The Similarity of Floral Scent Composition in Two Breynia Species Pollinated by the Same Host-Specific Epicephala Moth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Floral Scent Collection
2.3. Chemical Analysis of Floral Scent
2.4. Statistical Analyses
3. Results
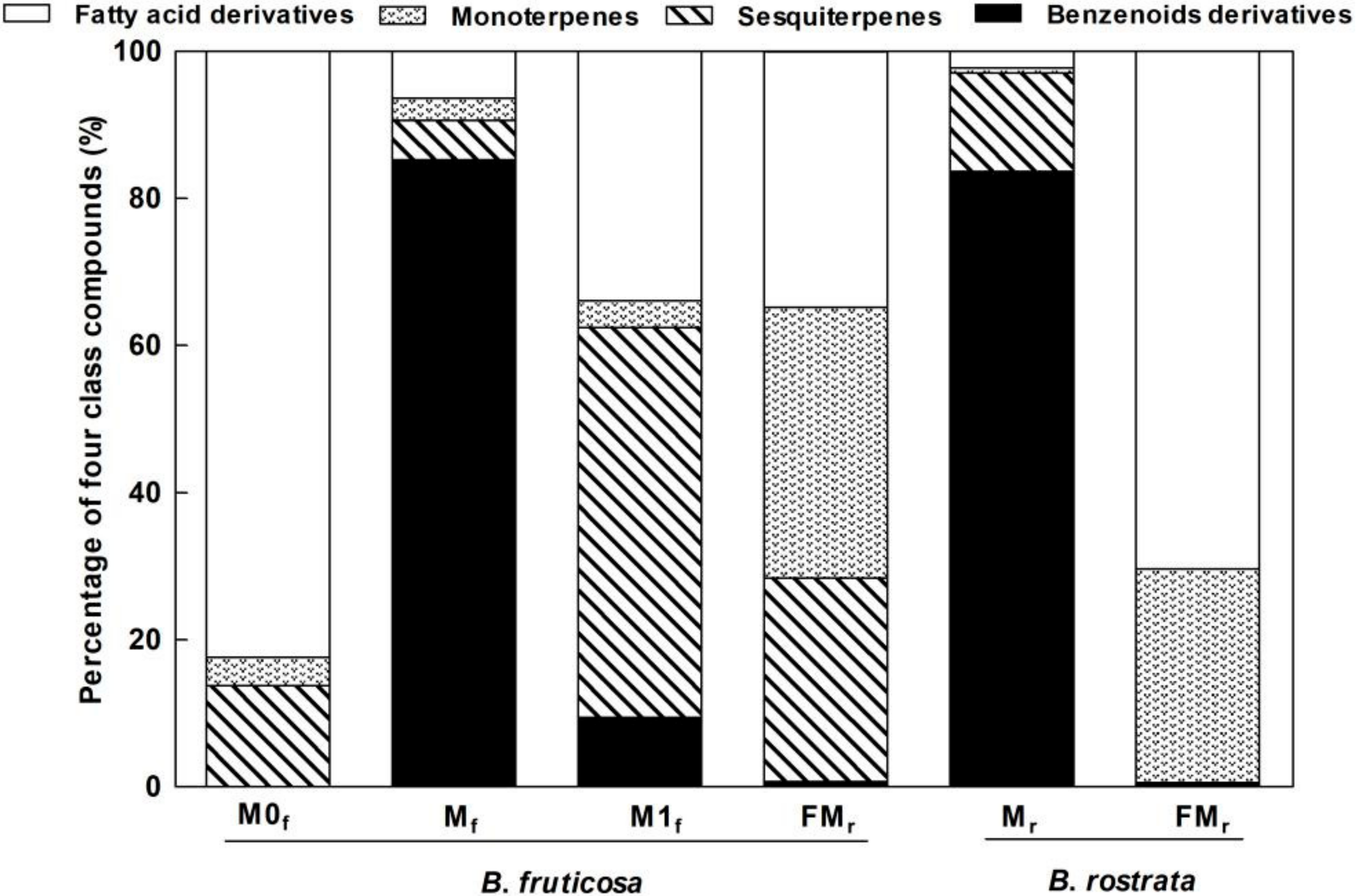
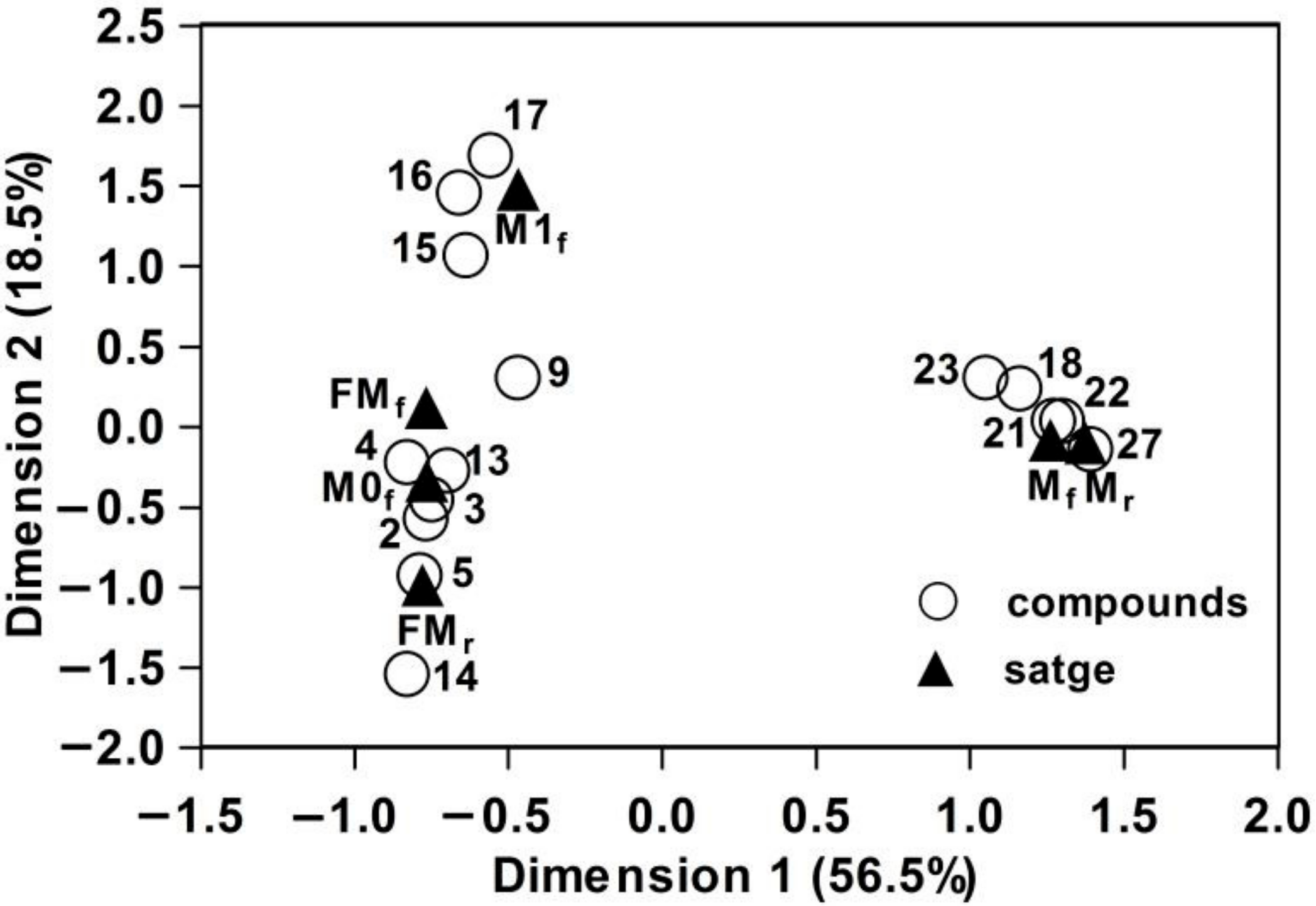
3.1. Chemical Composition of Floral Scent
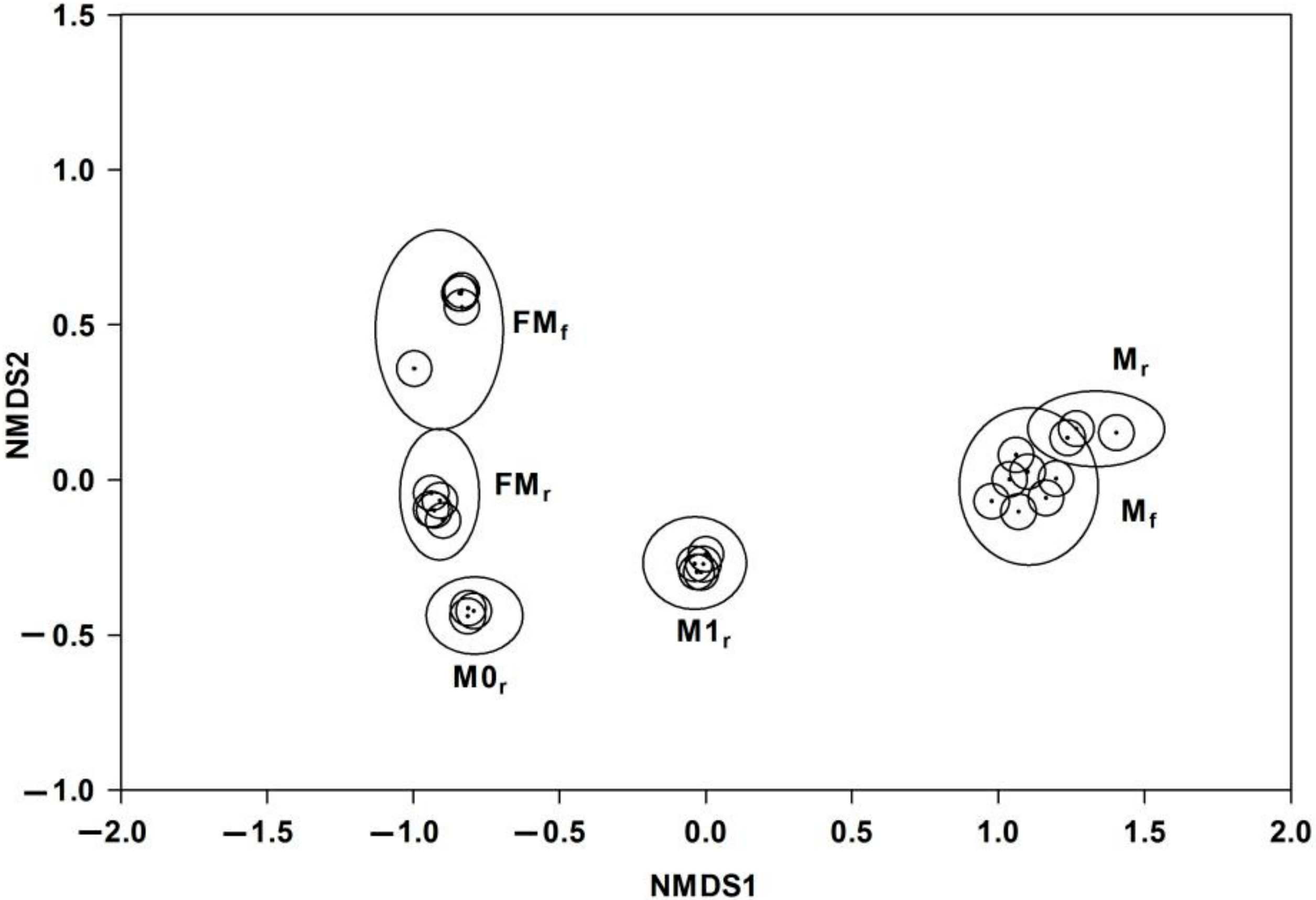
3.2. Interspecific, Intersexual, and Temporal Variation of Floral Scents
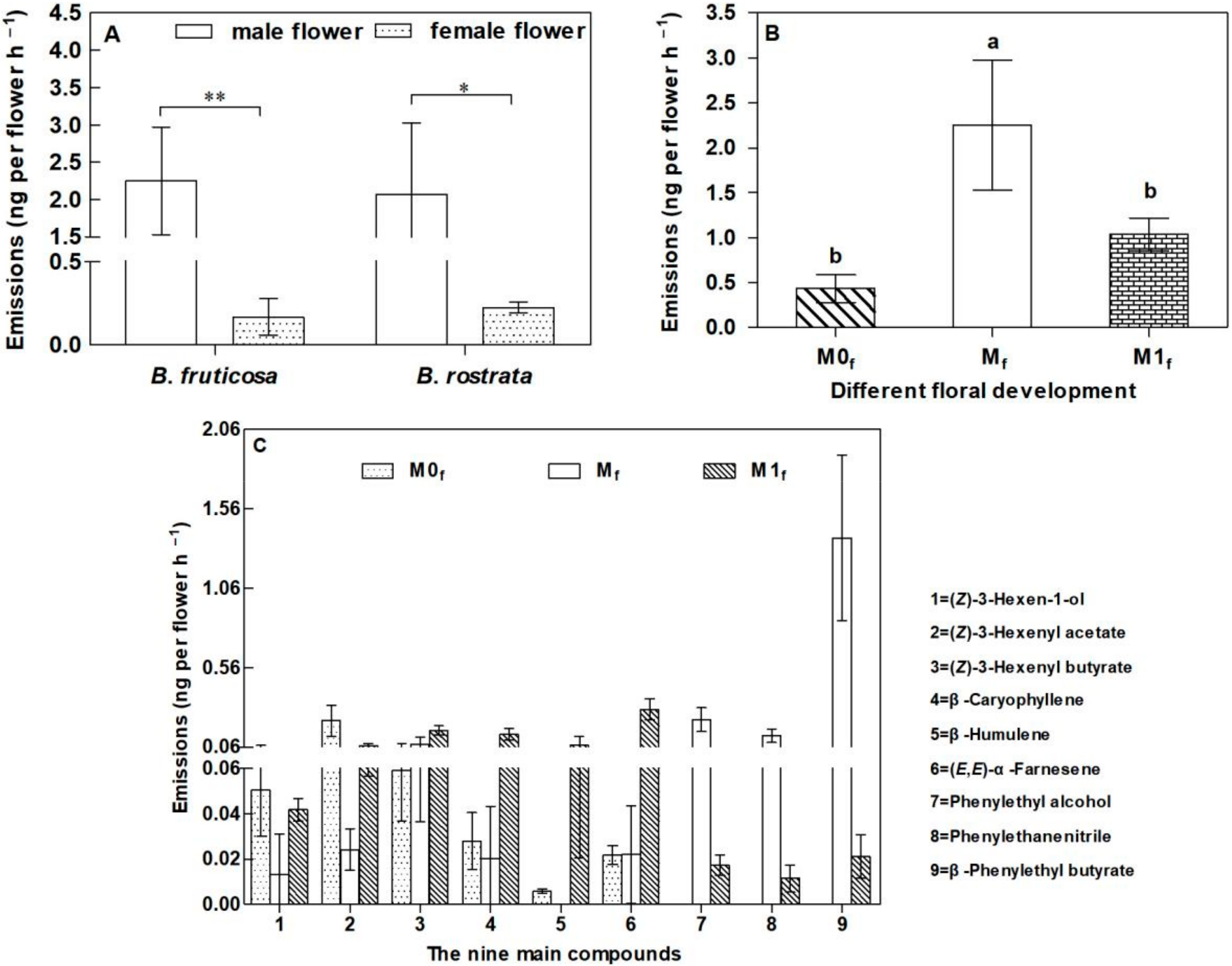
3.3. Emission of Floral Scents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dufay, M.; Anstett, M.C. Conflicts between plants and pollinators that reproduce within inflorescences, evolutionary variations on a theme. Oikos 2003, 100, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Weiblen, G.D. How to be a fig wasp. Annu. Rev. Entomol. 2002, 47, 299–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellmyr, O. Yuccas, yucca moths, and coevolution, a review. Ann. Mo. Bot. Gard. 2003, 90, 35–55. [Google Scholar] [CrossRef]
- Kato, M.; Takimura, A.; Kawakita, A. An obligate pollination mutualism and reciprocal diversification in the tree genus Glochidion (Euphorbiaceae). Proc. Biol. Sci. 2003, 100, 5264–5267. [Google Scholar] [CrossRef] [Green Version]
- Kawakita, A.; Kato, M. Evolution of obligate pollination mutualism in New Caledonian Phyllanthus (Euphorbiaceae). Am. J. Bot. 2004, 91, 410–415. [Google Scholar] [CrossRef]
- Kawakita, A.; Kato, M. Obligate pollination mutualism in Breynia (Phyllanthaceae), further documentation of pollination mutualism involving Epicephala moth (Gracillariidae). Am. J. Bot. 2004, 91, 1319–1325. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, B.B.; Wang, S.X.; Li, H.H. Six new species of the genus Epicephala Meyrick, 1880 (Lepidoptera, Gracillariidae) associated with Euphorbiaceae plants. Zootaxa 2012, 3275, 43–54. [Google Scholar] [CrossRef]
- Pellmyr, O.; Thien, L.B. Insect reproduction and floral fragrances: Keys to the evolution of the angiosperms? Taxon 1986, 35, 76–85. [Google Scholar] [CrossRef]
- Fleming, T.H.; Holland, J.N. The evolution of obligate pollination mutualisms: Senita cactus and senita moth. Oecologia 1998, 114, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raguso, R.A. Wake up and smell the roses, the ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Andrews, E.S.; Theis, N.; Adler, L.S. Pollinator and her-bivore attraction to cucurbita floral volatiles. J. Chem. Ecol. 2007, 33, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Milet-Pinheiro, P.; Silva, J.B.F.; Navarro, D.M.; Machado, I.C.; Gerlach, G. Notes on pollination ecology and floral scent chemistry of the rare neotropical orchid Catasetum galeritum Rchb.f. Plant Spec. Biol. 2018, 33, 158–163. [Google Scholar] [CrossRef]
- Theis, N.; Lerdau, M.; Raguso, R.A. The challenge of attracting pollinators while evading floral herbivores: Patterns of fragrance emission in Cirsium arvense and Cirsium repandum (Asteraceae). Int. J. Plant Sci. 2007, 168, 587–601. [Google Scholar] [CrossRef] [Green Version]
- Willmer, P.G.; Nuttman, C.V.; Raine, N.E.; Stone, G.N.; Pattrick, J.G.; Henson, K.; Stillman, P.; Mcllroy, L.; Potts, S.G.; Knudsen, J.T. Floral volatiles controllingant behaviour. Funct. Ecol. 2009, 23, 888–900. [Google Scholar] [CrossRef]
- Hossaert-McKey, M.; Soler, C.; Schatz, B.; Proffit, M. Floral scents, their role in nursery pollination mutualisms. Chemoecology 2010, 20, 75–88. [Google Scholar] [CrossRef]
- Dufaÿ, M.; Hossaert-McKey, M.; Anstett, M.C. When leavesact like flowers: How dwarf palms attract their pollinators. Ecol. Lett. 2003, 6, 28–34. [Google Scholar] [CrossRef]
- Grison, L.; Edwards, A.A.; Hossaert-McKey, M. Interspecies variation in floral fragrances emitted by tropical Ficus species. Phytochemistry 1999, 52, 1293–1299. [Google Scholar] [CrossRef]
- Song, Q.S.; Yang, D.R.; Zhang, G.M.; Yang, C.R. Volatiles from Ficus hispida and their attractiveness to fig wasps. J. Chem. Ecol. 2001, 27, 1929–1942. [Google Scholar] [CrossRef]
- Grison-Pige, L.; Hossaert-McKey, M.; Greeff, J.M.; Bessiere, J.M. Fig volatile compounds—A first comparative study. Phytochemistry 2002, 61, 61–71. [Google Scholar] [CrossRef]
- Proffit, M.; Chen, C.; Soler, C.; Bessiere, J.M.; Schatz, B.; Hossaert-McKey, M. Can chemical signals responsible for mutualistic partner encounter promote the specific exploitation of nursery pollination mutualisms? The case of figs and fig wasps. Entomol. Exp. Appl. 2009, 131, 46–57. [Google Scholar] [CrossRef]
- Proffit, M.; Schatz, B.; Bessiere, J.M. Signalling receptivity, comparison of the emission of volatile compounds by figs of Ficus hispida before, during and after the phase of receptivity to pollinators. Symbiosis 2008, 45, 15–24. [Google Scholar]
- Gu, D.; Peng, Y.; Yang, D. Convergence in host recognition behavior between obligate pollinating fig wasps and non-pollinating fig wasps. Biodivers. Sci. 2012, 20, 324–329. [Google Scholar]
- Okamoto, T.; Su, Z.H. Chemical analysis of floral scents in sympatric Ficus species: Highlighting different compositions of floral scents in morphologically and phylogenetically close species. Plant Syst. Evol. 2021, 307, 45. [Google Scholar] [CrossRef]
- Svensson, G.P.; Hickman, M.O.; Bartram, S.; Boland, W.; Pellmyr, O.; Raguso, R.A. Chemistry and geographic variation of floral scent in Yucca filamentosa (Agavaceae). Am. J. Bot. 2005, 92, 1624–1631. [Google Scholar] [CrossRef] [Green Version]
- Svensson, G.P.; Pellmyr, O.; Raguso, R.A. Strong conservation of floral scent composition in two allopatric yuccas. J. Chem. Ecol. 2006, 32, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Svensson, G.P.; Pellmyr, O.; Raguso, R.A. Pollinator attraction to volatiles from virgin and pollinated host flowers in a yucca moth obligate mutualism. Oikos 2011, 120, 1577–1583. [Google Scholar] [CrossRef]
- Okamoto, T.; Kawakita, A.; Kato, M. Interspecific variation of floral scent composition in Glochidion and its association with host-specific pollinating seed parasite (Epicephala). J. Chem. Ecol. 2007, 33, 1065–1081. [Google Scholar] [CrossRef]
- Svensson, G.P.; Okamoto, T.; Kawakita, A.; Goto, R.; Kato, M. Chemical ecology of an obligate pollination mutualism, testing the ‘private channel’ hypothesis in the Breynia-Epicephala association. New Phytol. 2010, 186, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.H.; Shi, F.C.; Chai, M.W.; Li, R.L.; Li, H.H. Interspecific and intersexual differences in the chemical composition of floral scent in Glochidion species (Phyllanthaceae) in south China. J. Chem. 2015, 2015, 865694. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, T.; Svensson, G.P.; Goto, R.; Kawakita, A.; Kato, M. Nocturnal emission and post-pollination change of floral scent in the leafflower tree, Glochidion rubrum, exclusively pollinated by seed-parasitic leafflower moths. Plant Spec. Biol. 2022, 37, 197–208. [Google Scholar] [CrossRef]
- Hossaert-McKey, M.; Gibernau, M.; Frey, J.E. Chemosensory attraction of fig wasps to substances produced by receptive figs. Entomol. Exp. Appl. 1994, 70, 185–191. [Google Scholar] [CrossRef]
- Ware, A.B.; Compton, S.G. Responses of fig wasps to host plant volatile cues. J. Chem. Ecol. 1994, 20, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Gibernau, M.; Hossaert-McKey, M.; Frey, J.; Kjellberg, F. Are olfactory signals sufficient to attract fig pollinators? Ecoscience 1998, 5, 306–311. [Google Scholar] [CrossRef]
- Grison-Pige, L.; Bessiere, J.M.; Hossaert-McKey, M. Specific attraction of fig-pollinating wasps, role of volatile compounds released by tropical figs. J. Chem. Ecol. 2002, 28, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, Q.S.; Proffit, M.; Bessiere, J.M.; Li, Z.B.; Hossaert-McKey, M. Private channel, a single unusual compound assures specific pollinator attraction in Ficus semicordata. Funct. Ecol. 2009, 23, 941–950. [Google Scholar] [CrossRef]
- Proffit, M.; Lapeyre, B.; Buatois, B.; Deng, X.; Arnal, P.; Gouzerh, F.; Gar-rasco, D.; Hossaert-McKey, M. Chemical signal is in the blend: Based of plant-pollinator encounter in a highly specialized interaction. Sci. Rep. 2020, 10, 10071. [Google Scholar]
- Zhang, J.; Wang, S.X.; Li, H.H.; Hu, B.B.; Yang, X.F.; Wang, Z.B. Diffuse coevolution between two Epicephala species (Gracillariidae) and two Breynia species (Phyllanthaceae). PLoS ONE 2012, 7, e35071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govaerts, R.; Fronding, R.G.; Randcliffe-Smith, A. World Checklist and Bibliography of Euphorbiaceae; Royal Botanic Gardens: London, UK, 2000. [Google Scholar]
- Li, B.T.; Qiu, H.X.; Ma, J.S.; Zhu, H.; Gilbert, M.G.; Esser, H.J.; Dressler, S.; Hoffmann, P.; Gillespie, L.J.; Maria, V.; et al. Flora of China; Science Press and Missouri Botanical Garden Press: Beijing, China, 2008; Volume 11, (Oxalidaceae through Aceraceae). [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Insect Pheromones and Semiochemicals. 2008. Available online: http://www.pherobase.com/ (accessed on 1 May 2015).
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: http://webbook.nist.gov (accessed on 1 May 2015).
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 20132280. [Google Scholar] [CrossRef]
- Okamoto, T.; Kawakita, A.; Goto, R.; Svensson, G.P.; Kato, M. Active pollination favours sexual dimorphism in floral scent. Proc. Biol. Sci. 2013, 280, 22–80. [Google Scholar] [CrossRef] [Green Version]
- Roxane, D.V.; Bertrand, S.; Mathilde, D. Understanding intraspecific variation of floral scent in light of evolutionary ecology. Ann. Bot. 2017, 120, 1–20. [Google Scholar]
- Cornille, A.; Underhill, J.G.; Cruaud, A.; Hossaert-McKey, M.; Tolley, K.A.; Kjellberg, F.; van Noort, S.; Proffit, M. Floral volatiles, pollinator sharing and diversification in the fig–wasp mutualism, insights from Ficus natalensis, and its two wasp pollinators (South Africa). Proc. R. Soc. B Biol. Sci. 2011, 19, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, J.T.; Lars, T. Floral scent in bat-pollinated plants: A case of convergent evolution. Bot. J. Linn. Soc. 1995, 119, 45–57. [Google Scholar] [CrossRef]
- Proctor, M.; Yeo, P.; Lack, A. The Natural History of Pollination; Timber Press: Portland, OR, USA, 1996. [Google Scholar]
- Chittka, L.; Thomson, J.D. Cognitive Ecology of Pollination: Animal Behaviour and Floral Evolution; Cambridge University Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Hemborg, A.M.; Bond, W.J. Different rewards in female and male flowers can explain the evolution of sexual dimorphism in plant. Biol. J. Linn. Soc. 2005, 85, 97–109. [Google Scholar] [CrossRef]
- Schiestle, F.P.; Ayasse, M.; Paulus, H.F. Variation of floral scent emission and post-pollination changes in individual flowers of Ophrys sphegodes subsp. sphegodes. J. Chem. Ecol. 1997, 23, 2281–2289. [Google Scholar]
- Schiestl, F.P.; Ayasse, M. Post-pollination emission of a repellent compound in a sexually deceptive orchid, a new mechanism for maximizing reproductive success? Oecologia 2001, 126, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, J.K.; Waelti, M.O.; Widmer, A.; Schiestl, F.P. Post-pollination changes in floral odor in Silene latifolia, adaptive mechanisms for seed-predator avoidance. J. Chem. Ecol. 2006, 8, 1855–1860. [Google Scholar] [CrossRef]
- Horn, K.C.; Holland, J.N. Discrimination among floral resources by an obligately pollinating seed-eating moth, host-marking signals and pollination and florivory cues. Evol. Ecol. Res. 2010, 12, 119–129. [Google Scholar]
- Grison-Pigé, J.M.; Bessière, T.C.J.; Turlings, F.; Kjellberg, J.; Roy, M.M.; Hossaert-McKey, M.M. Limited intersex mimicry of floral odour in Ficus carica. Funct. Ecol. 2001, 15, 551–558. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Compton, S.G.; Peng, Y.Q.; Yang, D.R. ‘Push’ and ‘pull’ responses by fig wasps to volatiles released by their host figs. Chemoecology 2012, 22, 217–227. [Google Scholar] [CrossRef]
- Bouchaib, K.; Marc, G.; Marie-Charlotte, A.; Finn, K.; Martine, H.M. When figs wait for pollinators, the length of fig receptivity. Am. J. Bot. 1995, 82, 992–999. [Google Scholar]
- Negre, F.; Kish, C.M.; Boatright, J.; Underwood, B.; Shibuya, K.; Wagner, C.; Clark, D.G.; Dudareva, N. Regulation of methylbenzoate emission after pollination in snapdragon and petunia flowers. Plant Cell 2003, 15, 2992–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theis, N.; Raguso, R.A. The effect of pollination on floral fragrance in thistles. J. Chem. Ecol. 2005, 31, 2581–2600. [Google Scholar] [CrossRef] [PubMed]
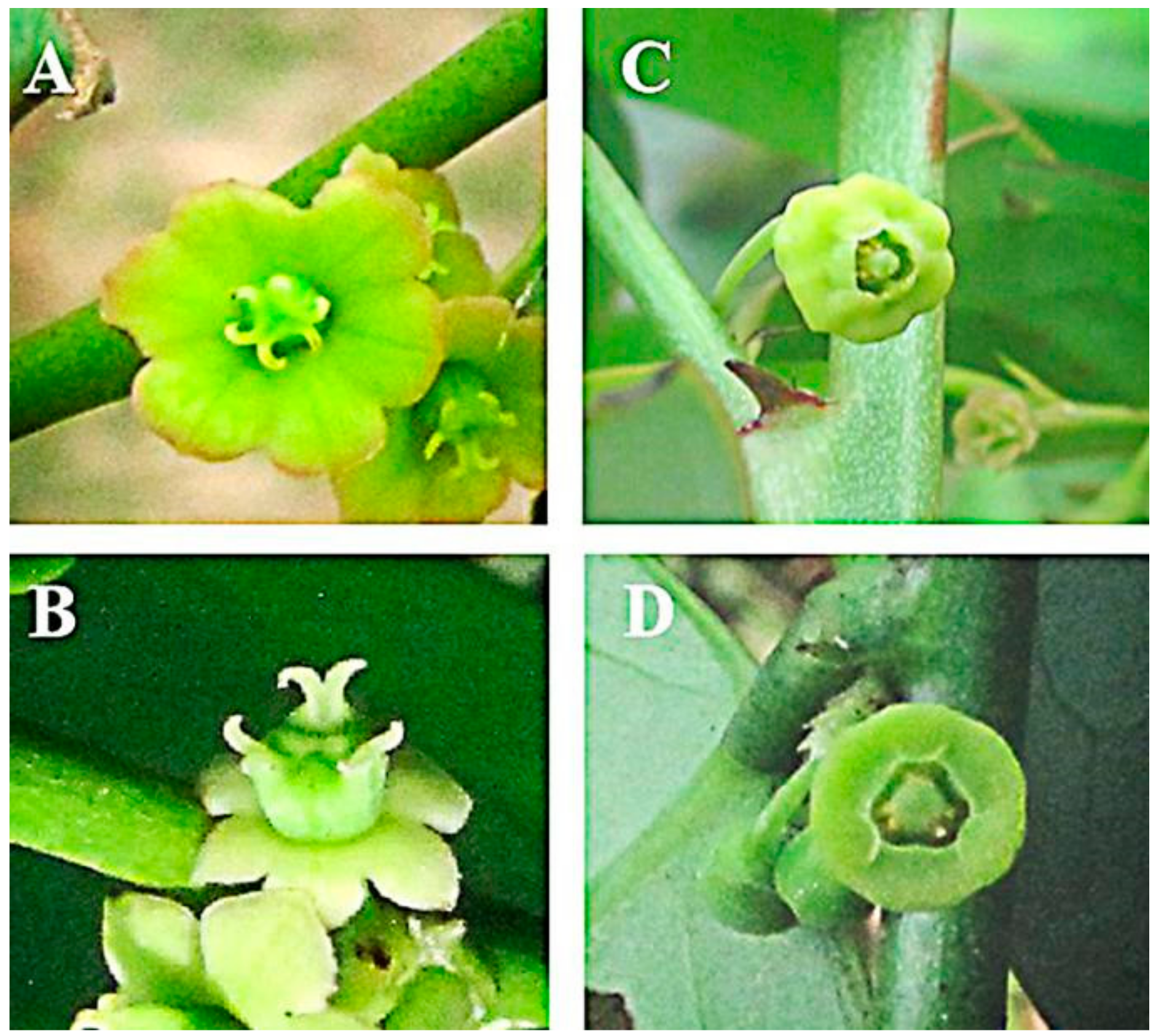
| Compounds | RT a | B. fruticosa | B. rostrata | |||||
|---|---|---|---|---|---|---|---|---|
| M0f d | Mf d | M1f d | FMf d | Mr d | FMr d | |||
| (N = 3) | (N = 7) | (N = 5) | (N = 6) | (N = 3) | (N = 4) | |||
| Fatty acid derivatives | ||||||||
| 1 | 3-Hexenal | 802 | – c | – | – | 2.04 ± 2.46 | – | 3.41 ± 2.42 |
| 2 | (E)-2-Hexenal b | 854 | 2.77 ± 1.01 | – | 3.05 ± 1.57 | 1.84 ± 2.19 | – | 14.86 ± 2.24 |
| 3 | (Z)-3-Hexen-1-ol b | 857 | 11.73 ± 2.65 | 0.51 ± 0.58 | 4.07 ± 0.40 | 3.56 ± 4.36 | 0.36 ± 0.42 | 6.09 ± 2.44 |
| 4 | Decane b | 1000 | – | – | – | 5.86 ± 1.13 | – | 1.53 ± 1.02 |
| 5 | (Z)-3-Hexenyl acetate b | 1007 | 50.96 ± 5.35 | 1.12 ± 0.39 | 6.64 ± 0.33 | 18.79 ± 6.06 | 0.70 ± 0.78 | 25.70 ± 3.93 |
| 6 | Hexyl acetate | 1011 | 1.51 ± 0.52 | – | – | – | – | – |
| 7 | (E)-2-Hexenyl acetate | 1012 | – | – | – | 0.76 ± 0.59 | – | 0.57 ± 0.14 |
| 8 | 3-Hexenyl acetate | 1015 | – | – | – | 0.92 ± 0.57 | – | 0.97 ± 0.12 |
| 9 | (Z)-3-Hexenyl butyrate b | 1185 | 13.59 ± 1.64 | 3.52 ± 1.14 | 15.95 ± 1.42 | 0.97 ± 0.37 | 1.03 ± 0.45 | 14.04 ± 2.81 |
| 10 | Hexyl butyrate | 1192 | – | 0.50 ± 0.30 | 2.96 ± 0.36 | – | 0.12 ± 0.10 | 0.49 ± 0.12 |
| 11 | (E)-2-Hexenyl butyrate | 1195 | – | 0.72 ± 0.56 | 1.20 ± 0.51 | – | 0.06 ± 0.08 | 0.68 ± 0.10 |
| Monoterpenes | ||||||||
| 12 | (Z)-β-Ocimene | 1038 | – | 0.89 ± 1.00 | 1.21 ± 0.85 | 0.79 ± 0.50 | 0.23 ± 0.31 | 0.62 ± 0.08 |
| 13 | (E)-β-Ocimene b | 1048 | 3.51 ± 0.45 | 1.44 ± 0.78 | 2.43 ± 0.46 | 34.19 ± 8.42 | 0.47 ± 0.23 | 21.31 ± 1.35 |
| 14 | cis-Linaloloxide(furanoid) b | 1088 | – | – | – | 1.62 ± 1.95 | – | 8.69 ± 3.22 |
| Sequiterpenes | ||||||||
| 15 | β-Caryophyllene b | 1418 | 6.40 ± 1.01 | 0.88 ± 0.67 | 13.44 ± 1.84 | 12.44 ± 8.85 | 0.01 ± 0.02 | – |
| 16 | β-Humulene b | 1454 | 1.42 ± 0.33 | – | 6.83 ± 3.64 | 4.74 ± 2.60 | – | – |
| 17 | (E,E)-α-Farnesene b | 1506 | 5.36 ± 1.31 | 0.88 ± 0.67 | 28.67 ± 4.34 | 10.42 ± 4.66 | 0.53 ± 0.51 | – |
| 18 | (Z)-Methyl jasmonate b | 1657 | – | 2.66 ± 0.66 | 2.50 ± 0.52 | – | 11.83 ± 1.29 | – |
| 19 | [1.α,2.α,(Z)]-3-oxo-2-(2-pentenyl)- Cyclopentaneacetic acid, methyl ester | 1685 | 0.55 ± 0.15 | 1.10 ±1.58 | 1.63 ± 0.63 | – | 0.97 ± 0.14 | – |
| Benzenoid dervatives | ||||||||
| 20 | Benzaldehyde | 962 | – | – | – | 0.56 ± 0.33 | – | 0.59 ± 0.20 |
| 21 | Phenylacetaldehyde b | 1043 | – | 1.44 ± 0.78 | 0.59 ± 0.25 | – | 5.45 ± 0.10 | – |
| 22 | Phenylethyl alcohol b | 1116 | – | 10.37 ± 1.03 | 1.67 ± 0.32 | – | 10.13 ± 1.28 | – |
| 23 | Phenylethanenitrile b | 1143 | – | 5.88 ± 0.81 | 1.16 ± 0.66 | – | 0.04 ± 0.06 | – |
| 24 | Methylsalicylate | 1190 | – | – | – | 0.22 ± 0.30 | – | 0.15 ± 0.06 |
| 25 | β-Phenylethy acetate | 1256 | – | 0.25 ± 0.20 | 2.25 ± 0.47 | – | 0.35 ± 0.22 | – |
| 26 | Eugenol | 1359 | – | 0.50 ± 0.98 | 1.80 ± 1.26 | – | 0.75 ± 0.09 | – |
| 27 | β-Phenylethyl butyrate b | 1447 | – | 66.79 ± 2.51 | 1.96 ± 0.57 | – | 66.99 ± 0.99 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Mamut, J.; Yang, X.; Shi, F.; Li, H. The Similarity of Floral Scent Composition in Two Breynia Species Pollinated by the Same Host-Specific Epicephala Moth. Diversity 2022, 14, 266. https://doi.org/10.3390/d14040266
Huang D, Mamut J, Yang X, Shi F, Li H. The Similarity of Floral Scent Composition in Two Breynia Species Pollinated by the Same Host-Specific Epicephala Moth. Diversity. 2022; 14(4):266. https://doi.org/10.3390/d14040266
Chicago/Turabian StyleHuang, Daihong, Jannathan Mamut, Xiaofei Yang, Fuchen Shi, and Houhun Li. 2022. "The Similarity of Floral Scent Composition in Two Breynia Species Pollinated by the Same Host-Specific Epicephala Moth" Diversity 14, no. 4: 266. https://doi.org/10.3390/d14040266
APA StyleHuang, D., Mamut, J., Yang, X., Shi, F., & Li, H. (2022). The Similarity of Floral Scent Composition in Two Breynia Species Pollinated by the Same Host-Specific Epicephala Moth. Diversity, 14(4), 266. https://doi.org/10.3390/d14040266






