Diversity and Distribution of Theileria Species and Their Vectors in Ruminants from India, Pakistan and Bangladesh
Abstract
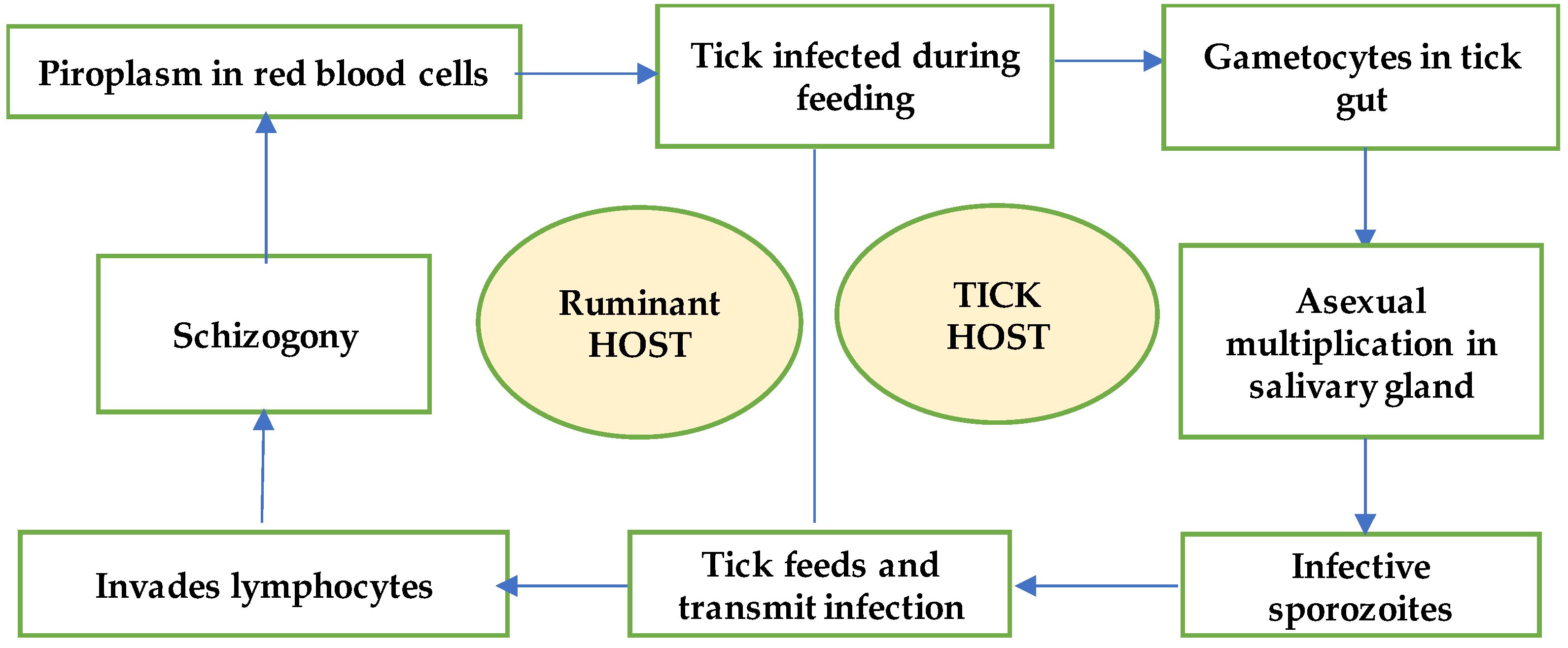
1. Introduction
2. Materials and Methods
2.1. Study Protocol
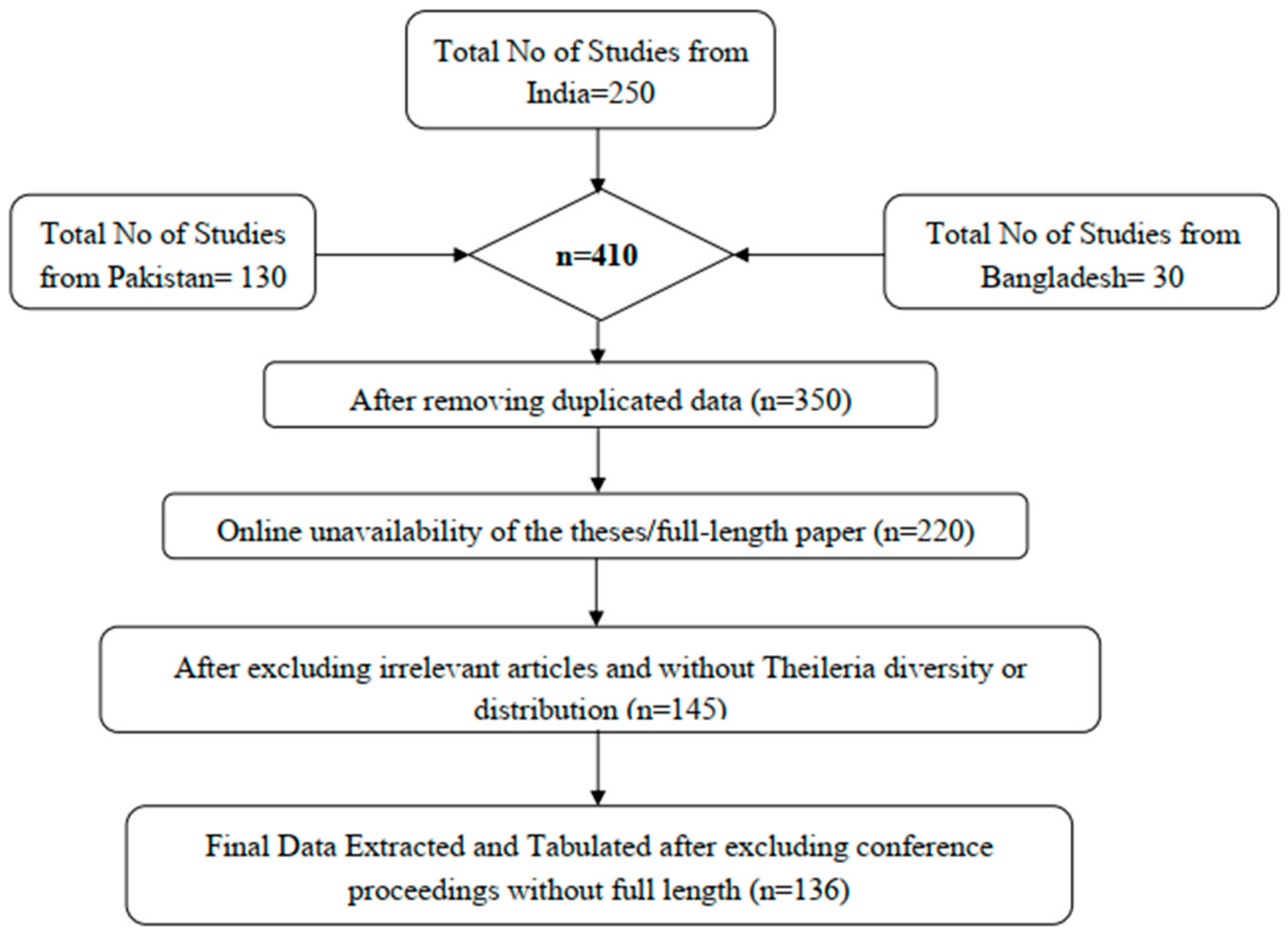
2.2. Literature Search Strategy
2.3. Data Extraction and Qualitative Assessment
2.4. Phylogenetic Analysis and Evolutionary Divergence
3. Results and Discussion
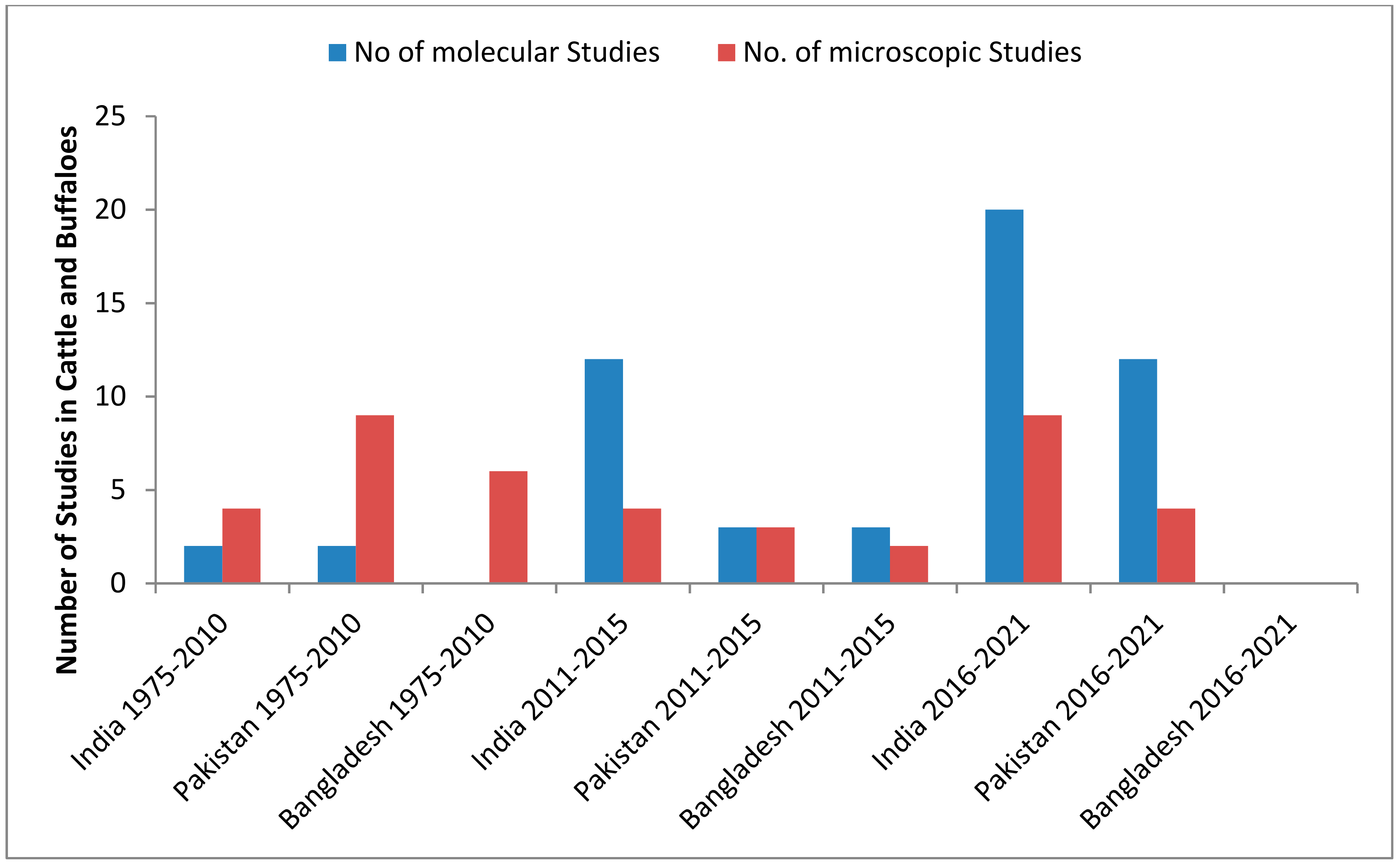
3.1. Diversity and Distribution of Theileria Species Infecting Livestock
3.2. Diversity and Distribution of Theileria Species Infecting Livestock in the Different Regions of India
3.3. Possible Tick Vectors for Theileria Species in India
3.4. Diversity and Distribution of Theileria Species in Different Regions of Pakistan
3.5. Tick Vectors for Transmission of Theileria in Pakistan
3.6. Regional Wise Distribution of Theileria Species and Its Possible Tick Vectors in Bangladesh
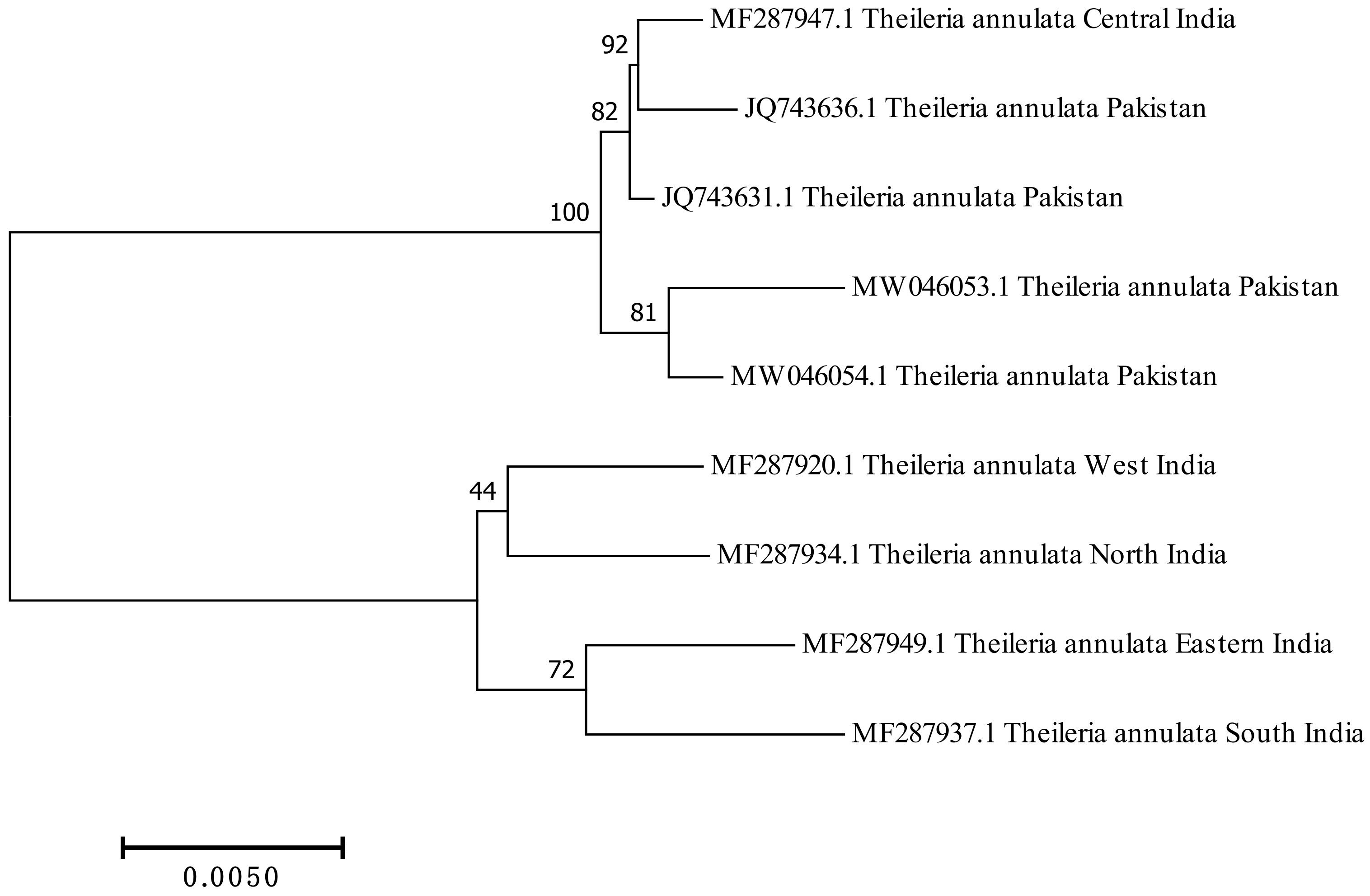
3.7. Phylogenetic Analysis, Genetic Divergence and Multiple Sequence Alignment
3.8. Overall Comparison (Why Vector-Borne Diseases like Theileria Are Increasing Day by Day)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lew-Tabor, A.E.; Valle, M.R. A review of reverse vaccinology approaches for the development of vaccines against ticks and tick borne diseases. Ticks Tick-Borne Dis. 2016, 7, 573–585. [Google Scholar] [CrossRef]
- Nene, V.; Kiara, H.; Lacasta, A.; Pelle, R.; Svitek, N.; Steinaa, L. The biology of Theileria parva and control of East Coast fever–current status and future trends. Ticks Tick-Borne Dis. 2016, 7, 549–564. [Google Scholar] [CrossRef]
- Ica, A.; Vatansever, Z.; Yildirim, A.; Duzlu, O.; Inci, A. Detection of Theileria and Babesia species in ticks collected from cattle. Vet. Parasitol. 2007, 148, 156–160. [Google Scholar] [CrossRef]
- Jenkins, C. Bovine theileriosis in Australia: A decade of disease. Microbiol. Aust. 2018, 39, 215–219. [Google Scholar] [CrossRef]
- Rashid, M.; Akbar, H.; Rashid, I.; Saeed, K.; Ahmad, L.; Ahmad, A.S.; Shehzad, W.; Islam, S.; Farooqi, S. Economic significance of tropical theileriosis on a Holstein Friesian dairy farm in Pakistan. J. Parasitol. 2018, 104, 310–312. [Google Scholar] [CrossRef]
- Gul, N.; Ayaz, S.; Gul, I.; Adnan, M.; Shams, S.; Akbar, N. Tropical theileriosis and east coast fever in cattle: Present, past and future perspective. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 1000–1018. [Google Scholar]
- Khan, M.A.; Khan, M.; Ahmad, I.; Khan, M.; Anjum, A.; Durrani, A.; Hameed, K.; Kakar, I.; Wajid, A.; Ramazan, M. Risk factors assessment and molecular characterization of Theileria in small ruminants of Balochistan. J. Anim. Plant Sci. 2017, 27, 1190–1196. [Google Scholar]
- Shaw, M.K.; Tilney, L.G. How individual cells develop from a syncytium: Merogony in Theileria parva (Apicomplexa). J. Cell Sci. 1992, 101, 109–123. [Google Scholar] [CrossRef]
- Bishop, R.; Musoke, A.; Morzaria, S.; Gardner, M.; Nene, V. Theileria: Intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitology 2004, 129, S271–S283. [Google Scholar] [CrossRef]
- Dobbelaere, D.; Heussler, V. Transformation of Leukocytes by Theileria parva and T. annulata. Annu. Rev. Microbiol. 1999, 53, 1–42. [Google Scholar] [CrossRef]
- Conrad, P.A.; Denham, D.; Brown, C.G.D. Intraerythrocytic multiplication of Theileria parva in vitro: An ultrastructural study. Int. J. Parasitol. 1986, 16, 223–229. [Google Scholar] [CrossRef]
- Kawamoto, S.; Takahashi, K.; Kurosawa, T.; Sonoda, M.; Onuma, M. Intraerythrocytic schizogony of Theileria sergenti in cattle. Jpn. J. Vet. Sci. 1990, 52, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Katzer, F.; Ngugi, D.; Oura, C.; Bishop, R.P.; Taracha, E.L.N.; Walker, A.R.; McKeever, D.J. Extensive genotypic diversity in a recombining population of the apicomplexan parasite Theileria parva. Infect. Immun. 2006, 74, 5456–5464. [Google Scholar] [CrossRef] [PubMed]
- Weir, W.; Ben-Miled, L.; Karagenç, T.; Katzer, F.; Darghouth, M.; Shiels, B.; Tait, A. Genetic exchange and sub-structuring in Theileria annulata populations. Mol. Biochem. Parasitol. 2007, 154, 170–180. [Google Scholar] [CrossRef] [PubMed]
- OIE. THEILERIOSIS Aetiology Epidemiology Diagnosis Prevention and Control References; OIE: Paris, France, 2021; Available online: https://www.oie.int/app/uploads/2021/03/theileriosis.pdf (accessed on 26 December 2021).
- Sivakumar, T.; Hayashida, K.; Sugimoto, C.; Yokoyama, N. Evolution and genetic diversity of Theileria. Infect. Genet. Evol. 2014, 27, 250–263. [Google Scholar] [CrossRef]
- Böse, R.; Jorgensen, W.K.; Dalgliesh, R.J.; Friedhoff, K.T.; De Vos, A.J. Current state and future trends in the diagnosis of babesiosis. Vet. Parasitol. 1995, 57, 61–74. [Google Scholar] [CrossRef]
- Passos, L.M.F.; Bell-Sakyi, L.; Brown, C.G.D. Immunochemical characterization of in vitro culture-derived antigens of Babesia bovis and Babesia bigemina. Vet. Parasitol. 1998, 76, 239–249. [Google Scholar] [CrossRef]
- Gubbels, J.M.; De Vos, A.P.; Van der Weide, M.; Viseras, J.; Schouls, L.M.; De Vries, E.; Jongejan, F. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J. Clin. Microbiol. 1999, 37, 1782–1789. [Google Scholar] [CrossRef]
- Rougemont, M.; Van Saanen, M.; Sahli, R.; Hinrikson, H.P.; Bille, J.; Jaton, K. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J. Clin. Microbiol. 2004, 42, 5636–5643. [Google Scholar] [CrossRef]
- Mens, P.F.; Schoone, G.J.; Kager, P.A.; Schallig, H.D.F.H. Detection and identification of human Plasmodium species with real-time quantitative nucleic acid sequence-based amplification. Malar. J. 2006, 5, 80. [Google Scholar] [CrossRef]
- Steenkeste, N.; Incardona, S.; Chy, S.; Duval, L.; Ekala, M.-T.; Lim, P.; Hewitt, S.; Sochantha, T.; Socheat, D.; Rogier, C. Towards high-throughput molecular detection of Plasmodium: New approaches and molecular markers. Malar. J. 2009, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, O.; Arango, E.; Maestre, A.; Carmona-Fonseca, J. Prevalence of gestational, placental and congenital malaria in north-west Colombia. Malar. J. 2013, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Haanshuus, C.G.; Mohn, S.C.; Mørch, K.; Langeland, N.; Blomberg, B.; Hanevik, K. A novel, single-amplification PCR targeting mitochondrial genome highly sensitive and specific in diagnosing malaria among returned travellers in Bergen, Norway. Malar. J. 2013, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Lai, Y.-H. Fabrication and Characterization of HER2 Cell Receptor-Targeted Indocyanine Green-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles. Peertechz J. Biomed. Eng. 2015, 1, 15–20. [Google Scholar]
- Bilgiç, H.B.; Karagenç, T.; Simuunza, M.; Shiels, B.; Tait, A.; Eren, H.; Weir, W. Development of a multiplex PCR assay for simultaneous detection of Theileria annulata, Babesia bovis and Anaplasma marginale in cattle. Exp. Parasitol. 2013, 133, 222–229. [Google Scholar] [CrossRef]
- Chaisi, M.E.; Janssens, M.E.; Vermeiren, L.; Oosthuizen, M.C.; Collins, N.E.; Geysen, D. Evaluation of a Real-Time PCR Test for the Detection and Discrimination of Theileria Species in the African Buffalo (Syncerus caffer). PLoS ONE 2013, 8, e75827. [Google Scholar] [CrossRef]
- Kundave, V.R.; Ram, H.; Banerjee, P.S.; Garg, R.; Mahendran, K.; Ravikumar, G.; Tiwari, A.K. Development of multiplex PCR assay for concurrent detection of tick borne haemoparasitic infections in bovines. Acta Parasitol. 2018, 63, 759–765. [Google Scholar] [CrossRef]
- Ayadi, O.; Gharbi, M.; Elfegoun, M.C.B. Milk losses due to bovine tropical theileriosis (Theileria annulata infection) in Algeria. Asian Pac. J. Trop. Biomed. 2016, 6, 801–802. [Google Scholar] [CrossRef]
- Kerario, I.I.; Simuunza, M.; Laisser, E.L.K.; Chenyambuga, S. Exploring knowledge and management practices on ticks and tick-borne diseases among agro-pastoral communities in Southern Highlands, Tanzania. Vet. World 2018, 11, 48. [Google Scholar] [CrossRef]
- Elsheikha, H. Management of ticks and tick-borne diseases: Challenges and opportunities. Vet. Nurse 2019, 10, 60–63. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Sangwan, A.K.; Chhabra, M.B.; Samantaray, S. Relative role of male and female Hyalomma anatolicum anatolicum ticks in Theileria transmission. Vet. Parasitol. 1989, 31, 83–87. [Google Scholar] [CrossRef]
- Naik, B.S.; Maiti, S.K.; Raghuvanshi, P.D.S. Prevalence of tropical theileriosis in cattle in Chhattisgarh State. J. Anim. Res. 2016, 6, 1043–1045. [Google Scholar] [CrossRef]
- Das, G.; Ray, D. PCR-based detection of Theileria annulata infection in ticks collected from cattle of West Bangal, India. J. Vet. Parasitol. 2003, 17, 11–14. [Google Scholar]
- Kartashov, M.Y.; Naidenova, E.V.; Zakharov, K.S.; Yakovlev, S.A.; Skarnovich, M.O.; Boumbaly, S.; Nikiforov, K.A.; Plekhanov, N.A.; Kritzkiy, A.A.; Ternovoi, V.A. Detection of Babesia caballi, Theileria mutans and Th. velifera in ixodid ticks collected from cattle in Guinea in 2017–2018. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100564. [Google Scholar] [CrossRef]
- Ananda, K.J.; D’Souza, P.E.; Puttalakshmamma, G.C. Prevalence of Haemoprotozoan diseases in crossbred cattle in Banglore north. Vet. World 2009, 2, 15. [Google Scholar] [CrossRef]
- Nair, A.S.; Ravindran, R.; Lakshmanan, B.; Kumar, S.S.; Tresamol, P.V.; Saseendranath, M.R.; Senthilvel, K.; Rao, J.R.; Tewari, A.K.; Ghosh, S. Haemoprotozoa of cattle in northern Kerala, India. Trop. Biomed. 2011, 28, 68–75. [Google Scholar]
- Maharana, B.R.; Kumar, B.; Prasad, A.; Patbandha, T.K.; Sudhakar, N.R.; Joseph, J.P.; Patel, B.R. Prevalence and assessment of risk factors for haemoprotozoan infections in cattle and buffaloes of South-West Gujarat, India. Indian J. Anim. Res. 2016, 50, 733–739. [Google Scholar]
- Aparna, M.; Vimalkumar, M.B.; Varghese, S.; Senthilvel, K.; Ajithkumar, K.G.; Raji, K.; Syamala, K.; Priya, M.N.; Deepa, C.K.; Jyothimol, G. Phylogenetic analysis of bovine Theileria spp. isolated in south India. Trop. Biomed. 2013, 30, 281–290. [Google Scholar]
- George, N.; Bhandari, V.; Reddy, D.P.; Sharma, P. Emergence of new genotype and diversity of Theileria orientalis parasites from bovines in India. Infect. Genet. Evol. 2015, 36, 27–34. [Google Scholar] [CrossRef]
- Ananda, K.J.; Adeppa, J. Prevalence of Haemoprotozoan infections in bovines of Shimoga region of Karnataka state. J. Parasit. Dis. 2016, 40, 890–892. [Google Scholar]
- Nimisha, M.; Devassy, J.K.; Pradeep, R.K.; Pakideery, V.; Sruthi, M.K.; Pious, A.; Kurbet, P.S.; Amrutha, B.M.; Chandrasekhar, L.; Deepa, C.K. Ticks and accompanying pathogens of domestic and wild animals of Kerala, South India. Exp. Appl. Acarol. 2019, 79, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.M.; Das, M.; Senapati, S.K.; Jena, G.R.; Mishra, C.; Mohanty, B.; Panda, S.K. Transplacental transmission of Theileria annulata in cattle confirmed by molecular techniques. J. Parasit. Dis. 2021, 45, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Dehuri, M.; Panda, M.; Sahoo, N.; Mohanty, B.; Behera, B. Nested PCR assay for detection of Theileria annulata in Hyalomma anatolicum infesting cattle from coastal Odisha, India. Anim. Biotechnol. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Banerji, P.K.; Samaddar, J.; Gupta, R.; Paul, J.; Guha, C. Natural infection with Theileria hirci in Jumnapari goat—A case report. Indian Vet. J. 1990, 67, 677–678. [Google Scholar]
- Shruthi, R.; Thimmareddy, P.M.; Mamatha, G.S.; Chandranaik, B.M.; Puttalakshmamma, G.C. Studies on theileriosis in goats from Karnataka, South India. J. Parasit. Dis. 2017, 41, 1082–1085. [Google Scholar] [CrossRef]
- Mamatha, G.S.; Shruthi, R.; Chandranaik, B.M.; D’Souza, P.E.; Thimmareddy, P.M.; Shivashankar, B.P.; Puttalakshmamma, G.C. Molecular epidemiology and phylogenetic characterisation of Theileria luwenshuni in India: A first report. Small Rumin. Res. 2017, 154, 52–57. [Google Scholar] [CrossRef]
- Begam, R.; Talukdar, S.K.; Sarmah, P.C.; Bulbul, K.H.; Kakati, P.; Tamuly, S.; Islam, S. Molecular and microscopic detection of Theileria luwenshuni infection in goats in and around Guwahati of Assam, India. Biol. Rhythm Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Begam, R.; Talukdar, S.; Sarmah, P.C.; Bulbul, K.H.; Kakati, P.; Neog, R.; Saleque, A.; Tamuly, S.; Tamuli, S.M. Emergence of Theileria luwenshuni infection in goats of Assam, India. J. Entomol. Zool. Stud. 2018, 6, 100. [Google Scholar]
- Bhosale, A.A.; Bhikane, A.U.; Chavhan, S.G.; Jadhav, R.K.; Mohan, A.; Kushwaha, N. Prevalence and Clinico-Therapeutic Management of Bubaline Theileriosis in Marathwada Region of Maharashtra. Int. J. Livest. Res. 2020, 10, 155–165. [Google Scholar] [CrossRef]
- Kundave, V.R.; Patel, A.K.; Patel, P.V.; Hasnani, J.J.; Joshi, C.G. Qualitative and quantitative assessment of Theileria annulata in cattle and buffaloes Polymerase Chain Reaction. Trop. Biomed. 2014, 31, 728–735. [Google Scholar] [PubMed]
- Kundave, V.R.; Patel, A.K.; Patel, P.V.; Hasnani, J.J.; Joshi, C.G. Detection of theileriosis in cattle and buffaloes by polymerase chain reaction. J. Parasit. Dis. 2015, 39, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, H.C.; Patel, B.K.; Bhagat, A.G.; Patel, M.V.; Patel, S.I.; Raval, S.H.; Panchasara, H.H.; Shrimali, M.D.; Patel, A.C.; Chandel, B.S. Comparison of molecular and microscopic technique for detection of Theileria annulata from the field cases of cattle. Vet. World 2015, 8, 1370–1374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kundave, V.R.; Ram, H.; Shahzad, M.; Garg, R.; Banerjee, P.S.; Nehra, A.K.; Rafiqi, S.I.; Ravikumar, G.; Tiwari, A.K. Genetic characterization of Theileria species infecting bovines in India. Infect. Genet. Evol. 2019, 75, 103962. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, S.D.; Kolte, S.W.; Ponnudurai, G.; Kurkure, N.; Magar, S.; Velusamy, R.; Rani, N.; Rubinibala, B.; Rekha, B.; Alagesan, A. The impact of tick-borne pathogen infection in Indian bovines is determined by host type but not the genotype of Theileria annulata. Infect. Genet. Evol. 2019, 75, 103972. [Google Scholar] [CrossRef]
- Azhahianambi, P.; Madhanmohan, M.; Madan, N.; Kumaran, D.; Priyadharshini, M.L.M.; Bharathi, R.; Senthilkumar, T.M.A.; Manoharan, S. Successful treatment of severe form of bovine tropical theileriosis in dairy cattle and genotyping of Theileria annulata isolates of Tamil Nadu, India. Vet. Parasitol. Reg. Stud. Rep. 2021, 26, 100628. [Google Scholar] [CrossRef]
- Kolte, S.W.; Larcombe, S.D.; Jadhao, S.G.; Magar, S.P.; Warthi, G.; Kurkure, N.V.; Glass, E.J.; Shiels, B.R. PCR diagnosis of tick-borne pathogens in Maharashtra state, India indicates fitness cost associated with carrier infections is greater for crossbreed than native cattle breeds. PLoS ONE 2017, 12, e0174595. [Google Scholar] [CrossRef]
- Manuja, A.; Malhotra, D.V.; Sikka, V.K.; Sangwan, A.K.; Sharma, R.; Kumar, B.; Mehta, B.D.; Gulati, B.R.; Nichani, A.K. Isolates of Theileria annulata collected from different parts of India show phenotypic and genetic diversity. Vet. Parasitol. 2006, 137, 242–252. [Google Scholar] [CrossRef]
- Velusamy, R.; Rani, N.; Ponnudurai, G.; Anbarasi, P. Prevalence of intestinal and haemoprotozoan parasites of small ruminants in Tamil Nadu, India. Vet. World 2015, 8, 1205. [Google Scholar] [CrossRef]
- Jayaram, A.S.; Soundararajan, C.; Latha, B.R.; Senthilkumar, T.M.A. Molecular detection of Theileria Luwenshuni in sheep and goats of Chennai, Tamil Nadu. Indian J. Small Rumin. 2019, 25, 242–246. [Google Scholar] [CrossRef]
- Dhaygude, V.S.; Kundu, K.; Kamdi, B.P.; Bagal, U.R.; Bhosale, S.B.; Sabharwal, D. Investigations on first confirmed outbreak of ovine theileriosis (Theileria luwenshuni) from Maharashtra state, India. Indian J. Anim. Res. 2021, 55, 951–955. [Google Scholar] [CrossRef]
- Devi, G.; Ajith, Y.; Mal, G.; Dimri, U.; Preena, P.; Jairath, G.; Kattoor, J.J.; Jacob, S.S.; Singh, B.; Dhar, J.B. Migratory Gaddi sheep and goats as potential carriers of Theileria infection: A molecular survey. Trop. Anim. Health Prod. 2021, 53, 302. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyulu, Y.; Chaudhri, R.P.; Gill, B.S. Transstadial transmission of Theileria annulata through common ixodid ticks infesting Indian cattle. Parasitology 1975, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.S.; Sharma, N.N. Potential of immunoprophylaxis using cobalt-60 irradiated Theileria annulata in salivary gland suspensions of the tick Hyalomma anatolicum. Vet. Parasitol. 1977, 3, 183–188. [Google Scholar] [CrossRef]
- Sudan, V.; Sharma, R.L.; Yadav, R.; Borah, M.K. Turning sickness in a cross bred cow naturally infected with Theileria annulata. J. Parasit. Dis. 2012, 36, 226–229. [Google Scholar] [CrossRef][Green Version]
- Sudan, V.; Singh, S.K.; Jaiswal, A.K.; Parashar, R.; Shanker, D. First molecular evidence of the transplacental transmission of Theileria annulata. Trop. Anim. Health Prod. 2015, 47, 1213–1215. [Google Scholar] [CrossRef]
- Kumar, A.; Gaur, G.K.; Gandham, R.K.; Panigrahi, M.; Ghosh, S.; Saravanan, B.C.; Bhushan, B.; Tiwari, A.K.; Sulabh, S.; Priya, B. Global gene expression profile of peripheral blood mononuclear cells challenged with Theileria annulata in crossbred and indigenous cattle. Infect. Genet. Evol. 2017, 47, 9–18. [Google Scholar] [CrossRef]
- Mohmad, A.; Chandra, D.; Saravanan, B.C.; Manjunathchar, H.V.; OR, V.K.; Fular, A.; Chigure, G.; Kaur, N.; Ghosh, S. Development of a recombinant TaSP-based Dot-ELISA for detection of Theileria annulata infection in cattle. Ticks Tick. Borne. Dis. 2018, 9, 1416–1420. [Google Scholar] [CrossRef]
- Aparna, M.; Ravindran, R.; Vimalkumar, M.B.; Lakshmanan, B.; Rameshkumar, P.; Kumar, K.G.A.; Promod, K.; Ajithkumar, S.; Ravishankar, C.; Devada, K. Molecular characterization of Theileria orientalis causing fatal infection in crossbred adult bovines of South India. Parasitol. Int. 2011, 60, 524–529. [Google Scholar] [CrossRef]
- Velusamy, R.; Rani, N.; Ponnudurai, G.; Harikrishnan, T.J.; Anna, T.; Arunachalam, K.; Senthilvel, K.; Anbarasi, P. Influence of season, age and breed on prevalence of haemoprotozoan diseases in cattle of Tamil Nadu, India. Vet. World 2014, 7, 574–578. [Google Scholar] [CrossRef]
- Dharanesha, N.K.; Giridhar, P.; Byregowda, S.M.; Venkatesh, M.D.; Ananda, K.J. Seasonal prevalence of blood parasitic diseases in crossbred cattle of Mysore and its surrounding districts of Karnataka. J. Parasit. Dis. 2017, 41, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, P.; Sudhagar, S.; Goudar, A.L.; Jacob, S.S.; Suresh, K.P. Molecular survey and phylogenetic analysis of tick-borne pathogens in ticks infesting cattle from two South Indian states. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100595. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, R.P.; Renukaprasad, C.; Keshvamurthy, B.S. A note on occurrence of outbreak of theileriosis in sheep in Karnataka. Indian J. Comp. Microbiol. Immunol. Infect. Dis. 1985, 6, 165–166. [Google Scholar]
- Hitaishi, V.N.; Lakshmanan, B.; Shameem, H.; Jose, P.E.; Jain Jose, K.; Sabu, L. Molecular identification of theileriosis in goats of Kerala. Int. J. Sci. Environ. Technol. 2017, 6, 1979–1984. [Google Scholar]
- Biradar, S.S.; D’Souza, P.E.; Mamatha, G.S.; Yathish, H.M.; Siju, S.J. Molecular epidemiology and phylogenetic analysis of Theileria species in sheep. Indian J. Small Rumin. 2019, 25, 192–198. [Google Scholar] [CrossRef]
- Satbige, A.S.; Patil, N.A. Therapeutic Management of Theileriosis in Sheep. Int. J. Livest. Res. 2020, 10, 168–170. [Google Scholar] [CrossRef]
- Kakati, P.; Sarmah, P.C.; Ray, D.; Bhattacharjee, K.; Sharma, R.K.; Barkalita, L.M.; Sarma, D.K.; Baishya, B.C.; Borah, P.; Stanley, B. Emergence of oriental theileriosis in cattle and its transmission through Rhipicephalus (Boophilus) microplus in Assam, India. Vet. World 2015, 8, 1099. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Behera, B.K.; Khuntia, H.K.; Dash, M. Prevalence of carrier state theileriosis in lactating cows. Vet. World 2017, 10, 1471. [Google Scholar] [CrossRef]
- Acharya, A.P.; Panda, S.K.; Prusty, B.K. Diagnosis and confirmation of Theileria annulata infection in cattle in Odisha, India. J. Entomol. Zool. Stud. 2017, 5, 1543–1546. [Google Scholar]
- Selim, A.M.; Senapati, S.K.; Das, M.; Mishra, C.; Patra, R.C.; Panda, S.K. Molecular, epidemiological and haematological evaluation in Theileria orientalis infected cattle from an endemic region in India. Anim. Biotechnol. 2020, 32, 663–670. [Google Scholar] [CrossRef]
- Kala, S.; Gopal Deo, B.; Kumari, N. Prevalence of Theileriosis in Buffaloes during Rainy Season in and Around Patna, Bihar. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2762–2766. [Google Scholar] [CrossRef]
- Roy, S.; Bhandari, V.; Dandasena, D.; Murthy, S.; Sharma, P. Genetic profiling reveals high allelic diversity, heterozygosity and antigenic diversity in the clinical isolates of the Theileria annulata from India. Front. Physiol. 2019, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.S.; Sarkar, S.; Lodh, C.; Gupta, A.R.; Batabyal, S.; Jas, R. Prevalence of Bovine Theileriosis in South Bihar. Pharma Innov. J. 2021, SP-10, 776–780. [Google Scholar]
- Prabhakaran, H.S.; Ghosh, K.K.; Kumari, R.R.; Kumar, P.; Kumar, M. Evaluation of sporozoite and macroschizont antigen (Spm2) of Theileria annulata for its diagnostic potential. Ticks Tick-Borne Dis. 2021, 12, 101691. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, R.D.; Nichani, A.K. Successful long-term in vitro cultivation of Theileria annulata schizonts in media supplemented with homologous and heterologous sera. Vet. Parasitol. 1998, 79, 135–141. [Google Scholar] [CrossRef]
- Govindarajan, R.; Pazhanivel, N.; Sunder, N.; Sekar, M.; Jawahar, T.P.; Purusothaman, V. An outbreak of concurrent infection of theileriosis and sheep pox in Tamil Nadu, India. Indian J. Anim. Sci. 2005, 75, 787–788. [Google Scholar]
- Haque, M.; Singh, N.K.; Rath, S.S. Prevalence of Theileria annulata infection in Hyalomma anatolicum anatolicum in Punjab state, India. J. Parasit. Dis. 2010, 34, 48–51. [Google Scholar] [CrossRef]
- Vahora, S.P.; Patel, J.V.; Patel, B.B.; Patel, S.B.; Umale, R.H. Seasonal incidence of Haemoprotozoal diseases in crossbred cattle and buffalo in Kaira and Anand districts of Gujarat, India. Vet. World 2012, 5, 223. [Google Scholar] [CrossRef]
- Singh, N.K.; Singh, H.; Haque, M.; Rath, S.S. Prevalence of parasitic infections in cattle of Ludhiana district, Punjab. J. Parasit. Dis. 2012, 36, 256–259. [Google Scholar] [CrossRef]
- Tuli, A.; Das Singla, L.; Sharma, A.; Bal, M.S.; Filia, G.; Kaur, P. Molecular epidemiology, risk factors and hematochemical alterations induced by Theileria annulata in bovines of Punjab (India). Acta Parasitol. 2015, 60, 378–390. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, N.K.; Singh, H.; Bhat, S.A.; Rath, S.S. Prevalence of Theileria annulata infection in Hyalomma anatolicum anatolicum collected from crossbred cattle of Ludhiana, Punjab. J. Parasit. Dis. 2015, 39, 57–61. [Google Scholar] [CrossRef][Green Version]
- Bhatnagar, C.S.; Bhardawaj, B.; Sharma, D.K.; Meena, S.K. Incidence of Haemoprotozoan diseases in cattle in Southern Rajasthan, India. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 509–514. [Google Scholar]
- Afifi, N.A.; Shihata, I.M.; El-Zorba, H.Y.; Ismail, I.M. Prevalence of theileriosis in cross-bred cattle: Its detection through blood smear examination and polymerase chain reaction in Dehradun district, Uttarakhand, India. Vet. World 2014, 7, 168. [Google Scholar]
- Ganguly, A.; Bisla, R.S.; Singh, H.; Bhanot, V.; Kumar, A.; Kumari, S.; Maharana, B.R.; Ganguly, I. Prevalence and haematobiochemical changes of tick borne haemoparasitic diseases in crossbred cattle of Haryana, India. Indian J. Anim. Sci. 2017, 87, 552–557. [Google Scholar]
- Ganguly, A.; Maharana, B.R.; Ganguly, I. Pentaplex PCR assay for rapid differential detection of Babesia bigemina, Theileria annulata, Anaplasma marginale and Trypanosoma evansi in cattle. Biologicals 2020, 63, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Kundave, V.R.; Nehra, A.K.; Ram, H.; Kumari, A.; Shahzad, M.; Vinay, T.S.; Garg, R.; Banerjee, P.S.; Singh, G.; Tiwari, A.K. Genetic diversity in the Tams1 gene of Theileria annulata (Duschunkowsky and Luhs, 1904) infecting cattle. Acta Trop. 2021, 224, 106121. [Google Scholar]
- Patial, V.; Gupta, T.; Angaria, S.; Bali, D.; Katoch, A.; Gautam, M.; Singh, N.K.; Sharma, M.; Chahota, R. Theileria orientalis outbreak in an organized cattle breeding farm. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100572. [Google Scholar] [CrossRef]
- Kamau, J.; de Vos, A.J.; Playford, M.; Salim, B.; Kinyanjui, P.; Sugimoto, C. Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasit. Vectors 2011, 4, 22. [Google Scholar]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Zeb, J.; Szekeres, S.; Takács, N.; Kontschán, J.; Shams, S.; Ayaz, S.; Hornok, S. Genetic diversity, piroplasms and trypanosomes in Rhipicephalus microplus and Hyalomma anatolicum collected from cattle in northern Pakistan. Exp. Appl. Acarol. 2019, 79, 233–243. [Google Scholar] [CrossRef]
- Ghosh, S.; Bansal, G.C.; Gupta, S.C.; Ray, D.; Khan, M.Q.; Irshad, H.; Shahiduzzaman, M.D.; Seitzer, U.; Ahmed, J.S. Status of tick distribution in Bangladesh, India and Pakistan. Parasitol. Res. 2007, 101, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, M.; Darghouth, M.A.; Elati, K.; AL-Hosary, A.A.T.; Ayadi, O.; Salih, D.A.; El Hussein, A.M.; Mhadhbi, M.; Khamassi Khbou, M.; Hassan, S.M.; et al. Current status of tropical theileriosis in Northern Africa: A review of recent epidemiological investigations and implications for control. Transbound. Emerg. Dis. 2020, 67, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Shahnawaz, S.; Ali, M.; Aslam, M.A.; Fatima, R.; Chaudhry, Z.I.; Hassan, M.U.; Iqbal, F. A study on the prevalence of a tick-transmitted pathogen, Theileria annulata, and hematological profile of cattle from Southern Punjab (Pakistan). Parasitol. Res. 2011, 109, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Maqbool, A.; Muhammad, K.; Khan, M.; Younis, M. Prevalence of Theileria annulata infected hard ticks of cattle and buffalo in Punjab, Pakistan. DNA 2013, 862, 846. [Google Scholar]
- Khan, M.K.; He, L.; Hussain, A.; Azam, S.; Zhang, W.-J.; Wang, L.-X.; Zhang, Q.-L.; Hu, M.; Zhou, Y.-Q.; Zhao, J. Molecular epidemiology of Theileria annulata and identification of 18S rRNA gene and ITS regions sequences variants in apparently healthy buffaloes and cattle in Pakistan. Infect. Genet. Evol. 2013, 13, 124–132. [Google Scholar] [CrossRef]
- Ghafar, A.; Cabezas-Cruz, A.; Galon, C.; Obregon, D.; Gasser, R.B.; Moutailler, S.; Jabbar, A. Bovine ticks harbour a diverse array of microorganisms in Pakistan. Parasit. Vectors 2020, 13, 1. [Google Scholar] [CrossRef]
- Gebrekidan, H.; Abbas, T.; Wajid, M.; Ali, A.; Gasser, R.B.; Jabbar, A. Molecular characterisation of Theileria orientalis in imported and native bovines from Pakistan. Infect. Genet. Evol. 2017, 47, 19–25. [Google Scholar] [CrossRef]
- Hassan, M.A.; Liu, J.; Sajid, M.S.; Mahmood, A.; Zhao, S.; Abbas, Q.; Guan, G.; Yin, H.; Luo, J. Molecular detection of Theileria annulata in cattle from different regions of Punjab, Pakistan, by using recombinase polymerase amplification and polymerase chain reaction. J. Parasitol. 2018, 104, 196–201. [Google Scholar] [CrossRef]
- Rehman, A.; Conraths, F.J.; Sauter-Louis, C.; Krücken, J.; Nijhof, A.M. Epidemiology of tick-borne pathogens in the semi-arid and the arid agro-ecological zones of Punjab province, Pakistan. Transbound. Emerg. Dis. 2019, 66, 526–536. [Google Scholar]
- Qayyum, M.; Farooq, U.; Samad, H.A.; Chauhdry, H.R. Prevalence, clinicotherapeutic and prophylactic studies on theileriosis in district Sahiwal (Pakistan). J. Anim. Plant Sci. 2010, 20, 266–270. [Google Scholar]
- Parveen, A.; Alkhaibari, A.M.; Asif, M.; Almohammed, H.I.; Naqvi, Z.; Khan, A.; Aktas, M.; Ozubek, S.; Farooq, M.; Iqbal, F. Molecular Epidemiology of Theileria annulata in Cattle from Two Districts in Punjab (Pakistan). Animals 2021, 11, 3443. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Ashraf, S.; Khan, A.; Asif, M.; Iqbal, F. Tick and tick-borne diseases in Pakistan. In The Entomological Guide to Rhipicephalus, 1st ed.; Kumar, S., Bayugar, R.C., Sharma, A.K., Miranda, E.M., Chaubey, A.K., Eds.; Nova Science Publishers: New York, NY, USA, 2021. [Google Scholar]
- Shahzad, W.; Haider, N.; Mansur-ud-Din, A.; Munir, R.; Saghar, M.S.; Mushtaq, M.H.; Ahmad, N.; Akbar, G.; Mehmood, F. Prevalence and molecular diagnosis of Babesia ovis and Theileria ovis in Lohi sheep at livestock experiment station (LES), Bahadurnagar, Okara, Pakistan. Iran. J. Parasitol. 2013, 8, 570. [Google Scholar] [PubMed]
- Riaz, M.; Tasawar, Z. Identification of Theileria species (Theileria ovis and Theileria lestoquardi) by PCR in apparently healthy small ruminants in and around Multan, Southern Punjab, Pakistan. Pakistan. J. Anim. Plant Sci. 2017, 27, 809–818. [Google Scholar]
- Habib, F.; Tabbasum, R.; Awais, T.; Sakhawat, A.; Khalil, R.; Sharif, A.; Yousaf, A.; Arshad, M.; Shahnawaz, R.; Shaheen, S.; et al. Prevalence of Bovine Tropical Theileriosis in Cattle in Quetta Balochistan-Pakistan. Arch. Anim. Husb. Dairy Sci. 2021, 2, 1–3. [Google Scholar]
- Chaudhry, U.; Ali, Q.; Rashid, I.; Shabbir, M.Z.; Ijaz, M.; Abbas, M.; Evans, M.; Ashraf, K.; Morrison, I.; Morrison, L.; et al. Development of a deep amplicon sequencing method to determine the species composition of piroplasm haemoprotozoa. Ticks Tick-Borne Dis. 2019, 10, 101276. [Google Scholar] [CrossRef]
- Riaz, M.; Nazir, M.M.; Tasawar, Z.; Ahmed, A.N.; Ayaz, M.M.; Akram, Q.; Lindsay, D.S. Molecular epidemiology and prevalence of Theileria lestoquardi and Theileria ovis infection in goats infested with tick vectors from Multan, Pakistan. J. Med. Entomol. 2019, 56, 844–848. [Google Scholar] [CrossRef]
- Abid, K.; Bukhari, S.; Asif, M.; Sattar, A.; Arshad, M.; Aktas, M.; Ozubek, S.; Shaikh, R.S.; Iqbal, F. Molecular detection and prevalence of Theileria ovis and Anaplasma marginale in sheep blood samples collected from Layyah district in Punjab, Pakistan. Trop. Anim. Health Prod. 2021, 53, 439. [Google Scholar] [CrossRef]
- Farooqi, S.; Ijaz, M.; Saleem, M.; Rashid, M.; Ahmad, S.; Islam, S. Prevalence and molecular diagnosis of Theileria annulata in bovine from three distinct zones of Khyber Pakhtunkhwa province, Pakistan. J. Anim. Plant Sci. 2017, 27, 1836–1841. [Google Scholar]
- Zeb, J.; Shams, S.; Din, I.U.; Ayaz, S.; Khan, A.; Nasreen, N.; Khan, H.; Khan, M.A.; Senbill, H. Molecular epidemiology and associated risk factors of Anaplasma marginale and Theileria annulata in cattle from North-western Pakistan. Vet. Parasitol. 2020, 279, 109044. [Google Scholar] [CrossRef]
- Ullah, R.; Shams, S.; Khan, M.A.; Ayaz, S.; ul Akbar, N.; ud Din, Q.; Khan, A.; Leon, R.; Zeb, J. Epidemiology and molecular characterization of Theileria annulata in cattle from central Khyber Pakhtunkhwa, Pakistan. PLoS ONE 2021, 16, e0249417. [Google Scholar] [CrossRef]
- Ullah, N.; Durrani, A.Z.; Avais, M.; Nisar, A.; Ullah, S.; Khan, M.S.; Mehmood, K.; Khan, M.A.; Haq, I. Prevalence, risk factors and host biomarkers of ovine theileriosis. Pak. J. Zool. 2018, 50, 1211–1216. [Google Scholar] [CrossRef]
- Khan, A.; Niaz, S.; Khan, A.; Ahmed, H.; Khattak, I.; Zeb, J.; Naeem, H.; Hassan, M.A.; Ulucesme, M.C.; Ozubek, S. Molecular detection of small ruminant piroplasmosis and first report of Theileria luwenshuni (Apicomplexa: Theileridae) in small ruminants of Pakistan. Exp. Parasitol. 2020, 212, 107872. [Google Scholar]
- Mohsin, M.; Hameed, K.; Kamal, M.; Ali, A.; Rafiq, N.; Usman, T.; Khan, W.; Abbasi, A.A.; Khan, R.U.; Yousafzai, G.J. Prevalence and risk factors assessment of theileriosis in livestock of Malakand Division, Pakistan. J. Saudi Soc. Agric. Sci. 2021. [Google Scholar] [CrossRef]
- Niaz, S.; Zia Ur Rahman, I.A.; Cossío-Bayúgar, R.; Amaro-Estrada, I.; Alanazi, A.D.; Khattak, I.; Zeb, J.; Nasreen, N.; Khan, A. Molecular prevalence, characterization and associated risk factors of Anaplasma spp. and Theileria spp. in small ruminants in Northern Pakistan. Parasite 2021, 28, 3. [Google Scholar] [CrossRef]
- Bhutto, B.; Gadahi, J.A.; Khuhro, A.; Rajput, H.M.; Bhutto, F.; Rajput, M.A.; Talpur, A.R. A survey on haemo-protozoan parasites in buffaloes of Landhi Dairy Colony, Karachi-Pakistan. Int. J. Agro Vet. Med. Sci. 2012, 6, 73–76. [Google Scholar] [CrossRef]
- Ghafar, A.; Koehler, A.V.; Hall, R.S.; Gauci, C.G.; Gasser, R.B.; Jabbar, A. Targeted next-generation sequencing and informatics as an effective tool to establish the composition of bovine piroplasm populations in endemic regions. Microorganisms 2021, 9, 21. [Google Scholar] [CrossRef]
- Durrani, S.; Khan, Z.; Khattak, R.M.; Andleeb, M.; Ali, M.; Hameed, H.; Taqddas, A.; Faryal, M.; Kiran, S.; Anwar, H.; et al. A comparison of the presence of Theileria ovis by PCR amplification of their SSU rRNA gene in small ruminants from two provinces of Pakistan. Asian Pac. J. Trop. Dis. 2012, 2, 43–47. [Google Scholar] [CrossRef]
- Karim, S.; Budachetri, K.; Mukherjee, N.; Williams, J.; Kausar, A.; Hassan, M.J.; Adamson, S.; Dowd, S.E.; Apanskevich, D.; Arijo, A. A study of ticks and tick-borne livestock pathogens in Pakistan. PLoS Negl. Trop. Dis. 2017, 11, e0005681. [Google Scholar] [CrossRef]
- Ashfaque, M.; Ajmal, M.; Ahmad, S. An outbreak of theileriosis in crossbred neonate calves. Pak. Vet. J. 1983, 3, 44–46. [Google Scholar]
- Muhammad, G. Clinico-epidemiological and therapeutic aspects of bovine theileriosis. Bull Calf Appear Female 1999, 36, 32. [Google Scholar]
- Khan, M.Q.; Zahoor, A.; Jahangir, M.; Mirza, M.A. Prevalence of blood parasites in cattle and buffaloes. Pak. Vet. J. 2004, 24, 193–194. [Google Scholar]
- Zahid, I.A.; Latif, M.; Baloch, K.B. Incidence and treatment of theileriasis and babesiasis. Pak. Vet. J. 2005, 25, 137. [Google Scholar]
- Durrani, A.; Kamal, N.; Khan, M.S. Incidence of theileriosis and estimation of packed cell volume, total erythrocyte count and hemoglobin in buffaloes. J. Anim. Plant Sci. 2006, 16, 85–88. [Google Scholar]
- Durrani, A.Z.; Kamal, N. Identification of ticks and detection of blood protozoa in friesian cattle by polmerase chain reacton test and estimation of blood parameters in district Kasur, Pakistan. Trop. Anim. Health Prod. 2008, 40, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Azizi, H.; Shiran, B.; Dehkordi, A.F.; Salehi, F.; Taghadosi, C. Detection of Theileria annulata by PCR and its comparison with smear method in native carrier cows. Biotechnology 2008, 7, 574–577. [Google Scholar] [CrossRef][Green Version]
- Atif, F.A.; Khan, M.S.; Iqbal, H.J.; Arshad, G.M.; Ashraf, E.; Ullah, S. Prevalence of Anaplasma marginale, Babesia bigemina and Theileria annulata infections among cattle in Sargodha District, Pakistan. Afr. J. Agric. Res. 2012, 7, 302–3307. [Google Scholar]
- Hassan, M.A.; Liu, J.; Sajid, M.S.; Rashid, M.; Mahmood, A.; Abbas, Q.; Guan, G.; Yin, H.; Luo, J. Simultaneous detection of Theileria annulata and Theileria orientalis infections using recombinase polymerase amplification. Ticks Tick-Borne Dis. 2018, 9, 1002–1005. [Google Scholar] [CrossRef]
- Irshad, N.; Qayyum, M.; Hussain, M.; Khan, M.Q. Prevalence of tick infestation and theileriosis in sheep and goats. Pak. Vet. J. 2010, 30, 178–180. [Google Scholar]
- Shabbir, M.Z.; Khan, J.A. Prevalence of theileriosis in sheep in Okara District, Pakistan. Pak. J. Zool. 2010, 42, 639–643. [Google Scholar]
- Durrani, A.Z.; Younus, M.; Kamal, N.; Mehmood, N.; Shakoori, A.R. Prevalence of ovine Theileria species in district Lahore, Pakistan. Pak. J. Zool. 2011, 43, 57–60. [Google Scholar]
- Naz, S.; Maqbool, A.; Ahmed, S.; Ashraf, K.; Ahmed, N.; Saeed, K.; Latif, M.; Iqbal, J.; Ali, Z.; Shafi, K. Prevalence of theileriosis in small ruminants in Lahore-Pakistan. J. Vet. Anim. Sci. 2012, 2, 16–20. [Google Scholar]
- Fatima, M.; Saeed, S.; Shaikh, R.S.; Ali, M.; Iqbal, F. A study on molecular detection of Theileria lestoquardi by PCR amplification in apparently healthy small ruminants from five districts of Southern Punjab. Pak. J. Zool. 2015, 47, 441–446. [Google Scholar]
- Riaz, M.; Tasawar, Z. A study on molecular surveillance of Theileria spp. infection and its impact on hematological and biochemical changes in naturally infected small ruminants at Multan, Pakistan. Pure Appl. Biol. 2017, 6, 1427–1435. [Google Scholar] [CrossRef]
- Afridi, Z.K.; Ahmad, I. Incidence of anaplasmosis, babesiosis and theileriosis in dairy cattlein Peshawar [Pakistan]. Sarhad J. Agric. 2005, 21, 311–316. [Google Scholar]
- Iqbal, F.; Khattak, R.M.; Ozubek, S.; Khattak, M.N.K.; Rasul, A.; Aktas, M. Application of the reverse line blot assay for the molecular detection of Theileria and Babesia sp. in sheep and goat blood samples from Pakistan. Iran. J. Parasitol. 2013, 8, 289. [Google Scholar]
- Zeb, J.; Shams, S.; Ayaz, S.; Din, I.U.; Khan, A.; Adil, N.; Ullah, H.; Raza, A. Epidemiology of ticks and molecular characterization of Rhipicephalus microplus in cattle population in North-Western Pakistan. Int. J. Acarol. 2020, 46, 335–343. [Google Scholar] [CrossRef]
- Khan, A.; Jamil, M.; Ali, A.; Ali, A.; Imdad, S.; Zeeshan, M. Prevalence of Theileriosis in Buffaloes at Government and Private Farms in Tehsil Paharpur, Dera Ismail Khan. Int. J. Mod. Agric. 2021, 10, 4360–4363. [Google Scholar]
- Saeed, S.; Jahangir, M.; Fatima, M.; Shaikh, R.S.; Khattak, R.M.; Ali, M.; Iqbal, F. PCR based detection of Theileria lestoquardi in apparently healthy sheep and goats from two districts in Khyber Pukhtoon Khwa (Pakistan). Trop. Biomed. 2015, 32, 225–232. [Google Scholar]
- Anwar, K.; Din, A. Epidemiology of Tick Borne Heamoprotozoan Infection in Ruminants in District Peshawar, and Periphery, Khyber Pakhtunkhwa,(Pakistan). Am. Sci. Res. J. Eng. Technol. Sci. 2017, 35, 191–200. [Google Scholar]
- Shah, S.S.A.; Khan, M.I.; Rahman, H.U. Epidemiological and hematological investigations of tick-borne diseases in small ruminants in Peshawar and Khyber agency. Pak. J. Adv. Parasitol. 2017, 4, 15–22. [Google Scholar]
- Ullah, N.; Durrani, A.Z.; Ullah, S.; Ullah, S.; Shah, M.K.; Khan, A.Z.; Khan, M.S.; Khan, N.U.; Khan, M.A. A study on potential factors and physiological biomarkers associated with the occurrence of ovine theileriosis. Small Rumin. Res. 2018, 168, 32–38. [Google Scholar] [CrossRef]
- Buriro, S.N.; Phulan, M.S.; Arijo, A.H.; Memon, A.B. Incidence of some haemo-protozoans in Bos indicus and Bubalis bubalis in Hyderabad. Pak. Vet. J. 1994, 14, 28–29. [Google Scholar]
- Abbasi, F.; Abbasi, I.H.R.; Nissa, T.F.; Bhutto, Z.A.; Arain, M.A.; Soomro, R.N.; Siyal, F.A.; Fazlani, S.A. Epidemiological study of tick infestation in buffalo of various regions of district Khairpur, Pakistan. Vet. World 2017, 10, 688–694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soomro, M.H.; Soomro, S.P.; Bhutto, M.B.; Akbar, Z.; Yaqoob, M.; Arijo, A.G. Prevalence of ticks in buffaloes in the upper Sindh Pakistan. Buffalo Bull. 2014, 33, 323–327. [Google Scholar]
- Durrani, A.Z.; Shakoori, A.R. Study on ecological growth conditions of cattle Hyalomma ticks in Punjab, Pakistan. Iran. J. Parasitol. 2009, 14, 19–25. [Google Scholar]
- Rehman, A.; Nijhof, A.M.; Sauter-Louis, C.; Schauer, B.; Staubach, C.; Conraths, F.J. Distribution of ticks infesting ruminants and risk factors associated with high tick prevalence in livestock farms in the semi-arid and arid agro-ecological zones of Pakistan. Parasit. Vectors 2017, 10, 190. [Google Scholar] [CrossRef]
- Zahida, T.; Sumaira, N.; Lashari, M.H. The prevalence of ixodid ticks on buffaloes at private animal farm Bibipur, Multan. Glob. Vet. 2014, 12, 154–157. [Google Scholar]
- Khan, M.N.; Hayar, C.S.; Iqbal, Z.; Hayat, B. Prevalence of ticks on livestock in Faisalabad (Pakistan). Pak. Vet. J. 1993, 13, 182. [Google Scholar]
- Ramzan, M.; Khan, M.S.; Avais, M.; Khan, J.A.; Pervez, K.; Shahzad, W. Prevalence of ecto parasites and comparative efficacy of different drugs against tick infestation in cattle. J. Anim. Plant Sci. 2008, 18, 17–19. [Google Scholar]
- Ahmed, S.; Numan, M.; Manzoor, A.W.; Ali, F.A. Investigations into Ixodidae ticks in cattle in Lahore, Pakistan. Vet. Ital. 2012, 48, 185–191. [Google Scholar]
- Nasreen, N.; Niaz, S.; Khan, A.; Ayaz, S.; Rashid, M.; Khattak, I.; Yu, Z.; Wang, T.; Al Sarraf, M.; Ali, A. Molecular characterization of ticks infesting livestock in Khyber Pakhtunkhwa Province, Pakistan. Int. J. Acarol. 2020, 46, 165–170. [Google Scholar] [CrossRef]
- Hussain, S.I.; Kumar, G.A. Prevalence of ticks (Ixodoidea, Ixodidae) of buffaloes at Thatta and its adjoining areas in the Province of Sindh, Pakistan. Pak. Congr. Zool. 1990, 10, 11–16. [Google Scholar]
- Rafique, N.; Kakar, A.; Iqbal, A.; Masood, Z.; Razzaq, W.; Iqbal, F. Impact assessment of tick species, Rhipicephalus (Boophilus) microplus on the milk productions of cattle’s in the Quetta City of Province Balochistan, Pakistan. Glob. Vet. 2015, 15, 19–23. [Google Scholar]
- Sajid, M.S.; Iqbal, Z.; Khan, M.N.; Muhammad, G. Point prevalence of hard ticks (Ixodids) infesting domestic ruminants of lower Punjab, Pakistan. Int. J. Agric. Biol. 2008, 10, 349–351. [Google Scholar]
- Rafiq, N.; Kakar, A.; Ghani, A.; Iqbal, A.; Achakzai, W.M.; Sadozai, S.; Shafiq, M.; Mengal, M.A. Ixodid ticks (Arachnida: Acari) prevalence associated with risk factors in the bovine host in District Quetta, Balochistan. Pak. J. Zool. 2017, 46, 2113–2121. [Google Scholar] [CrossRef]
- Bibi, S.; Rafique, N.; Kareem, A.; Taj, M.K.; Iqbl, K.; Bibi, A.; Shafiq, M.; Ghafoor, G.; Ghafoor, A.; Ijaz, A. 15. Prevalence and taxonomic identification of hard ticks (Ixodidea) found in livestock of Harnai District, Balochistan, Pakistan. Pure Appl. Biol. 2020, 9, 2330–2338. [Google Scholar] [CrossRef]
- Cruz, D.D.; Arellano, E.; Denis Ávila, D.; Ibarra-Cerdeña, C.N. Identifying Chagas disease vectors using elliptic Fourier descriptors of body contour: A case for the cryptic dimidiata complex. Parasit. Vectors 2020, 13, 332. [Google Scholar] [CrossRef]
- Batool, M.; Nasir, S.; Rafique, A.; Yousaf, I.; Yousaf, M. Prevalence of tick infestation in farm animals from Punjab, Pakistan. Pak. Vet. J. 2019, 39, 406–410. [Google Scholar] [CrossRef]
- Sajid, M.S.; Iqbal, Z.; Khan, M.N.; Muhammad, G.; Needham, G.; Khan, M.K. Prevalence, associated determinants, and in vivo chemotherapeutic control of hard ticks (Acari: Ixodidae) infesting domestic goats (Capra hircus) of lower Punjab, Pakistan. Parasitol. Res. 2011, 108, 601–609. [Google Scholar] [CrossRef]
- Iqbal, A.; Siddique, F.; Mahmood, M.S.; Shamim, A.; Zafar, T.; Rasheed, I.; Saleem, I.; Ahmad, W. Prevalence and impacts of ectoparasitic fauna infesting goats (Capra hircus) of district Toba Tek Singh, Punjab Pakistan. Glob. Vet. 2014, 12, 158–164. [Google Scholar]
- Ramzan, M.; Naeem-Ullah, U.; Abbas, H.; Adnan, M.; Rasheed, Z.; Khan, S. Diversity of hard ticks in goats and sheep in Multan, Punjab, Pakistan. Int. J. Agric. Biol. Res. 2019, 35, 7–9. [Google Scholar]
- Ramzan, M.; Naeem-Ullah, U.; Saba, S.; Iqbal, N.; Saeed, S. Prevalence and identification of tick species (Ixodidae) on domestic animals in district Multan, Punjab Pakistan. Int. J. Acarol. 2020, 46, 83–87. [Google Scholar] [CrossRef]
- Siddiqi, M.N.; Jan, A.H. Ixodid ticks (ixodidae) of NWFP (Pakistan). Pak. Vet. J. 1986, 6, 124–126. [Google Scholar]
- Shah, A.; Shah, S.R.; Rafi, M.A.; Noorrahim, M.S.; Mitra, A. Identification of the prevalent ticks (Ixodid) in goats and sheep in Peshawar, Pakistan. J. Entomol. Zool. Stud. 2015, 3, 11–14. [Google Scholar]
- Ali, A.; Khan, M.A.; Zahid, H.; Yaseen, P.M.; Qayash Khan, M.; Nawab, J.; Ur Rehman, Z.; Ateeq, M.; Khan, S.; Ibrahim, M. Seasonal Dynamics, Record of Ticks Infesting Humans, Wild and Domestic Animals and Molecular Phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front. Physiol. 2019, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Shah, S.F.; Amin, F.; Khan, M.A.; Ahmad, M. Taxonomic Study of Arthropod Pests of Livestock in District Peshawar, Khyber Pakhtunkhwa. Pak. J. Life Soc. Sci. 2018, 16, 85–96. [Google Scholar]
- Khatoon, N.; Noureen, S.; Khan, Z.; Gul, S.U.; Ur, H. Domestic animals ectoparasite fauna of district Karak, KP, Pakistan. Int. J. Biosci. 2018, 13, 384–388. [Google Scholar]
- Khan, A.; Nasreen, N.; Niaz, S.; Sajjad Ali Shah, S.; Mitchell III, R.D.; Ayaz, S.; Naeem, H.; Khan, L.; De León, A.P. Tick burden and tick species prevalence in small ruminants of different agencies of the Federally Administered Tribal Areas (FATA), Pakistan. Int. J. Acarol. 2019, 45, 374–380. [Google Scholar] [CrossRef]
- Hussain, S.I.; Kumar, G.A. Prevalence of ixodid ticks (Ixodoidea, Ixodidae) of goats at Khairpur Mir’s and its adjoining areas [Pakistan]. Pak. J. Zool. 1983, 15, 51–55. [Google Scholar]
- Hussain, S.I.; Kumar, G.A. The incidence of ticks (Ixodoidea: Ixodidae) infesting sheep and goats in Sind province, Pakistan. Pak. J. Zool. 1985, 17, 89–97. [Google Scholar]
- Iqbal, A.; Nawaz, M. Taxonomic studies of Haemaphysalis flava (Neumann), its seasonal prevalence and role in parasitic diseases of sheep/goat in Balochistan. Pak. Entomol. 2007, 29, 1–4. [Google Scholar]
- Haneef, M.; Kakar, A.; Naseem, M.; Kurd, A.; Rafiq, N.; Kakar, B.; Uddin, S. 40. Incidence of ectoparasite in chiltan wild goat (Artiodactyla: Caprinae) native of Hazarganji chiltan national park (HCNP), Balochistan, Pakistan. Pure Appl. Biol. 2019, 8, 389–396. [Google Scholar]
- Kasi, K.K.; von Arnim, F.; Schulz, A.; Rehman, A.; Chudhary, A.; Oneeb, M.; Sas, M.A.; Jamil, T.; Maksimov, P.; Sauter-Louis, C. Crimean-Congo haemorrhagic fever virus in ticks collected from livestock in Balochistan, Pakistan. Transbound. Emerg. Dis. 2020, 67, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Ahmed AKNU Blood parasites of domestic animals in Bangladesh. Bangladesh Vet. J. 1976, 10, 69–71.
- Al Mahmud, M.A.; Belal, S.M.S.H.; Hossain, M.A. Prevalence of theileriosis and babesiosis in cattle in Sirajganj district of Bangladesh. Res. Agric. Livest. Fish. 2015, 2, 79–86. [Google Scholar] [CrossRef]
- Roy, B.C.; Estrada-Peña, A.; Krücken, J.; Rehman, A.; Nijhof, A.M. Morphological and phylogenetic analyses of Rhipicephalus microplus ticks from Bangladesh, Pakistan and Myanmar. Ticks Tick-Borne Dis. 2018, 9, 1069–1079. [Google Scholar] [CrossRef]
- Islam, M.F.; Rudra, P.G.; Singha, S.; Das, T.; Gebrekidan, H.; Uddin, M.B.; Chowdhury, M.Y.E. Molecular Epidemiology and Characterization of Theileria in Goats. Protist 2021, 172, 125804. [Google Scholar] [CrossRef]
- Siddiki, A.Z.; Uddin, M.B.; Hasan, M.B.; Hossain, M.F.; Rahman, M.M.; Das, B.C.; Sarker, M.S.; Hossain, M.A. Coproscopic and Haematological Approaches to Determine the Prevalence of Helminthiasis and Protozoan Diseases of Red Chittagong Cattle (RCC) Breed in Bangladesh. Pak. Vet. J. 2010, 30, 1–6. [Google Scholar]
- Kispotta, S.; Islam, M.F.; Hoque, M.F.; Rahman, M.S.; Borman, A.; Haque, M.A.; Rahman, M.R. Study of prevalence and associated risk factors of anaplasmosis and theileriasis in cattle. Asian J. Med. Biol. Res. 2016, 2, 567–576. [Google Scholar] [CrossRef]
- Ali, M.W.; Alauddin, M.; Azad, M.T.A.; Hasan, M.A.; Appiah-Kwarteng, C.; Takasu, M.; Baba, M.; Kitoh, K.; Rahman, M.; Takashima, Y. Theileria annulata seroprevalence among different cattle breeds in Rajshahi Division, Bangladesh. J. Vet. Med. Sci. 2016, 78, 1577–1582. [Google Scholar] [CrossRef]
- Moni, M.I.Z.; Hayashi, K.; Sivakumar, T.; Rahman, M.; Nahar, L.; Islam, M.Z.; Yokoyama, N.; Kitoh, K.; Appiah-Kwarteng, C.; Takashima, Y. First Molecular detection of Theileria annulata in Bangladesh. J. Vet. Med. Sci. 2019, 81, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Z.; Giasuddin, M.; Rahman, M.M.; Ershaduzzaman, M.; Hasan, M. Identification of vector-borne blood protozoa in cattle and sheep in Bangladesh. J. Virol. Antivir. 2019, 2, 4. [Google Scholar]
- Samad, A.; Dhar, S.; Gautam, O.P. Prevalence of Theileria annulata infection among cattle of Bangladesh. Indian J. Parasitol. 1983, 7, 61–63. [Google Scholar]
- Samad, M.A.; Bashar, S.A.; Shahidullah, M.; Ahmed, M.U. Prevalence of haemoprotozoan parasites in cattle of Bangladesh. Indian Vet. Med. J. 1989, 13, 50–51. [Google Scholar]
- Dhar, S.; Gautam, O.P. Theileria annulata infection of cattle I complement fixation and coagulating absorption tests for serodiagnosis. Indian J. Anim. Sci. 1977, 47, 389–394. [Google Scholar]
- Ros-García, A.; Nicolás, A.; García-Pérez, A.L.; Juste, R.A.; Hurtado, A. Development and evaluation of a real-time PCR assay for the quantitative detection of Theileria annulata in cattle. Parasit. Vectors 2012, 5, 171. [Google Scholar] [CrossRef]
- Chae, J.; Allsopp, B.A.; Waghela, S.D.; Park, J.; Kakuda, T.; Sugimoto, C.; Allsopp, M.T.E.P.; Wagner, G.G.; Holman, P.J. A study of the systematics of Theileria spp. based upon small-subunit ribosomal RNA gene sequences. Parasitol. Res. 1999, 85, 877–883. [Google Scholar] [CrossRef]
- Glidden, C.K.; Koehler, A.V.; Hall, R.S.; Saeed, M.A.; Coppo, M.; Beechler, B.R.; Charleston, B.; Gasser, R.B.; Jolles, A.E.; Jabbar, A. Elucidating cryptic dynamics of Theileria communities in African buffalo using a high-throughput sequencing informatics approach. Ecol. Evol. 2020, 10, 70–80. [Google Scholar] [CrossRef]
- Ogden, N.H.; Ben Beard, C.; Ginsberg, H.S.; Tsao, J.I. Possible Effects of Climate Change on Ixodid Ticks and the Pathogens They Transmit: Predictions and Observations. J. Med. Entomol. 2021, 58, 1536–1545. [Google Scholar] [CrossRef]



| Country | Host | Theileria Species Name |
|---|---|---|
| India | Cattle & Buffaloes | Theileria spp. T. annulata, T. orientalis, T. mutans & T. velifera |
| Goats & Sheep | Theileria spp. T. lestoquardi T. luwenshuni & T. ovis | |
| Pakistan | Cattle & Buffaloes | T. annulata, T. orientalis & Theileria spp. |
| Goats & Sheep | T. annulata, T. lestoquardi, T. luwenshuni T. ovis & Theileria spp. T. lestoquardi-like spp. | |
| Bangladesh | Cattle & Buffaloes | Theileria spp. T. annulata, T. orientalis, T. Mutans, |
| Goats & Sheep | T. annulata |
| India | |||||
|---|---|---|---|---|---|
| Province/State | Theileria spp. | Identification Method | Host | Year of Study | References |
| Central India | |||||
| Cattle & Buffaloes | |||||
| Central India | T. annulata | Microscopy | Ticks & Cattle | 1975 | [64] |
| Uttar Pradesh | T. annulata | Molecular | Cattle | 1977 | [65] |
| Uttar Pradesh | T. annulata | Microscopy | Cattle | 2012 | [66] |
| Uttar Pradesh | T. annulata | Molecular | Cattle | 2015 | [67] |
| Chhattisgarh | T. annulata | Microscopy | Cattle | 2016 | [34] |
| Central India | T. annulata | Molecular | Cattle | 2017 | [68] |
| Uttar Pradesh | T. annulata | Molecular | Cattle | 2018 | [69] |
| Hisar | T. annulata | Microscopy | Cattle | 1989 | [33] |
| Eastern India | |||||
| West Bangal | T. annulata | Molecular | Cattle | 2003 | [35] |
| Guinea | T. mutans & T. velifera | Molecular | Cattle | 2021 | [36] |
| South India | |||||
| North Banglore | T. annulata | Microscopy | Cattle | 2009 | [37] |
| Kerala | Theileria spp. & T. annulata | Microscopy & Molecular | Cattle | 2011 | [38] |
| South India | T. orientalis | Molecular | Cattle | 2011 | [70] |
| South India | T. annulata & Theileria spp. | Molecular | Cattle | 2013 | [40] |
| Tamil Nadu | Theileria spp. | Microscopy | Cattle | 2014 | [71] |
| Telangana and Andhra Pradesh | T. orientalis | Molecular | Cattle | 2015 | [41] |
| Karnataka | T. annulata | Microscopy | Cattle | 2016 | [42] |
| Southwest India | T. annulata | Microscopy | Buffalo & Cattle | 2016 | [39] |
| Karnataka | T. annulata | Microscopy | Cattle | 2017 | [72] |
| Kerala | T. orientalis | Molecular | R. annulatus Ticks | 2019 | [43] |
| South India | Theileria spp. | Molecular | Ticks & Cattle | 2021 | [73] |
| Goats & Sheep | |||||
| West Bengal | T. hirci (T. lestoquardi) | Microscopy | Goat | 1990 | [46] |
| Karnataka | Theileria spp. | Microscopy | Sheep | 1985 | [74] |
| South India | Theileria spp. | Microscopy | Goat & Ticks | 2017 | [47] |
| Kerala | Theileria spp. | Microscopy & Molecular | Goats | 2017 | [75] |
| Karnataka | T. luwenshuni | Molecular | Goats & Sheep | 2017 | [48] |
| Karnataka | T. luwenshuni & T. ovis | Molecular | Sheep | 2019 | [76] |
| Karnataka | Theileria spp. | Microscopy | Sheep | 2020 | [77] |
| Assam | T. annulata & T. orientalis | Molecular | Cattle | 2015 | [78] |
| Odisha | T. annulata & T. orientalis | Microscopy & Molecular | Cattle | 2017 | [79] |
| Odisha | T. annulata | Microscopy and Molecular | Cattle | 2017 | [80] |
| Odisha | T. orientalis | Molecular | Cattle | 2020 | [81] |
| Odisha | T. annulata | Molecular | Cattle | 2021 | [44] |
| Odisha | T. annulata | Molecular | Cattle | 2021 | [45] |
| Goats & Sheep | |||||
| Assam | T. luwenshuni | Microscopy & Molecular | Goat | 2018 | [50] |
| Guwahati of Assam | T. luwenshuni | Microscopy & Molecular | Goat | 2019 | [49] |
| West India | |||||
| Anand | T. annulata | Molecular | Buffalo & Cattle | 2014 | [52] |
| Gujrat | T. annulata | Microscopy and Molecular | Cattle | 2015 | [54] |
| Gujrat | T. annulata | Microscopy & Molecular | Cattle & Buffalo | 2015 | [53] |
| Maharashtra | T. annulata & T. orientalis | Molecular | Cattle | 2017 | [58] |
| Bihar | T. annulata | Microscopy | Buffalo | 2018 | [82] |
| Anand | T. annulata & T. orientalis | Molecular | Cattle | 2019 | [55] |
| Maharashtra & tamil Nadu | T. annulata | Molecular | Buffalo & Cattle | 2019 | [58] |
| Telangana, Gujarat, Haryana, and Bihar | T. annulata | Molecular | Vaccine Isolate | 2019 | [83] |
| Maharashtra | Theileria spp. | Microscopy | Buffalo | 2020 | [51] |
| Bihar | Theileria spp. | Microscopy | Cattle | 2021 | [84] |
| Tamil Nadu | T. annulata | Molecular | Cattle | 2021 | [57] |
| Bihar | T. annulata | Microscopy & Molecular | Cattle | 2021 | [85] |
| Haryana | T. annulata | Microscopy | Goat, cattle, sheep Sera | 1998 | [86] |
| Tamil Nadu | Theileria Spp. | Microscopy | Sheep | 2005 | [87] |
| Haryana | T. annulata | Molecular | Tick | 2006 | [59] |
| Tamil Nadu | Theileria spp. | Microscopy | Goats & Sheep | 2015 | [60] |
| Tamil Nadu | T. luwenshuni | Molecular | Gaots & Sheep | 2019 | [61] |
| Maharashtra | T. luwenshuni | Microscopy & Molecular | Sheep | 2021 | [62] |
| Punjab | T. annulata | Microscopy | Tick Hy. anatolicum | 2010 | [88] |
| Gujrat | Theileria spp. | Microscopy | Buffalo & Cattle | 2021 | [89] |
| Ludhiana Punjab | T. annulata | Microscopy | Cattle | 2012 | [90] |
| Punjab | T. annulata | Molecular | Cattle | 2015 | [91] |
| Ludhiana Punjab | T. annulata | Molecular | Tick & Cattle | 2015 | [92] |
| Rajasthan | Theileria | Microscopy | Cattle | 2015 | [93] |
| Uttara hand | Theileria genus | Microscopy & Molecular | Cattle | 2014 | [94] |
| Haryana | T. annulata | Microscopy | Cattle | 2017 | [95] |
| Haryana | T. annulata | Molecular | Cattle | 2020 | [96] |
| Telangana, Gujarat, Haryana, and Bihar | T. annulata | Molecular | Vaccine | 2021 | [83] |
| Gujrat | T. annulata | Molecular | Cattle | 2021 | [97] |
| Himachal Pradesh | T. orientalis | Molecular | Cattle | 2021 | [98] |
| Goats & Sheep | |||||
| Himachal Pradesh | T. luwenshuni | Molecular | Goats & Sheep | 2021 | [63] |
| Tick Species | Host | States/Region | References |
|---|---|---|---|
| R. microplus. R. haemaphysaloides | Cattle & Buffalo | It is found in all places except Manipur, Kerala, Nagaland, Tripura & Maharashtra | [78,102] |
| Hy. anatolicum | Ruminants | It may be present in all parts except Andhra Pradesh, Jharkhand, Manipur, Mega laya, Stkin, Tripuri | [33,46,103] |
| Hae. Bispinosa | Goats & Sheep | Widely distributed except Delhi, Haryana, Kerala, Nagaland, Uttar Pradesh and Chhattisgarh | [47,64,70,99] |
| Hy. truncatum | Goats & Sheep | It is restricted to only Gujrat, Maharashtra & Uttar Pradesh | [102] |
| Hy. dromedarii | Goats & Sheep | It can be found only in Andhra Pradesh, Delhi, Gujrat, Haryana, Himachal Pradesh, Jammu & Kashmir, Odisha, Punjab, Rajasthan, Uttar Pradesh | [102] |
| R. Sanguineus | Goats & Sheep | It is reported from all places except Gora, Delhi, Manipur, Megha laya, Nagaland, Tripuri and Uttar Pradesh | [47,64,102] |
| Pakistan | |||||
|---|---|---|---|---|---|
| Province/State | Theileria Species | Identification Method | Host | Year | Reference |
| Punjab | |||||
| Cattle & Buffaloes | |||||
| Punjab | Theileria spp. | Microscopy | Cattle | 1983 | [131] |
| Faisalabad | Theileria spp. | Microscopy | Cattle | 1999 | [132] |
| Faisalabad | T. annulata | Microscopy | Buffaloes & Cattle | 2004 | [133] |
| Kasur | Theileria spp. | Microscopy | Cattle | 2005 | [134] |
| Punjab | T. annulata | Microscopy | Buffaloes | 2006 | [135] |
| Kasur | T. annulata | Molecular | Cattle | 2008 | [136] |
| Punjab | T. annulata | Microscopy & Molecular | Cattle | 2008 | [137] |
| Sahiwal | Theileria spp. | Microscopy | Cattle | 2010 | [111] |
| Southern Punjab | T. annulata | Microscopy | Cattle | 2011 | [104] |
| Sargodha | T. annulata | Microscopy | Cattle | 2012 | [138] |
| Faisalabad, Jhang, Khanewal | T. annulata | Molecular | Ticks of Cattle & Buffaloes | 2013 | [105] |
| Faisalabad | T. annulata | Molecular | Cattle & Buffaloes | 2013 | [106] |
| Punjab | T. orientalis | Molecular | Cattle | 2021 | [108] |
| Punjab | T. annulata | Molecular | Cattle | 2018 | [109] |
| Punjab | T. annulata & T. orientalis | Molecular | Cattle | 2018 | [139] |
| Lahore | T. annulata | Microscopy | Cattle | 2018 | [5] |
| Agro-ecological Zones Punjab | T. orientalis & T. annulata | Molecular | Ruminants | 2019 | [110] |
| Agro-ecological Zones Punjab | T. annulata | Molecular | Cattle | 2020 | [107] |
| Layyah | T. annulata & T. orientalis | Molecular | Cattle | 2021 | [113] |
| Dera Ghazi Khan & Lodhran | T. annulata | Molecular | Cattle | 2021 | [112] |
| Attock | Theileria spp. | Microscopy | Goats & Sheep | 2010 | [140] |
| Okara | Theileria spp. | Microscopy | Sheep | 2010 | [141] |
| Lahore | T. lestoquardi & T. ovis | Microscopy & Molecular | Sheep | 2011 | [142] |
| Lahore | Theileria spp. | Microscopy | Goats & Sheep | 2011 | [143] |
| Okara | T. ovis | Molecular | Sheep | 2013 | [114] |
| Southern Punjab | T.lestoquardi | Molecular | Goats & Sheep | 2015 | [144] |
| Multan | T. ovis & T. lestoquardi | Microscopy & Molecular | Goats & Sheep | 2017 | [115] |
| Multan | T. lestoquardi & T. ovis | Microscopy & Molecular | Goats & Sheep | 2017 | [145] |
| Punjab | T. annulata T.ovis & T. lestoquardi | Molecular | Ruminants | 2019 | [117] |
| Multan | T. lestoquardi && T. ovis | Molecular & Microscopy | Goats | 2019 | [118] |
| Layyah | T. annulata | Molecular | Sheep | 2021 | [119] |
| Lahore | Theileria Spp. | Microscopy | Goats & Sheep | 2021 | [116] |
| Peshawar | T. annulata | Microscopy | Buffalo & Cattle | 2005 | [146] |
| KPK (Southern KP) | T. annulata | Molecular | Cattle | 2012 | [147] |
| KPK | T. annulata | Molecular | Cattle | 2017 | [120] |
| Northern Pakistan | T. annulata | Molecular | Cattle (Ticks) | 2019 | [101] |
| North-Western Pakistan | T. annulata | Molecular | Cattle | 2021 | [148] |
| DI Khan | Theileria spp. | Microscopy | Cattle | 2021 | [149] |
| Central KPK | T. annulata | Microscopy & Molecular | Cattle | 2021 | [122] |
| KPK | T. lestoquardi & T. ovis | Molecular | Goats & Sheep | 2013 | [147] |
| KPK | T.lestoquardi | Molecular | Goats & Sheep | 2015 | [150] |
| Peshawar & Periphery | Theileria spp. | Microscopy | Ruminants | 2017 | [151] |
| Peshawar & Khyber Agency | Theileria | Microscopy | Goats & Sheep | 2017 | [152] |
| Southern KPK | T. ovis T. lestoquardi | Molecular | Goats & Sheep | 2018 | [123] |
| Southern KPK | Theileria spp. | Microscopy | Sheep | 2018 | [153] |
| KPK | T. annulata, T. lestoquardi, T. luwenshuni T. ovis & Theileria spp. | Molecular | Goats & Sheep | 2020 | [124] |
| Malakand Division | Theileria spp. | Microscopy | Buffalo, Cattle, Goat & Sheep | 2021 | [125] |
| KPK | Theileria Spp. T. annulata, T. lestoquardi, T. ovis | Molecular | Goats & Sheep | 2021 | [126] |
| Sindh | |||||
| Hyderabad | Theileria spp. | Microscopy | Cattle | 1994 | [154] |
| Karachi | Theileria | Microscopy | Buffalo | 2012 | [127] |
| Quetta | T. annulata | Microscopy | Cattle | 2021 | [116] |
| Baluchistan | Theileria ovis & T. lestoquardi | Molecular | Goats & Sheep | 2017 | [7] |
| FATA | T. ovis | Molecular | Goats & Sheep | 2020 | [107] |
| Punjab & KPK | T. ovis | Microscopy & Molecular | Goats & Sheep | 2012 | [129] |
| Pakistan/Punjab-KPK | T. annulata | Molecular | Ruminants | 2017 | [130] |
| Sindh & Punjab | T. lestoquardi-like spp., T. orientalis & T. annulata | Molecular | Cattle | 2021 | [128] |
| Ticks | Host | References |
|---|---|---|
| Punjab | ||
| R. microplus, Hy. anatolicum, Hy. aegyptium, Hy. dromedarii, R. appendiculatus, R. sanguineus | Cattle and buffaloes | [102,105,138,147,153,157,158,159,160,161,162] |
| Khyber Pakhtunkhwa | ||
| R. microplus, R. appendiculatus, Hy. anatolicum | Cattle & buffaloes | [101,163] |
| Sindh | ||
| Hy. hussaini, Hy. scupense, R. annulatus, R. microplus, Hy. anatolicum, Hy. scupense, Hy. excavatum | Buffaloes | [155,156,164] |
| Balochistan | ||
| R. microplus, Hy. anatolicum, Hy. scupense, Hy. aegyptium, Haemaphysalis | Cattle & buffaloes | [107,165,166,167,168,169] |
| Punjab | ||
| Hy. anatolicum, Hy. excavatum R. appendiculatus, Hy. dromedarii, R. microplus, R. sanguineus, R. Turanicus | Goats & Sheep | [158,160,170,171,172,173,174] |
| Khyber Pakhtunkhwa | ||
| Hy. anatolicum, Hy. detritum, Hy. excavatum, Hy. scupense, Haemaphysalis longicornis, Hyalomma impeltatum, R. appendiculatus, R. microplus | Goats & Sheep | [107,175,176,177,178,179,180] |
| Sindh | ||
| Hae. bispinosa, Hy. anatolicum, Hy. detritum, Hy. dromedarii, Hy. hussaini, Hy. impeltatum, Hy. marginatum isaaci, R. microplus, R. Sanguineus | Goats & Sheep | [181,182] |
| Balochistan | ||
| Hy. anatolicum, Hy. dromedarii, Hy. excavatum, Hy. scupense, R. microplus | Goats & Sheep | [168,183,184,185] |
| Bangladesh | |||||
|---|---|---|---|---|---|
| City | Theileria spp. | Identification Method | Host | Year of Study | References |
| Central Region | |||||
| Dhaka Targil | T. annulata | Microscopy | Cattle | 1983 | [195] |
| Dhaka | T. annulata and T. mutans | Microscopy | Cattle | 1989 | [196] |
| Dhaka, Sirajganj and Nikhangsori | T. annulata | Microscopy & Molecular | Cattle goats & Sheep | 2019 | [194] |
| Goats & Sheep | |||||
| Dhaka | Theileria spp. | Microscopy & Molecular | Goats | 2021 | [189] |
| South Eastern Region | |||||
| Chittagong | Theileria spp. | Microscopy | Cattle | 2010 | [190] |
| North Central Region | |||||
| Sirajganj | Theileria spp. | Microscopy | Cattle | 2015 | [187] |
| Sirajganj | T. annulata & T. mutans | Microscopy | Cattle | 1976 | [186] |
| Sirajganj | T. annulata | Molecular | Cattle | 1977 | [197] |
| Mymensingh | T. orientalis | Molecular | Cattle | 2018 | [188] |
| Northern Region | |||||
| Dinajpur | Theileria spp. | Microscopy | Cattle | 2016 | [191] |
| Western region | |||||
| Rajshahi | T. annulata | Molecular | Cattle | 2016 | [192] |
| Natores | T. annulata | Molecular | Cattle | 2019 | [193] |
| Divisions | Possible Tick Vector | Reference |
|---|---|---|
| Braisel, Dhaka, Savar, Narayanganj, Tangali | R. microplus | [102] |
| Dhaka, Rajshahi, Savar | Hae. bispinosa | [102] |
| North western dry Region (Rajshahi, Rangpur, and Dinajpur districts) | Hy. anatolicum anatolicum | [102] |
| Savar | Hy. Truncatum | [102] |
| Braisal, Chitagang, Dhaka, Narayanganj, Tangail, Rangpur, Sylhet | R. sanguineus | [102] |
| Possible Tick Vectors for TT | Host | References |
|---|---|---|
| R. microplus, Hae. bispinosa, R. sanguineus, Hy. anatolicum anatolicum | Cattle & Buffaloes | [102] |
| R. sanguineus, Hy. anatolicum anatolicum | Goats & Sheep | [102] |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| MF287947.1 T annulata Central India | |||||||||
| MF287920.1 T annulata West India | 0.58 | ||||||||
| MF287949.1 T annulata Eastern India | 0.62 | 0.22 | |||||||
| MF287937.1 T annulata South India | 0.63 | 0.25 | 0.19 | ||||||
| MF287934.1 T annulata North India | 0.58 | 0.16 | 0.19 | 0.28 | |||||
| JQ743631.1 T annulata Pakistan | 0.4 | 0.55 | 0.59 | 0.6 | 0.56 | ||||
| JQ743636.1 T annulata Pakistan | 0.7 | 0.6 | 0.63 | 0.065 | 0.6 | 0.5 | |||
| MW046053.1 T annulata Pakistan | 2.14 | 1.65 | 4.7 | 4.7 | 0.65 | 0.12 | 0.15 | ||
| MW046054.1 T annulata Pakistan | 0.9 | 0.57 | 2.63 | 3.62 | 0.57 | 3.8 | 0.11 | 0.0 | _ |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeb, J.; Song, B.; Aziz, M.U.; Hussain, S.; Zarin, R.; Sparagano, O. Diversity and Distribution of Theileria Species and Their Vectors in Ruminants from India, Pakistan and Bangladesh. Diversity 2022, 14, 82. https://doi.org/10.3390/d14020082
Zeb J, Song B, Aziz MU, Hussain S, Zarin R, Sparagano O. Diversity and Distribution of Theileria Species and Their Vectors in Ruminants from India, Pakistan and Bangladesh. Diversity. 2022; 14(2):82. https://doi.org/10.3390/d14020082
Chicago/Turabian StyleZeb, Jehan, Baolin Song, Muhammad Umair Aziz, Sabir Hussain, Riaz Zarin, and Olivier Sparagano. 2022. "Diversity and Distribution of Theileria Species and Their Vectors in Ruminants from India, Pakistan and Bangladesh" Diversity 14, no. 2: 82. https://doi.org/10.3390/d14020082
APA StyleZeb, J., Song, B., Aziz, M. U., Hussain, S., Zarin, R., & Sparagano, O. (2022). Diversity and Distribution of Theileria Species and Their Vectors in Ruminants from India, Pakistan and Bangladesh. Diversity, 14(2), 82. https://doi.org/10.3390/d14020082








