Human-Wildlife Conflict at a Suburban–Wildlands Interface: Effects of Short- and Long-Distance Translocations on Red Diamond Rattlesnake (Crotalus ruber) Activity and Survival
Abstract
:1. Introduction
2. Materials and Methods
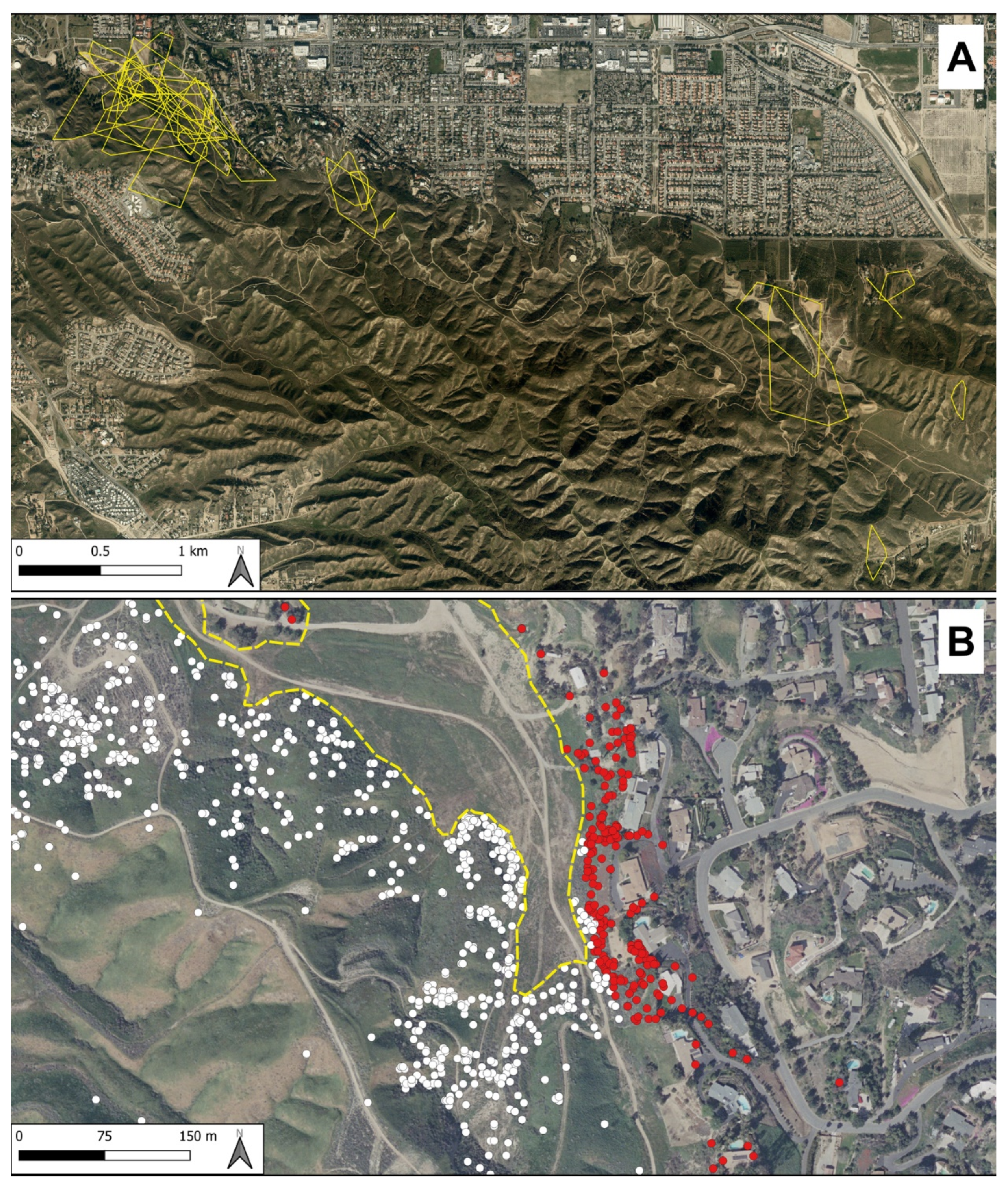
2.1. Study Site
2.2. Legal Issues
2.3. Radiotelemetry
2.4. Translocation Protocol
2.5. Activity Range and Movements
2.6. Statistical Analyses
2.6.1. Activity Area and Mean Daily Movement
2.6.2. Snake Mass and Mortality
2.6.3. Risk of Human Conflict and Return to Capture Site after Translocation
3. Results
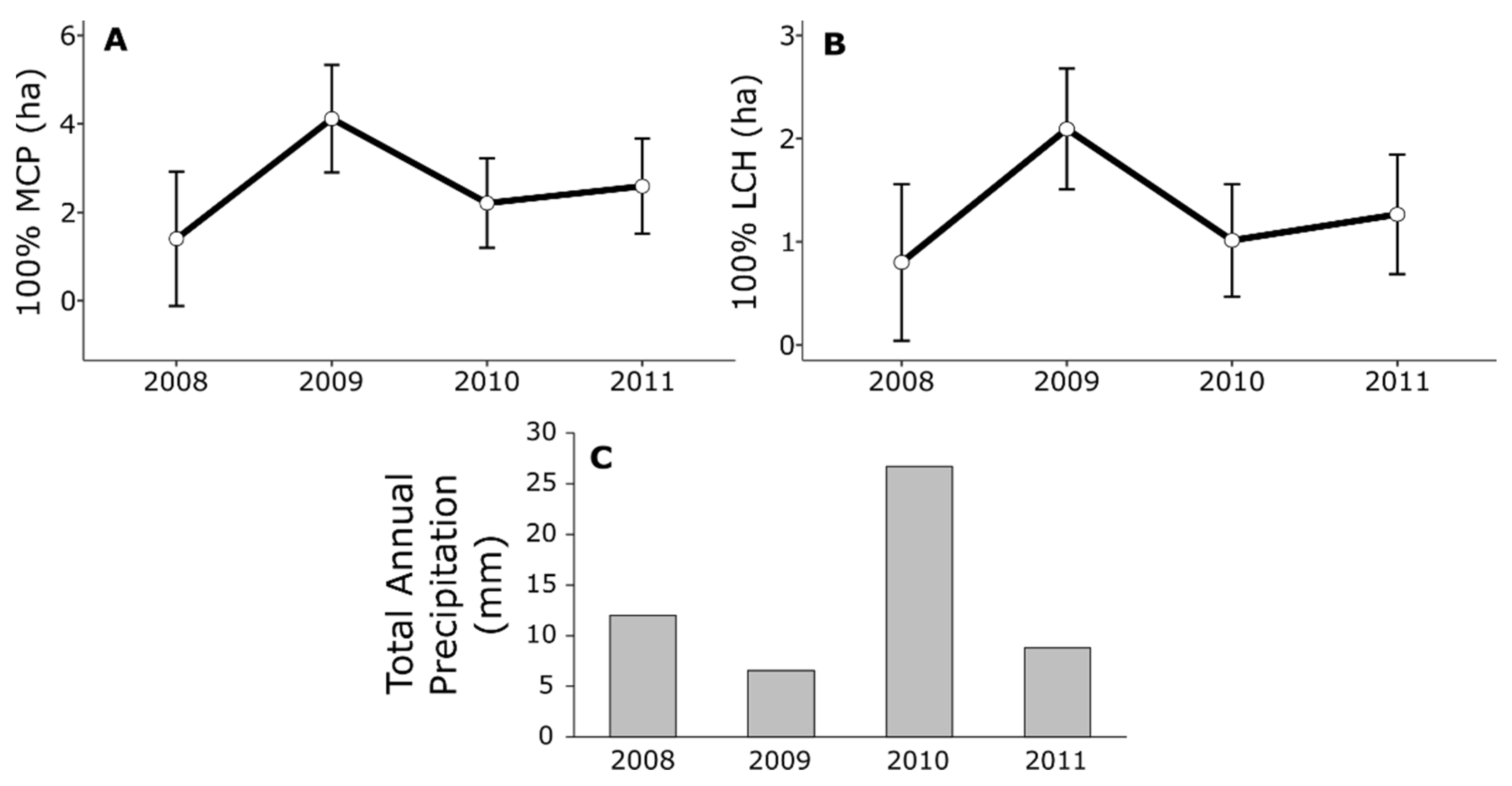
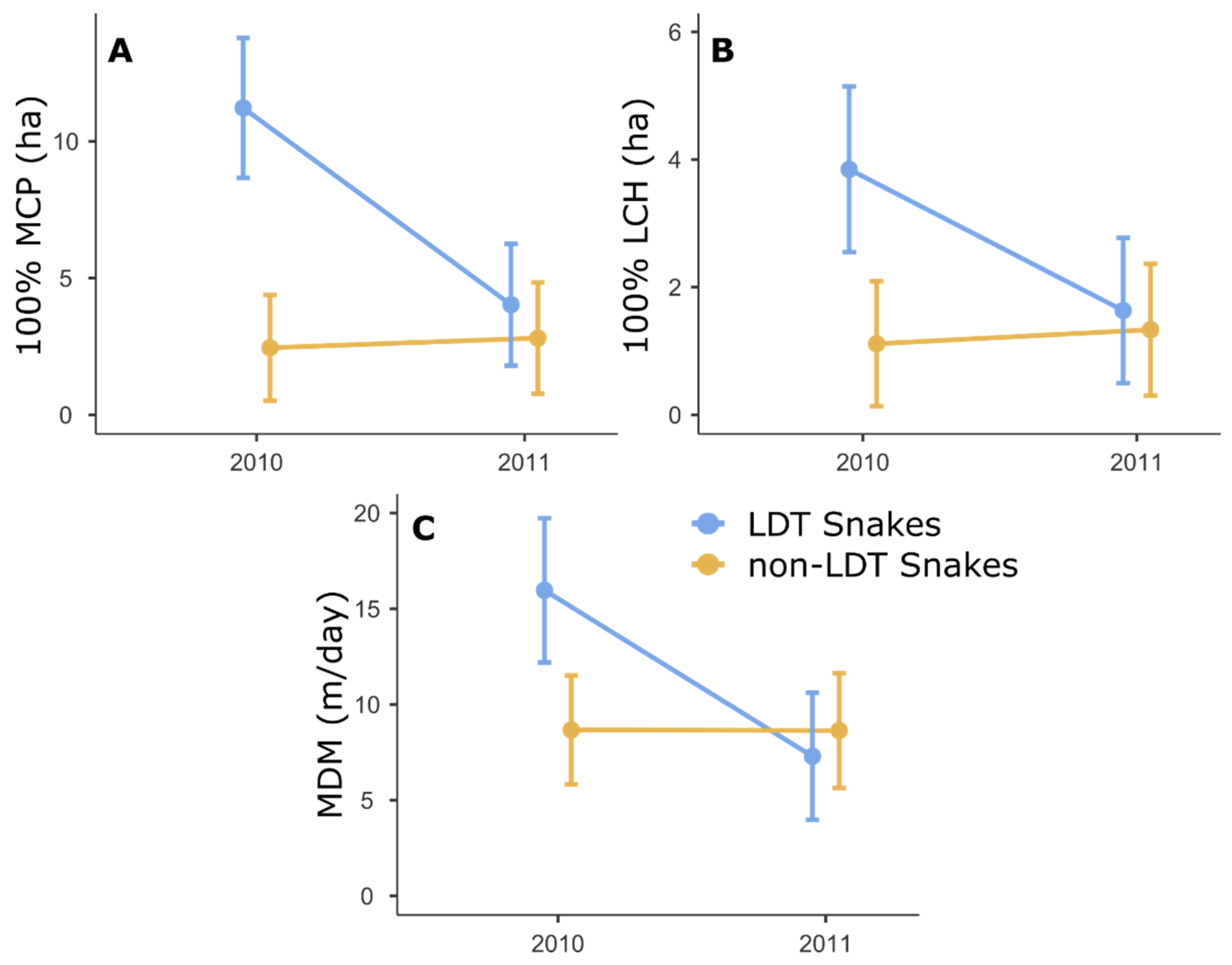
3.1. Activity Ranges and Movements
3.2. Snake Mass and Mortality
3.3. Risk of Return to Human-Modified Areas
3.4. Risk of Return to Area of Capture after Translocation
4. Discussion
4.1. Translocation Effects on the Snake
4.2. Risks Associated with Human-Snake Conflict
4.3. Implications for Managing Nuisance Rattlesnakes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conover, M.R. Resolving Human-Wildlife Conflicts: The Science of Wildlife Damage Management; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Woodroffe, R.; Thirgood, S.; Rabinowitz, A. The impact of human-wildlife conflict on natural systems. In People and Wildlife, Conflict or Coexistence? Woodroffe, R., Thirgood, S., Rabinowitz, A., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 1–12. [Google Scholar]
- Frank, B.; Glikman, J.A.; Marchini, S. (Eds.) Human-Wildlife Interactions: Turning Conflict into Coexistence; Cambridge University Press: Cambridge, UK, 2019; Volume 23. [Google Scholar]
- Bhatia, S.; Redpath, S.M.; Suryawanshi, K.; Mishra, C. Beyond conflict: Exploring the spectrum of human-wildlife interactions and their underlying mechanisms. Oryx 2020, 54, 621–628. [Google Scholar] [CrossRef]
- Malhotra, A.; Wüster, W.; Owens, J.B.; Hodges, C.W.; Jesudasan, A.; Ch, G.; Kartik, A.; Christopher, P.; Louies, J.; Naik, H.; et al. Promoting co-existence between humans and venomous snakes through increasing the herpetological knowledge base. Toxicon X 2021, 12, 100081. [Google Scholar] [CrossRef] [PubMed]
- Benn, B.; Herrero, S. Grizzly Bear mortality and human access in Banff and Yoho National Parks, 1971–1998. Ursus 2002, 13, 213–221. [Google Scholar]
- Fernández-Juricic, E.; Vaca, R.; Schroeder, N. Spatial and temporal responses of forest birds to human approaches in a protected area and implications for two management strategies. Biol. Cons. 2004, 117, 407–416. [Google Scholar] [CrossRef]
- Carter, E.T.; Attum, O.; Eads, B.C.; Hoffman, A.S.; Kingsbury, B.A. Reducing the potential for human-snake encounters in a recreational park. Hum.-Wildl. Interact. 2014, 8, 158–167. [Google Scholar]
- Craven, S.; Barnes, T.; Kania, G. Toward a professional position on the translocation of problem wildlife. Wildl. Soc. Bull. 1998, 26, 171–177. [Google Scholar]
- Griffith, B.; Scott, J.M.; Carpenter, J.W.; Reed, C. Translocation as a species conservation tool: Status and strategy. Science 1989, 245, 477–480. [Google Scholar] [CrossRef]
- Reinert, H.K. Translocation as a conservation strategy for amphibians and reptiles: Some comments, concerns, and observation. Herpetologica 1991, 47, 357–363. [Google Scholar]
- IUCN/SSC. Guidelines for Reintroductions and Other Conservation Translocations; IUCN: Gland, Switzerland, 2013. [Google Scholar]
- Sullivan, B.K.; Nowak, E.M.; Kwiatkowski, M.A. Problems with mitigation translocation of herpetofauna. Cons. Biol. 2015, 29, 12–18. [Google Scholar] [CrossRef]
- Bradley, H.S.; Tomlinson, S.; Craig, M.D.; Cross, A.T.; Bateman, P.W. Mitigation translocation as a management tool. Cons. Biol. 2022. [Google Scholar] [CrossRef]
- Fitzgerald, L.A.; Walkup, D.; Chyn, K.; Buchholtz, E.; Angeli, N.; Parker, M. The future for reptiles: Advances and challenges in the Anthropocene. Encycl. Anthr. 2018, 3, 163–174. [Google Scholar]
- Massei, G.; Quy, R.J.; Gurney, J.; Cowan, D.P. Can translocations be used to mitigate human-wildlife conflicts? Wildlife Res. 2010, 37, 428–439. [Google Scholar] [CrossRef]
- Devan-Song, A.; Martelli, P.; Dudgeon, D.; Crow, P.; Ades, G.; Karraker, N.E. Is long-distance translocation an effective mitigation tool for white-lipped pit vipers (Trimeresurus albolabris) in South China? Biol. Cons. 2016, 204, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, A.K.; Fleming, P.A.; Bateman, P.W. Impacts of translocation on a large urban-adapted venomous snake. Wildl. Res. 2018, 45, 316–324. [Google Scholar] [CrossRef]
- Cornelis, J.; Parkin, T.; Bateman, P.W. Killing them softly: A review on snake translocation and an Australian case study. Herpetol. J. 2021, 31, 118–131. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, D. Spatial ecology of translocated and resident Amur ratsnakes (Elaphe schrenckii) in two mountain valleys of South Korea. Asian Herpetol. Res. 2011, 2, 223–229. [Google Scholar]
- Nash, D.J.; Griffiths, R.A. Ranging behaviour of adders (Vipera berus) translocated from a development site. Herpetol. J. 2018, 28, 155–159. [Google Scholar]
- Plummer, M.V.; Mills, N.E. Spatial ecology and survivorship of resident and translocated hognose snakes (Heterodon platirhinos). J. Herpetol. 2000, 34, 565–575. [Google Scholar] [CrossRef]
- Read, J.L.; Johnston, G.R.; Morley, T.P. Predation by snakes thwarts trial reintroduction of the endangered woma python Aspidites ramsayi. Oryx 2011, 45, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Roe, J.H.; Frank, M.R.; Gibson, S.E.; Attum, O.; Kingsbury, B.A. No place like home: An experimental comparison of reintroduction strategies using snakes. J. Appl. Ecol. 2010, 47, 1253–1261. [Google Scholar] [CrossRef]
- Sullivan, B.K.; Kwiatkowski, M.A.; Schuett, G.W. Translocation of urban Gila monsters: A problematic conservation tool. Biol. Cons. 2004, 117, 235–242. [Google Scholar] [CrossRef]
- Newman, B.C.; Henke, S.E.; Wester, D.B.; Shedd, T.M.; Perotto-Baldivieso, H.L.; Rudolph, D.C. Determining the suitability of the Jamaican Boa (Chilabothrus subflavus) for short-distance translocation in Cockpit Country, Jamaica. Caribb. J. Sci. 2019, 49, 222–238. [Google Scholar] [CrossRef]
- Roe, J.H.; Frank, M.R.; Kingsbury, B.A. Experimental evaluation of captive-rearing practices to improve success of snake reintroductions. Herpetol. Cons. Biol. 2015, 10, 711–722. [Google Scholar]
- Heiken, K.H.; Brusch IV, G.A.; Gartland, S.; Escallón, C.; Moore, I.T.; Taylor, E.N. Effects of long distance translocation on corticosterone and testosterone levels in male rattlesnakes. Gen. Comp. Endocrinol. 2016, 237, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, A.A. Translocations of wildlife disease risks. Cons. Biol. 1996, 10, 349–353. [Google Scholar] [CrossRef]
- Linnell, J.D.C.; Aanes, R.; Swenson, J.E.; Odden, J.; Smith, M.E. Translocation of carnivores as a method for managing problem animals: A review. Biodivers. Cons. 1997, 6, 1245–1257. [Google Scholar] [CrossRef]
- Hardy, D.L.; Greene, H.W.; Tomberlin, B.; Webster, M. Relocation of nuisance rattlesnakes: Problems using short-distance translocation in a small rural community. Newslet. Col. Herpetol. Soc. 2001, 28, 62–63. [Google Scholar]
- Butler, H.; Malone, B.; Clemann, N. The effects of translocation on the spatial ecology of tiger snakes (Notechis scutatus) in a suburban landscape. Wildlife Res. 2005, 32, 165–171. [Google Scholar] [CrossRef]
- Hodges, C.W.; Barnes, C.H.; Patungtaro, P.; Strine, C.T. Deadly dormmate: A case study on Bungarus candidus living among a student dormitory with implications for human safety. Ecol. Solut. Evid. 2021, 2, e12047. [Google Scholar] [CrossRef]
- Burke, R.L. Relocations, repatriations, and translocations of amphibians and reptiles: Taking a broader view. Herpetologica 1991, 47, 350–357. [Google Scholar]
- Chipman, R.; Slate, D.; Rupprecht, C.; Mendoza, M. Downside risk of wildlife translocation. Devel. Biol. 2008, 131, 223–232. [Google Scholar]
- Suarez, M.B.; Ewen, J.G.; Groombridge, J.J.; Beckmann, K.; Shotton, J.; Masters, N.; Hopkins, T.; Sainsbury, A.W. Using qualitative disease risk analysis for herpetofauna conservation translocations transgressing ecological and geographical barriers. Ecohealth 2017, 14, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Nowak, E.M.; van Riper, C., III. Effects and Effectiveness of Rattlesnake Relocation at Montezuma Castle National Monument; USGS Forest and Rangeland Ecosystem Science Center: Flagstaff, AZ, USA, 1999. [Google Scholar]
- Mccrystal, H.K.; Ivanyi, C.S. Translocation of venomous reptiles in the southwest: A solution—Or part of the problem? In The Biology of Rattlesnakes; Hayes, W.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 395–402. [Google Scholar]
- Gibbons, J.W.; Dorcas, M.E. Defensive behavior of Cottonmouths (Agkistrodon piscivorus) toward humans. Copeia 2002, 2002, 195–198. [Google Scholar] [CrossRef]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [Green Version]
- Dart, R.C.; McNally, J.T.; Spaite, D.W.; Gustafson, R. The sequelae of pitviper poisoning in the United States. In Biology of the Pitvipers; Campbell, J.A., Brodie, E.D., Eds.; Selva: Tyler, TX, USA, 1992; pp. 395–404. [Google Scholar]
- Corneille, M.G.; Larson, S.; Stewart, R.M.; Dent, D.; Myers, J.G.; Lopez, P.P.; McFarland, M.J.; Cohn, S.M. A large single-center experience with treatment of patients with crotalid envenomations: Outcomes with and evolution of antivenin therapy. Am. J. Surg. 2006, 192, 848–852. [Google Scholar] [CrossRef]
- Smith, J.; Bush, S.P. Envenomations by reptiles in the United States. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 475–490. [Google Scholar]
- Braband, L.A.; Clark, K.D. Perspectives on wildlife nuisance control: Results of a wildlife damage control firm’s customer survey. Proc. East. Wildl. Damage Control Conf. 1991, 5, 34–37. [Google Scholar]
- Shine, R.; Koenig, J. Snakes in the garden: An analysis of reptiles “rescued” by community-based wildlife carers. Biol. Cons. 2001, 102, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Estes, J.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic downgrading of planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Sealy, J.B. Ecology and behavior of the timber rattlesnake (Crotalus horridus) in the upper Piedmont of North Carolina: Identified threats and conservation recommendations. In Biology of the Vipers; Schuett, G.W., Höggren, M., Douglas, M.E., Greene, H.W., Eds.; Eagle Mountain Publishing LC: Eagle Mountain, UT, USA, 2002; pp. 561–578. [Google Scholar]
- Nowak, E.M.; Hare, T.; McNally, J.T. Management of “nuisance” effects of translocation on Western Diamond-backed Rattlesnakes (Crotalus atrox). In Biology of the Vipers; Schuett, G.W., Höggren, M., Douglas, M.E., Greene, H.W., Eds.; Eagle Mountain Publishing LC: Eagle Mountain, UT, USA, 2002; pp. 533–560. [Google Scholar]
- Brown, T.K.; Lemm, J.M.; Montagne, J.-P.; Tracey, J.A.; Alberts, A.C. Spatial ecology, habitat use, and survivorship of resident and translocated Red Diamond Rattlesnakes (Crotalus ruber). In The Biology of Rattlesnakes; Hayes, W.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 377–394. [Google Scholar]
- Brown, J.R.; Bishop, C.A.; Brooks, R.J. Effectiveness of short-distance translocation and its effects on Western Rattlesnakes. J. Wildl. Manag. 2009, 73, 419–425. [Google Scholar] [CrossRef]
- Johnson, B. Eastern Massasauga Rattlesnake conservation program at Metro Toronto Zoo. In Proceedings of the International Symposium and Workshop on the Conservation of the Eastern Massasauga Rattlesnake; Johnson, B., Menzie, V., Eds.; Metropolitan Toronto Zoo: West Hill, ON, Canada, 1993; pp. 89–93. [Google Scholar]
- Hare, T.; McNally, J.T. Evaluation of a rattlesnake relocation program in the Tucson, Arizona, area. Sonoran Herpetol. 1997, 10, 26–31. [Google Scholar]
- McNally, J.T. Evaluation of a Rattlesnake Relocation Program (U93009); Report; Arizona Game and Fish Heritage Fund: Phoenix, AZ, USA, 1995. Available online: http://azmemory.azlibrary.gov/cdm/ref/collection/statepubs/id/16661 (accessed on 31 December 2021).
- King, R.S.; Berg, C.; Hay, B. A Repatriation study of the Eastern Massasauga (Sistrurus catenatus catenatus) in Wisconsin. Herpetologica 2004, 60, 429–437. [Google Scholar] [CrossRef]
- Walker, M.L.; Dorr, J.A.; Benjamin, R.J.; Pisani, G.R. Successful relocation of a threatened suburban population of Timber Rattlesnakes (Crotalus horridus): Combining snake ecology, politics, and education. IRCF Reptiles Amphib. 2009, 16, 210–221. [Google Scholar]
- Harvey, D.S.; Lentini, A.M.; Cedar, K.; Weatherhead, P.J. Moving massasaugas: Insight into rattlesnake relocation using Sistrurus c. catenatus. Herpetol. Cons. Biol. 2014, 9, 67–75. [Google Scholar]
- Jungen, M.T. Eastern Diamondback Rattlesnake (Crotalus adamanteus) Telemetry Techniques and Translocation. Master’s Thesis, Marshall University, Huntington, WV, USA, 2018. [Google Scholar]
- Kelley, A.G. The Effectiveness of Long-Distance Translocation of Eastern Diamondback Rattlesnakes (Crotalus adamanteus). Master’s Thesis, Marshall University, Huntington, WV, USA, 2020. [Google Scholar]
- Reinert, H.K.; Rupert, R.R. Impacts of translocation on behavior and survival of Timber Rattlesnakes, Crotalus horridus. J. Herpetol. 1999, 33, 45–61. [Google Scholar] [CrossRef]
- Holding, M.L. Short-Distance Translocation of the Northern Pacific Rattlesnake (Crotalus o. oreganus): Effects on Volume and Neurogenesis in the Cortical Forebrain, Steroid Hormone Concentrations, and Behaviors. Master’s Thesis, California Polytechnic State University, San Louis Obispo, CA, USA, 2011. [Google Scholar]
- Holding, M.L.; Frazier, J.A.; Taylor, E.N.; Strand, C.R. Experimentally altered navigational demands induce changes in the cortical forebrain of free-ranging Northern Pacific Rattlesnakes (Crotalus o. oreganus). Brain Behav. Evol. 2012, 79, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Holding, M.L.; Frazier, J.A.; Taylor, E.N.; Strand, C.R. Physiological and behavioral effects of repeated handling and short-distance translocations on free-ranging Northern Pacific Rattlesnakes (Crotalus oreganus oreganus). J. Herpetol. 2014, 48, 233–239. [Google Scholar] [CrossRef]
- Jennings, M.R.; Hayes, M.P. Amphibian and Reptile Species of Special Concern in California; California Department of Fish and Game, Inland Fisheries Division: Rancho Cordova, CA, USA, 1994. [Google Scholar]
- Dugan, E.A.; Figueroa, A.; Hayes, W.K. Home range size, movements, and mating phenology of sympatric Red Diamond (Crotalus ruber) and Southern Pacific (C. oreganus helleri) rattlesnakes in southern California. In The Biology of Rattlesnakes; Hayes, W.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 353–364. [Google Scholar]
- Halama, K.J.; Malisch, A.J.; Aspell, M.; Rotenberry, J.T.; Allen, M.F. Modeling the landscape niche characteristics of Red Diamond Rattlesnakes (Crotalus ruber): Implications for biology and conservation. In The Biology of Rattlesnakes; Hayes, W.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 463–472. [Google Scholar]
- Cowling, R.M.; Rundel, P.W.; Lamont, B.B.; Arroyo, M.K.; Arianoutsou, M. Plant diversity in Mediterranean-climate regions. Trends Ecol. Evol. 1996, 11, 362–366. [Google Scholar] [CrossRef]
- Western Regional Climate Center (undated). Redlands, California (047306): Period of Record Monthly Climate Summary: 04/01/1898 to 05/17/2016. Available online: https://wrcc.dri.edu/cgi-bin/cliMAIN.pl?ca7306 (accessed on 31 December 2021).
- Quinn, H.; Jones, J. Squeeze box technique for measuring snakes. Herpetol. Rev. 1974, 5, 35. [Google Scholar]
- Rasband, W.S. ImageJ; National Institutes of Health: Bethesda, MD, USA, 1997. [Google Scholar]
- Hardy, D.L.; Greene, H.W. Surgery on rattlesnakes in the field for implantation of transmitters. Sonoran Herpetol. 1999, 12, 25–27. [Google Scholar]
- Reinert, H.K.; Cundall, D. An improved surgical implantation method for radio-tracking snakes. Copeia 1982, 1982, 702–705. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Calenge, C. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Getz, W.M.; Wilmers, C.C. A local nearest-neighbor convex-hull construction of home ranges and utilization distributions. Ecography 2004, 27, 489–505. [Google Scholar] [CrossRef] [Green Version]
- Getz, W.M.; Fortmann-Roe, S.; Cross, P.C.; Lyons, A.J.; Ryan, S.J.; Wilmers, C.C. LoCoH: Nonparameteric kernel methods for constructing home ranges and utilization distributions. PLoS ONE 2007, 2, e207. [Google Scholar] [CrossRef] [Green Version]
- Gregory, P.T.; Macartney, J.M.; Larsen, K.W. Spatial patterns and movements. In Snakes: Ecology and Evolutionary Biology; Seigel, R.A., Collins, J.T., Novak, S.S., Eds.; Blackburn Press: Caldwell, NJ, USA, 2001; pp. 366–395. [Google Scholar]
- Gallucci, M. GAMLj: General Analysis for Linear Models. 2019. Available online: https://gamlj.github.io/ (accessed on 31 December 2021).
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Meth. Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Bates, D.; Macchler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Scheipl, F.; Grothendieck, G.; Green, P.; et al. Test Fitted Model for (near) Singularity. R Documentation. 2015. Available online: https://search.r-project.org/CRAN/refmans/lme4/html/isSingular.html (accessed on 31 December 2021).
- Therneau, T. A Package for Survival Analysis in R. 2021. Available online: https://cran.r-project.org/package=survival (accessed on 31 December 2021).
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457. [Google Scholar] [CrossRef]
- Pollock, K.H.; Winterstein, S.R.; Bunck, C.M.; Curtis, P.D. Survival analysis in telemetry studies: The staggered entry design. J. Wildl. Manag. 1989, 53, 7. [Google Scholar] [CrossRef]
- Robertson, H.A.; Westbrooke, I.M. A Practical Guide to the Management and Analysis of Survivorship Data from Radio-Tracking Studies; Science & Technical Publishing, Department of Conservation: Wellington, New Zealand, 2005.
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Ser. B (Methodol.) 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Miller, H.W.; Johnson, D.H. Interpreting the results of nesting studies. J. Wildl. Manag. 1978, 42, 471–576. [Google Scholar] [CrossRef]
- Mathew, A.; Pandey, M.; Murthy, N.S. Survival analysis: Caveats and pitfalls. Eur. J. Surg. Oncol. 1999, 25, 321–329. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Duvall, D.; Schuett, G. Straight-line movement and competitive mate searching in prairie rattlesnakes, Crotalus viridis viridis. Anim. Behav. 1997, 54, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Glaudas, X.; Rodríguez-Robles, J.A. Vagabond males and sedentary females: Spatial ecology and mating system of the Speckled Rattlesnake (Crotalus mitchellii). Biol. J. Linn. Soc. 2011, 103, 681–695. [Google Scholar] [CrossRef]
- Jellen, B.C.; Shepard, D.B.; Dreslik, M.J.; Phillips, C.A. Male movement and body size affect mate acquisition in the Eastern Massasauga (Sistrurus catenatus). J. Herpetol. 2007, 41, 451–457. [Google Scholar] [CrossRef]
- Petersen, C.E.; Goetz, S.M.; Dreslik, M.J.; Kleopfer, J.D.; Savitzky, A.H. Sex, mass, and monitoring effort: Keys to understanding spatial ecology of Timber Rattlesnakes (Crotalus horridus). Herpetologica 2019, 75, 162–174. [Google Scholar] [CrossRef]
- Aldridge, R.D.; Duvall, D. Evolution of the mating season in the pitvipers of North America. Herpetol. Monogr. 2022, 16, 1–25. [Google Scholar] [CrossRef]
- Dreslik, M.J.; Shepard, D.B.; Jellen, B.C.; Phillips, C.A. Movement and home range of the Eastern Massasauga (Sistrurus catenatus) at its southern range limit. In The Biology of Rattlesnakes II; Dreslik, M.J., Hayes, W.K., Beaupre, S.J., Mackessy, S.P., Eds.; ECO Herpetological Publishing and Distribution: Rodeo, NM, USA, 2017; pp. 168–178. [Google Scholar]
- Figueroa, A.; Dugan, E.A.; Hayes, W.K. Behavioral ecology of neonate Southern Pacific Rattlesnakes (Crotalus oreganus helleri) tracked with externally-attached transmitters. In The Biology of Rattlesnakes; Hayes, W.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 365–376. [Google Scholar]
- Stamps, J.A.; Swaisgood, R.R. Someplace like home: Experience, habitat selection and conservation biology. Appl. Anim. Behav. Sci. 2007, 102, 392–409. [Google Scholar] [CrossRef]
- Nowak, E.M.; Greene, H.W. Rattlesnake conservation in the 21st century. In Rattlesnakes of Arizona, Conservation, Behavior, Venom, and Evolution; Schuett, G.W., Feldner, M.J., Smith, C.F., Reiserer, R.S., Eds.; ECO Herpetological Publishing and Distribution: Rodeo, NM, USA, 2017; Volume 2, pp. 413–454. [Google Scholar]
- Bateman, H.L.; Brown, J.A.; Larson, K.L.; Andrade, R.; Hughes, B. Unwanted residential wildlife: Evaluating social-ecological patterns for snake removals. Glob. Ecol. Cons. 2021, 27, e01601. [Google Scholar] [CrossRef]
- Bonnet, X.; Lecq, S.; Lassay, J.L.; Ballouard, J.M.; Barbraud, C.; Souchet, J.; Mullin, S.J.; Provost, G. Forest management bolsters native snake populations in urban parks. Biol. Cons. 2016, 193, 1–8. [Google Scholar] [CrossRef]
- Hayes, W.K. Book review: Rattlesnakes of Arizona, Volume 2: Conservation, Behavior, Venom, and Evolution. Herpetol. Rev. 2017, 48, 870–873. [Google Scholar]
- Bonnet, X.; Naulleau, G.; Shine, R. The dangers of leaving home: Dispersal and mortality in snakes. Biol. Cons. 1999, 89, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Shepard, D.B.; Dreslik, M.K.; Jellen, B.C.; Phillips, C.A. Reptile road mortality around an oasis in the Illinois Corn Desert with emphasis on the endangered Eastern Massasauga. Copeia 2008, 2008, 350–359. [Google Scholar] [CrossRef]
- Cardwell, M.D.; Bush, S.P.; Clark, R.T.; Dugan, E.A. Males Biting Males: Does Testosterone Shape Both Sides of the Snakebite Equation? Undated Poster. Available online: https://www.researchgate.net/profile/Michael-Cardwell-2/publication/266470728_Males_biting_males_does_testosterone_shape_both_sides_of_the_snakebite_equation/links/54dd0ba10cf25b09b912e9cd/Males-biting-males-does-testosterone-shape-both-sides-of-the-snakebite-equation.pdf (accessed on 31 December 2021).
- Macartney, J.M.; Gregory, P.T.; Larsen, K.W. A tabular survey of data on movements and home ranges of snakes. J. Herpetol. 1988, 22, 61–73. [Google Scholar] [CrossRef]

| I.D. | Sex | LDT | SDTs (#) | First Date | Last Date | Days Tracked | Total Fixes | Δ Mass (%) | Fate |
|---|---|---|---|---|---|---|---|---|---|
| 2 | ♀ | No | 3 | 8/2/2008 | 6/10/2009 | 313 | 62 | −21.4 | Translocated to become snake 2.1 |
| 2.1 a | ♀ | Yes | 0 | 10/14/2009 | 10/21/2009 | 14 | 5 | Died—killed by human action | |
| 3 | ♀ | No | 6 | 8/3/2008 | 10/12/2011 | 1145 | 180 | −7.1 | Transmitter removed, snake released |
| 4 | ♀ | No | 0 | 8/10/2008 | 8/23/2009 | 384 | 68 | 25.0 | Died—found depredated d |
| 5 | ♂ | No | 1 | 8/27/2008 | 2/16/2010 | 539 | 92 | −19.2 | Died—second surgery complications |
| 6 | ♂ | No | 13 | 8/27/2008 | 10/12/2011 | 1121 | 151 | 13.2 | Transmitter removed, snake released |
| 7 | ♀ | No | 1 | 9/1/2008 | 9/15/2009 | 382 | 63 | Lost—suspected transmitter failure c | |
| 10 | ♂ | No | 1 | 9/16/2008 | 12/8/2008 | 88 | 3 | Died—injury/surgery complications | |
| 11 | ♂ | No | 4 | 10/9/2008 | 10/14/2009 | 350 | 52 | 0.0 | Translocated to become snake 11.1 |
| 11.1 a | ♂ | Yes | 0 | 10/21/2009 | 10/18/2011 | 721 | 90 | 18.8 | Transmitter removed, snake released |
| 12 | ♀ | No | 2 | 10/9/2008 | 5/7/2009 | 212 | 34 | Lost—suspected transmitter failure c | |
| 13 | ♂ | No | 1 | 10/21/2008 | 11/21/2008 | 36 | 6 | Lost—suspected transmitter failure c | |
| 14 | ♂ | No | 1 | 1/11/2009 | 1/27/2009 | 26 | 3 | Died—suspected depredation d | |
| 15 | ♀ | No | 1 | 1/26/2009 | 7/31/2009 | 193 | 31 | Lost—suspected transmitter failure c | |
| 16 | ♀ | No | 1 | 3/13/2009 | 10/11/2011 | 929 | 165 | −32.1 | Transmitter removed, snake released |
| 17 | ♀ | No | 3 | 3/19/2009 | 1/28/2010 | 215 | 42 | −35.2 | Died—illness |
| 18 | ♂ | No | 0 | 3/19/2009 | 3/27/2009 | 11 | 2 | Lost—suspected transmitter failure c | |
| 19 b | ♀ | Yes | 8 | 10/14/2009 | 10/17/2011 | 706 | 131 | 27.3 | Transmitter removed, snake released |
| 20 | ♂ | No | 3 | 10/21/2009 | 7/26/2011 | 631 | 122 | Died—killed by human action | |
| 21 | ♀ | Yes | 13 | 4/7/2010 | 10/11/2011 | 559 | 80 | Transmitter removed, snake released | |
| 22 | ♂ | Yes | 14 | 4/7/2010 | 4/12/2011 | 382 | 79 | 25.8 | Died—unknown cause |
| 23 | ♂ | No | 1 | 4/9/2010 | 8/14/2011 | 424 | 78 | 15.9 | Transmitter removed, snake released |
| 24 | ♀ | No | 1 | 5/13/2010 | 12/1/2010 | 204 | 45 | Transmitter failed early; found alive on 10/21/2013 | |
| 25 | ♂ | Yes | 5 | 4/27/2010 | 10/11/2011 | 516 | 92 | 10.0 | Lost—suspected transmitter failure c |
| 26 | ♂ | No | 10 | 4/27/2010 | 12/30/2011 | 608 | 103 | 13.3 | Not recaptured |
| 27 | ♂ | No | 8 | 6/10/2010 | 12/30/2011 | 550 | 83 | 102.9 | Not recaptured |
| 28 | ♂ | Yes | 1 | 6/10/2010 | 10/18/2011 | 480 | 46 | −2.6 | Transmitter removed, snake released |
| 29 | ♀ | No | 3 | 7/6/2010 | 6/15/2011 | 350 | 65 | Lost—suspected transmitter failure c | |
| 30 | ♀ | No | 3 | 7/27/2010 | 8/23/2011 | 391 | 68 | 22.2 | Transmitter removed, snake released |
| 31 | ♂ | Yes | 0 | 8/5/2010 | 9/27/2011 | 423 | 62 | 23.1 | Lost—suspected transmitter failure c |
| 32 | ♂ | Yes | 4 | 9/29/2010 | 9/19/2011 | 357 | 53 | Lost—suspected death by human action | |
| 33 | ♂ | Yes | 2 | 11/3/2010 | 10/24/2011 | 356 | 54 | 21.7 | Transmitter removed, snake released |
| 100% MCP | 100% LCH | MDM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Num df | Den df | p | F | Num df | Den df | p | F | Num df | Den df | p | |
| SDTs | 6.24 | 1 | 18.41 | 0.022 | 0.09 | 1 | 25.00 | 0.770 | 15.31 | 1 | 19.60 | <0 001 |
| Sex | 1.90 | 1 | 15.69 | 0.187 | 5.66 | 1 | 25.00 | 0.025 | 0.01 | 1 | 16.99 | 0.927 |
| Year | 4.01 | 3 | 18.18 | 0.024 | 3.13 | 3 | 25.00 | 0.044 | 1.26 | 3 | 19.22 | 0.316 |
| SVL | 7.42 | 1 | 12.04 | 0.018 | 6.25 | 1 | 25.00 | 0.019 | 0.90 | 1 | 13.44 | 0.359 |
| Sex × Year | 1.48 | 3 | 19.34 | 0.252 | 2.11 | 3 | 25.00 | 0.125 | 0.78 | 3 | 20.24 | 0.518 |
| Sex × SVL | 5.72 | 1 | 12.97 | 0.033 | 2.67 | 1 | 25.00 | 0.115 | 1.84 | 1 | 14.39 | 0.196 |
| R2 Marginal | 0.61 | 0.55 | 0.52 | |||||||||
| R2 Conditional | 0.63 | 0.55 | 0.54 | |||||||||
| 100% MCP | 100% LCH | MDM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Num df | Den df | p | F | Num df | Den df | p | F | Num df | Den df | p | |
| LDT | 21.28 | 1 | 25.00 | <0.001 | 7.17 | 1 | 9.14 | 0.025 | 3.07 | 1 | 11.62 | 0.106 |
| SDTs | 2.90 | 1 | 25.00 | 0.101 | 5.39 | 1 | 8.35 | 0.052 | 13.46 | 1 | 10.77 | 0.007 |
| Sex | 7.74 | 1 | 25.00 | 0.010 | 3.72 | 1 | 12.33 | 0.047 | 9.12 | 1 | 14.41 | 0.004 |
| Year | 10.51 | 1 | 25.00 | 0.003 | 0.13 | 1 | 8.57 | 0.077 | 0.03 | 1 | 11.00 | 0.009 |
| SVL | 0.27 | 1 | 25.00 | 0.608 | 4.17 | 1 | 24.02 | 0.727 | 8.64 | 1 | 24.69 | 0.856 |
| LDT × Year | 12.82 | 1 | 25.00 | 0.001 | 5.58 | 1 | 12.25 | 0.036 | 9.02 | 1 | 14.33 | 0.009 |
| Sex × SVL | 0.37 | 1 | 25.00 | 0.547 | 0.55 | 1 | 9.12 | 0.475 | 0.54 | 1 | 11.61 | 0.477 |
| R2 marginal | 0.64 | 0.47 | 0.59 | |||||||||
| R2 conditional | 0.64 | 0.52 | 0.66 | |||||||||
| Group | N | Survived (%) | Lost (%) | Confirmed Dead (%) | Survival Rate (%) |
|---|---|---|---|---|---|
| Without Human Mortality | |||||
| Non-LDT | 21 | 61.9 | 28.6 | 9.5 | 86.7 |
| LDT | 9 | 88.9 | 11.1 | 0.0 | 100.0 |
| With Human Mortality | |||||
| Non-LDT | 21 | 61.9 | 28.6 | 9.5 | 86.7 |
| LDT | 10 | 80.0 | 10.0 | 10.0 | 88.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbit, A.G.; Hayes, W.K. Human-Wildlife Conflict at a Suburban–Wildlands Interface: Effects of Short- and Long-Distance Translocations on Red Diamond Rattlesnake (Crotalus ruber) Activity and Survival. Diversity 2022, 14, 130. https://doi.org/10.3390/d14020130
Corbit AG, Hayes WK. Human-Wildlife Conflict at a Suburban–Wildlands Interface: Effects of Short- and Long-Distance Translocations on Red Diamond Rattlesnake (Crotalus ruber) Activity and Survival. Diversity. 2022; 14(2):130. https://doi.org/10.3390/d14020130
Chicago/Turabian StyleCorbit, Aaron G., and William K. Hayes. 2022. "Human-Wildlife Conflict at a Suburban–Wildlands Interface: Effects of Short- and Long-Distance Translocations on Red Diamond Rattlesnake (Crotalus ruber) Activity and Survival" Diversity 14, no. 2: 130. https://doi.org/10.3390/d14020130
APA StyleCorbit, A. G., & Hayes, W. K. (2022). Human-Wildlife Conflict at a Suburban–Wildlands Interface: Effects of Short- and Long-Distance Translocations on Red Diamond Rattlesnake (Crotalus ruber) Activity and Survival. Diversity, 14(2), 130. https://doi.org/10.3390/d14020130







