Are the Interactions between Oaks and Pre-Dispersal Seed Predators Retained in Urban Environments? An Analysis of Two Quercus Species in Southern Mexico City
Abstract
:1. Introduction
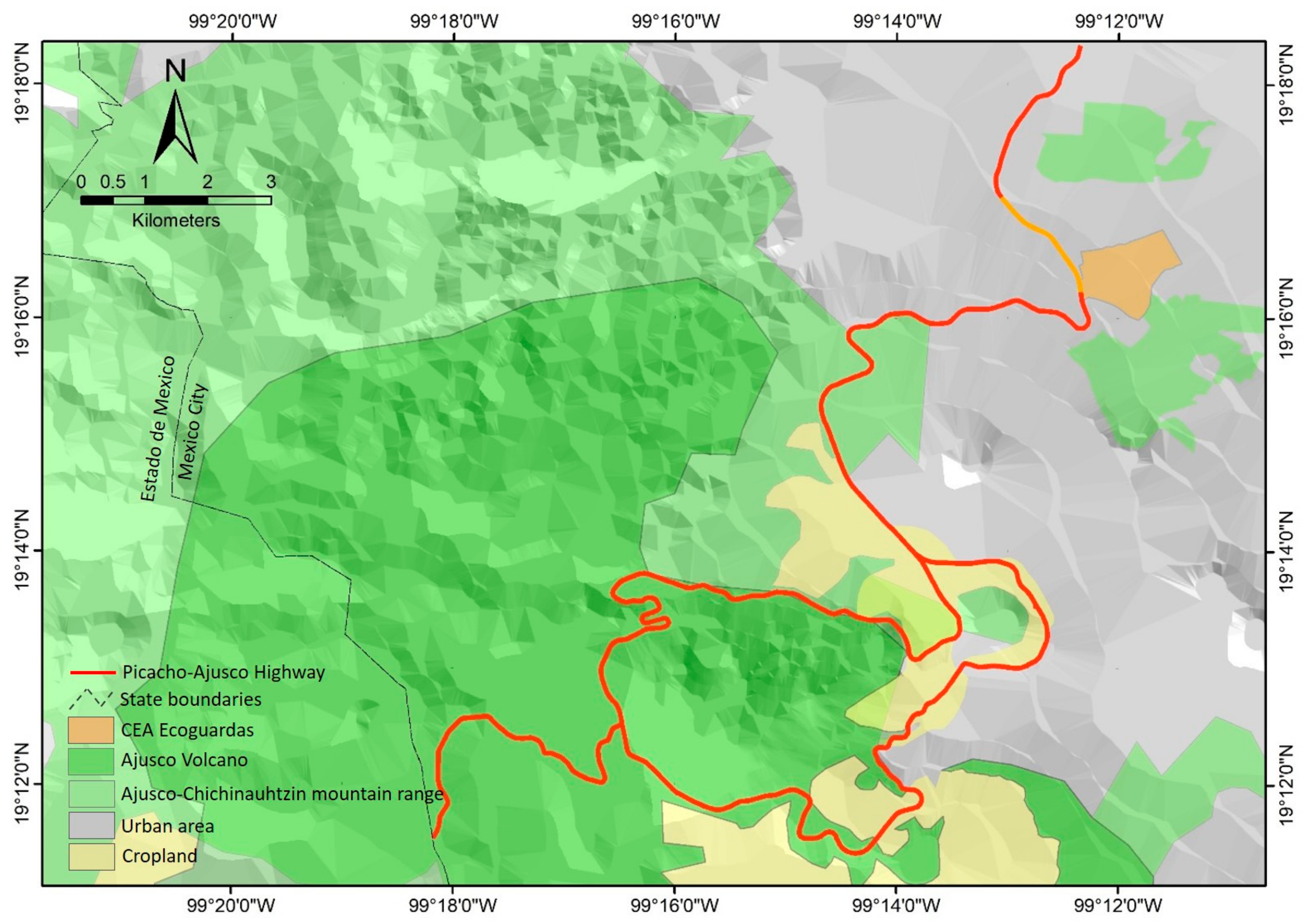
2. Materials and Methods
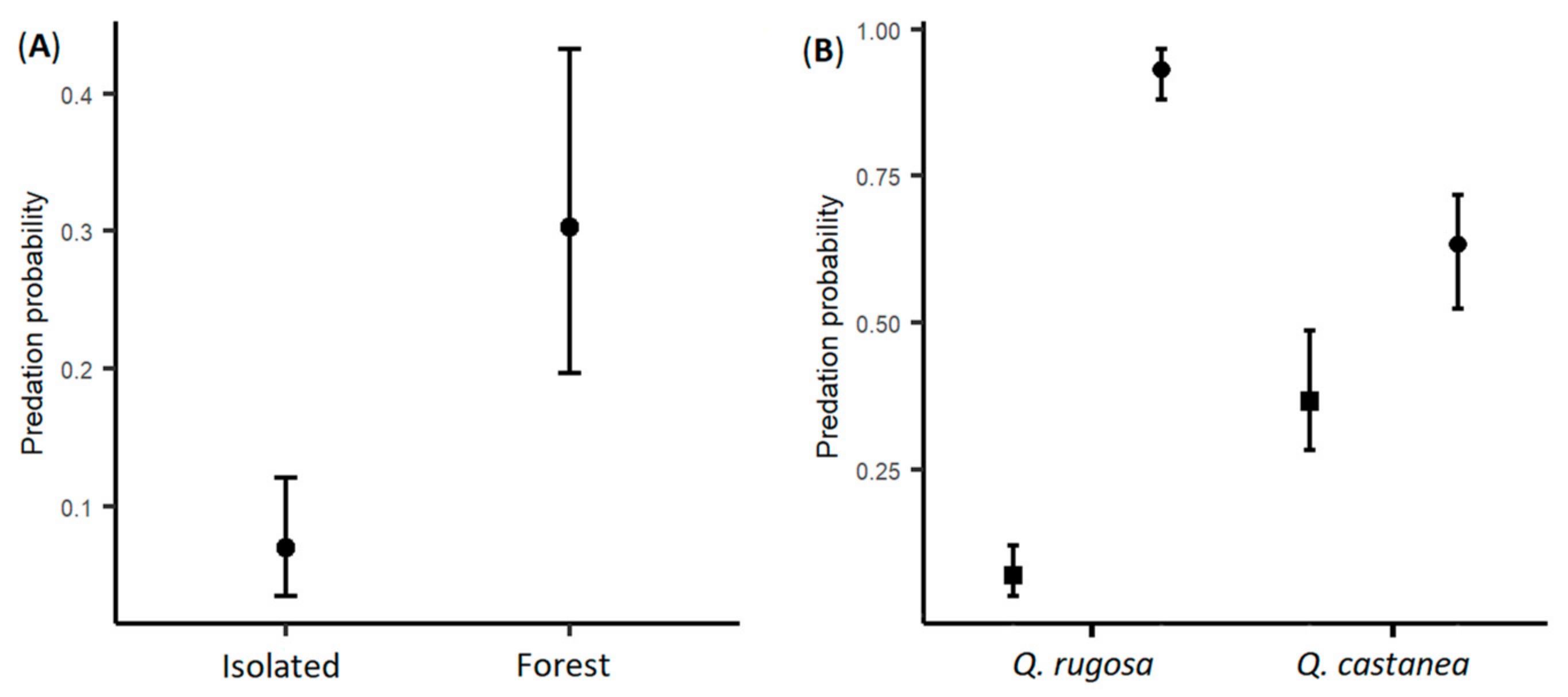
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manning, A.D.; Fischer, J.; Lindenmayer, D.B. Scattered trees are keystone structures–implications for conservation. Biol. Conserv. 2006, 132, 311–321. [Google Scholar] [CrossRef]
- Dunn, R.R. Isolated trees as foci of diversity in active and fallow fields. Biol. Conserv. 2000, 95, 317–321. [Google Scholar] [CrossRef]
- Laborde, J.; Guevara, S.; Sánchez-Ríos, G. Tree and shrub seed dispersal in pastures: The importance of rainforest trees outside forest fragments. Ecoscience 2008, 15, 6–16. [Google Scholar] [CrossRef]
- Cadavid-Florez, L.; Laborde, J.; Mclean, D.J. Isolated trees and small woody patches greatly contribute to connectivity in highly fragmented tropical landscapes. Landsc. Urban Plan. 2020, 196, 103745. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E. Carbon storage and sequestration by urban trees in the USA. Environ. Pollut. 2002, 116, 381–389. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air pollution removal by urban trees and shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Grote, R.; Samson, R.; Alonso, R.; Amorim, J.H.; Cariñanos, P.; Churkina, G.C.; Calfapietra, C. Functional traits of urban trees: Air pollution mitigation potential. Front. Ecol. Environ. 2016, 14, 543–550. [Google Scholar] [CrossRef]
- Georgi, N.J.; Zafiriadis, K. The impact of park trees on microclimate in urban areas. Urban Ecosyst. 2006, 9, 195–209. [Google Scholar] [CrossRef]
- Linden, J.; Fonti, P.; Esper, J. Temporal variations in microclimate cooling induced by urban trees in Mainz, Germany. Urban For. Urban Green. 2016, 20, 198–209. [Google Scholar] [CrossRef]
- Galindo-González, J.; Guevara, S.; Sosa, V.J. Bat-and bird-generated seed rains at isolated trees in pastures in a tropical rainforest. Conserv. Biol. 2000, 14, 1693–1703. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B. The conservation value of paddock trees for birds in a variegated landscape in southern New South Wales. 1. Species composition and site occupancy patterns. Biodivers. Conserv. 2002, 11, 807–832. [Google Scholar] [CrossRef]
- Lumsden, L.F.; Bennett, A.F. Scattered trees in rural landscapes: Foraging habitat for insectivorous bats in south-eastern Australia. Biol. Conserv. 2005, 122, 205–222. [Google Scholar] [CrossRef]
- Helden, A.J.; Stamp, G.C.; Leather, S.R. Urban biodiversity: Comparison of insect assemblages on native and non-native trees. Urban Ecosyst. 2012, 15, 611–624. [Google Scholar] [CrossRef]
- McIntyre, N.E. Ecology of urban arthropods: A review and a call to action. Ann. Entomol. Soc. Am. 2000, 93, 825–835. [Google Scholar] [CrossRef]
- Kennedy, C.E.J.; Southwood, T.R.E. The number of species of insects associated with British trees: A re-analysis. J. Anim. Ecol. 1984, 53, 455–478. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Wint, G.W.; Kennedy, C.E.; Greenwood, S.R. Seasonality abundance, species richness and specificity of the phytophagous guild of insects on oak (Quercus) canopies. Eur. J. Entomol. 2004, 101, 43–50. [Google Scholar] [CrossRef]
- Tovar-Sánchez, E.; Cano-Santana, Z.; Oyama, K. Canopy arthropod communities on Mexican oaks at sites with different disturbance regimes. Biol. Conserv. 2004, 115, 79–87. [Google Scholar] [CrossRef]
- Valencia-Cuevas, L.; Tovar-Sánchez, E. Oak canopy arthropod communities: Which factors shape its structure? Rev. Chil. de Hist. Nat. 2015, 88, 15. [Google Scholar] [CrossRef]
- Bonal, R.; Muñoz, A. Seed weevils living on the edge: Pressures and conflicts over body size in the endoparasitic Curculio larvae. Ecol. Entomol. 2009, 34, 304–309. [Google Scholar] [CrossRef]
- Yi, X.F.; Yang, Y.Q. Large acorns benefit seedling recruitment by satiating weevil larvae in Quercus aliena. Plant Ecol. 2010, 209, 291–300. [Google Scholar] [CrossRef]
- Bonal, R.; Espelta, J.M.; Vogler, A. Complex selection on life-history traits and the maintenance of variation in exaggerated rostrum length in acorn weevils. Oecologia 2011, 167, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Bonal, R.; Hernández, M.O.; Muñoz, A.; Espelta, J.M. Positive cascade effects of forest fragmentation on acorn weevils mediated by seed size enlargement. Insect Conserv. Divers. 2012, 5, 381–388. [Google Scholar] [CrossRef]
- Bonal, R.; Espelta, J.M.; Munoz, A.; Ortego, J.; Aparicio, J.M.; Gaddis, K.; Sork, V.L. Diversity in insect seed parasite guilds at large geographical scale: The roles of host specificity and spatial distance. J. Biogeogr. 2016, 43, 1620–1630. [Google Scholar] [CrossRef] [Green Version]
- Mezquida, E.T.; Caputo, P.; Acebes, P. Acorn crop, seed size and chemical defenses determine the performance of specialized insect predators and reproductive output in a Mediterranean oak. Insects 2021, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Peguero, G.; Bonal, R.; Sol, D.; Muñoz, A.; Sork, V.L.; Espelta, J.M. Tropical insect diversity: Evidence of greater host specialization in seed-feeding weevils. Ecology 2017, 98, 2180–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.Y.; Hu, X.H.; Liu, D.C.; Ouyang, A.; Tong, X.; Wang, Y.J.; Chen, X.Y. High diversity and strong variation in host specificity of seed parasitic acorn weevils. Insect Conserv. Divers. 2021, 14, 367–376. [Google Scholar] [CrossRef]
- Carrillo-Trueba, C. El Pedregal de San Ángel, 1st ed.; Universidad Nacional Autónoma de México: Mexico City, Mexico, 1995; p. 177. [Google Scholar]
- Palma, M.; Cram, S.; Bocco, G.; Velázquez, A. Caracterización abiótica de la región de la montaña del sur de la Cuenca de México. In Biodiversidad de la cuenca de México; Universidad Autónoma Metropolitana: Mexico City, Mexico, 1999. [Google Scholar]
- Bonfil, C.; Soberón, J. Quercus rugosa seedling dynamics in relation to its re-introduction in a disturbed Mexican landscape. Appl. Veg. Sci. 1999, 2, 189–200. [Google Scholar] [CrossRef]
- González-Hidalgo, B.; Orozco-Segovia, A.; Diego-Pérez, N. La vegetación de la reserva ecológica Lomas del Seminario, Ajusco, México. Bol. Soc. Bot. Mex. 2001, 69, 77–99. [Google Scholar] [CrossRef] [Green Version]
- Cano-Zantana, Z.; Pisanty, I.; Segura, S.; Mendoza-Hernández, P.E.; Martínez-Ballesté, A. Ecología, conservación, restauración y manejo de áreas naturales y protegidas del Pedregal del Xitle. In Manejo, Conservación y Restauración de Recursos Naturales en México; Oyama, K., Castillo, A., Eds.; Universidad Nacional Autónoma de México and Siglo XXI.: Mexico City, Mexico, 2006; pp. 203–226. [Google Scholar]
- Rohlfs, D.A. A Study of Acorn Feeding Insects: Filbert Weevil (Curculio occidentalis (Casey)) and Filbertworm (Cydia latiferreana (Walsingham)) on Garry Oak (Quercus garryana) (Dougl.) in the Southeastern Vancouver Island Area. Master’s Thesis, Faculty of Graduate Studies, University of British Columbia, Vancouver, BC, Canada, April 1999. [Google Scholar]
- Lewis, V.R. Within-Tree Distribution of Acorns Infested by Curculio occidentalis (Coleoptera: Curculionidae) and Cydia latiferreana (Lepidoptera: Tortricidae) on the Coast Live Oak. Enviroment. Entomol. 1992, 21, 975–982. [Google Scholar] [CrossRef]
- Jiménez, A.; Soria, F.; Villagrán, M. Seguimiento del ciclo biológico de Cydia fagiglandana (Zeller) (Lepidoptera: Tortricidae) en un encinar del sur de España. Boletín De Sanid. Veg. Plagas 2006, 32, 157–168. [Google Scholar]
- Ganhao, E.; Siva Dias, L. Seed volume dataset—An ongoing inventory of seed size expressed by volume. Data 2019, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Maechler, B.; Bolker, B.M.; Walker, S. Fitting linear mixed-effects models using Ime4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Trexler, J.C.; Travis, J. Nontraditional regression analyses. Ecology 1993, 74, 1629–1637. [Google Scholar] [CrossRef]
- Santos, T.; Tellería, J. Pérdida y fragmentación de hábitat: Efecto sobre la conservación de las especies. Ecosistemas 2006, 15, 3–12. [Google Scholar]
- Fernández-Juricic, E. Bird community composition patterns in urban parks of Madrid: The role of age, size and isolation. Ecol. Res. 2000, 15, 373–383. [Google Scholar] [CrossRef]
- Burghardt, K.T.; Tallamy, D.W.; Gregory Shriver, W. Impact of native plants on bird and butterfly biodiversity in suburban landscapes. Conserv. Biol. 2009, 23, 219–224. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Escobar-Ibáñez, J.F.; Schondube, J.E.; Zuria, I.; Ortega-Álvarez, R.; Sosa-López, J.R.; Vega-Rivera, J.H. The urban contrast: A nationwide assessment of avian diversity in Mexican cities. Sci. Total Environ. 2021, 753, 141915. [Google Scholar] [CrossRef]
- Helden, A.J.; Leather, S.R. Biodiversity on urban roundabouts—Hemiptera, management and the species–area relationship. Basic Appl. Ecol. 2004, 5, 367–377. [Google Scholar] [CrossRef]
- Soria, F.; Jiménez, A.; Villagrán, M.; Ocete, M. Influencia de la infestación de Cydia fagiglandana (Zeller) (Lepidoptera:Tortricidae) en la caída del fruto de la encina. Boletín de Sanid. Veg. Plagas 2002, 28, 213–216. [Google Scholar]
- Debouzie, E.; Desouhant, E.; Oberli, F.; Menu, F. Resouce limitation in natural populations of phytophagous insects. A long-term study case whit the chestnut weevil. Acta Oecol. 2002, 23, 31–39. [Google Scholar] [CrossRef]
- Debouzie, D.; Heizmann, A.; Desouhant, E.; Menu, F. Interference at several temporal and spatial scales between two chestnut insects. Oecologia 1996, 108, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Chacoff, N.; Morales, J.Y.; Vaquera, M. Efectos de la fragmentación sobre la aborción y depredación de semillas en el Chaco Serrano. Biotropica 2004, 36, 109–117. [Google Scholar] [CrossRef]
- Savilaakso, S.; Koivisto, J.; Veteli, T.; Roininen, H. Microclimate and tree community linked to differences in lepidopteran larval communities, between forest fragments and continuous forest. Divers. Distrib. 2009, 15, 356–365. [Google Scholar] [CrossRef]
- Morán-López, T.; Forner, A.; Flores-Rentería, D.; Díaz, M.; Valladares, F. Some positive effects of the fragmentation of holm oak forests: Attenuation of water stress and enhancement of acorn production. For. Ecol. Manag. 2016, 370, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, P.; Weyeneth, A.; Weber, D.; Dorn, S. Long flights in Cydia pomonella L. (Lepidoptera:Tortricidae) measured by a flight mill: Influence of sex, mated status and age. Physiol. Entomol. 1997, 22, 149–160. [Google Scholar] [CrossRef]
- Díaz, M. Distribución del arbolado y persistencia a largo plazo de las dehesas: Patrones y procesos. Rev. Ecosistemas 2014, 23, 5–12. [Google Scholar] [CrossRef]
- Pélisson, P.F.; Bernstein, C.; Debias, F.; Menu, F.; Venner, S. Dispersal and dormancy strategies among insect species competing for a pulsed resource. Ecol. Entomol. 2013, 38, 470–477. [Google Scholar] [CrossRef]
- Espelta, J.M.; Bonal, R.; Sánchez-Humanes, B. Pre-dispersal acorn predation in mixed oak forests: Interspecific differences are driven by the interplay among seed phenology, seed size and predator size. J. Ecol. 2009, 97, 1416–1423. [Google Scholar] [CrossRef]
- Díaz-Guzmán, H.; Bonfil, C. Depredación predispersión en tres especies de Quercus del pie de monte del Ajusco. Rev. Mex. de Biodivers. 2020, 91, e91.3242. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; García de la Cruz, Y.; Gómez-Aparicio, L. Contrasting responses of insects and vertebrates as seed consumers in two neotropical oak species: The interactive effects of individual crop size and seed mass. For. Ecol. Manag. 2017, 401, 99–106. [Google Scholar] [CrossRef]
- Weckerly, F.W.; Sugg, D.W.; Semlitsch, R.D. Germination success of acorns (Quercus): Insect predation and tannins. Can. J. For. Res. 1989, 19, 811–815. [Google Scholar] [CrossRef]
- Valencia, S. Diversidad del género Quercus (Fagaceae) en México. Boletín de La Soc. Botánica De México 2004, 75, 33–53. [Google Scholar] [CrossRef] [Green Version]

| Response Variable | GLMM Model | p |
|---|---|---|
| (undamaged, predated) = location × species (undamaged, predated) = location + species | 0.706 | |
| Total predation proportion | (undamaged, predated) = location + species (undamaged, predated) = species | <0.001 |
| (undamaged, predated) = location + species (undamaged, predated) = location | 0.113 |
| Response Variable | GLMM Model | p |
|---|---|---|
| (weevils, moths) = location × species (weevils, moths) = location + species | 0.402 | |
| Predation proportion | (weevils, moths) = location + species (weevils, moths) = species | 0.154 |
| (weevils, moths) = location + species (weevils, moths) = location | <0.001 |
| Response Variable | GLMM Model | p |
|---|---|---|
| Predation by weevils | (undamaged, predated) = volume × oak species (undamaged, predated) = volume + oak species | 0.016 |
| Response Variable | GLMM Model | p |
|---|---|---|
| (undamaged, predated) = volume × oak sp. (undamaged, predated) = volume + oak sp. | 0.277 | |
| Predation by moths | (undamaged, predated) = volume + oak sp. (undamaged, predated) = volume (undamaged, predated) = volume (undamaged, predated) = 1 | 0.124 0.622 |
| (undamaged, predated) = volume + oak sp. (undamaged, predated) = oak sp. (undamaged, predated) = oak sp. + tree (undamaged, predated) = 1 + tree | 0.783 0.112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Guzmán, H.; González, E.J.; Bonfil, C. Are the Interactions between Oaks and Pre-Dispersal Seed Predators Retained in Urban Environments? An Analysis of Two Quercus Species in Southern Mexico City. Diversity 2022, 14, 351. https://doi.org/10.3390/d14050351
Díaz-Guzmán H, González EJ, Bonfil C. Are the Interactions between Oaks and Pre-Dispersal Seed Predators Retained in Urban Environments? An Analysis of Two Quercus Species in Southern Mexico City. Diversity. 2022; 14(5):351. https://doi.org/10.3390/d14050351
Chicago/Turabian StyleDíaz-Guzmán, Hilda, Edgar J. González, and Consuelo Bonfil. 2022. "Are the Interactions between Oaks and Pre-Dispersal Seed Predators Retained in Urban Environments? An Analysis of Two Quercus Species in Southern Mexico City" Diversity 14, no. 5: 351. https://doi.org/10.3390/d14050351
APA StyleDíaz-Guzmán, H., González, E. J., & Bonfil, C. (2022). Are the Interactions between Oaks and Pre-Dispersal Seed Predators Retained in Urban Environments? An Analysis of Two Quercus Species in Southern Mexico City. Diversity, 14(5), 351. https://doi.org/10.3390/d14050351






