High Variability and Dual Strategy in the Wintering Red Kites (Milvus milvus)
Abstract
1. Introduction
2. Methods
2.1. Tagging and Data Collection
2.2. Spatial Parameters and Analysis
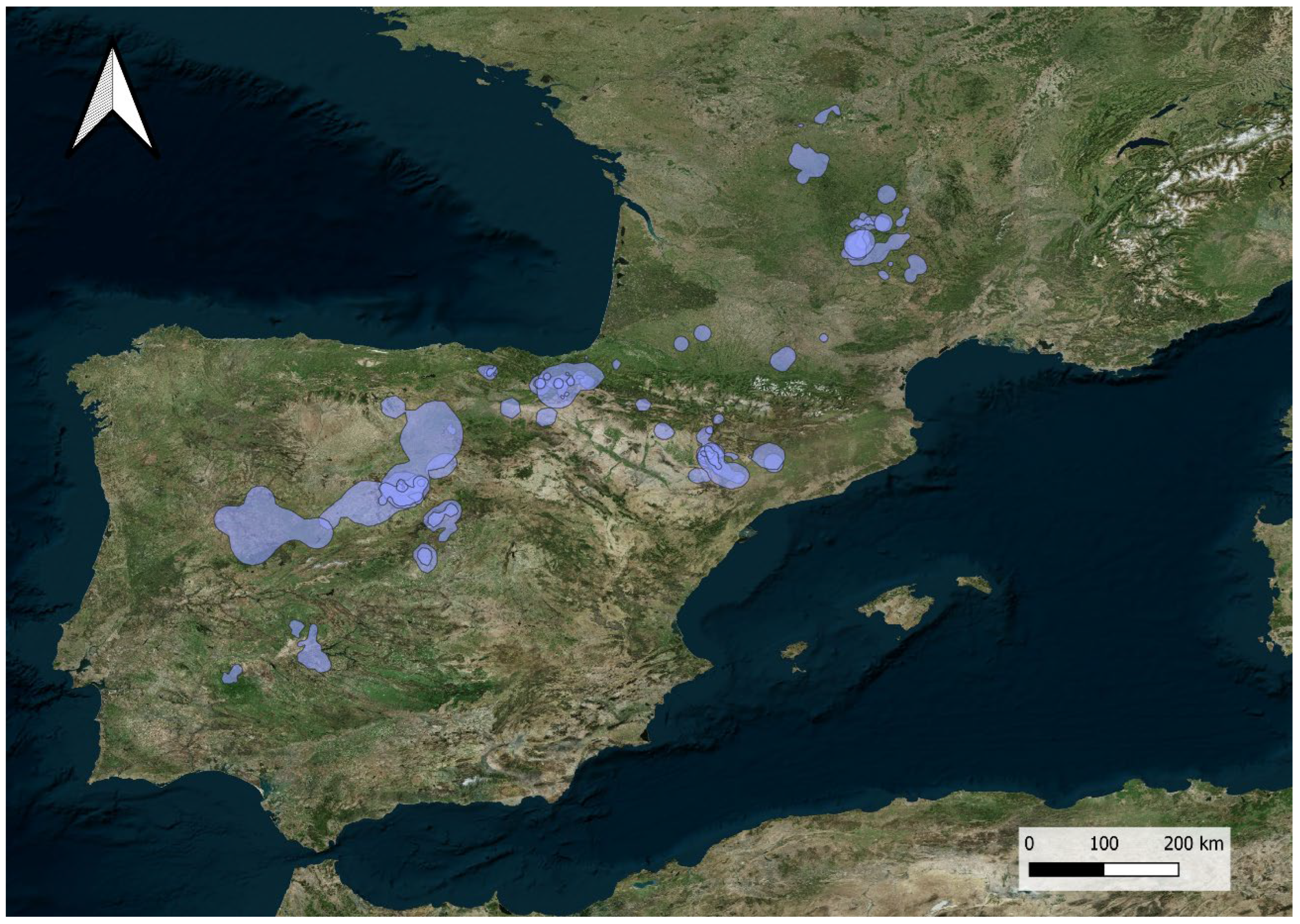
3. Results
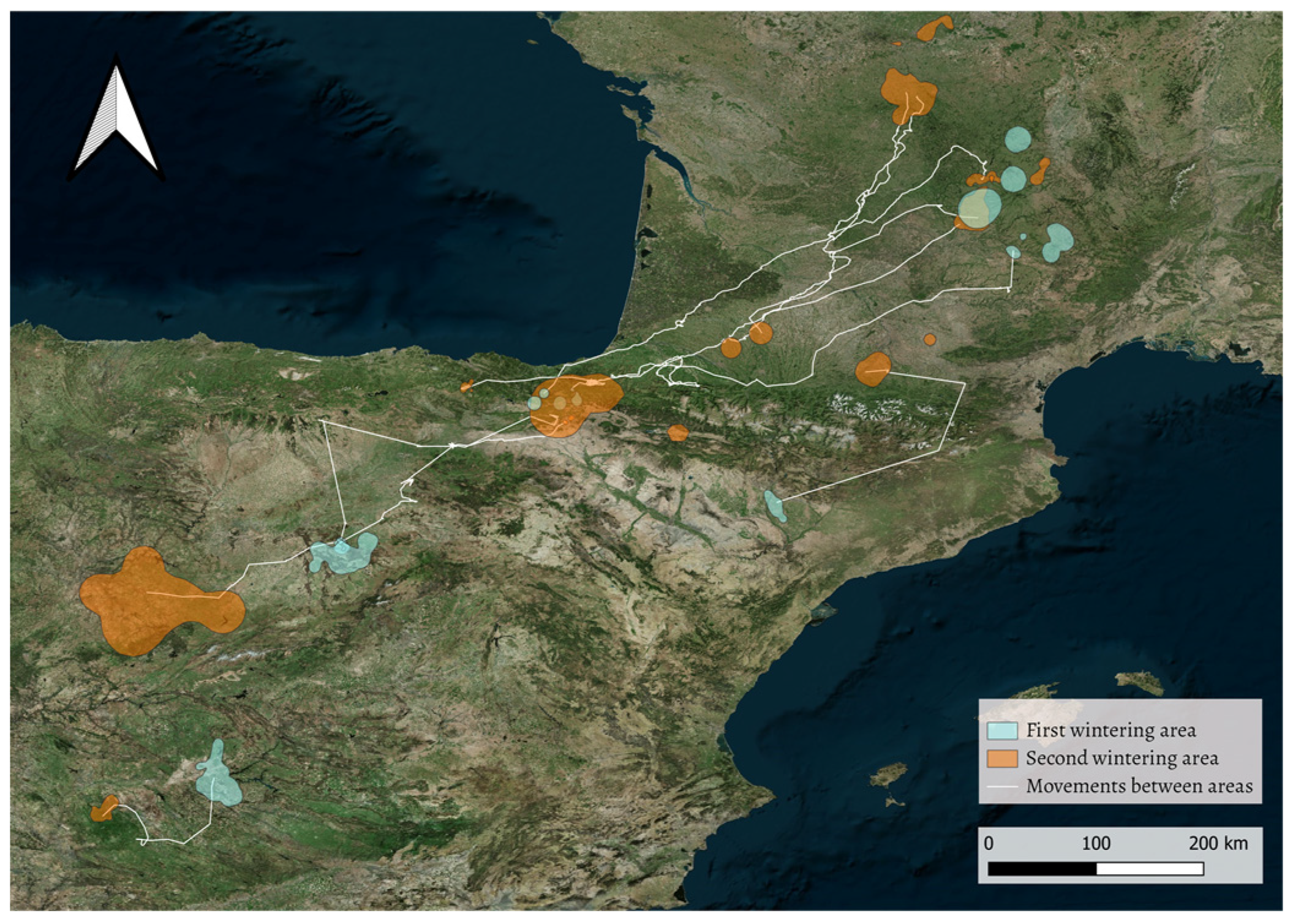
3.1. Dual Strategy: One or Two Wintering Areas
3.2. Home Range Size
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Both, C.; Bouwhuis, S.; Lessells, C.M.; Visser, M.E. Climate change and population declines in a long distance migratory bird. Nature 2006, 441, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Newton, I. The Migration Ecology of Birds; Academic Press: London, UK, 2008. [Google Scholar]
- Jones, T.; Cresswell, W. The phenology mismatch hypothesis: Are declines of migrant birds linked to uneven global climate change? J. Anim. Ecol. 2010, 79, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Urios, V.; Romero, M.; Mellone, U. The Use of Satellite Telemetry for the Study of the Movement Ecology of Raptors; Publicaciones de la Universidad de Alicante: Alicante, Spain, 2015. [Google Scholar]
- Limiñana, R.; Arroyo, B.; Terraube, J.; McGrady, M.; Mougeot, F. Using satellite telemetry and environmental niche modelling to inform conservation targets for a long-distance migratory raptor in its wintering grounds. Oryx 2014, 49, 329–337. [Google Scholar] [CrossRef]
- Mellone, U.; López-López, P.; Limiñana, R.; Urios, V. Wintering habitats of Eleonora’s Falcons Falco eleonorae in Madagascar. Bird Study 2012, 59, 29–36. [Google Scholar] [CrossRef]
- Kassara, C.; Gangoso, L.; Mellone, U.; Piasevoli, G.; Hadjikyriakou, T.G.; Tsiopelas, N.; Giokas, S.; López-López, P.; Urios, V.; Figuerola, J. Current and future suitability of wintering grounds for a long-distance migratory raptor. Sci. Rep. 2017, 7, 8798. [Google Scholar] [CrossRef]
- García-Ripollés, C.; López-López, P.; Urios, V. First description of migration and wintering of adult Egyptian vultures Neophron percnopterus tracked by GPS satellite telemetry. Bird Study 2010, 57, 261–265. [Google Scholar] [CrossRef]
- Vidal-Mateo, J.; Urios, V. Ecología espacial en el período invernal. In Migración y Ecología Espacial de la Población Española de Águila Calzada. Monografía No. 2 del Programa Migra; Urios, V., Bermejo, A., Vidal-Mateo, J., De la Puente, J., Eds.; SEO/Birdlife: Madrid, Spain, 2017; pp. 63–72. [Google Scholar]
- Blanco, J.C.; Hiraldo, F.; Heredia, B. Variations in the diet and foraging behaviour of a wintering Red Kite (Milvus milvus) population in response to changes in food availability. Ardeola 1990, 37, 267–278. [Google Scholar]
- Heredia, B.; Alonso, J.C.; Hiraldo, F. Space and habitat use by Red Kites Milvus milvus during winter in the Guadalquivir marshes: A comparison between resident and wintering populations. Ibis 1991, 133, 374–381. [Google Scholar] [CrossRef]
- Hiraldo, F.; Heredia, B.; Alonso, J.C. Communal Roosting of Wintering Red Kites Milvus milvus (Aves, Accipitridae): Social Feeding Strategies for the Exploitation of Food Resources. Ethology 1993, 93, 117–124. [Google Scholar] [CrossRef]
- García, J.T.; Viñuela, J.; Sunyer, C. Geographic variation of the winter diet of the Red Kite Milvus milvus in the Iberian Peninsula. Ibis 1998, 140, 302–309. [Google Scholar] [CrossRef]
- Nachtigall, W.; Stubbe, M.; Herrmann, S. Aktionsraum und Habitatnutzung des Rotmilans (Milvus milvus) im Winter—Eine telemetrische Studie im Nordharzvorland. J. Ornithol. 2003, 144, 284–294. [Google Scholar] [CrossRef]
- Literák, I.; Raab, R.; Petretto, M.; Škrábal, J.; Spakovszky, P.; Steindl, J. Diverse natal dispersal in four sibling Red Kites originating from Austria, including wintering in Tunisia. Biología 2020, 75, 1399–1407. [Google Scholar] [CrossRef]
- Panter, C.T.; Literák, I.; Raab, R.; Tolhurst, B.A.; White, R.L. Age, landscape, and arrival date explain ranging behavior of wintering Red Kites in southwest Europe. J. Wildl. Manag. 2021, 86, e22147. [Google Scholar] [CrossRef]
- Molina, B. El Milano Real en España. III Censo Nacional. Población Invernante y Reproductora en 2014 y Método de Censo; SEO/BirdLife: Madrid, Spain, 2015. [Google Scholar]
- Viñuela, J.; Martí, R.; Ruiz, A. El Milano Real en España. Monografía No. 6; SEO/BirdLife: Madrid, Spain, 1999. [Google Scholar]
- Madroño, A.; González, C.; Atienza, J.C. Libro Rojo de las Aves de España; Dirección General para la Biodiversidad-SEO/BirdLife: Madrid, Spain, 2004. [Google Scholar]
- Seoane, J.; Viñuela, J.; Díaz-Delgado, R.; Bustamante, J. The effects of land use and climate on red kite distribution in the Iberian Peninsula. Biol. Conserv. 2003, 111, 401–414. [Google Scholar] [CrossRef][Green Version]
- Monclús, L.; Ballesteros-Cano, R.; De La Puente, J.; Lacorte, S.; Lopez-Bejar, M. Influence of persistent organic pollutants on the endocrine stress response in free-living and captive Red Kites (Milvus milvus). Environ. Pollut. 2018, 242, 329–337. [Google Scholar] [CrossRef]
- BirdLife International. Milvus milvus. The IUCN Red List of Threatened Species 2020, e.T22695072A181651010. 2021. Available online: https://www.birdlife.org/projects/iucn-red-list/ (accessed on 22 January 2022).
- Life Eurokite. Red Kite. Available online: https://www.life-eurokite.eu/en/projects/red-kite.html (accessed on 5 November 2021).
- IUCN Red List—Red Kite (Milvus milvus). Available online: https://www.iucnredlist.org/es/species/22695072/181651010#population (accessed on 5 October 2021).
- Del Hoyo, J.; Elliott, A.; Sargatal, J. Handbook of the Birds of the World. Volume 2: New World Vultures to Guineafowl; Lynx Editions: Barcelona, Spain, 1994. [Google Scholar]
- García-Macía, J.; Vidal-Mateo, J.; De La Puente, J.; Bermejo, A.; Raab, R.; Urios, V. Seasonal differences in migration strategies of Red Kites (Milvus milvus) wintering in Spain. J. Ornithol. 2022, 163, 27–36. [Google Scholar] [CrossRef]
- Aebischer, A. Distribution and recent population changes of the Red Kite in the Western Palaearctic—Results of a recent comprehensive inquiry. In Proceedings of the International Red Kite Symposium 2009, Monbéliard, France, 17–18 October 2009; LPO: Paris, France, 2010; pp. 12–14. [Google Scholar]
- Snow, D.W.; Perrins, C.M. The Birds of the Western Palearctic. Volume 1: Non-Passerines; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Visser, M.E.; Perdeck, A.C.; Van Balen, J.H.; Both, C. Climate change leads to decreasing bird migration distances. Glob. Change Biol. 2009, 15, 1859–1865. [Google Scholar] [CrossRef]
- Heath, J.A.; Steenhof, K.; Foster, M.A. Shorter migration distances associated with higher winter temperatures suggest a mechanism for advancing nesting phenology of American Kestrels Falco sparverius. J. Avian Biol. 2012, 43, 376–384. [Google Scholar] [CrossRef]
- Martín, B.; Onrubia, A.; Ferrer, M.A. Effects of climate change on the migratory behavior of the common buzzard Buteo buteo. Clim. Res. 2014, 60, 187–197. [Google Scholar] [CrossRef]
- García, V.; Iglesias-Lebrija, J.J.; Moreno-Opo, R. Null effects of Garcelon harnessing method and transmitter type on soaring raptors. Ibis 2021, 163, 899–912. [Google Scholar] [CrossRef]
- Kenward, R. A Manual for Wildlife Radio Tagging, 2nd ed.; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Bodey, T.W.; Cleasby, I.R.; Bell, F.; Parr, N.; Schultz, A.; Votier, S.C.; Bearhop, S. A phylogenetically controlled meta-analysis of biologging device effects on birds: Deleterious effects and a call for more standardized reporting of study data. Methods Ecol. Evol. 2018, 9, 946–955. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Pfeiffer, T.; Meyburg, B.U. Satellitentelemetrische Untersuchungen zum Zug- und Überwinterungsverhalten thüringischer Rotmilane Milvus milvus. Vogelwarte 2009, 47, 171–187. [Google Scholar]
- Mellone, U.; De La Puente, J.; López-López, P.; Limiñana, R.; Bermejo, A.; Urios, V. Migration routes and wintering areas of Booted Eagles Aquila pennata breeding in Spain. Bird Study 2013, 60, 409–413. [Google Scholar] [CrossRef]
- Jaffré, M.; Beaugrand, G.; Goberville, É.; Jiguet, F.; Kjellén, N.; Troost, G.; Dubois, P.J.; Leprêtre, A.; Luczak, C. Long-Term Phenological Shifts in Raptor Migration and Climate. PLoS ONE 2013, 8, e79112. [Google Scholar] [CrossRef]
- Washburn, B.E.; Martell, M.S.; Bierregaard, R.O.; Henny, C.J.; Dorr, B.S.; Olexa, T.J. Wintering ecology of adult North American Ospreys. J. Raptor Res. 2014, 48, 325–333. [Google Scholar] [CrossRef]
- Kocina, M.; Aagaard, K. A Review of Home Range Sizes of Four Raptor Species of Regional Conservation Concern. West. N. Am. Nat. 2021, 81, 87–96. [Google Scholar] [CrossRef]
- Meyburg, B.U.; Mendelsohn, S.; Mendelsohn, J.; De Klerk, H.M. Revealing unexpected uses of space by wintering Aquila pomarina: How does satellite telemetry identify behaviour at different scales? J. Avian Biol. 2015, 46, 648–657. [Google Scholar] [CrossRef]
- Marucci, G.; Romano, A.C.; Interisano, M.; Toce, M.; Pietragalla, I.; Collazzo, G.P.; Palazzo, L. Trichinella pseudospiralis in a Red Kite (Milvus milvus) from Italy. Parasitol. Res. 2021, 120, 2287–2290. [Google Scholar] [CrossRef]


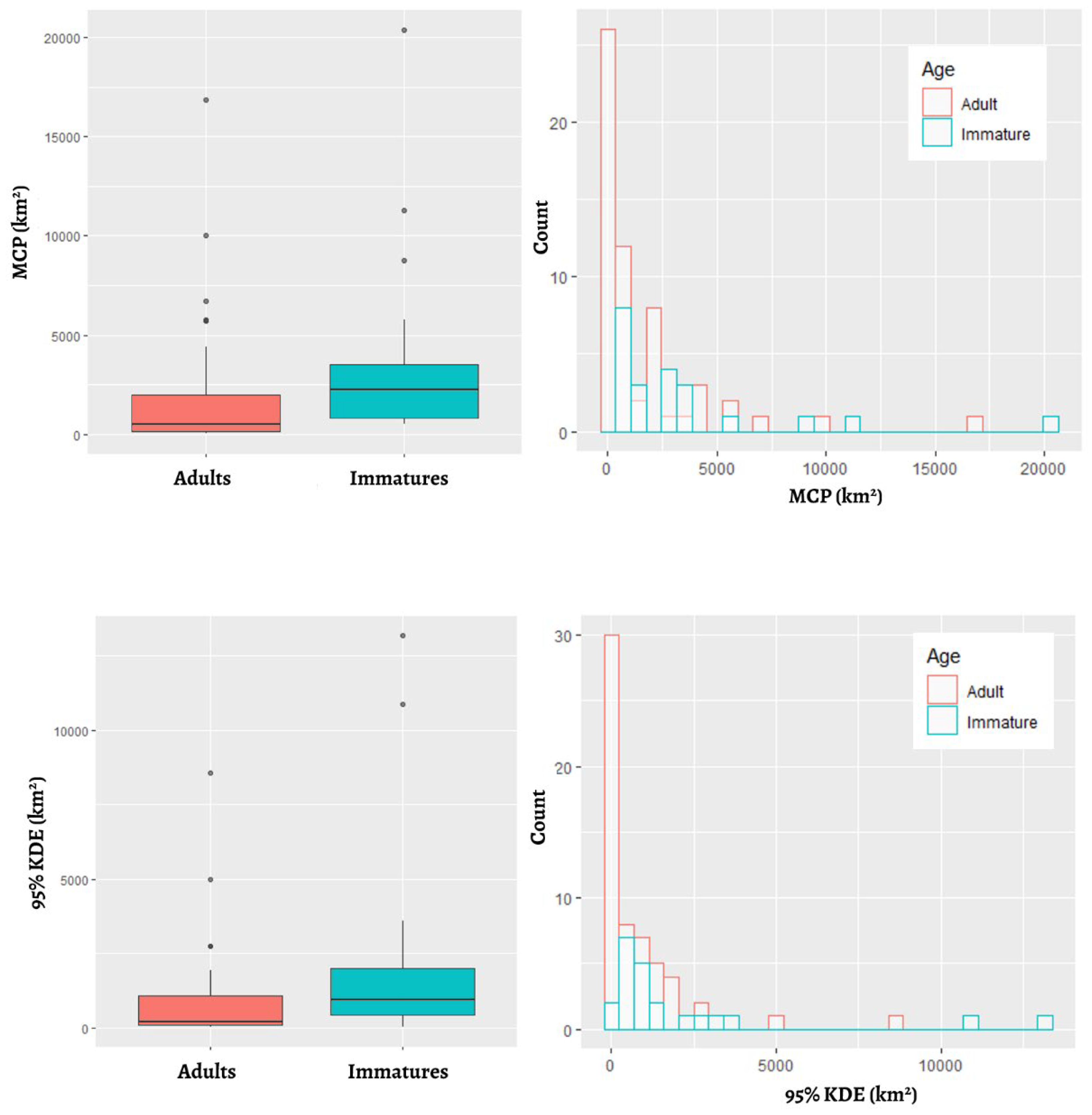
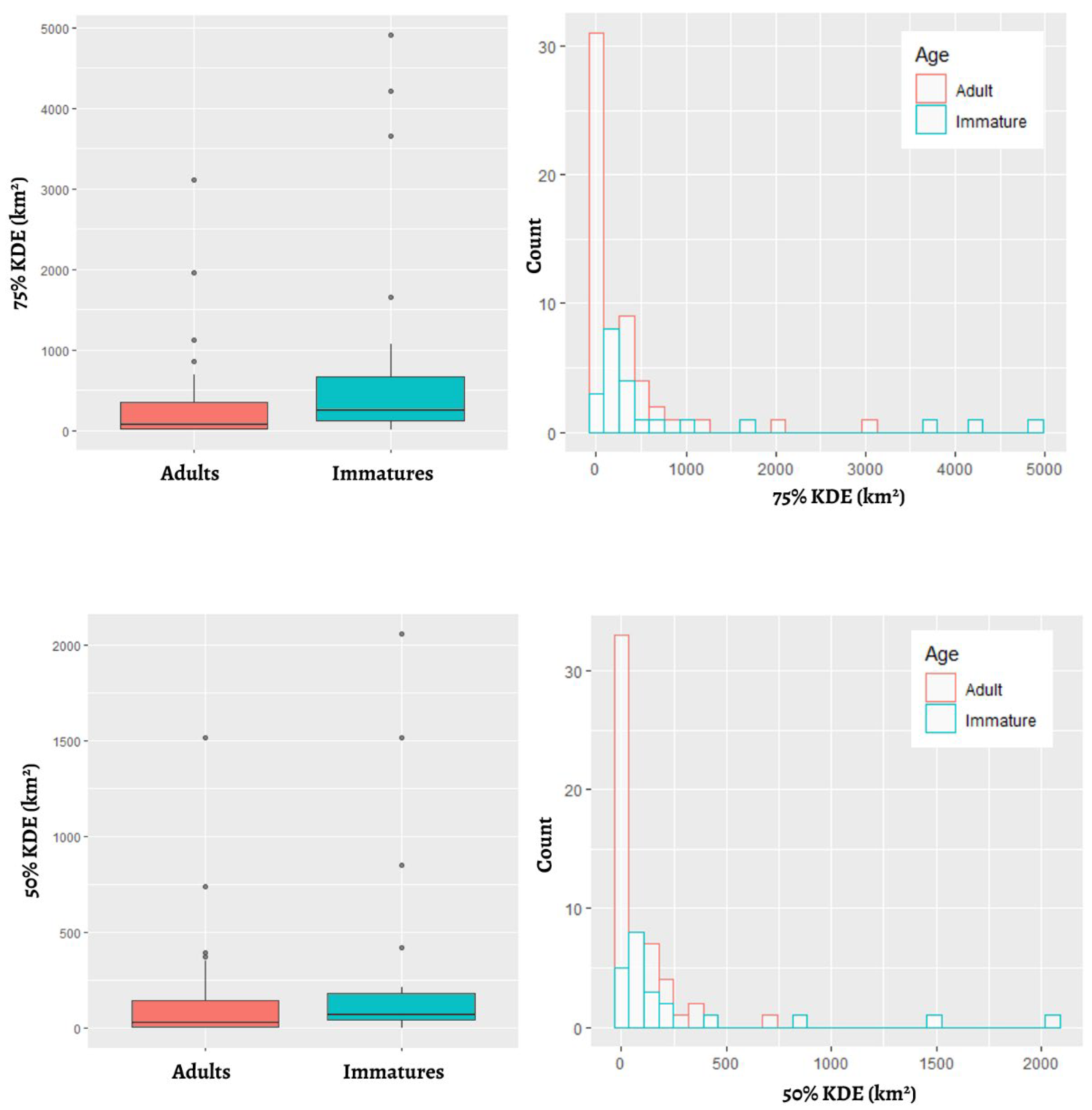
| n | Days of Wintering * | MCP (km2) | 95% Kernel (km2) | 75% Kernel (km2) | 50% Kernel (km2) | |
|---|---|---|---|---|---|---|
| overall | 82 | 95 ± 30 (32–139) | 2173 ± 3519 (27–20340) | 1158 ± 2182 (11–13176) | 438 ± 899 (5–4904) | 158 ± 344 (0.1–2056) |
| one area | 62 | 96 ± 27 (33–138) | 2315 ± 3776 (27–20340) | 979 ± 2030 (11–13176) | 373 ± 810 (5–4216) | 130 ± 291 (0.1–1517) |
| two areas (considered separate) | 10*2 | 97 ± 34 (39–139) | 1655 ± 2377 (54–9998) | 1774 ± 2609 (45–10891) | 659 ± 1155 (11–4904) | 257 ± 482 (3–2056) |
| adults | 60 | 94 ± 29 (32–138) | 1614 ± 2825 (27–16827) | 796 ± 1380 (21–8550) | 271± 504 (5–3107) | 111 ± 230 (0.08–1518) |
| immatures | 22 | – – | 3586 ± 4632 (521–20340) | 2111 ± 3379 (11–13175) | 877 ± 1440 (8–4904) | 282 ± 528 (3–2056) |
| males | 19 | 119± 13 (101–127) | 2828 ± 4510 (32–16828) | 918 ± 1270 (21–4995) | 292 ± 475 (7–1959) | 98 ± 181 (0.1–743) |
| females | 14 | 92 ± 29 (33–139) | 830 ± 1204 (27–4381) | 646 ± 921 (53–2753) | 223 ± 323 (26–1128) | 86 ± 121 (7–396) |
| Individual (Age) | Season | First Area | Days in First Area | Second Area | Days in Second Area | Travelling Days Between Areas | Distance Between Areas (km) | Direction | When Area Changed |
|---|---|---|---|---|---|---|---|---|---|
| Segovia 03 (A) | 2012/2013 | Segovia | - | Pamplona | 61 | 1 | 290 | S -> N | before spring migration |
| Álava 04 (A) | 2014/2015 | Southern France | 48 | Álava | 60 | 1 | 530 | N -> S | after autumn migration |
| Álava 05 (A) | 2016/2017 | Southern France | 45 | Southern France | 37 | 2 | 267 | N -> S | after autumn migration |
| Toledo 01 (A) | 2016/2017 | Toledo | 27 | Badajoz | 111 | 1 | 108 | NE -> SW | before spring migration |
| Ecotone 31 (I) | 2018/2019 | Huesca | >99 | Southern France | 23 | 1 | 187 | S -> N | before spring migration |
| Ecotone 106 (I) | 2018/2019 | Segovia | >66 | Salamanca | 23 | 2 | 207 | N-> S | before spring migration |
| Ornitela 79 (A) | 2019/2020 | Segovia | 25 | Pamplona | 28 | 5 | 250 | S -> N | before spring migration |
| Ornitela 154 (I) | 2019/2020 | Álava | - | Southern France | - | - | ~400 | S -> N -> S | wandering movements;first, it moved to the French area; then, it returned to first area before migration |
| Ornitela 157 (I) | 2019/2020 | Álava | - | Southern France | 43 | 2 | 465 | S -> N | before spring migration |
| Ornitela 194 (A) | 2019/2020 | Álava | - | Southern France | 27 | 10 | 412 | S -> N | before spring migration |
| Fixed Factor (n) | Variable | Factor | Estimate | SE | Df | t Value | p Value |
|---|---|---|---|---|---|---|---|
| Number of areas (one/two) (n = 82). | kernel 95% | intercept | 1396.10 | 303.86 | 32.33 | 4.595 | <0.001 |
| no areas (1) | −400.88 | 296.08 | 61.27 | −1.354 | 0.181 | ||
| kernel 75% | intercept | 529.17 | 128.25 | 24.46 | 4.126 | <0.001 | |
| no areas (1) | −140.40 | 123.46 | 55.65 | −1.137 | 0.260 | ||
| kernel 50% | intercept | 195.69 | 47.84 | 30.26 | 4.090 | <0.001 | |
| no areas (1) | −63.10 | 46.64 | 59.76 | −1.353 | 0.181 | ||
| MCP | intercept | 2101.89 | 532.26 | 45.07 | 3.95 | <0.001 | |
| no areas (1) | 250.05 | 488.71 | 72.57 | 0.512 | 0.61 | ||
| Age (n = 82) | kernel 95% | intercept | 796.10 | 277.50 | 78.0 | 2.869 | 0.0053 |
| age (immatures) | 1315.10 | 529.20 | 78.0 | 2.485 | 0.0151 | ||
| kernel 75% | intercept | 271.10 | 113.2 | 78.0 | 2.394 | 0.0191 | |
| age (immatures) | 605.80 | 215.9 | 78.0 | 2.806 | 0.0063 | ||
| kernel 50% | intercept | 111.42 | 44.29 | 78.0 | 2.516 | 0.0139 | |
| age (immatures) | 170.93 | 84.45 | 78.0 | 2.024 | 0.0464 | ||
| MCP | intercept | 1553.90 | 528.37 | 44.93 | 2.941 | 0.005 | |
| age (immatures) | 2140.75 | 861.46 | 77.61 | 2.485 | 0.015 | ||
| Sex (n = 32) | kernel 95% | intercept | 889.215 | 333.246 | 10.521 | 2.668 | 0.0226 |
| sex (female) | −329.996 | 552.939 | 7.105 | −0.597 | 0.5692 | ||
| kernel 75% | intercept | 283.624 | 121.982 | 10.886 | 2.325 | 0.0404 | |
| sex (female) | −92.647 | 202.523 | 7.418 | −0.457 | 0.6604 | ||
| kernel 50% | intercept | 95.548 | 45.931 | 11.033 | 2.080 | 0.0616 | |
| sex (female) | −21.549 | 76.115 | 7.463 | −0.283 | 0.7848 | ||
| MCP | intercept | 2664.53 | 1144.579 | 11.143 | 2.328 | 0.0397 | |
| sex (female) | −1982.23 | 1982.92 | 9.382 | −0.987 | 0.3484 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Macía, J.; De La Puente, J.; Bermejo-Bermejo, A.; Raab, R.; Urios, V. High Variability and Dual Strategy in the Wintering Red Kites (Milvus milvus). Diversity 2022, 14, 117. https://doi.org/10.3390/d14020117
García-Macía J, De La Puente J, Bermejo-Bermejo A, Raab R, Urios V. High Variability and Dual Strategy in the Wintering Red Kites (Milvus milvus). Diversity. 2022; 14(2):117. https://doi.org/10.3390/d14020117
Chicago/Turabian StyleGarcía-Macía, Jorge, Javier De La Puente, Ana Bermejo-Bermejo, Rainer Raab, and Vicente Urios. 2022. "High Variability and Dual Strategy in the Wintering Red Kites (Milvus milvus)" Diversity 14, no. 2: 117. https://doi.org/10.3390/d14020117
APA StyleGarcía-Macía, J., De La Puente, J., Bermejo-Bermejo, A., Raab, R., & Urios, V. (2022). High Variability and Dual Strategy in the Wintering Red Kites (Milvus milvus). Diversity, 14(2), 117. https://doi.org/10.3390/d14020117







