DNA Barcoding to Enhance Conservation of Sunshine Coast Heathlands
Abstract
1. Introduction
Aims of This Study
- Is there variation in species richness, phylogenetic diversity, and composition among heath regional ecosystems of the Sunshine Coast, which may be important for assessing conservation priorities?
- Is there any evidence in the diversity metrics to inform on the ecological and evolutionary history of the heaths on the Sunshine Coast?
- Do the species composition and phylogenetic metrics provide insights into the community assembly dynamics of the heath; are the regional ecosystems operating as distinct and discreet communities or is there overlap in species composition?
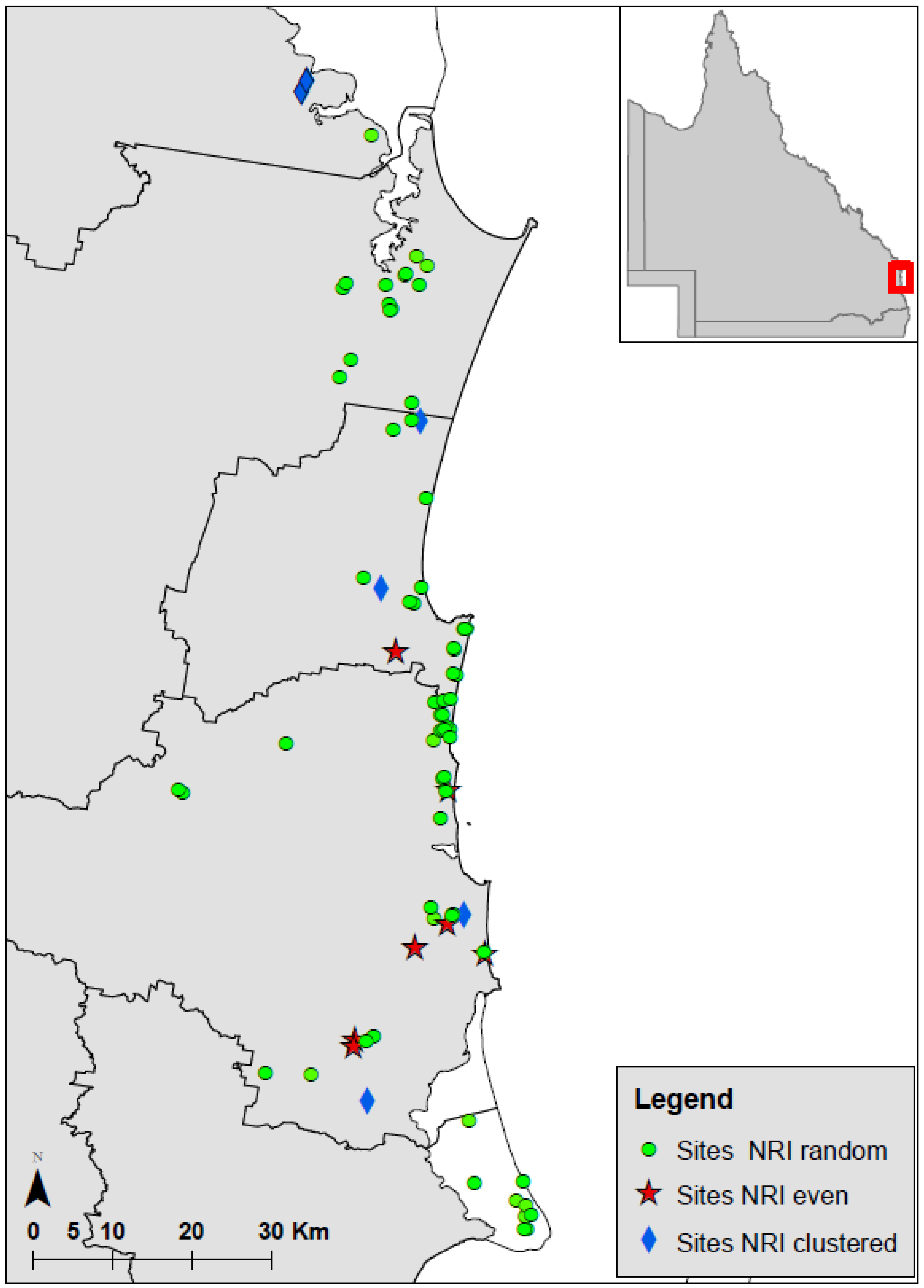
- Is there evidence of sites of “refugia” or centres of diversity where are these located, and do they warrant consideration of enhanced protection?
2. Materials and Methods
2.1. Design and Data Collection
2.2. DNA Barcoding and Sequence Alignment
2.3. Phylogenetic Reconstruction
2.4. Diversity Measures and Analyses
3. Results
3.1. Phylogenetic Position
3.2. Species Richness and Phylogenetic Diversity Metrics
3.3. Species and Phylogenetic Composition
4. Discussion
4.1. Value of a Range of Diversity Metrics
4.2. Data Consistent with Theory of Evolutionary History of Heath Flora
4.3. Phylogenetic Clustering
4.4. Community Assembly
4.5. A Refugial Environment?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Convention on Biological Diversity. First Draft of the post-2020 Global Biodiversity Framework. In Proceedings of the Open Ended Working Group on the Post 2020 Global Biodiversity Framework, Third Meeting, Online, 23 August–3 September 2021; Available online: https://www.cbd.int/doc/c/abb5/591f/2e46096d3f0330b08ce87a45/wg2020-03-03-en.pdf (accessed on 27 March 2022).
- Department of Agriculture Water and the Environment. Australia’s Sixth National Report to the Convention on Biological Diversity 2014–2018; Department of Agriculture Water and the Environment, Commonwealth of Australia: Canberra, Australia, 2020.
- Watson, J.E.M.; Watson, J.E.M.; Simmonds, J.S.; Narain, D.; Ward, M.; Maron, M.; Maxwell, S.L. Talk is cheap: Nations must act now to achieve long-term ambitions for biodiversity. One Earth 2021, 4, 897–900. [Google Scholar] [CrossRef]
- Maron, M.; Juffe-Bignoli, D.; Krueger, L.; Kiesecker, J.; Kumpel, N.F.; Kate, K.; Milner-Gulland, E.J.; Arlidge, W.N.S.; Booth, H.; Bull, J.W.; et al. Setting robust biodiversity goals. Conserv. Lett. 2021, 14, e12816. [Google Scholar] [CrossRef]
- Maron, M.; Simmonds, J.S.; Watson, J.E.M. Bold nature retention targets are essential for the global environment agenda. Nat. Ecol. Evol. 2018, 2, 1194–1195. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.L.; Cazalis, V.; Dudley, N.; Hoffmann, M.; Rodrigues, A.S.L.; Stolton, S.; Visconti, P.; Woodley, S.; Kingston, N.; Lewis, E.; et al. Area-based conservation in the twenty-first century. Nature 2020, 586, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, A.H.; Mishler, B.D.; Knerr, N.J.; Gonzalez-Orozco, C.E.; Costion, C.M.; Crayn, D.M.; Laffan, S.W.; Miller, J.T. Continental-scale spatial phylogenetics of Australian angiosperms provides insights into ecology, evolution and conservation. J. Biogeogr. 2016, 43, 2085–2098. [Google Scholar] [CrossRef]
- Orme, C.D.L.; Davies, R.G.; Burgess, M.; Eigenbrod, F.; Pickup, N.; Olson, V.A.; Webster, A.J.; Ding, T.S.; Rasmussen, P.C.; Ridgely, R.S.; et al. Global hotspots of species richness are not congruent with endemism or threat. Nature 2005, 436, 1016–1019. [Google Scholar] [CrossRef]
- Fleishman, E.; Noss, R.F.; Noon, B.R. Utility and limitations of species richness metrics for conservation planning. Ecol. Indic. 2006, 6, 543–553. [Google Scholar] [CrossRef]
- Brooks, T.M.; Cuttelod, A.; Faith, D.P.; Garcia-Moreno, J.; Langhammer, P.; Perez-Espona, S. Why and how might genetic and phylogenetic diversity be reflected in the identification of key biodiversity areas? Philos. Trans. R. Soc. B-Biol. Sci. 2015, 370, 20140019. [Google Scholar] [CrossRef]
- Forest, F.; Crandall, K.A.; Chase, M.W.; Faith, D.P. Phylogeny, extinction and conservation: Embracing uncertainties in a time of urgency. Philos. Trans. R. Soc. B-Biol. Sci. 2015, 370, 20140002. [Google Scholar] [CrossRef]
- Laity, T.; Laffan, S.W.; Gonzalez-Orozco, C.E.; Faith, D.P.; Rosauer, D.F.; Byrne, M.; Miller, J.T.; Crayn, D.; Costion, C.; Moritz, C.C.; et al. Phylodiversity to inform conservation policy: An Australian example. Sci. Total Environ. 2015, 534, 131–143. [Google Scholar] [CrossRef]
- Gonzalez-Orozco, C.E.; Pollock, L.J.; Thornhill, A.H.; Mishler, B.D.; Knerr, N.; Laffan, S.; Miller, J.T.; Rosauer, D.F.; Faith, D.P.; Nipperess, D.A.; et al. Phylogenetic approaches reveal biodiversity threats under climate change. Nat. Clim. Chang. 2016, 6, 1110–1114. [Google Scholar] [CrossRef]
- Veron, S.; Saito, V.; Padilla-Garcia, N.; Forest, F.; Bertheau, Y. The Use of Phylogenetic Diversity in Conservation Biology and Community Ecology: A Common Base but Different Approaches. Q. Rev. Biol. 2019, 94, 123–148. [Google Scholar] [CrossRef]
- Shapcott, A.; Liu, Y.N.; Howard, M.; Forster, P.I.; Kress, W.J.; Erickson, D.L.; Faith, D.P.; Shimizu, Y.; McDonald, W.J.F. Comparing Floristic Diversity and Conservation Priorities across South East Queensland Regional Rain Forest Ecosystems Using Phylodiversity Indexes. Int. J. Plant Sci. 2017, 178, 211–229. [Google Scholar] [CrossRef]
- Howard, M.G.; McDonald, W.J.F.; Forster, P.I.; Kress, W.J.; Erickson, D.; Faith, D.P.; Shapcott, A. Patterns of Phylogenetic Diversity of Subtropical Rainforest of the Great Sandy Region, Australia Indicate Long Term Climatic Refugia. PLoS ONE 2016, 11, e0153565. [Google Scholar] [CrossRef]
- Shapcott, A.; Forster, P.I.; Guymer, G.P.; McDonald, W.J.F.; Faith, D.P.; Erickson, D.; Kress, W.J. Mapping Biodiversity and Setting Conservation Priorities for SE Queensland’s Rainforests Using DNA Barcoding. PLoS ONE 2015, 10, e0122164. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Tucker, C.M. Difficult decisions: Strategies for conservation prioritization when taxonomic, phylogenetic and functional diversity are not spatially congruent. Biol. Conserv. 2018, 225, 128–133. [Google Scholar] [CrossRef]
- Forest, F.; Grenyer, R.; Rouget, M.; Davies, T.J.; Cowling, R.M.; Faith, D.P.; Balmford, A.; Manning, J.C.; Proches, S.; van der Bank, M.; et al. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 2007, 445, 757–760. [Google Scholar] [CrossRef]
- Pollock, L.J.; Thuiller, W.; Jetz, W. Large conservation gains possible for global biodiversity facets. Nature 2017, 546, 141–144. [Google Scholar] [CrossRef]
- Pollock, L.J.; Rosauer, D.F.; Thornhill, A.H.; Kujala, H.; Crisp, M.D.; Miller, J.T.; McCarthy, M.A. Phylogenetic diversity meets conservation policy: Small areas are key to preserving eucalypt lineages. Philos. Trans. R. Soc. B-Biol. Sci. 2015, 370, 20140007. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and Community Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Swenson, N.G. Phylogenetic Analyses of Ecological Communites Using DNA Barcode Data. In DNA Barcoding Methods and Protocols; Kress, W.A., Erickson, D.L., Eds.; Springer: New York, NY, USA, 2012; pp. 409–419. [Google Scholar]
- Gerhold, P.; Cahill, J.F.; Winter, M.; Bartish, I.V.; Prinzing, A. Phylogenetic patterns are not proxies of community assembly mechanisms (they are far better). Funct. Ecol. 2015, 29, 600–614. [Google Scholar] [CrossRef]
- Gerhold, P.; Carlucci, M.B.; Proches, S.; Prinzing, A. The Deep Past Controls the Phylogenetic Structure of Present, Local Communities. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 477–497. [Google Scholar] [CrossRef]
- Costion, C.M.; Edwards, W.; Ford, A.J.; Metcalfe, D.J.; Cross, H.B.; Harrington, M.G.; Richardson, J.E.; Hilbert, D.W.; Lowe, A.J.; Crayn, D.M. Using phylogenetic diversity to identify ancient rain forest refugia and diversification zones in a biodiversity hotspot. Divers. Distrib. 2015, 21, 279–289. [Google Scholar] [CrossRef]
- Tucker, C.M.; Cadotte, M.W.; Carvalho, S.B.; Davies, T.J.; Ferrier, S.; Fritz, S.A.; Grenyer, R.; Helmus, M.R.; Jin, L.S.; Mooers, A.O.; et al. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. 2017, 92, 698–715. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.; Stock, E. Pleistocene occupation in the southeast Queensland coastal region. Nature 1986, 323, 618–621. [Google Scholar] [CrossRef]
- Griffith, S.J.; Bale, C.; Adam, P.; Wilson, R. Wallum and related vegetation on the NSW North Coast: Description and phytosociological analysis. Cunninghamia 2003, 8, 202–252. [Google Scholar]
- Reed, A.W. Aboriginal Place Names; Reed Books: French Forests, NSW, Australia, 1970. [Google Scholar]
- Australian Bureau of Statistics. Table 3.2 Australian Historical Population Statistics. 2019. Available online: https://www.abs.gov.au/statistics/people/population/historical-population/latest-release#data-download (accessed on 1 December 2021).
- Green, E. Piece by Piece: Conservation and Development on the Sunshine Coast 1960–2020; Wildlife Preservation Society of Queensland, Sunshine Coast & Hinterland Inc.: Caloundra, QLD, Australia, 2021. [Google Scholar]
- Wellington, T. Noosa and Cooloola; Beaut Books: Tinbeerwah, QLD, Australia, 2014. [Google Scholar]
- Keith, D.; Lindenmayer, D.; Lowe, A.; Russell-Smith, J.; Barrett, S.; Enright, N.; Fox, B.; Guerin, G.; Paton, D.; Tozer, M.; et al. Heathlands. In Biodiversity and Environmental Change: Monitoring, Challenges and Direction; Lindenmayer, D., Burns, E., Thurgate, N., Lowe, A., Eds.; CSIRO Publishing: Collingwood, VIC, Australia, 2014. [Google Scholar]
- Coaldrake, J.E. The Ecosystem of the Coastal Lowlands (“Wallum”) of Southern Queensland; Commonwealth Scientific and Industrial Research Organisation: Melbourne, VIC, Australia, 1961. [Google Scholar]
- Specht, R.L. The Sclerophyllous (Heath) Vegetation of Australia: The Eastern and Central States. In Ecosystems of the World: Heathlands and Related Shurblands; Specht, R.L., Ed.; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Byrne, M.; Steane, D.A.; Joseph, L.; Yeates, D.K.; Jordan, G.J.; Crayn, D.; Aplin, K.; Cantrill, D.J.; Cook, L.G.; Crisp, M.D.; et al. Decline of a biome: Evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. J. Biogeogr. 2011, 38, 1635–1656. [Google Scholar] [CrossRef]
- Barlow, B.A.; Clifford, H.T.; George, A.S.; Kanis, A.; McClusker, A. Flora of Australia Volume 1; AGPS: Canberra, Australia, 1981; Volume 1. [Google Scholar]
- Hill, R.S. Origins of the southeastern Australian vegetation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 1537–1549. [Google Scholar] [CrossRef]
- Scanlan, L.; McDonald, W.J.F.; Shapcott, A. Phylogenetic diversity and conservation of rainforests in the Sunshine Coast region, Queensland, Australia. Aust. J. Bot. 2018, 66, 518–530. [Google Scholar] [CrossRef]
- Specht, R.L.; Clifford, H.T.; Arianoutsou, M.; Bird, L.H.; Bolton, M.P.; Forster, P.I.; Grundy, R.I.; Hegarty, E.E.; Specht, A. Structure, floristics and species richness of plant communities in southeast Queensland. Proc. R. Soc. Qld. 1991, 101, 27–78. [Google Scholar]
- Young, P. How significant is the plant biodiversity of localised patches of heathy vegetation growing on low fertility soils on the hills and ranges and adjacent inland of southern Queensland? In Faculty of Science, Health, Education and Engineering; University of the Sunshine Coast: Sippy Downs, QLD, Australia, 2015. [Google Scholar]
- Filer, A.; Beyer, H.L.; Meyer, E.; Van Rensburg, B.J. Distribution mapping of specialized amphibian species in rare, ephemeral habitats: Implications for the conservation of threatened “acid” frogs in south-east Queensland. Conserv. Sci. Pract. 2020, 2, e143. [Google Scholar] [CrossRef]
- Baker, J.; Whelan, R.J.; Evans, L.; Moore, S.; Norton, M. Managing the Ground Parrot in its fiery habitat in south-eastern Australia. Emu Austral Ornithol. 2010, 110, 279–284. [Google Scholar] [CrossRef][Green Version]
- Willmott, W. Rocks and Landscapes of the Sunshine Coast, 2nd ed.; Geological Society of Australia, Queensland Division: Brisbane, QLD, Australia, 2007. [Google Scholar]
- Pickett, J.W.; Thompson, C.H.; Kelley, R.A.; Romans, D. Evidence of high sea-levl during isotope stage-5C in Queensland, Australia. Quat. Res. 1985, 24, 103–114. [Google Scholar] [CrossRef]
- Lamont, R.W.; Stokoe, R.L.; Shapcott, A. Ecological genetics of the wind-pollinated, tetraploid, Allocasuarina emuina L. Johnson (Casuarinaceae) from southeast Queensland reveals montane refugia for coastal heath during the last interglacial. Aust. J. Bot. 2012, 60, 718–734. [Google Scholar] [CrossRef]
- Reside, A.E.; Welbergen, J.A.; Phillips, B.L.; Wardell-Johnson, G.W.; Keppel, G.; Ferrier, S.; Williams, S.E.; Vanderwal, J. Characteristics of climate change refugia for Australian biodiversity. Austral Ecol. 2014, 39, 887–897. [Google Scholar] [CrossRef]
- Crisp, M.D.; Laffan, S.; Linder, H.P.; Monro, A. Endemism in the Australian flora. J. Biogeogr. 2001, 28, 183–198. [Google Scholar] [CrossRef]
- Keppel, G.; Mokany, K.; Wardell-Johnson, G.W.; Phillips, B.L.; Welbergen, J.A.; Reside, A.E. The capacity of refugia for conservation planning under climate change. Front. Ecol. Environ. 2015, 13, 106–112. [Google Scholar] [CrossRef]
- Nelder, V.J.; Butler, D.W.; Guymer, G.P. Queensland’s Regional Ecosystems: Building and Maintaining a Biodiversity Inventory, Planning Framework and Information System for Queensland Version 2.0; Queensland Herbarium; Queensland Department of Environment and Science: Brisbane, QLD, Australia, 2019. [Google Scholar]
- Queensland Herbarium. Regional Ecosystem Description Database (REDD). Version 10.1 (March 2018); DSITI; Queensland Herbarium: Brisbane, QLD, Australia, 2018. [Google Scholar]
- Queensland Herbarium. Queensland CORVEG Database, Version 5/2019; Department of Environment and Science; TERN AEKOS: Brisbane, QLD, Australia, 2012. Available online: http://aekos.org.au/collection/qld.gov.au/corveg (accessed on 24 September 2020).
- Esri Inc. ArcGIS Version 10.5; Esri Inc.: Redlands, CA, USA, 2016. [Google Scholar]
- Queensland Herbarium. Biodiversity Status of Pre-Clearing and 2015 Remnant Regional Ecosystems—Version 10.0; Department of Environment and Science; Queensland Government: Brisbane, QLD, Australia, 2017. [Google Scholar]
- Queensland Parks and Wildlife Service. Protected Areas of Queensland; Department of Environment and Science; Queensland Government: Brisbane, QLD, Australia, 2017. [Google Scholar]
- Nelder, V.J.; Wilson, B.A.; Dillewaard, H.A.; Ryan, T.S.; Butler, D.W. Methodology for Survey and Mapping of Regional Ecosystems and Vegetation Communities in Queensland. Version 4.0; I.T.a.I. Queensland Department of Science; Queensland Herbarium: Brisbane, QLD, Australia, 2017. [Google Scholar]
- Elphick, C.S. How you count counts: The importance of methods research in applied ecology. J. Appl. Ecol. 2008, 45, 1313–1320. [Google Scholar] [CrossRef]
- Leiper, G.; Glazebrook, J.; Cox, D.; Rathie, K. Mangroves to Mountains, 2nd ed.; Queensland, Society of Growing Australian Plants (Queensland Region) Inc.: Logan River Branch, QLD, Australia, 2017. [Google Scholar]
- Harrold, A. Wildflowers of the Noosa-Cooloola Area; Noosa Parks Association Inc.: Noosa Heads, QLD, Australia, 1994. [Google Scholar]
- Bostock, P.D.; Holland, A.E. Census of the Queensland Flora 2017. 2017. Available online: https://data.qld.gov.au/dataset/census-of-the-queensland-flora-2015 (accessed on 22 January 2016).
- Carr, A. A Field Guide to Native Plants of Bribie Island and Nearby Coastal South-East Queensland; Caboolture Daytime Branch, Native Plants Queensland: Caboolture, QLD, Australia, 2018. [Google Scholar]
- MacRae, I.C. Wildflowers of Bribie Island; Bribie Island Environmental Protection Association Inc.: Bribie Island, QLD, Australia, 1996. [Google Scholar]
- AVH. The Australasian Virtual Herbarium. 2018. Available online: https://avh.chah.org.au/ (accessed on 1 September 2020).
- Department of Natural Resources, Mines and Energy. Mainland-Queensland. 2007. Available online: http://qldspatial.information.qld.gov.au/catalogue/custom/search.page?q=%22Mainland%20-%20Queensland%22 (accessed on 28 October 2021).
- Etherington, R.; Shapcott, A. Do habitat fragmentation and fire influence variation of plant species composition, structure and diversity within three regional ecosystems on the Sunshine Coast, Queensland, Australia? Aust. J. Bot. 2014, 62, 36–47. [Google Scholar] [CrossRef]
- Kress, W.J.; Erickson, D.L.; Jones, F.A.; Swenson, N.G.; Perez, R.; Sanjur, O.; Bermingham, E. Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc. Natl. Acad. Sci. USA 2009, 106, 18621–18626. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Holder, M.T.; Sukumaran, J.; Mirarab, S.; Oaks, J. SATé Version 2.2.7. 2013. Available online: http://phylo.bio.ku.edu/software/sate/sate.html (accessed on 23 April 2020).
- Bremer, B.; Bremer, K.; Chase, M.W.; Fay, M.F.; Reveal, J.L.; Soltis, D.E.; Soltis, P.S.; Stevens, P.F.; Anderberg, A.A.; Moore, M.J.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef]
- Webb, C.O.; Donoghue, M.J. Phylomatic: Tree assembly for applied phylogenetics. Mol. Ecol. Notes 2005, 5, 181–183. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.61. Available online: http://www.mesquiteproject.org (accessed on 1 May 2020).
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE) 2010, New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Britton, T.; Anderson, C.L.; Jacquet, D.; Lundqvist, S.; Bremer, K. Estimating divergence times in large phylogenetic trees. Syst. Biol. 2007, 56, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R Project for Statistical Computing. 2020. Available online: https://www.r-project.org/ (accessed on 1 May 2021).
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). 2014. Available online: https://cran.r-project.org/web/packages/PMCMR/index.html (accessed on 1 May 2021).
- Warnes, G.R.; Bolker, B.; Lumley, T.; Johnson, R.C. Gmodels: Various R Programming Tools for Model Fitting. 2018. Available online: https://cran.r-project.org/web/packages/gmodels/index.html (accessed on 1 May 2021).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, R.; O’Hara, B.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package. 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 1 May 2021).
- Lozupone, C.; Hamady, M.; Knight, R. UniFrac—An online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinform. 2006, 7, 371. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Diniz, J.A.F.; Soares, T.N.; Lima, J.S.; Dobrovolski, R.; Landeiro, V.L.; Telles, M.P.D.; Rangel, T.F.; Bini, L.M. Mantel test in population genetics. Genet. Mol. Biol. 2013, 36, 475–485. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life v2: Online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011, 39, W475–W478. [Google Scholar] [CrossRef]
- Noss, R.F. Indicators for monitoring biodiversity—A hierarchical approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]
- Specht, R.L.; Specht, A. Species richness of sclerophyll (heathy) plant -communities in Australia—The influence of overstory cover. Aust. J. Bot. 1989, 37, 337–350. [Google Scholar] [CrossRef]
- Specht, R.L. Heathlands. In Australian Vegetation; Groves, R.H., Ed.; Cambridge University Press: Cambridge, UK, 1994; pp. 321–344. [Google Scholar]
- Myerscough, P.J.; Clarke, P.J.; Skelton, N.J. Plant coexistence in coastal heaths: Floristic patterns and species attributes. Aust. J. Ecol. 1995, 20, 482–493. [Google Scholar] [CrossRef]
- Sniderman, J.M.K.; Jordan, G.J.; Cowling, R.M. Fossil evidence for a hyperdiverse sclerophyll flora under a non-Mediterranean-type climate. Proc. Natl. Acad. Sci. USA 2013, 110, 3423–3428. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Murphy, D.J. The origins and evolutionary history of xerophytic vegetation in Australia. Aust. J. Bot. 2020, 68, 195–207. [Google Scholar] [CrossRef]
- Crisp, M.; Cook, L.; Steane, D. Radiation of the Australian flora: What can comparisons of molecular phylogenies across multiple taxa tell us about the evolution of diversity in present-day communities? Philos. Trans. R. Soc. Lond. Ser. B-Biol. Sci. 2004, 359, 1551–1571. [Google Scholar] [CrossRef]
- Carpenter, R.J.; Macphail, M.K.; Jordan, G.J.; Hill, R.S. Fossil evidence for open, Proteaceae-dominated heathlands and fire in the Late Cretaceous of Australia. Am. J. Bot. 2015, 102, 2092–2107. [Google Scholar] [CrossRef]
- Brunbjerg, A.K.; Cavender-Bares, J.; Eiserhardt, W.L.; Ejrnaes, R.; Aarssen, L.W.; Buckley, H.L.; Forey, E.; Jansen, F.; Kattge, J.; Lane, C.; et al. Multi-scale phylogenetic structure in coastal dune plant communities across the globe. J. Plant Ecol. 2014, 7, 101–114. [Google Scholar] [CrossRef]
- Vamosi, J.C.; Magallon, S.; Mayrose, I.; Otto, S.P.; Sauquet, H. Macroevolutionary Patterns of Flowering Plant Speciation and Extinction. Annu. Rev. Plant Biol. 2018, 69, 685–706. [Google Scholar] [CrossRef]
- Specht, R.L. Plant communities of North Stradbroke Island: Development of structure and species richness. Proc. R. Soc. Qld. 2011, 117, 181–191. [Google Scholar]
- Letten, A.D.; Keith, D.A.; Tozer, M.G. Phylogenetic and functional dissimilarity does not increase during temporal heathland succession. Proc. R. Soc. B-Biol. Sci. 2014, 281, 20142102. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Davies, T.J.; Peres-Neto, P.R. Why phylogenies do not always predict ecological differences. Ecol. Monogr. 2017, 87, 535–551. [Google Scholar] [CrossRef]
- Munkemuller, T.; Gallien, L.; Pollock, L.J.; Barros, C.; Carboni, M.; Chalmandrier, L.; Mazel, F.; Mokany, K.; Roquet, C.; Smycka, J.; et al. Dos and don’ts when inferring assembly rules from diversity patterns. Glob. Ecol. Biogeogr. 2020, 29, 1212–1229. [Google Scholar] [CrossRef]
- Prinzing, A.; Ozinga, W.A.; Brandle, M.; Courty, P.E.; Hennion, F.; Labandeira, C.; Parisod, C.; Pihain, M.; Bartish, I.V. Benefits from living together? Clades whose species use similar habitats may persist as a result of eco-evolutionary feedbacks. New Phytol. 2017, 213, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Brundrett, M.C.; Raven, J.A.; Hopper, S.D. Plant mineral nutrition in ancient landscapes: High plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 2011, 348, 7–27. [Google Scholar] [CrossRef]
- Zemunik, G.; Turner, B.L.; Lambers, H.; Laliberte, E. Increasing plant species diversity and extreme species turnover accompany declining soil fertility along a long-term chronosequence in a biodiversity hotspot. J. Ecol. 2016, 104, 792–805. [Google Scholar] [CrossRef]
- Clements, F.E. Nature and structure of the climax. J. Ecol. 1936, 24, 252–284. [Google Scholar] [CrossRef]
- Gleason, H.A. The Individualistic Concept of the Plant Association. Am. Midl. Nat. 1939, 21, 92–110. [Google Scholar] [CrossRef]
- Loreau, M. The Ecosystem: Superorganism, or Collection of Individuals? In Unsolved Problems in Ecology; Dobson, A., Holt, R.D., Tilman, D., Eds.; Princeton University Press: Princeton, NJ, USA, 2020; pp. 218–224. [Google Scholar]
- Keith, D. Mosaics in Sydney heathland vegetation: The roles of fire, competition and soils. CALMSci. Suppl. 1995, 4, 199–206. [Google Scholar]
- Specht, R.L. Conservation: Australian Heathlands. In Ecosystems of the World: Heathlands and Related Shrublands; Specht, R.L., Ed.; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1981. [Google Scholar]
- Keppel, G.; Van Niel, K.P.; Wardell-Johnson, G.W.; Yates, C.J.; Byrne, M.; Mucina, L.; Schut, A.G.T.; Hopper, S.D.; Franklin, S.E. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr. 2012, 21, 393–404. [Google Scholar] [CrossRef]
- Keppel, G.; Ottaviani, G.; Harrison, S.; Wardell-Johnson, G.W.; Marcantonio, M.; Mucina, L. Towards an eco-evolutionary understanding of endemism hotspots and refugia. Ann. Bot. 2018, 122, 927–934. [Google Scholar] [CrossRef]
- Morelli, T.L.; Barrows, C.W.; Ramirez, A.R.; Cartwright, J.M.; Ackerly, D.D.; Eaves, T.D.; Ebersole, J.L.; Krawchuk, M.A.; Letcher, B.H.; Mahalovich, M.F.; et al. Climate-change refugia: Biodiversity in the slow lane. Front. Ecol. Environ. 2020, 18, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, M.; Kooyman, R. Conserving Refugia: What Are We Protecting and Why? Diversity 2021, 13, 67. [Google Scholar] [CrossRef]
- Kooyman, R.; Rossetto, M.; Cornwell, W.; Westoby, M. Phylogenetic tests of community assembly across regional to continental scales in tropical and subtropical rain forests. Glob. Ecol. Biogeogr. 2011, 20, 707–716. [Google Scholar] [CrossRef]
- Tibby, J.; Barr, C.; Marshall, J.C.; McGregor, G.B.; Moss, P.T.; Arnold, L.J.; Page, T.J.; Questiaux, D.; Olley, J.; Kemp, J.; et al. Persistence of wetlands on North Stradbroke Island (south-east Queensland, Australia) during the last glacial cycle: Implications for Quaternary science and biogeography. J. Quat. Sci. 2017, 32, 770–781. [Google Scholar] [CrossRef]
- Hopper, S.D.; Gioia, P. The Southwest Australian Floristic Region: Evolution and conservation of a global hot spot of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 623–650. [Google Scholar] [CrossRef]
- Schut, A.G.T.; Wardell-Johnson, G.W.; Yates, C.J.; Keppel, G.; Baran, I.; Franklin, S.E.; Hopper, S.D.; Van Niel, K.P.; Mucina, L.; Byrne, M.; et al. Rapid Characterisation of Vegetation Structure to Predict Refugia and Climate Change Impacts across a Global Biodiversity Hotspot. PLoS ONE 2014, 9, e0082778. [Google Scholar] [CrossRef]
- Buerki, S.; Callmander, M.W.; Bachman, S.; Moat, J.; Labat, J.N.; Forest, F. Incorporating evolutionary history into conservation planning in biodiversity hotspots. Philos. Trans. R. Soc. B-Biol. Sci. 2015, 370, 20140014. [Google Scholar] [CrossRef][Green Version]
- Reside, A.E.; Briscoe, N.J.; Dickman, C.R.; Greenville, A.C.; Hradsky, B.A.; Kark, S.; Kearney, M.R.; Kutt, A.S.; Nimmo, D.G.; Pavey, C.R.; et al. Persistence through tough times: Fixed and shifting refuges in threatened species conservation. Biodivers. Conserv. 2019, 28, 1303–1330. [Google Scholar] [CrossRef]



| Regional Ecosystem | Short Description | Biodiversity Status | No. Sites | SR | PD | MPD | MNTD | NRI | NTI |
|---|---|---|---|---|---|---|---|---|---|
| 12.2.9 | Banksia aemula low open woodland on dunes and sand plains, usually deeply leached soils | No concern at present | 12 | 121 | 3632.73 *L | 208.48 | 32.94 | 0.63 | 2.91 *H |
| 12.2.12 | Closed heath on seasonally waterlogged sand plains | Of concern | 22 | 151 | 4258.21 *L | 209.17 | 33.10 | 0.09 | 2.24 *H |
| 12.2.13 | Open or dry heath on dunes and beaches | Endangered | 6 | 61 | 2376.66 *L | 203.59 | 45.79 | 2.41 *C | 1.80 *H |
| 12.3.13 | Closed heathland on seasonally waterlogged alluvial plains usually near coast | No concern at present | 7 | 89 | 3004.52 *L | 210.71 | 38.66 | −0.76 | 2.36 *H |
| 12.3.14 | Banksia aemula low woodland on alluvial plains usually near coast | Of concern | 7 | 95 | 3153.69 *L | 209.56 | 39.56 | −0.16 | 1.94 *H |
| 12.5.9 | Sedgeland to heathland in low lying areas on complex of remnant Tertiary surface and Tertiary sedimentary rocks | Of concern | 6 | 114 | 3564.05 *L | 208.40 | 39.14 | 0.60 | 1.50 |
| 12.8.19 | Heath and rock pavement with scattered shrubs or open woodland on Cainozoic igneous hills and mountains | Of concern | 9 | 106 | 3295.21 *L | 207.88 | 36.92 | 0.88 | 2.36 *H |
| 12.9-10.22 | Closed sedgeland/shrubland on sedimentary rocks, generally coastal | Of concern | 8 | 123 | 3849.04 *L | 208.68 | 38.48 | 0.41 | 1.49 |
| 12.12.10 | Shrubland of rocky peaks on Mesozoic to Proterozoic igneous rocks | Of concern | 3 | 61 | 2743.24 | 205.66 | 63.85 | 1.52 | −0.88 |
| RE | No. Site | SR | GR | FR | PD | MPD | MNTD | NRI | NTI |
|---|---|---|---|---|---|---|---|---|---|
| 12.12.10 | 3 | 30.3 (4.2) | 27.0 (3.7) | 18.3 (3.1) | 1616.9 (126.7) | 206.8 (0.7) | 72.3 (7.5) | 0.61 (0.20) ab | 0.48 (0.51) |
| 12.2.12 | 22 | 34.5 (12.1) | 30.7 (10.0) | 17.5 (5.0) | 1754.6 (402.0) | 211.1 (3.8) | 68.7 (14.5) | −0.45 (0.94) ab | 0.67 (0.64) |
| 12.2.13 | 6 | 36.3 (2.7) | 32.0 (1.4) | 17.8 (1.0) | 1771.3 (71.7) | 206.0 (2.2) | 56.1 (6.5) | 0.87 (0.60) a | 1.48 (0.52) |
| 12.2.9 | 12 | 30.1 (11.2) | 26.1 (9.2) | 15.5 (4.3) | 1570.0 (390.6) | 205.5 (6.9) | 70.8 (25.9) | 0.92 (1.29) a | 0.99 (0.77) |
| 12.3.13 | 7 | 31.0 (7.9) | 27.7 (6.7) | 17.0 (3.7) | 1652.2 (344.6) | 213.5 (2.6) | 66.2 (9.3) | −1.08 (0.74) b | 0.90 (0.89) |
| 12.3.14 | 7 | 28.7 (4.3 | 26.4 (4.0) | 14.9 (2.9) | 1464.3 (251.3) | 208.3 (8.4) | 60.4 (15.5) | 0.09 (1.79) ab | 1.47 (1.15) |
| 12.5.9 | 6 | 43.3 (7.0) | 37.3 (5.8) | 21.2 (3.8) | 1923.3 (302.0) | 208.2 (5.2) | 55.2 (5.8) | 0.41 (1.62) ab | 1.28 (0.79) |
| 12.8.19 | 9 | 31.2 (16.5) | 27.9 (14.2) | 16.7 (8.7) | 1564.9 (756.5) | 206.6 (9.6) | 61.9 (9.3) | −0.09 (1.34) ab | 1.24 (1.10) |
| 12.9-10.22 | 8 | 42.5 (12.3) | 37.1 (11.2) | 21.0 (5.9) | 1991.1 (514.2) | 208.9 (5.1) | 62.9 (10.2) | −0.12 (1.05) ab | 0.48 (1.16) |
| NS | NS | NS | NS | NS | NS | KW = 20.9 p = 0.007 | NS |
| Regional Ecosystem | Site | SR | GR | FR | PD | MPD | MNTD | NRI | NTI |
|---|---|---|---|---|---|---|---|---|---|
| RE 12.2.9 Banksia aemula, low open woodland on dunes and sand plains, usually deeply leached soils | 15248 | 34 | 32 | 19 | 1866.9 | 212.0 | 69.1 | −0.72 | 0.41 |
| 15250 | 9 | 9 | 8 | 824.5 | 214.5 | 143.7 | −0.41 | −0.7 | |
| 15621 | 20 | 17 | 9 | 1061.0 *L | 189.5 | 60.9 | 3.56 *C | 1.90 *H | |
| 16488 | 28 | 25 | 16 | 1687.7 | 207.2 | 76.7 | 0.42 | 0.27 | |
| 16491 | 25 | 22 | 13 | 1433.6 | 211.8 | 75.0 | −0.49 | 0.65 | |
| BI29-63 | 37 | 33 | 18 | 1774.9 | 208.9 | 58.9 | 0.13 | 1.12 | |
| BI29-65 | 16 | 15 | 11 | 1122.2 | 197.5 | 91.2 | 1.8 | 0.51 | |
| COO29-44 | 40 | 34 | 19 | 1817.0 *L | 200.9 | 52.7 | 2.54 *C | 1.68 | |
| COO29-62 | 47 | 40 | 20 | 2081.9 | 205.9 | 49.9 | 1.11 | 1.69 *H | |
| LCOO29-43 | 40 | 34 | 19 | 1881.3 | 204.5 | 56.3 | 1.46 | 1.25 | |
| MCNP29-60 | 28 | 23 | 15 | 1458.6 | 207.4 | 61.0 | 0.5 | 1.49 | |
| NNP29-61 | 37 | 29 | 19 | 1830.7 | 205.7 | 54.2 | 1.13 | 1.63 *H | |
| RE 12.2.12 Closed heath on seasonally waterlogged sand plains | 15228 | 28 | 26 | 12 | 1449.0 | 205.7 | 66.9 | 0.8 | 0.97 |
| 15252 | 29 | 27 | 19 | 1827.5 | 212.9 | 80.4 | −0.87 | −0.14 | |
| 16443 | 17 | 16 | 11 | 1262.6 | 213.2 | 101.2 | −0.59 | −0.15 | |
| 16450 | 23 | 21 | 12 | 1264.4 | 205.6 | 65.0 | 0.74 | 1.49 | |
| 16493 | 24 | 22 | 14 | 1514.1 | 216.2 | 91.2 | −1.3 | −0.46 | |
| BI212-50 | 38 | 34 | 16 | 1778.2 *L | 204.5 | 58.4 | 1.38 | 1.15 | |
| BI212-51 | 28 | 24 | 17 | 1648.7 | 215.5 | 76.3 | −1.4 | 0.32 | |
| BI212-53 | 45 | 37 | 21 | 1878.1 *L | 207.7 | 51.3 | 0.57 | 1.61 | |
| COO212-24 | 44 | 39 | 21 | 2052.1 | 209.1 | 64.5 | 0.12 | 0.17 | |
| COO212-45 | 22 | 19 | 12 | 1341.1 | 214.1 | 78.7 | −0.9 | 0.61 | |
| COO212-46 | 57 | 47 | 25 | 2585.6 | 213.0 | 57.2 | −1.43 | 0.29 | |
| ES212-10 | 17 | 17 | 11 | 1160.5 | 215.8 | 94.5 | −0.94 | 0.26 | |
| ES212-5 | 39 | 35 | 21 | 2045.6 | 212.1 | 70.7 | −0.79 | −0.12 | |
| ES59-40 | 23 | 22 | 13 | 1467.9 | 213.2 | 83.9 | −0.71 | 0.22 | |
| KMCP212-31 | 48 | 41 | 21 | 2053.3 *L | 209.7 | 51.7 | −0.13 | 1.45 | |
| KMCP212-7 | 58 | 51 | 28 | 2528.4 | 213.5 | 51.6 | −1.66 *E | 1.04 | |
| MCNP212-39 | 41 | 39 | 24 | 2243.9 | 217.2 | 65.5 | −2.38 *E | 0.2 | |
| MCNP212-42 | 27 | 23 | 12 | 1359.7 *L | 208.3 | 59.4 | 0.18 | 1.73 *H | |
| ME212-2 | 27 | 24 | 16 | 1445.6 | 205.1 | 70.4 | 0.85 | 0.84 | |
| NNS212-33 | 41 | 38 | 19 | 1930.6 | 212.0 | 59.2 | −0.8 | 0.9 | |
| PEP212-27 | 36 | 33 | 18 | 1793.7 | 209.6 | 61.1 | −0.11 | 0.99 | |
| PEP212-49 | 47 | 40 | 22 | 1969.6 *L | 211.0 | 52.7 | −0.57 | 1.3 | |
| RE 12.2.13 Open or dry heath on dunes and beaches | MHD213-3 | 32 | 30 | 17 | 1761.5 | 208.8 | 64.0 | 0.09 | 0.98 |
| MHD213-35 | 38 | 34 | 19 | 1846.3 | 208.8 | 54.6 | 0.14 | 1.51 | |
| NNP213-13 | 38 | 32 | 17 | 1686.3 *L | 205.4 | 45.9 | 1.04 | 2.35 *H | |
| NNP213-14 | 38 | 33 | 18 | 1764.1 | 204.1 | 52.6 | 1.43 | 1.78 *H | |
| NNP213-32 | 34 | 32 | 17 | 1706.3 | 203.8 | 60.9 | 1.34 | 1.15 | |
| NNP213-47 | 38 | 31 | 19 | 1863.2 | 205.0 | 58.6 | 1.19 | 1.1 | |
| 12.3.13 Closed heathland on seasonally waterlogged alluvial plains, usually near coast | 16454 | 22 | 22 | 14 | 1316.3 | 211.3 | 69.1 | −0.38 | 1.32 |
| BSA313-4 | 29 | 26 | 17 | 1588.2 | 214.9 | 73.3 | −1.25 | 0.43 | |
| COO313-17 | 25 | 21 | 13 | 1277.7 *L | 208.6 | 53.8 | 0.19 | 2.22 *H | |
| MRNP313-16 | 35 | 33 | 21 | 1939.0 | 215.3 | 74.0 | −1.63 *E | −0.14 | |
| MRNP313-59 | 26 | 22 | 13 | 1380.0 | 213.8 | 66.4 | −1.03 | 1.17 | |
| PV313-37 | 35 | 32 | 21 | 1959.8 | 215.3 | 74.1 | −1.60 *E | −0.15 | |
| TNP313-36 | 45 | 38 | 20 | 2104.3 | 215.2 | 52.8 | −1.85 *E | 1.49 | |
| 12.3.14 Banksia aemula, low woodland on alluvial plains, usually near coast | 15622 | 24 | 22 | 12 | 1405.8 | 196.6 | 81.7 | 2.65 *C | 0.21 |
| BSA314-38 | 28 | 28 | 17 | 1610.3 | 215.9 | 76.0 | −1.53 *E | 0.27 | |
| BSA314-6 | 31 | 29 | 17 | 1702.5 | 215.6 | 66.0 | −1.54 *E | 0.9 | |
| BSA314-8 | 28 | 24 | 14 | 1385.5 *L | 215.2 | 48.0 | −1.37 | 2.38 *H | |
| MRNP314-15 | 23 | 21 | 10 | 962.8 *L | 197.3 | 38.0 | 2.27 *C | 3.34 *H | |
| MRNP314-57 | 35 | 31 | 17 | 1530.9 *L | 206.7 | 52.5 | 0.63 | 1.89 *H | |
| MRNP314-58 | 32 | 30 | 17 | 1652.0 | 211.1 | 60.9 | −0.45 | 1.28 | |
| 12.5.9 Sedgeland to heathland in low lying areas on complex of remnant Tertiary surface and Tertiary sedimentary rocks | COO59-19 | 46 | 40 | 23 | 2094.7 | 209.3 | 57.7 | 0.05 | 0.89 |
| COO59-25 | 39 | 36 | 20 | 1891.7 | 212.6 | 59.9 | −0.97 | 1.01 | |
| COO59-26 | 32 | 28 | 16 | 1499.8 *L | 212.1 | 54.4 | −0.69 | 1.89 *H | |
| COO59-56 | 51 | 45 | 26 | 2264.9 | 208.7 | 59.0 | 0.19 | 0.37 | |
| LC59-37 | 49 | 40 | 24 | 2147.3 | 208.1 | 55.7 | 0.34 | 0.96 | |
| LC59-48 | 43 | 36 | 18 | 1641.3 *L | 198.3 | 44.2 | 3.55 *C | 2.54 *H | |
| 12.8.19 Heath and rock pavement with scattered shrubs or open woodland on Cainozoic igneous hills and mountains | 13962 | 6 | 6 | 4 | 443.7 *L | 203.2 | 66.1 | 0.34 | 2.46 *H |
| MB819-12 | 12 | 11 | 7 | 721.5 *L | 197.7 | 74.0 | 1.22 | 1.73 *H | |
| MCNP819-40 | 44 | 38 | 23 | 2234.2 | 213.6 | 68.9 | −1.35 | −0.32 | |
| MCNP819-41 | 38 | 33 | 20 | 1916.6 | 213.4 | 62.2 | −1.14 | 0.73 | |
| ME212-29 | 45 | 41 | 24 | 2121.7 | 211.6 | 58.4 | −0.76 | 0.84 | |
| ME819-1 | 34 | 31 | 21 | 1824.7 | 213.4 | 72.1 | −1.07 | 0.13 | |
| ME819-11 | 42 | 37 | 21 | 1961.2 | 212.5 | 52.4 | −1 | 1.66 | |
| ME819-30 | 48 | 42 | 25 | 2286.6 | 208.2 | 56.6 | 0.35 | 0.84 | |
| WHM819-9 | 12 | 12 | 5 | 573.8 *L | 185.4 | 46.4 | 2.60 *C | 3.11 *H | |
| 12.9-10.22 Closed sedgeland/shrubland on sedimentary rocks, generally coastal | COO910-20 | 49 | 45 | 20 | 1975.0 *L | 210.7 | 46.9 | −0.48 | 1.97 *H |
| COO910-54 | 45 | 40 | 23 | 2087.8 | 205.2 | 64.8 | 1.32 | 0.03 | |
| COO910-55 | 50 | 44 | 26 | 2458.4 | 211.8 | 69.3 | −0.94 | −0.88 | |
| COO910-70 | 44 | 41 | 22 | 2025.4 | 210.1 | 60.0 | −0.3 | 0.67 | |
| ES910-33 | 53 | 41 | 27 | 2402.4 | 210.2 | 60.5 | −0.4 | 0.08 | |
| ES910-71 | 50 | 44 | 23 | 2255.8 | 210.9 | 59.8 | −0.59 | 0.37 | |
| NNS910-34 | 16 | 12 | 8 | 818.2 *L | 198.1 | 59.6 | 1.67 | 2.34 *H | |
| NNS910-72 | 33 | 30 | 19 | 1906.3 | 214.5 | 82.6 | −1.29 | −0.74 | |
| 12.12.10 Shrubland of rocky peaks on Mesozoic to Proterozoic igneous rocks | MNP1210-30 | 27 | 25 | 15 | 1483.3 | 206.2 | 69.9 | 0.69 | 0.91 |
| MNP1210-66 | 29 | 25 | 19 | 1632.1 | 207.7 | 80.7 | 0.38 | −0.08 | |
| SP1210-31 | 35 | 30 | 21 | 1735.4 | 206.6 | 66.3 | 0.76 | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pearl, H.; Ryan, T.; Howard, M.; Shimizu, Y.; Shapcott, A. DNA Barcoding to Enhance Conservation of Sunshine Coast Heathlands. Diversity 2022, 14, 436. https://doi.org/10.3390/d14060436
Pearl H, Ryan T, Howard M, Shimizu Y, Shapcott A. DNA Barcoding to Enhance Conservation of Sunshine Coast Heathlands. Diversity. 2022; 14(6):436. https://doi.org/10.3390/d14060436
Chicago/Turabian StylePearl, Hilary, Tim Ryan, Marion Howard, Yoko Shimizu, and Alison Shapcott. 2022. "DNA Barcoding to Enhance Conservation of Sunshine Coast Heathlands" Diversity 14, no. 6: 436. https://doi.org/10.3390/d14060436
APA StylePearl, H., Ryan, T., Howard, M., Shimizu, Y., & Shapcott, A. (2022). DNA Barcoding to Enhance Conservation of Sunshine Coast Heathlands. Diversity, 14(6), 436. https://doi.org/10.3390/d14060436






