Abstract
Anoxia (Protanoxia) orientalis is a beetle of the family Melolonthidae for which Italy represents the western limit of its distribution. The ecology of this species is little known from the quantitative point of view. The ecological correlates of A. orientalis presence in the whole European range and, more specifically, at its western border in Sicily, are analyzed in this paper to develop a potential distribution map for Sicily and to define the habitat selection of this species. There was a clear non-random habitat selection by A. orientalis at both the European and the Sicilian scales and a clear difference in the factors affecting the presence of this species in the larger spatial scale compared to Sicily. At the European scale, the bioclimatic factors were more important than landscape factors, whereas the same was not true at the Sicilian scale. In Sicily, the populations were statistically influenced by a combination of predictors that make their potential optimal distribution very narrow and mostly limited to a few coastal areas, suggesting a region-specific ecological diversification. Since A. orientalis is in strong decline in Italy due to the degradation of coastal environments, it is necessary to minimize the degradation of the dune and back dune environments in Sicily to achieve better management for the populations of this beetle species.
1. Introduction
Factors associated with occurrence and habitat preferences are not constant across the whole distribution range of a species but may change locally and in general may be different at the peripheral areas than in the core areas of a species’ range [1]. Thus, analyzing these factors at the borders versus the core areas of a species’ range is important to better understand the ecological and biogeographical dynamics behind the apparent distribution patterns of a given species [2].
Anoxia (Protanoxia) orientalis Krynicki, 1832 (Figure 1) is a beetle of the family Melolonthidae, characterized by an overall length of 21–32 mm, with an Eastern European chorotype (sensu [3]). Its wide range includes Austria, Bosnia and Herzegovina, Bulgaria, Croatia, Greece, Hungary, Italy, Macedonia, Romania, Russia, Turkey [4,5], Ukraine, Serbia, Slovenia, Syria, Lebanon and Israel [6,7,8]. This species also occurs in Italy. That is interesting from the ecological and biogeographical point of view (sensu [1]) as it represents the western limit of its distribution. Indeed, A. orientalis was found in a small, disjointed population near the marshes of Orbetello in Tuscany [9], in a few stations in Calabria [10,11,12,13], and in a comparatively higher number of sites in Sicily [14,15,16]. The ecology of this species is little known from the quantitative point of view, but it is known that the flight period runs from May to the end of July depending on the geographical area considered [5]. In Sicily, non-quantitative studies revealed that the species’ phenology peaks between the third week of May and the first week of June. At dusk, swarms of this beetle can be observed flying on the top of tamarisks and reed beds, remaining active for about 30/40 min before disappearing under the sand or, more rarely, clinging to the shrubs or bushes of Onopordum where they can also be observed in the daylight hours [17]. Studies carried out in Central Europe (Hungary, see [18]) have shown that oviposition occurs about a week after mating, with eggs being laid in the roots of the host plants. The larva hatches the following year and takes another three years before reaching the imago stage. No data concerning the phenology of reproduction of A. orientalis are available for the western Mediterranean range, where it is likely that the life cycle is shorter. Empirical evidence suggests that A. orientalis inhabits a wide range of habitats across its range, from sandy coasts to deciduous lowland forests and orchards, e.g., see [18].

Figure 1.
Anoxia orientalis from “Foce del Simeto”, Sicily.
In this study, we (i) analyze the ecological correlates of A. orientalis presence at different spatial scales, i.e., in the whole European range and, more specifically, at its western border in Sicily, (ii) develop a potential distribution map for Sicily, (iii) define landscape and land-use characteristics where the species is present compared to random sites and (iv) outline the main conservation implications of our study.
2. Materials and Methods
2.1. Study Area and Protocol
This study is based on both field and literature data. Original field data were collected only throughout Sicily during the period 2012–2021. Sicily has a typical Mediterranean climate, with a long hot and dry summer and with precipitation concentrated in autumn and spring. Specimens were collected by hand or by light traps, and/or photographed during the twilight flight (between about 20:45 and 21:30) or immediately after whilst clinging to vegetation, alone or in copulation. Light traps consisted of a white sheet resting on the ground and illuminated with a black light lamp (Wood’s light) and a cold white light (color temperature 6500 degrees Kelvin)—both 20w and powered by lithium batteries from 20,000 mha. This system was combined with another mobile trap system consisting of a white umbrella illuminated by a 390–405 nm UV LED. The presence of the species at given sites was also ascertained by elytra or fragments of specimens opportunistically encountered in the field. Some captured specimens were dry prepared and stored.
In case of bibliographic data, only those studies reporting precise geographic coordinates were included (see Figure 2). These bibliographic records included localities appearing in a search on the main naturalistic forums (www.entomologiitaliani.net; www.naturamediterraneo.com; both lastly accessed on 14 November 2021), online databases (www.inaturalist.org; last accessed on 14 November 2021) and groups present on Facebook (“Faunasiciliana”, “Entomology”). In all cases, reports were critically screened by selecting only those from which it was possible to precisely determine the point of collection and the species’ identity through unambiguous photos. However, it cannot be excluded that there would be some cryptic taxa, currently identified as A. orientalis, within the wide species’ range.
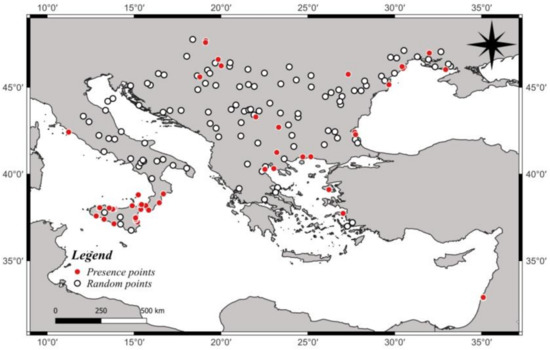
Figure 2.
Distribution of the presence and random points of Anoxia orientalis in Europe.
2.2. Statistical Analyses
We downloaded bioclimatic rasters for the whole European scale (Maximum temperature, minimum temperature, average temperature, precipitation, solar irradiation and wind) from the world climate database available at: https://www.worldclim.org/data/bioclim.html (last accessed on 3 May 2022). For each occurrence point we created a buffer area of 8,5 Km radius. To select the random points at the European scale, we produced a convex polygon with the creation of minimum bounding geometry around the selected points. The extension of the convex (11.1666666666650034, 32.8333333333280066:35.1666666651290072, 47.6666666657120075) polygon was used to clip all the rasters. Climatic data of Sicily (12.224950443, 15.713481331, 36.605915330, 38.876360611 [EPSG:4326]) was downloaded from CFSR Global Weather Data for SWAT 1979–2014 (https://swat.tamu.edu/data/; last accessed on 3 March 2022) [19,20]. Climatic data were processed with Excel 2016 and with QGIS 3.22; for the spatial data, the cubic interpolation with Delaunay triangulation was used to obtain the climatic raster in the time span 1979–2014 of the maximum temperature, the minimum temperature, the average temperature for May, June and July, winds, relative humidity, precipitation and solar irradiation. For Sicily, land use was obtained from Copernicus Global Land Operations (https://land.copernicus.eu/global/products/lc; last accessed on 3 May 2022) [21]. The discrete classification map provides 23 classes and is defined using the land cover classification system (LCCS) developed by the United Nations (UN) Food and Agriculture Organization (FAO). The Land Cover V2.0 products were delivered in a regular latitude/longitude grid (EPSG:4326) with the ellipsoid WGS 1984 (terrestrial radius = 6378 km). The resolution of the grid was 1°/1008 or approximately 100 m at the equator. The coordinate reference system of the project was reprojected with SCRWGS 84 EPSG 4326. Fourteen land-use variables that were not autocorrelated were used for this paper (Table 1). To provide a potential distribution map for Anoxia orientalis in both Sicily and Europe, we used the MAXENT version 3.4.4, with the entropy being defined as (Phillips et al. 2006):
where x are the pixels of the study area, π = the probability distribution, and is the entropy. The raster format used in the MAXENT of the land use had the same cell size, extent and projection system as that used for the GLM model. The C-Log-Log function was performed with parameters of presence points and the land use raster. Then, the MAXENT model was set with 10,000 maximum random points, 5000 maximum iterations, the random test being 25%, and the resampling technique used being the cross validation. For both the European and the Sicilian scales, we used both climatic and land use data for the presence-only sites to build the MAXENT models. For the European scale, raw data outputs and control parameters were: (i) regularized training gain = 2.236, training AUC = 0.969, unregularized training gain = 2.561; (ii) unregularized test gain = 2.214; (iii) test AUC = 0.962, standard deviation = 0.009. The model algorithm converged after 560 iterations, with 69 presence records used for training and 23 for testing. Overall, 10,069 points were used to determine the MAXENT distribution (background points and presence points). As for the regularization values: linear/quadratic/product = 0.139, categorical = 0.250, threshold = 1.310, hinge = 0.500 and the random test points = 25. For the Sicilian scale, raw data outputs and control parameters were: (i) regularized training gain = 1.862, training AUC = 0.853, unregularized training gain = 1.449; (ii) unregularized test gain = 1.763; (iii) test AUC = 0.976, standard deviation = 0.054 (calculated as in DeLong et al., 1988, Equation (2). The model algorithm converged after 380 iterations, with 18 presence records used for training and 6 for testing. Overall, 10,018 points were used to determine the MAXENT distribution (background points and presence points). As for the regularization values: linear/quadratic/product = 0.481, categorical = 0.250, threshold = 1.820, hinge = 0.500, and the random test points = 25.

Table 1.
List of the CORINE land cover variables used for both the Anoxia orientalis presence points and the random points used for the analyses in the present paper.
For the large scale (European) analysis, we downloaded the species’ occurrence points from the GBIF Database (https://doi.org/10.15468/dl.tv9sm6; last accessed on 14 November 2021) and selected only the presence point labelled as “This observation is Research Grade! It can now be used for research and featured on other websites”. In this way, it was possible to select 45 presence points which were added to the 48 original presence points used for the Sicilian distribution modeling.
The land use of Europe was created with the alignment of a set of rasters downloaded from Copernicus Global Land Operations (https://land.copernicus.eu/global/products/lc; last accessed on 14 November 2021) [21] with the same parameters mentioned above for Sicily land use. A total of 114 random points were created within a MultiPolygon (Extent 10.6877552918004284, 32.4143404291684689:35.5075368339517539, 48.1279291305885693). A buffer zone with a radius of 300 m was created for both the presence points and the random points.
For the raster layer analysis, the algorithm “Zonal histogram” was used. This algorithm appends fields representing counts of each unique land-use variable value from a raster layer containing the polygons. The resulting output table has as many fields as the unique value of the raster intersecting the polygons. We then calculated the relative percentage of each land-use variable within each polygon, with a same procedure being applied for both the presence and the random points.
The effect of the various land use/climatic variables on the presence/absence of the study species was explored by logistic regression using a forward stepwise addition. Thus, whereas for the MAXENT models we used only presence data, for the logistic regression analyses we used the presence versus random data as the dependent variable, as this type of analysis can be performed only with a binary dependent variable [22]. Three logistic regression models were run: (i) all data included, (ii) Europe without Sicily, and (iii) only Sicily. In this modelling procedure, the variables were presented in the final equation by order of entrance in the models. For this analysis, 11 CORINE land cover (CLC) variables that were not autocorrelated (p > 0.05) were considered (Table 1). Individual variables were tested for explanatory power with G-statistics in univariate logistic regression analysis, and presence data were contrasted with the random data that were chosen from the same geographic area constituting the species’ range [22]. We equilibrated the impact of presences and random (=absences) data through a weighting procedure [23], and we used a correct classification score (CCS) to show the percentage of correctly described presences and absences at π(x) = 0.5. Models were derived with the Bonferroni correction [24,25]. Logistic regressions were made using PASW 18.0 statistical software. All tests were two-tailed with alpha set at 5%.
3. Results
3.1. Ecological Modelling at Two Spatial Scales
A total of 93 presence sites were uncovered during the present study, including 47 from our field surveys throughout Sicily and 46 from the literature (Appendix A), whereas a total of 114 random points were used for building the distribution models. Some of these presence sites were relative to very nearby sites and are therefore presented only once in Appendix A. The distribution of the presence and random points at the two spatial scales is given in Figure 2.
A logistic regression model applied to the whole available dataset (Wald = 0.237, df = 13, p = 0.569) uncovered six significant variables that were positively correlated with the presence of the species, with three variables being by far the most important (i.e., HISTO_50, HISTO_40 and HISTO_114; Table 2). After the Bonferroni correction, the significance of HISTO_20 and HISTO_110 disappeared from the model. Thus, the final logit equation for A. orientalis at the European scale was as follows:
G(x) = −0.068 + 48.735 × HISTO_50 + 30.951 × HISTO_40 + 29.139 × HISTO_114 + 23.615 × HISTO_126 + 16.985 × HISTO_80 + 12.828 × HISTO_114.

Table 2.
Results of a logistic regression analysis on the relationship between Anoxia orientalis occurrence and habitat characteristics (explained as land use CORINE categories) at different spatial scales (overall, in its European range and in Sicily). Significance is in boldface. Note that despite their p-value being <0.05, the significance of some variables disappeared after the Bonferroni correction was applied. Codes for the variables are as in Table 1.
Considering only Europe (without Sicily), the logistic regression model (Wald = 22.168, p < 0.0001) uncovered four variables significantly and positively affecting the species’ presence, with only one (HISTO_50) being by far the most important (Table 2). The resulting equation was:
G(x)i= −0.809 + 49.044 × HISTO_50 + 13.39 × HISTO_114 + 12.485 × HISTO_126 + 7.551 × HISTO_40.
At the Sicilian scale, the logistic regression (Wald = 25.273, df = 13, p < 0.00001) revealed that after the Bonferroni correction, nine variables were correlated with the presence of the species (Table 2) according to the following logit:
G(x)ii= −0.208 + 45.693 × HISTO_80 + 22.166 × HISTO_50 + 18 − 176 × HISTO_121 + 11.421 × HISTO_200 + 10.266 × HISTO_90 + 8.406 × HISTO_20 + 8.406 × HISTO_124 + 8.215 × HISTO_115 + 11.421 × HISTO_40+.
Comparing G(x)i with G(x)ii, there were remarkable differences (Table 2) with seven variables that were significant for Sicily but not for Europe (HISTO_20, HISTO_80, HISTO_90, HISTO_115, HISTO_121, HISTO_124, HISTO_200) and two variables that were significant for Europe but not for Sicily (HISTO_114 and HISTO_126). Instead, two variables were significant at either spatial scale (HISTO_40, HISTO_50), and the remaining three variables were not significant at any spatial scale (Table 2).
3.2. Graphical Model Map for Anoxia Orientalis in Europe
The distribution of A. orientalis presence points in relation to the investigated variables resulted in a valid MAXENT model according to the ROC pattern (Figure 3). The most important variables affecting the presence were bioclimatic (Table 3), with a graphical spatial model showing that the most suitable areas of presence were along the coast (Figure 4).
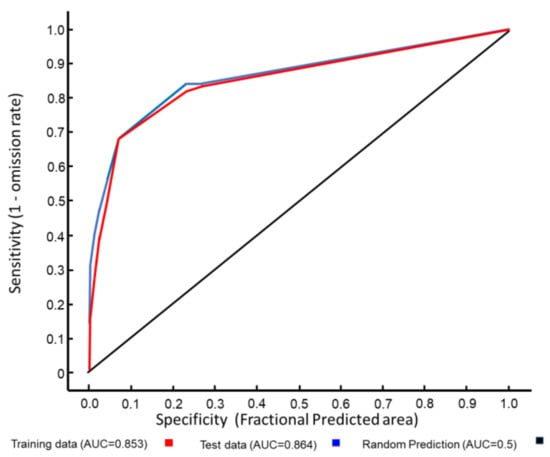
Figure 3.
ROC curve for the MAXENT modelling of the potential distribution of Anoxia orientalis in Europe.

Table 3.
Relative importance of the various factors associated with the presence points in a MAXENT modelling on the potential distribution of Anoxia orientalis in Europe.
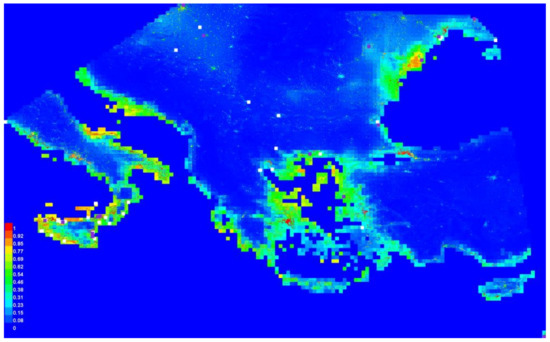
Figure 4.
Graphical model map of Anoxia orientalis in Europe. Different colors would indicate the probability of occurrence of the species on the basis of the given ensemble of predictors.
3.3. Graphical Model Map for Anoxia Orientalis in Sicily
The distribution of A. orientalis presence points in relation to land use and bioclimate in Sicily is given in Appendix B, whereas the climate ensemble is given in Appendix C. The MAXENT model appeared statistically valid according to the ROC pattern (Figure 5), with the land-use characteristics and the relative humidity being the two most important factors associated with the presence points of A. orientalis in Sicily (Table 3). The resulting graphical model map showed that this species had very narrow areas of optimal suitability in Sicily, almost entirely situated along the coast (Figure 6), with the landuse variables being far more important than the bioclimatic variables (Table 4).
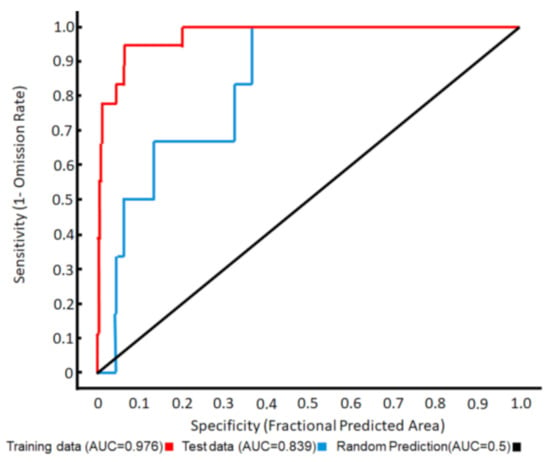
Figure 5.
ROC curve for the MAXENT modelling of the potential distribution of Anoxia orientalis in Sicily.
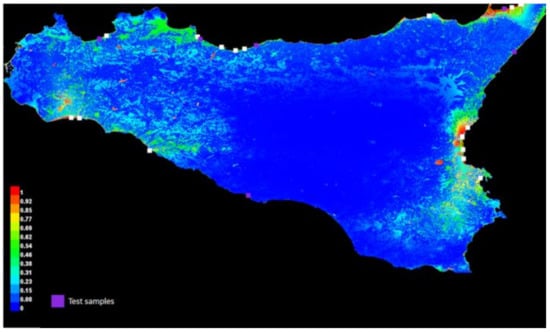
Figure 6.
Graphical model map of Anoxia orientalis in Sicily. Different colors would indicate the probability of occurrence of the species on the basis of the given ensemble of predictors.

Table 4.
Relative importance of the various factors associated with the presence points in a MAXENT modelling on the potential distribution of Anoxia orientalis in Sicily.
4. Discussion
This study showed two main patterns: (i) an obvious non-random habitat (=land use category) selection by A. orientalis at both the European and the Sicilian scales, and (ii) a clear difference in the factors affecting the presence of this species in Sicily compared to the larger spatial scale. Interestingly, the factors associated with the presence of A. orientalis were partially different at the two spatial scales, thus suggesting a region-specific ecological diversification of this widely distributed species in this Mediterranean island. More specifically, the Sicilian populations were statistically influenced by the presence of a combination of seven variables that make their potential optimal distribution very narrow and mostly limited to a few coastal areas. At the European scale, the bioclimatic factors were more important than landscape factors, whereas the same was not true at the Sicilian scale. At present, there are no data to evaluate why the Sicilian populations of A. orientalis are different from those of the rest of Europe in terms of habitat (=land use category) characteristics. However, it should be mentioned that apparently similar habitat use patterns are also observed in other sympatric populations of melolonthinid beetles in Sicily, including the endemic Polyphylla ragusae [26], Anoxia (Mesanoxia) matutinalis and Anoxia (Anoxia) scutellaris (Muscarella et al., unpublished data).
Using statistical analyses and a MAXENT graphical model map, we also showed that A. orientalis could be a habitat generalist in Europe, but in Sicily, the populations were statistically influenced by a combination of predictors that make their potential optimal distribution very narrow and mostly limited to a few coastal areas. Indeed, Anoxia orientalis inhabits sandy coasts, both dunes and back dunes in almost all Sicily. Today, we can find this species only in dunes because the back dune environment has been modified faster and more extensively, thus the only remaining suitable habitat for this species is the terminal sandy strip near the sea. A similar distribution and habitat patterns were observed in the endemic Polyphylla ragusae [26]. We should, however, take into consideration that our MAXENT model at the Sicilian scale suggests that it may poorly predict the distribution of A. orientalis since most of the recorded localities (presence data) lie in the area of very low probability of the occurrence of the species. We suppose that the scarce predictive spatial power may depend on a combination of a relatively low number of presence cases and the relatively inconsistent characteristics of some sites in terms of characterizing variables.
We hypothesize that the habitat selection patterns by the Sicilian A. orientalis is due to the fact that this species is here at the edge of its distribution range and in a condition of peninsularity (southern Italy) and/or insularity (Sicily). However, the available information on the habitat requirements of A. orientalis in Italy and in Europe are just anecdotal and purely descriptive, so they are difficult to be compared with our quantitative data. However, the available literature suggests that in Italy, the A. orientalis habitat of choice is intact dune environments located mostly near river mouths that have a rich riparian vegetation where it lives in coexistence with Polyphylla ragusae, Anoxia matutinalis, Anoxia scutellaris and other species linked to the dunes, whereas it can rarely go towards the inland (Valle del Crati, Trasi [13]). However, some differences in the distribution and type of habitat preferred in Sicily by these species should be underlined. Indeed, Anoxia scutellaris sicula and A. scutellaris argentea are exclusively dune-dwellers and limited to the northwestern beaches [27] both in the northernmost (A. scutellarsi sicula) and southernmost territories (A. scutellaris argentea). Polyphilla ragusae, instead, is widely diffused in all Sicilian coasts, except a northeastern portion, with the nominal subspecies (from Palermo to Gela) and with the ssp. aliquoi (from Gela to Syracuse). Moreover, it also prefers the back dune, moving away from the coast, where possible, even for several kilometers [26]. Anoxia matutinalis matutinalis is more sporadic on the coasts of all of Sicily but is present in various inland locations [16] even at higher altitudes on Etna [28]. It also has a marked photophilic behavior, thus being easily attracted to light traps (our unpublished observations). Considered in Central Europe as a harmful species for orchards and vineyards [29], A. orientalis is, however, in strong decline in Italy due to the degradation of coastal environments [30]. Thus, the degradation of the dune and back dune environments should be considered as the first cause of rarefaction of this species in Sicily, where almost all bibliographic and our recent observations refer to locally abundant populations. The destruction of the localities where A. orientalis lives may cause the disappearance of this species which, however, tends to recolonize these environments if conditions for even partial renaturalization are created. This is the case of the populations of the Buonfornello plain, between the mouths of the Imera River and the River Torto [31], where Anoxia orientalis disappeared after 1973–1975, then returned around 1990 and is now in a phase of further decline due to new environmental alterations.
At the moment, the most abundant A. orientalis populations in Sicily are found at the mouth of the Simeto River. Already known since the first half of the 1800s [31], they have not shown any significant decrease in the last 50 years of observations in this locality that is today even protected by law (“Oasi del Simento Nature Reserve”).
Author Contributions
Conceptualization, L.L., D.D., methodology, C.M., I.S., D.D.; formal analysis, D.D., L.L.; investigation, C.M., I.S., D.D.; data curation, D.D.; writing—original draft preparation, L.L., D.D., M.D.V.; writing—review and editing, all authors; supervision, L.L., D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The research protocol was approved by IDECC ethical committee on 27 June 2016 (approval code: 2016-3-VI/col/11). No individuals were killed or damaged during the field studies.
Data Availability Statement
Data will be available to interested readers from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Summary of the Presence Records for Anoxia Orientalis on the Basis of Our Original Field Surveys and Literature Data. LAT = latitude; LON = longitude.
Table A1.
Summary of the Presence Records for Anoxia Orientalis on the Basis of Our Original Field Surveys and Literature Data. LAT = latitude; LON = longitude.
Appendix B
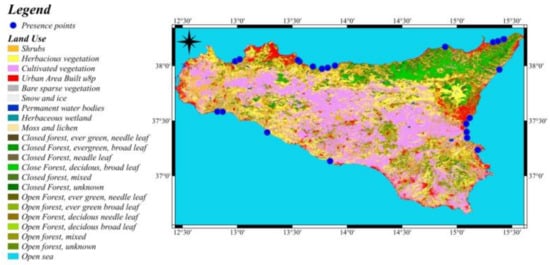
Figure A1.
Distribution of Anoxia Orientalis Presence Points in Relation to Land Use in Sicily. The various CORINE landcover categories are given in the legend.
Appendix C
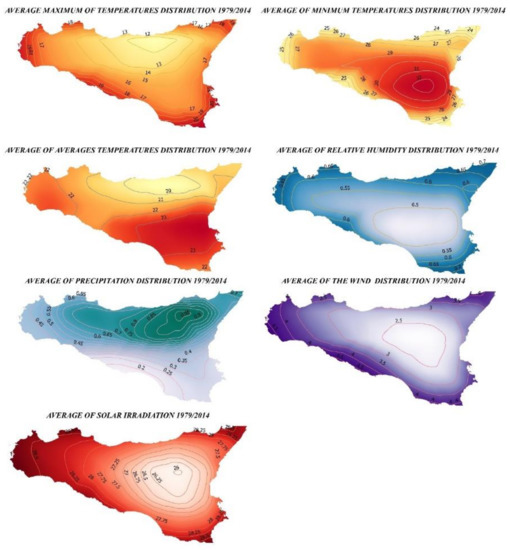
Figure A2.
Spatial Distribution of the Climatic Variables of Sicily.
References
- Hengeveld, R. Dynamic Biogeography; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Franklin, J. Moving beyond static species distribution models in support of conservation biogeography. Div. Distrib. 2010, 16, 321–330. [Google Scholar] [CrossRef]
- Vigna Taglianti, A.; Audisio, P.; Belfiore, C.M.; Biondi, M.; Bologna, M.A.; Carpaneto, G.M.; De Biase, A.; De Felici, S.; Piattella, E.; Racheli, T.; et al. Riflessioni di gruppo sui corotipi fondamentali della fauna W-paleartica ed in particolare italiana. Biogeographia 1992, 16, 159–179. [Google Scholar] [CrossRef] [Green Version]
- Carpaneto, G.M.; Piattella, E.; Pittino, R. The scarab beetles of Turkey: An updated checklist and chorotype analysis (Coleoptera, Scarabaeoidea). Biogeographia 2000, 21, 218–240. [Google Scholar] [CrossRef] [Green Version]
- Çobanoğlu, S.; Çakmak, I.; Başpinar, H. Hypoaspis krameri (Canestrini, 1881) (Mesostigmata: Laelapidae) an ectoparasitic mite associated with Anoxia orientalis Kryn. (Col., Scarabaeidae) from Turkey. Entomol. Mon. Mag. 2003, 139, 97–101. [Google Scholar]
- Baraud, J. Révision des Anoxia Castelnau d’Europe et d’Asie 1re note: Le sous-genre Protanoxia Medvedev (Coleoptera, Melolonthidae). Bull. Soc. Entomol. Fr. 1989, 93, 273–284. [Google Scholar] [CrossRef]
- Bezdek, A. Scarabaeidae: Melolonthinae: Melolonthini. In Catalogue of Palaearctic Coleoptera, Vol. 3. Scarabaeoidea–Scirtoidea–Dascilloidea–Buprestoidea–Byrrhoidea; Löbl, I., Löbl, D., Eds.; Brill: Leiden, The Netherlands, 2016; pp. 226–236. [Google Scholar]
- Rittner, O. Synopsis of the Melolonthini (Scarabaeidae: Melolonthinae) of Israel, with a first description of the female of Anoxia (Protanoxia) laevimacula Petrovitz, 1973. Israel J. Entomol. 2016, 46, 99–108. [Google Scholar]
- Perazzini, G. Osservazioni morfologiche e geonemiche su alcuni Scarabaeidae floricoli italiani (Coleoptera, Scarabaeidae). Acta Coleopt. 1987, 3, 33–40. [Google Scholar]
- Luigioni, P. I Coleotteri d’Italia. Catalogo sinonimico-topografico-bibliografico. Mem. Pontif. Accad. Sci. Nuovi Lincei (Ser. II) 1912, 13, 1–1159. [Google Scholar]
- Mariani, G. Ricerche coleotterologiche sul litorale ionico di Puglia, Lucania e Calabria. Campagne 1956-1957-1958. II. Coleoptera Lamellicornia. Mem. Soc. Entomol. Ital. Suppl. 1959, 38, 143–184. [Google Scholar]
- Mikšic, R. Beitrag zur kenntnis der Lamellicornia-Fauna der Apenninen. IV. Aspromonte. Mem. Mus. Civ. St. Nat. Verona 1961, 9, 5–25. [Google Scholar]
- Tassi, F. Reperti 5. Anoxia orientalis Kryn. (Col. Scarabaeidae). Boll. Assoc. Romana Entomol. 1967, 22, 46. [Google Scholar]
- Arnone, M. Quinto contributo alla revisione della collezione coleotterologica di Enrico Ragusa: Scarabaeoidea. Nat. Sicil. 2010, 34, 61–172. [Google Scholar]
- Sabella, G.; Sparacio, I. Il ruolo dei Parchi siciliani nella conservazione di taxa di Insetti di particolare interesse naturalistico (Insecta Coleoptera et Lepidoptera Rhopalocera). Nat. Sicil. 2004, 28, 447–508. [Google Scholar]
- Lapiana, F.; Sparacio, I. Coleotteri Lamellicorni delle Madonie (Sicilia) (Insecta Coleoptera Lucanoidea et Scarabaeoidea). Naturalista Sicil. (Ser. IV) 2006, 30, 227–292. [Google Scholar]
- Schatzmayr, A. Appunti coleotterologici. 12. Natura 1944, 35, 54–59. [Google Scholar]
- Homonnay, F. Data on the biology and development of soil-inhabiting pests in Hungary. Növényvédelem 1989, 25, 492–499. [Google Scholar]
- Dile, Y.T.; Srinivasan, R. Evaluation of CFSR climate data for hydrologic prediction in data-scarce watersheds: An application in the Blue Nile River Basin. J. Am. Water Resour. Assoc. 2014, 1, 1226–1241. [Google Scholar] [CrossRef]
- Fuka, D.R.; MacAllister, C.A.; Degaetano, A.T.; Easton, Z.M. Using the Climate Forecast System Reanalysis dataset to improve weather input data for watershed models. Hydrol. Proc. 2013, 30, 5613–5623. [Google Scholar] [CrossRef]
- Buchhorn, M.; Smets, B.; Bertels, L.; Lesiv, M.; Tsendbazar, N.E.; Herold, M.; Fritz, S. Copernicus Global Land Service: Land Cover 100m: Epoch 2015: Globe; (Version V2.0.2); Zenodo: Geneva, Switzerland, 2019. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Teixeira, J.; Ferrand, N.; Arntzen, J.W. Biogeography of the golden-striped salamander Chioglossa lusitanica: A field survey and spatial modelling approach. Ecography 2001, 24, 618–624. [Google Scholar] [CrossRef]
- Arntzen, J.W.; Alexandrino, J. Ecological modelling of genetically differentiated forms of the Iberian endemic golden-striped salamander, Chioglossa lusitanica. Herpetol. J. 2004, 14, 137–141. [Google Scholar]
- Holm, S. A simple sequential rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Muscarella, C.; Luiselli, L.; Di Vittorio, M.; Sparacio, I.; Amori, G.; Dendi, D. Factors associated with occurrence, potential distribution and conservation of Polyphylla ragusae, an endemic Scarabaeidae (Melolonthinae) from Sicily. J. Insect. Conserv. 2022, in press. [Google Scholar]
- Aliquò, V.; Massa, B. Contributo allo studio di Anoxia scutellaris s. l. e descrizione di Anoxia scutellaris argentea n. ssp. di Sicilia. Boll. Soc. Ent. Ital. Genova 1976, 108, 151–157. [Google Scholar]
- Sabatinelli, G. Note su alcuni Scarabeoidea floricoli dell’ Italia meridionale e descrizione di Amphimallon pseudomajale n.sp. (Coleoptera). Boll. Ass. Romana Entomol. 1976, 31, 35–46. [Google Scholar]
- Tóth, J. Damage caused by cockchafers (Melolontha spp.) in Hungary during the last 30 years. In Proceedings of the Population Dynamics, Impacts, and Integrated Management of Forest Defoliating Insects, Banska Stiavnica, Slovakia, 18–23 August 1996; McManus, M.L., Liebhold, A.M., Eds.; Department of Agriculture, Forest Service, Northeastern Research Station: Radnor, PA, USA, 1998; p. 341. [Google Scholar]
- Lapiana, F.; Sparacio, I. Lo studio degli Insetti nella valutazione della naturalità degli ambienti dunali costieri in Sicilia: Coleoptera e Orthoptera. Nat. Sicil. 2008, 32, 411–434. [Google Scholar]
- Aliquo, V.; Massa, B.; Mignani, R. Brevi note sulla fauna coleotterologica di un particolare biotopo costiero del Palermitano (Coleoptera). Boll. Soc. Entomol. Ital. 1973, 105, 59–68. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).