Mitogenomics and the Global Dispersion of Vespula germanica: A Case Study from South Africa Shows Evidence for Two Separate Invasion Events
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Collection and DNA Extraction
2.2. Next-Generation Sequencing, Assembly and Annotation of Mitogenomes
2.3. Phylogenetic Tree
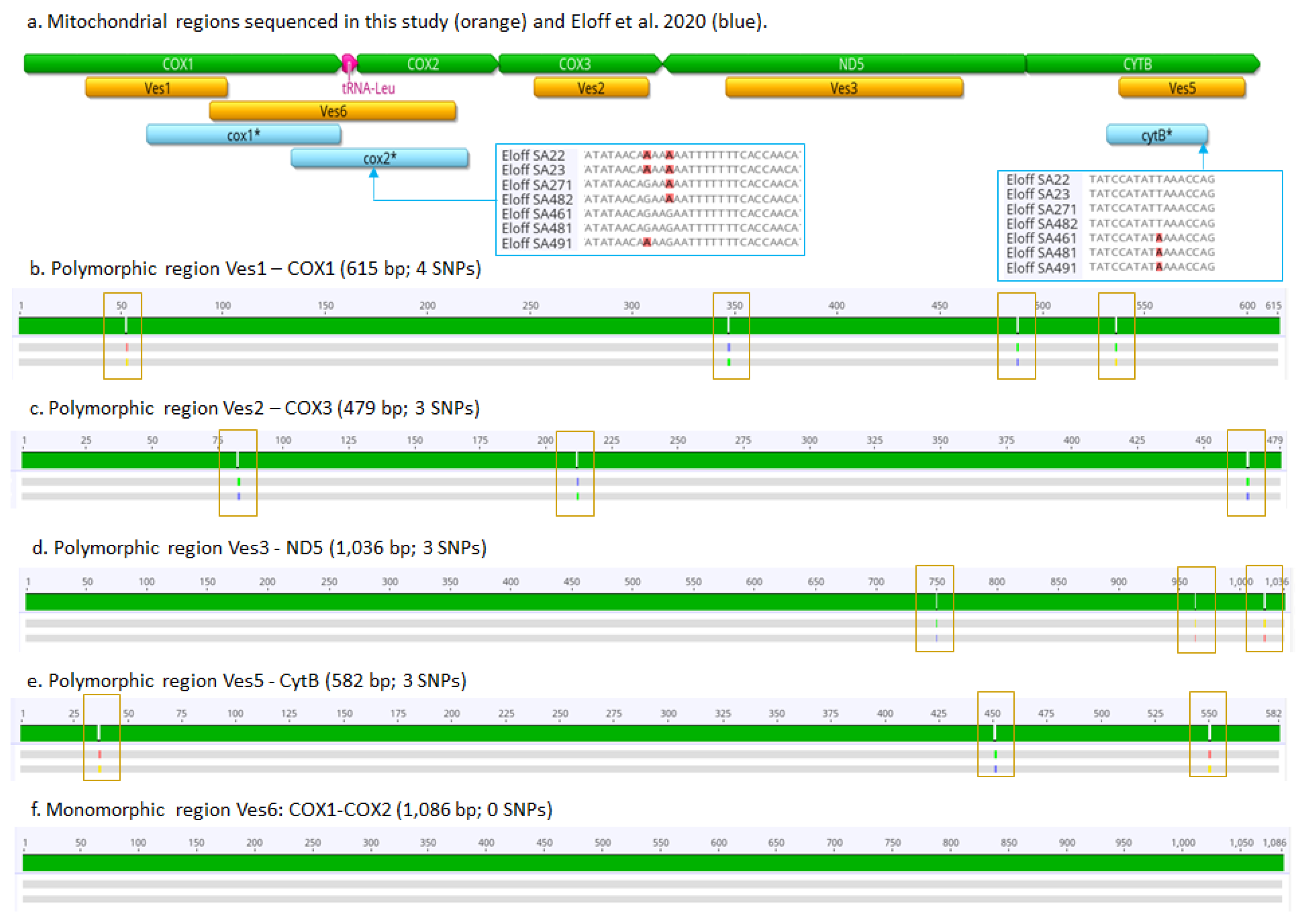
2.4. Identification of Mitochondrial Polymorphic Regions
2.5. PCR Amplification and Sanger Sequencing of Polymorphic Regions
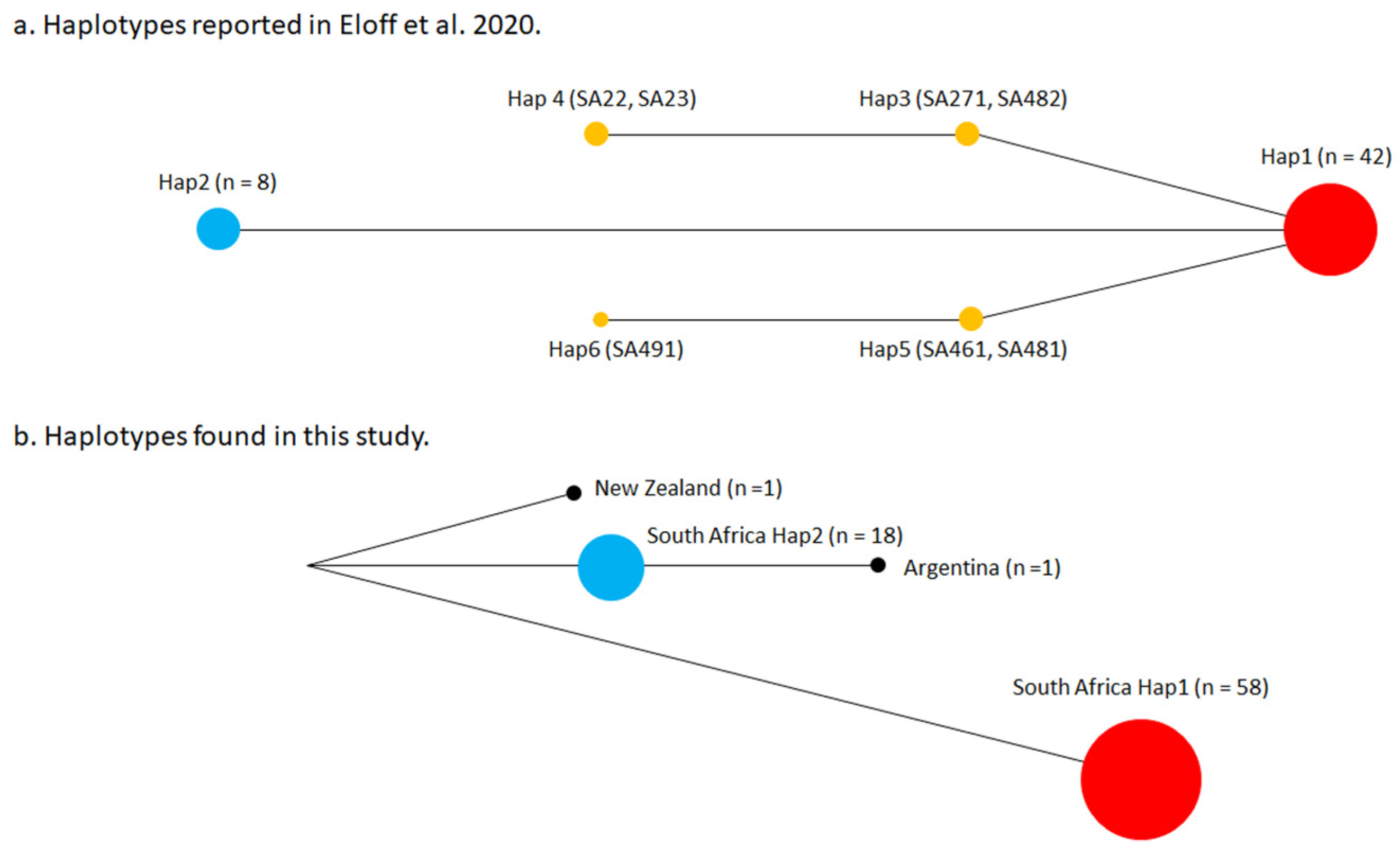
2.6. Haplotype Analyses
3. Results
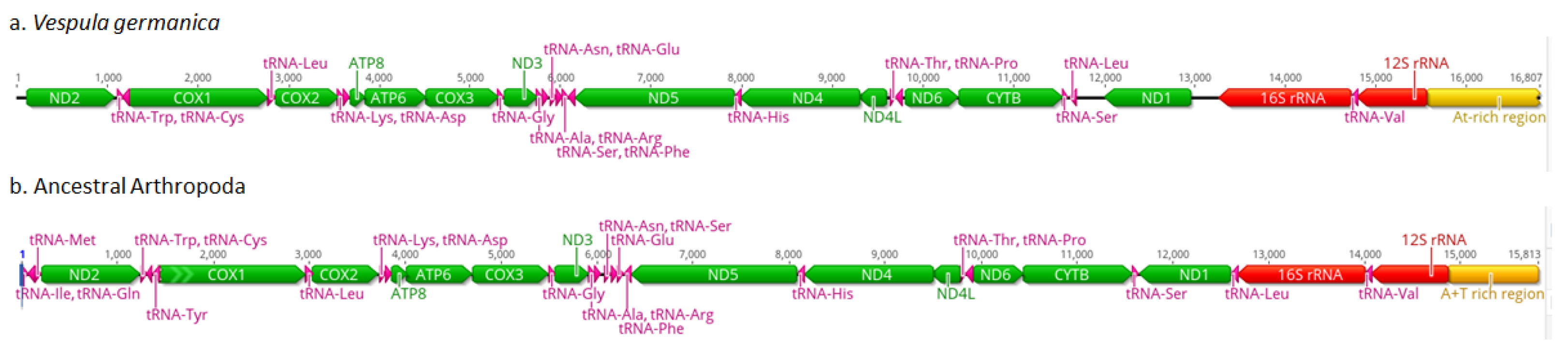
3.1. Mitochondrial Genomes, Phylogeny of Vespula germanica, and Polymorphic Regions
3.2. Haplotypes of Vespula germanica in South Africa
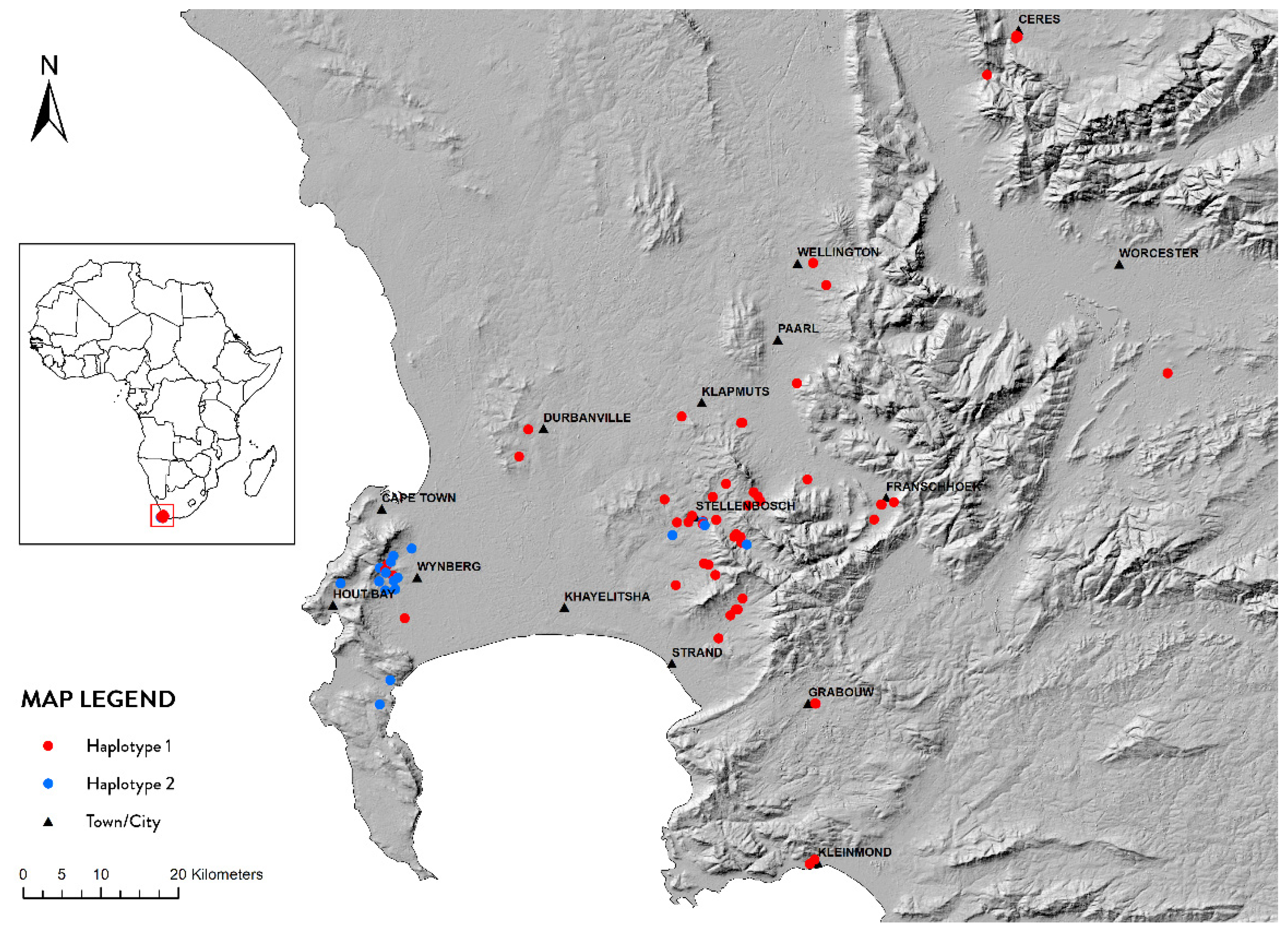
3.3. Geographic Distribution of Haplotypes in the Western Cape
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nature Comm. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The population biology of invasive species. Annu. Rev. Ecol. Systemat. 2001, 32, 305–332. [Google Scholar] [CrossRef] [Green Version]
- Sanders, N.J.; Barton, K.E.; Gordon, D.M. Long-term dynamics of the distribution of the invasive Argentine ant, Linepithema humile, and native ant taxa in northern California. Oecologia 2001, 127, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bayles, B.R.; Thomas, S.M.; Simmons, G.S.; Grafton-Cardwell, E.E.; Daugherty, M.P. Spatiotemporal dynamics of the SouthernCalifornia Asian citrus psyllid (Diaphorina citri) invasion. PLoS ONE 2017, 12, e0173226. [Google Scholar] [CrossRef] [Green Version]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Capinha, C.; Brotons, L.; Anastácio, P. Geographical variability in propagule pressure and climatic suitability explain the European distribution of two highly invasive crayfish. J. Biogeogr. 2013, 40, 548–558. [Google Scholar] [CrossRef]
- Hanna, C.; Cook, E.D.; Thompson, A.R.; Dare, L.E.; Palaski, A.L.; Foote, D.; Goodisman, M.A.D. Colony social structure in native and invasive populations of the social wasp Vespula pensylvanica. Biol. Invasions 2014, 16, 283–294. [Google Scholar] [CrossRef]
- Châu, L.M.; Hanna, C.; Jenkins, L.T.; Kutner, R.E.; Burns, E.A.; Kremen, C.; Goodisman, M.A.D. Population genetic structure of the predatory, social wasp Vespula pensylvanica in its native and invasive range. Ecol. Evol. 2015, 5, 5573–5587. [Google Scholar] [CrossRef]
- Eloff, J.; Veldtman, R.; Bulgarella, M.; Lester, P.J. Population genetics of the invasive wasp Vespula germanica in South Africa. Insectes Sociaux 2020, 67, 229–238. [Google Scholar] [CrossRef]
- Schmack, J.M.; Brenton-Rule, E.C.; Veldtman, R.; Wenseleers, T.; Beggs, J.R.; Lester, P.J.; Bulgarella, M. Lack of genetic structuring, low effective population sizes and major bottlenecks characterise common and German wasps in New Zealand. Biol. Invasions 2019, 21, 3185–3201. [Google Scholar] [CrossRef]
- De Villiers, M.; Kriticos, D.J.; Veldtman, R. Including irrigation in niche modelling of the invasive wasp Vespula germanica (Fabricius) improves model fit to predict potential for further spread. PLoS ONE 2017, 12, e0181397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorvari, J. Habitat preferences and spring temperature-related abundance of German wasp Vespula germanica in its northern range. Insect Conserv. Divers. 2018, 11, 363–369. [Google Scholar] [CrossRef]
- Hammer, S.; Jensen, J.-K. The invasion of two species of social wasps (Hymenoptera, Vespidae) to the Faroe Islands. BioInvasion Rec. 2019, 8, 558–567. [Google Scholar] [CrossRef]
- Farji-Brener, A.; Corley, J.C. Successful invasions of Hymenopteran insects into NW Patagonia. Ecol. Austral 1998, 8, 237–249. Available online: https://hdl.handle.net/20.500.12110/ecologiaaustral_v008_n02_p237 (accessed on 15 April 2021).
- Clapperton, B.K.; Moller, H.; Sandlant, G.R. Distribution of social wasps (Hymenoptera: Vespidae) in New Zealand in 1987. N. Z. J. Zool. 1989, 16, 315–323. [Google Scholar] [CrossRef]
- Whitehead, V.B.; Prins, A.J. The European Wasp, Vespula germanica (F.), in the Cape Peninsula. J. Entomol. Soc. South. Am. 1975, 38, 39–42. Available online: https://hdl.handle.net/10520/AJA00128789_3335 (accessed on 1 June 2021).
- Veldtman, R.; Addison, P.; Tribe, G.D. Current status and potential future impact of invasive vespid wasps (Vespula germanica and Polistes dominulus) in South Africa. In Working Group ‘Landscape Management for Functional Biodiversity’, Proceedings of the Meeting at Lleida, Lleida, Spain, 7–10 May 2012; Holland, J., Gerowitt, B., Alomar, O., Bianchi, F., Egenschwiler, L., van Helden, M., Moonen, C., Poehling, H.-M., Rossing, W., Eds.; IOBC/Wprs Bulletin: Lleida, Spain, 2012; Volume 75, pp. 217–221. ISBN 978-92-9067-252-4. [Google Scholar]
- Davies, S.J.; Jordaan, M.; Karsten, M.; Terblanche, J.S.; Turner, A.A.; van Wilgen, N.J.; Veldtman, R.; Zengeya, T.A.; Measey, J. Experience and Lessons from Alien and Invasive Animal Control Projects in South Africa. In Biological Invasions in South Africa; van Wilgen, B., Measey, J., Richardson, D., Wilson, J., Zengeya, T., Eds.; Invading Nature-Springer Series in Invasion Ecology; Springer: Cham, Switzerland, 2020; p. 14. [Google Scholar]
- Veldtman, R.; Daly, D.; Bekker, G.F.H.V.G. Spatio–environmental analysis of Vespula germanica nest records explains slow invasion in South Africa. Insects 2021, 12, 732. [Google Scholar] [CrossRef]
- Rankin, E.E.W. Emerging patterns in social wasp invasions. Curr. Opin. Insect Sci. 2021, 46, 72–77. [Google Scholar] [CrossRef]
- Brenton-Rule, E.C.; Dobelmann, J.; Baty, J.W.; Brown, R.L.; Dvorak, L.; Grangier, J.; Masciocchi, M.; McGrannachan, C.; Shortall, C.R.; Schmack, J.; et al. The origins of global invasions of the German wasp (Vespula germanica) and its infection with four honey bee viruses. Biol. Invasions 2018, 20, 3445–3460. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Isolation of high-molecular-weight DNA using organic solvents. Cold Spring Harb. Protoc. 2017, 4, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogen. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canbäck, B. ARWEN: A program to detect TRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Lefort, V.; Longueville, J.E.; Gascuel, O. SMS: Smart model selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef] [Green Version]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Dobelmann, J.; Alexander, A.; Baty, J.W.; Gemmell, N.J.; Gruber, M.A.M.; Quinn, O.; Wenseleers, T.; Lester, P.J. The association between mitochondrial genetic variation and reduced colony fitness in an invasive wasp. Mol. Ecol. 2019, 28, 3324–3338. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, C.Y.; Wang, J.D.; He, Z.; Xu, H.C.; Feng, Y. The complete mitochondrial genome of an edible wasp Vespula flaviceps (Hymenoptera, Vespidae). Mitochondrial DNA Part B 2019, 4, 1085–1086. [Google Scholar] [CrossRef] [Green Version]
- Ilyasov, R.A.; Poskryakov, A.V.; Petukhov, A.V.; Nikolenko, A.G. New approach to the mitotype classification in black honeybee Apis mellifera mellifera and Iberian honeybee Apis mellifera iberiensis. Russ. J. Genet. 2016, 52, 281–291. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Nikolenko, A.; Tuktarov, V.; Goto, K.; Takahashi, J.I.; Kwon, H.W. Comparative analysis of mitochondrial genomes of the honey bee subspecies A. m. caucasica and A. m. carpathica and refinement of their evolutionary lineages. J. Apic. Res. 2019, 58, 567–579. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Housley, D.J.E.; Zalewski, Z.A.; Beckett, S.E.; Venta, P.J. Design factors that influence PCR amplification success of cross-species primers among 1147 mammalian primer pairs. BMC Genom. 2006, 7, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Hu, Y.L.; Xu, Z.F.; Wei, S.J. The mitochondrial genome of the German wasp Vespula germanica (Fabricius, 1793) (Hymenoptera: Vespoidea: Vespidae). Mitochondrial DNA 2016, 27, 2917–2918. [Google Scholar] [CrossRef] [PubMed]



| Region | Location | Primer | Sequence (5′-3′) | Size (bp) |
|---|---|---|---|---|
| Ves1 | COX1 | Ves1-F | GATTTTGATTATTACCTCCATCC | 686 |
| Ves1-R * | ACCGTAAATTGTTGCTAATCATC | |||
| Ves2 | COX3 | Ves2-F | ACCAATGATGACGAGACGTAG | 550 |
| Ves2-R * | GCAGCTTCGTATCCAATATGG | |||
| Ves3 | ND5 | Ves3-F * | CCTCATATTCTAGAAAGATATAAC | 1145 |
| Ves3-R * | TGTCCAAATATTATGGGTTTATTG | |||
| Ves5 | CytB | Ves5-F * | TCTGCTATTCCTTATATTGGCC | 608 |
| Ves5-R * | TTTGAGTTAATTCAATGAAAGGTG | |||
| Ves6 | COX1-COX2 | Ves6-F * | CTCGTGCATATTTTACATCTGC | 1189 |
| Ves6-R * | ATCTGTTGATGTTGTTAGAATTC |
| Eloff et al. [9] | n | Freq |
|---|---|---|
| Hap1 | 43 | 0.74 |
| Hap2 | 8 | 0.14 |
| Hap3 | 2 | 0.03 |
| Hap4 | 2 | 0.03 |
| Hap5 | 2 | 0.03 |
| Hap6 | 1 | 0.02 |
| Total number of individuals | 58 | 1.00 |
| Total number of nests | 41 | - |
| This study | n | Freq |
| Hap1 | 58 | 0.76 |
| Hap2 | 18 | 0.24 |
| Total number of individuals | 76 | 1.00 |
| Total number of nests | 72 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Asch, B.; Wolf, M.; Marais, I.; Daly, D.; Veldtman, R. Mitogenomics and the Global Dispersion of Vespula germanica: A Case Study from South Africa Shows Evidence for Two Separate Invasion Events. Diversity 2022, 14, 154. https://doi.org/10.3390/d14030154
van Asch B, Wolf M, Marais I, Daly D, Veldtman R. Mitogenomics and the Global Dispersion of Vespula germanica: A Case Study from South Africa Shows Evidence for Two Separate Invasion Events. Diversity. 2022; 14(3):154. https://doi.org/10.3390/d14030154
Chicago/Turabian Stylevan Asch, Barbara, Michael Wolf, Inès Marais, Derek Daly, and Ruan Veldtman. 2022. "Mitogenomics and the Global Dispersion of Vespula germanica: A Case Study from South Africa Shows Evidence for Two Separate Invasion Events" Diversity 14, no. 3: 154. https://doi.org/10.3390/d14030154
APA Stylevan Asch, B., Wolf, M., Marais, I., Daly, D., & Veldtman, R. (2022). Mitogenomics and the Global Dispersion of Vespula germanica: A Case Study from South Africa Shows Evidence for Two Separate Invasion Events. Diversity, 14(3), 154. https://doi.org/10.3390/d14030154







