Community Structure of Eukaryotic Phytoplankton in Wetland of Golmud River and Its Lower Reaches and Relative Environmental Factors
Abstract
:1. Introduction
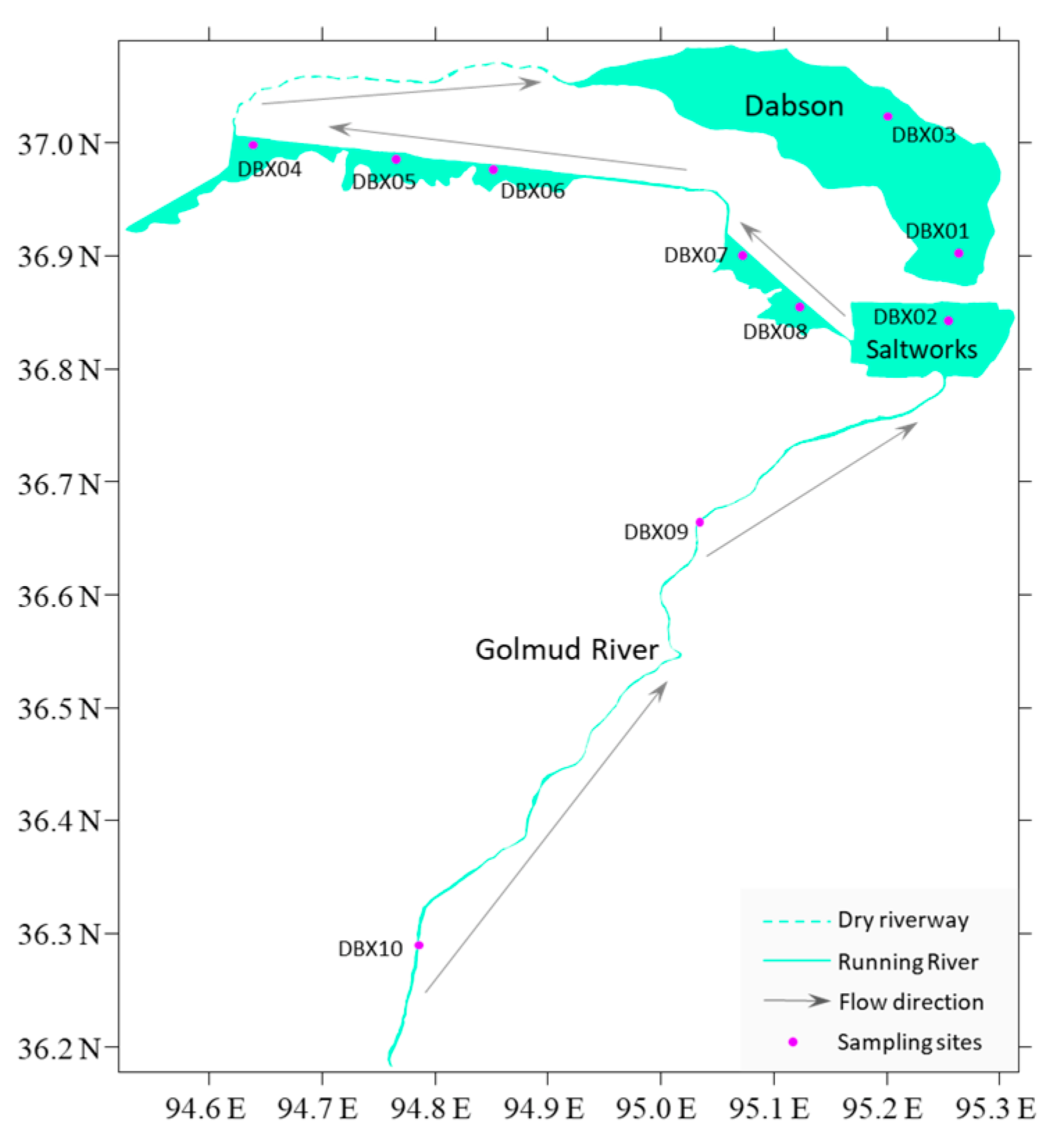
2. Materials and Methods
3. Results
3.1. Results of Microscopic Observation
3.2. Results of High-Throughput Sequencing
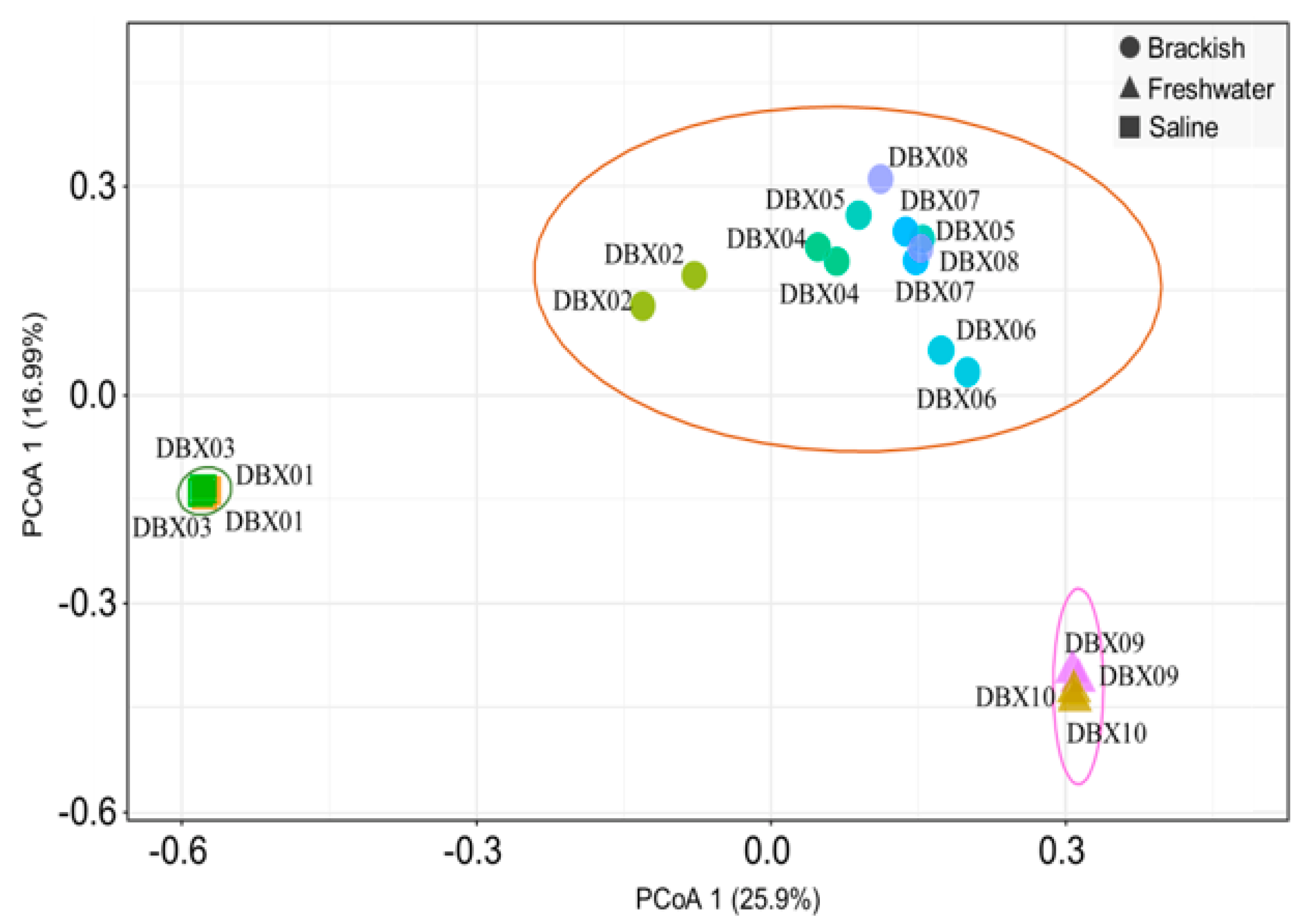
3.3. Analysis of Alpha and Beta Diversity Index
3.4. Correlation between the Phytoplankton Community and Environmental Factors
4. Discussion
5. Conclusions
- (1)
- Bacillariophyta and Chlorophyta were dominant phyla in the Golmud area. The density of the eukaryotic phytoplankton in this area was 0.09–12.08 × 105 cells/L and the biomass was 0.002–0.55 mg/L.
- (2)
- Dunaliella sp. was the dominant species in saline water, Teleaulax sp. and Parvodinium mixtum were the dominant species in brackish water, and Lindavia radiosa was dominant in freshwater. Moreover, Parvodinium mixtum only exists in high-altitude and cold-water areas.
- (3)
- Differences in the eukaryotic phytoplankton community structure between water bodies with different salinity were identified. Salinity influences the alpha and beta diversity of phytoplankton communities.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Larsson, U.; Nyberg, S.; Walve, J. Phytoplankton Primary Production: 14C- In Situ and 14C-Incubator Methods Compared. ICES J. Mar. Sci. 2021, 78, 3592–3602. [Google Scholar] [CrossRef]
- Egorov, V.N.; Popovichev, V.N.; Gulin, S.B.; Bobko, N.I.; Rodionova, N.Y.; Tsarina, T.V.; Marchenko, Y.G. The Influence of Phytoplankton Primary Production on the Cycle of Biogenic Elements in the Coastal Waters off Sevastopol, Black Sea. Russ. J. Mar. Biol. 2018, 44, 240–247. [Google Scholar] [CrossRef]
- Perkins, D.M.; Perna, A.; Adrian, R.; Cermeño, P.; Gaedke, U.; Huete-Ortega, M.; White, E.P.; Yvon-Durocher, G. Energetic Equivalence Underpins the Size Structure of Tree and Phytoplankton Communities. Nat. Commun. 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Luan, Q.; Wu, Q. Phytoplankton Community Succession and Its Relationship with The Density Distribution of Major Pelagic Fishes in Laizhou Bay. J. Fish. Sci. China 2020, 27, 547–558. [Google Scholar] [CrossRef]
- Stenger-Kovács, C.; Buczkó, K.; Hajnal, É.; Padisák, J. Epiphytic, Littoral Diatoms as Bioindicators of Shallow Lake Trophic Status: Trophic Diatom Index for Lakes (TDIL) Developed in Hungary. Hydrobiologia 2007, 589, 141–154. [Google Scholar] [CrossRef]
- Tan, X.; Sheldon, F.; Bunn, S.E.; Zhang, Q. Using Diatom Indices for Water Quality Assessment in a Subtropical River, China. Environ. Sci. Pollut. Res. 2013, 20, 4164–4175. [Google Scholar] [CrossRef] [Green Version]
- Rhee, G.-Y. Effects of Environmental Factors and Their Interactions on Phytoplankton Growth. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Advances in Microbial Ecology; Springer: Boston, MA, USA, 1982; Volume 6, pp. 33–74. ISBN 978-1-4615-8320-2. [Google Scholar]
- Liu, T.; Zhang, A.N.; Wang, J.; Liu, S.; Jiang, X.; Dang, C.; Ma, T.; Liu, S.; Chen, Q.; Xie, S.; et al. Integrated Biogeography of Planktonic and Sedimentary Bacterial Communities in the Yangtze River. Microbiome 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Sun, J.; Zhang, G.; Wang, X.; Wang, F. Environmental Factors Controlling the Dynamics of Phytoplankton Communities during Spring and Fall Seasons in the Southern Sunda Shelf. Environ. Sci. Pollut. Res. 2020, 27, 23222–23233. [Google Scholar] [CrossRef]
- Larson, C.A.; Belovsky, G.E. Salinity and Nutrients Influence Species Richness and Evenness of Phytoplankton Communities in Microcosm Experiments from Great Salt Lake, Utah, USA. J. Plankton Res. 2013, 35, 1154–1166. [Google Scholar] [CrossRef]
- Songqi, Y.; Xingliang, G.; Lijuan, W.; Tingxun, Z.; Danxia, W.; Guanghong, L. China Phytoplankton Community Structure and Driving Factors in Typical Reservoirs of Arid Region of Northwest China. J. Lake Sci. 2021, 33, 377–387. [Google Scholar] [CrossRef]
- Leray, M.; Knowlton, N. Censusing Marine Eukaryotic Diversity in the Twenty-First Century. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150331. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Glöckner, G.; Jahn, R.; Enke, N.; Gemeinholzer, B. Metabarcoding vs. Morphological Identification to Assess Diatom Diversity in Environmental Studies. Mol. Ecol. Resour. 2015, 15, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Priscu, J.C.; Xiong, J.; Conrad, R.; Vick-Majors, T.; Chu, H.; Hou, J. Salinity Drives Archaeal Distribution Patterns in High Altitude Lake Sediments on the Tibetan Plateau. FEMS Microbiol. Ecol. 2016, 92, fiw033. [Google Scholar] [CrossRef] [PubMed]
- An, S.M.; Choi, D.H.; Lee, J.H.; Lee, H.; Noh, J.H. Identification of Benthic Diatoms Isolated from the Eastern Tidal Flats of the Yellow Sea: Comparison between Morphological and Molecular Approaches. PLoS ONE 2017, 12, e0179422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axmacher, J.C.; Liu, Y.; Wang, C.; Li, L.; Yu, Z. Spatial α-Diversity Patterns of Diverse Insect Taxa in Northern China: Lessons for Biodiversity Conservation. Biol. Conserv. 2011, 144, 2362–2368. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Múrria, C.; Bonada, N.; Arnedo, M.A.; Prat, N.; Vogler, A.P. Higher β- and γ-Diversity at Species and Genetic Levels in Headwaters than in Mid-Order Streams in Hydropsyche (Trichoptera). Freshw. Biol. 2013, 58, 2226–2236. [Google Scholar] [CrossRef]
- Chen, F.; Yi, L.; Zhang, R.; Han, F.; Liu, X.; Lu, X.; Ma, Z. Trend and Abrupt Change Features of Surface Runoff of Golmud River. J. Salt Lake Res. 2021, 29, 30–42. [Google Scholar]
- Chen, Y.; Shen, Z.; Liu, Y. Study on Community Structure of Phytoplankton in Geermu River of Chaidamu River System in Autumn. Sci. Technol. Qinghai Agric. For. 2012, 2, 10–15. [Google Scholar]
- Khatoon, H.; Haris, N.; Banerjee, S.; Rahman, N.A.; Begum, H.; Mian, S.; Abol-Munafi, A.B.; Endut, A. Effects of Different Salinities on the Growth and Proximate Composition of Dunaliella Sp. Isolated from South China Sea at Different Growth Phases. Process Saf. Environ. Prot. 2017, 112, 280–287. [Google Scholar] [CrossRef]
- Kretschmann, J.; Owsianny, P.M.; Žerdoner Čalasan, A.; Gottschling, M. The Hot Spot in a Cold Environment: Puzzling Parvodinium (Peridiniopsidaceae, Peridiniales) from the Polish Tatra Mountains. Protist 2018, 169, 206–230. [Google Scholar] [CrossRef] [PubMed]
- Xi, F.; Zheng, T.; Jiao, N.; Zhang, Y. A Preliminary Analysis of Mechanism of Deep Sea Microorganisms Diversity. Adv. Earth Sci. 2004, 19, 39–46. [Google Scholar]
- Jonsson, M.; Snäll, T.; Asplund, J.; Clemmensen, K.E.; Dahlberg, A.; Kumordzi, B.B.; Lindahl, B.D.; Oksanen, J.; Wardle, D.A. Divergent Responses of Β-diversity among Organism Groups to a Strong Environmental Gradient. Ecosphere 2016, 7, e01535. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.-J.; Banerjee, S.; Zhou, N.; Zhao, Z.-Y.; Zhang, K.; Tian, C.-Y. Soil PH Is Equally Important as Salinity in Shaping Bacterial Communities in Saline Soils under Halophytic Vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef] [PubMed]
- Hepp, L.U.; Landeiro, V.L.; Melo, A.S. Experimental Assessment of the Effects of Environmental Factors and Longitudinal Position on Alpha and Beta Diversities of Aquatic Insects in a Neotropical Stream. Int. Rev. Hydrobiol. 2012, 97, 157–167. [Google Scholar] [CrossRef]
- Yang, J.; Ma, L.; Jiang, H.; Wu, G.; Dong, H. Salinity Shapes Microbial Diversity and Community Structure in Surface Sediments of the Qinghai-Tibetan Lakes. Sci. Rep. 2016, 6, 25078. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Xie, G.; Shao, K.; Tian, W.; Gao, G.; Qin, B. Aquatic Bacterial Diversity, Community Composition and Assembly in the Semi-Arid Inner Mongolia Plateau: Combined Effects of Salinity and Nutrient Levels. Microorganisms 2021, 9, 208. [Google Scholar] [CrossRef]
- Ji, M.; Kong, W.; Yue, L.; Wang, J.; Deng, Y.; Zhu, L. Salinity Reduces Bacterial Diversity, but Increases Network Complexity in Tibetan Plateau Lakes. FEMS Microbiol. Ecol. 2019, 95, fiz190. [Google Scholar] [CrossRef]
- Li, S.; Hou, C.; Huang, S.; Wang, X. Camparisons in Plankton Community During Wet and Dry Seasons in Fuxian Lake. Environ. Prot. 2021, 49, 31–34. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Zhu, H.; Xiong, X.; Liu, G. Community Structure of Eukaryotic Phytoplankton in Wetland of Golmud River and Its Lower Reaches and Relative Environmental Factors. Diversity 2022, 14, 269. https://doi.org/10.3390/d14040269
Dong X, Zhu H, Xiong X, Liu G. Community Structure of Eukaryotic Phytoplankton in Wetland of Golmud River and Its Lower Reaches and Relative Environmental Factors. Diversity. 2022; 14(4):269. https://doi.org/10.3390/d14040269
Chicago/Turabian StyleDong, Xiaoqi, Huan Zhu, Xiong Xiong, and Guoxiang Liu. 2022. "Community Structure of Eukaryotic Phytoplankton in Wetland of Golmud River and Its Lower Reaches and Relative Environmental Factors" Diversity 14, no. 4: 269. https://doi.org/10.3390/d14040269
APA StyleDong, X., Zhu, H., Xiong, X., & Liu, G. (2022). Community Structure of Eukaryotic Phytoplankton in Wetland of Golmud River and Its Lower Reaches and Relative Environmental Factors. Diversity, 14(4), 269. https://doi.org/10.3390/d14040269





