6):442. https://doi.org/10.3390/d14060442
Chicago/Turabian StyleLelana, Neo Endra, Sri Utami, Enny Widyati, Dwi Murti Puspitaningtyas, Yulianti, Bambang Supriadi, Seva Oktarina, and Deni Priatna. 2022. "The Bacterial Composition and Diversity in a Eucalyptus pellita Plantation in South Sumatra, Indonesia" Diversity 14, no. 6: 442. https://doi.org/10.3390/d14060442
APA StyleLelana, N. E., Utami, S., Widyati, E., Puspitaningtyas, D. M., Yulianti, Supriadi, B., Oktarina, S., & Priatna, D. (2022). The Bacterial Composition and Diversity in a Eucalyptus pellita Plantation in South Sumatra, Indonesia. Diversity, 14(6), 442. https://doi.org/10.3390/d14060442







