Discovery of the First Blattinopsids of the Genus Glaphyrophlebia Handlirsch, 1906 (Paoliida: Blattinopsidae) in the Upper Carboniferous of Southern France and Spain and Hypothesis on the Diversification of the Family
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
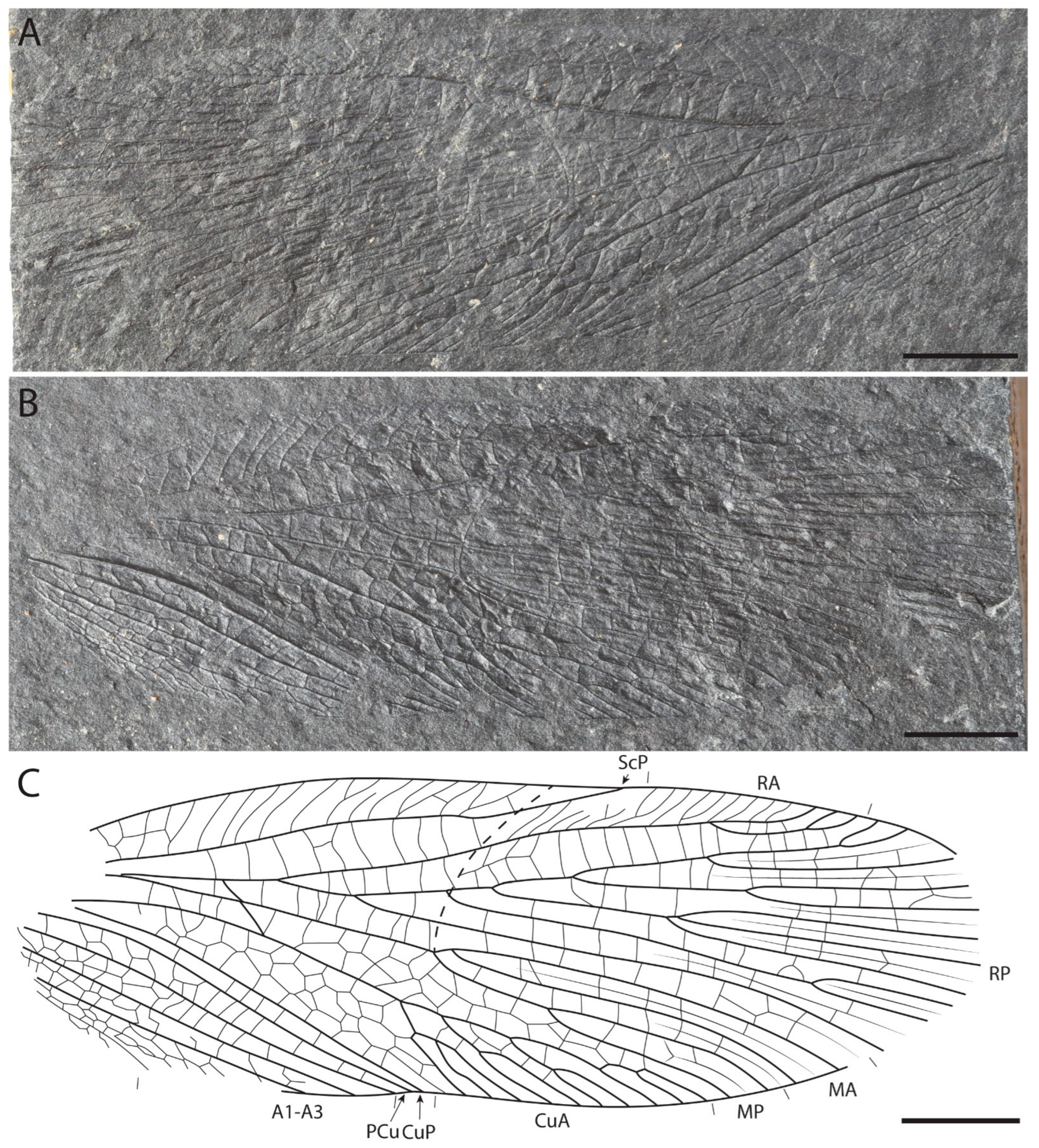
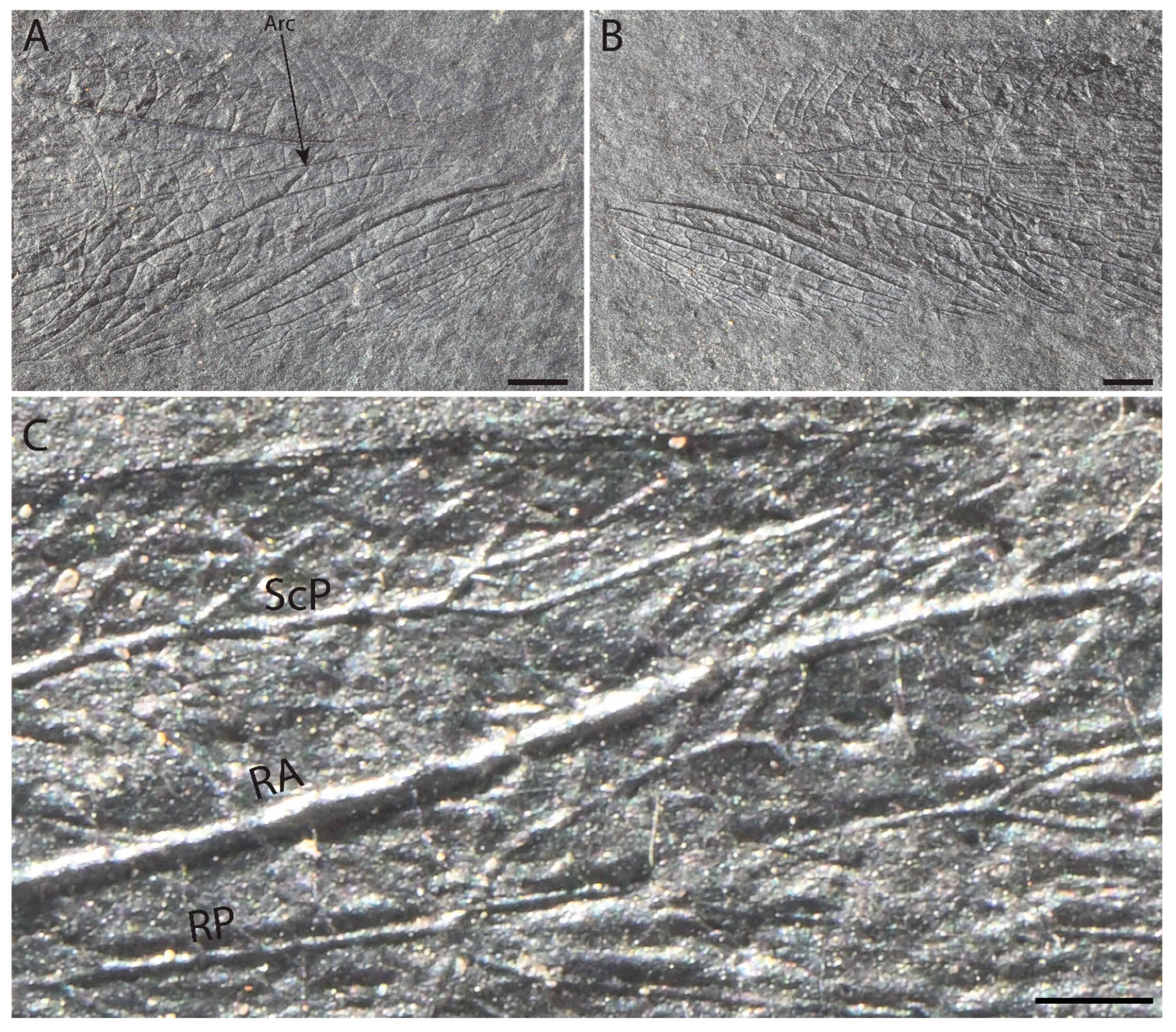
3. Results
Differences from Other Species of Glaphyrophlebia
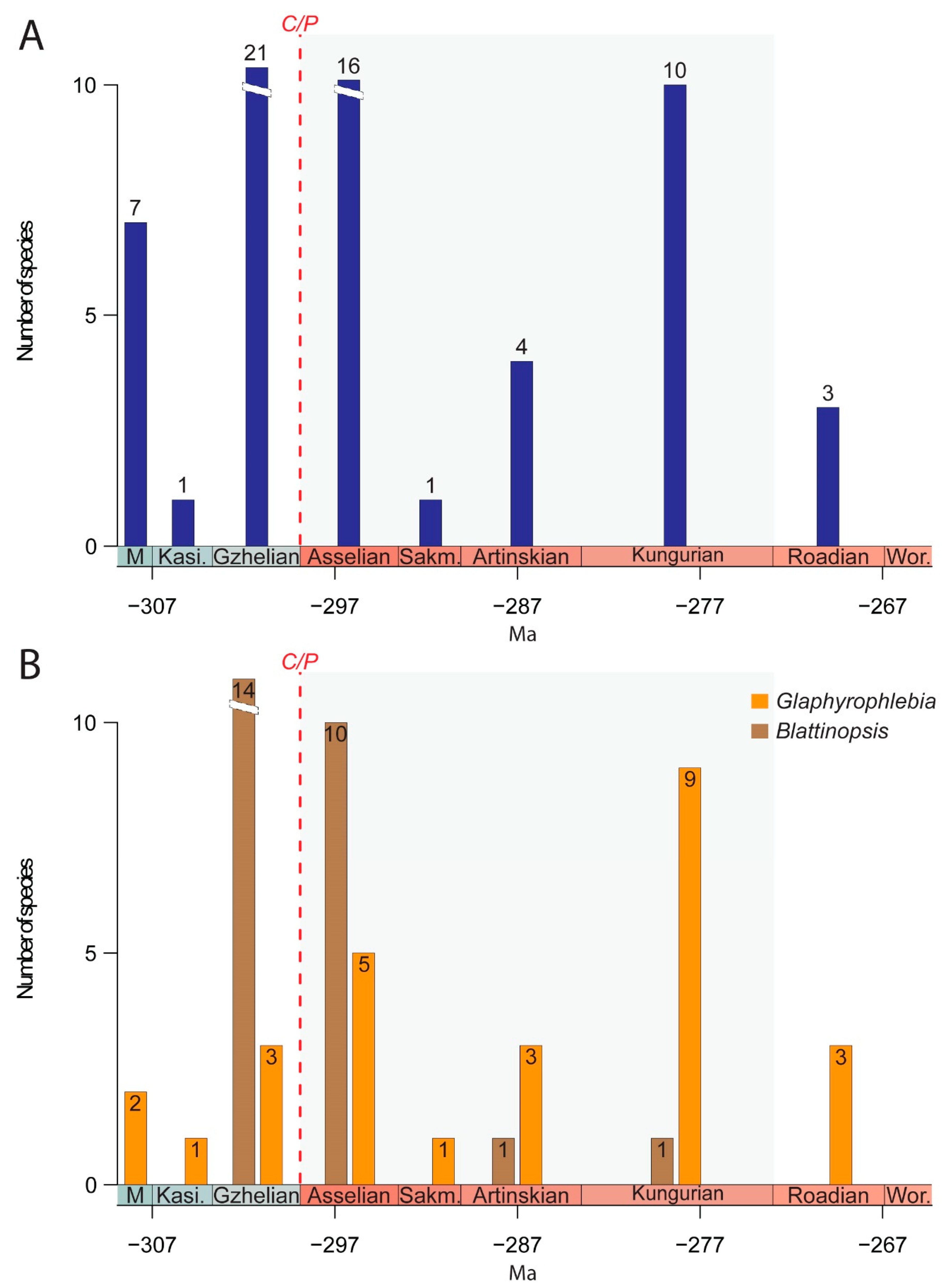
4. Macroevolutionary, Taphonomic, and Palaeoecological Comments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quispe, L.; Roques, P.; Garrouste, R.; Nel, A. Carboniferous Blattinopsidae: Revision of Klebsiella and new genus and species from Avion (Insecta, Paoliida). Hist. Biol. 2021, 34, 383–389. [Google Scholar] [CrossRef]
- Prokop, J.; Krzeminski, W.; Krzeminska, E.; Hörnschemeyer, T.; Ilger, J.-M.; Brauckmann, C.; Grandcolas, P.; Nel, A. Late Palaeozoic Paoliida is the sister group of Dictyoptera (Insecta: Neoptera). J. Syst. Palaeontol. 2014, 12, 601–622. [Google Scholar] [CrossRef]
- Rasnitsyn, A.P.; Aristov, D.S. New species of the genus Blattinopsis Giebel, 1867 (Insecta: Blattinopsida: Blattinopsidae) from the Permian of Taimyr Peninsula, Russia. Far East. Entomol. 2021, 437, 6–9. [Google Scholar] [CrossRef]
- Aristov, D.S.; Rasnitsyn, A.P. New and little known Blattinopsidae (Insecta: Blattinopsida) from the Middle Permian of Russia. Paleontol. J. 2021, 55, 288–293. [Google Scholar] [CrossRef]
- Aristov, D.S.; Rasnitsyn, A.P. New species of the genus Glaphyrophlebia (Insecta, Blattinopsida: Blattinopsidae) from the Upper Carboniferous of Ukraine. Far East. Entomol. 2022, 450, 9–11. [Google Scholar] [CrossRef]
- Aristov, D.S.; Rasnitsyn, A.P. New Blattinopsidae (Insecta: Blattinopsida) from the Middle Permian of European Russia. Paleontol. J. 2022, 56, 187–193. [Google Scholar] [CrossRef]
- Aristov, D.S.; Rasnitsyn, A.P.; Naugolnykh, S.V. New Blattinopsidae (Insecta: Blattinopsida) in the Permian of the Pechora Basin (Komi Republic, Russia) in the context of landscape and vegetation evolution. Paleontol. J. 2021, 55, 641–649. [Google Scholar] [CrossRef]
- Aristov, D.S.; Rasnitsyn, A.P.; Naugolnykh, S.V. A review of Blattinopsida (Insecta) and flora of Latest Early and Early Middle Permian in European Russia. Paleontol. J. 2022, 56, 548–558. [Google Scholar] [CrossRef]
- Pardo, J.D.; Small, B.J.; Milner, A.R.; Huttenlocker, A.K. Carboniferous–Permian climate change constrained early land vertebrate radiations. Nat. Ecol. Evol. 2019, 3, 200–206. [Google Scholar] [CrossRef]
- Santos, A.A.; Hernández-Orúe, A.; Wappler, T.; Peñalver, E.; Diez, J.B.; Nel, A. Late Carboniferous insects from the Iberian Peninsula: State of the art and new taxa. Palaeontogr. Abt. A 2022, 1–27. [Google Scholar] [CrossRef]
- Nel, A.; Santos, A.A.; Hernández-Orúe, A.; Wappler, T.; Diez, J.B.; Peñalver, E. The first representative of the roachoid family Spiloblattinidae (Insecta, Dictyoptera) from the Late Pennsylvanian of the Iberian Peninsula. Insects 2022, 13, 828. [Google Scholar] [CrossRef]
- Fernández García, L.G.; Moro Gómez, C.; Gómez Prieto, J.A.; Álvarez del Campo, C. Revisión y síntesis geológico-minera de la cuenca carbonífera de “El Bierzo” (León). Memoria 1984, 1–98. [Google Scholar]
- Wagner, R.H.; Álvarez-Vázquez, C. The Carboniferous floras of the Iberian Peninsula: A synthesis with geological connotations. Rev. Palaeobot. Palynol. 2010, 162, 239–324. [Google Scholar] [CrossRef]
- Knight, J.A.; Álvarez-Vázquez, C. A summary of upper Pennsylvanian regional substages defined in NW Spain—the chrono-stratigraphic legacy of Robert H. Wagner. Newsl. Stratigr. 2021, 54, 275–300. [Google Scholar] [CrossRef]
- Schubnel, T.; Desutter-Grandcolas, L.; Legendre, F.; Prokop, J.; Mazurier, A.; Garrouste, R.; Grandcolas, P.; Nel, A. To be or not to be: Postcubital vein in insects revealed by microtomography. Syst. Entomol. 2019, 45, 327–336. [Google Scholar] [CrossRef]
- Laurentiaux, D. Les insectes des bassins houillers du Gard et de la Loire. [Insects from the Gard and Loire coalfields]. Ann. Paleontol. 1950, 36, 63–84. [Google Scholar]
- Beckemeyer, R.J. A new species of Glaphyrophlebia Handlirsch, 1906 (Insecta: Neoptera: Blattinopsidae) from the Lower Permian Wellington Formation of Noble County, Oklahoma, USA, The Carboniferous-Permian transition. Bull. New Mex. Mus. Nat. Hist. Sci. 2013, 60, 7–11. [Google Scholar]
- Hörnschemeyer, T.; Stapf, H. Review of Blattinopsidae (Prothortoptera) with description of new species from the Lower Permian of Niedermoschel (Germany). Neues Jahrb. Geol. Palaontol. Abh. 2001, 221, 81–109. [Google Scholar] [CrossRef]
- Martynov, A.V. New fossil insects from Tikhie Gory. 2. Neoptera (excluding Miomoptera). Tr. Geol. Muzeya Akad. Nauk. SSSR 1931, 8, 149–212. [Google Scholar]
- Handlirsch, A. Revision of American Paleozoic insects. Proc. Natl. United States Mus. 1906, 29, 661–820. [Google Scholar] [CrossRef]
- Fritsch, A. Oberste Schichten des Steinkohlen-Gebirges in Schladebach (Wettiner Schichten) [Beschreibung einiger neuen fossilen Insekten in Schleferton des Steinkohlengebirge v Wettin]. In Das Jüngere Steinkohlengebirge und das Rothliegende in der Provinz Sachsen und den Angrenzenden Gebieten; Beyschlag, F.; Fritsch, K.W., Eds. Abhandlungen der Königlich Preußischen-Geologischen Landesanstalt, (N.S.) 1899, 10, 1–263. [Google Scholar]
- Bolton, H. New forms from the insect fauna of British coal measures. Q. J. Geol. Soc. London 1934, 90, 277–301. [Google Scholar] [CrossRef]
- Wilson, J.P.; Montanez, I.P.; White, J.D.; DiMichele, W.A.; McElwain, J.C.; Poulsen, C.J.; Hren, M.T. Dynamic Carboniferous tropical forests: New views of plant function and potential for physiological forcing of climate. New Phytol. 2017, 215, 1333–1353. [Google Scholar] [CrossRef] [Green Version]
- Cleal, C.J.; Uhl, D.; Cascales-Miñana, B.; Thomas, B.A.; Bashforth, A.R.; King, S.C.; Zodrow, E.I. Plant biodiversity changes in Carboniferous tropical wetlands. Earth. Sci. Rev. 2012, 114, 124–155. [Google Scholar] [CrossRef]
- Prevec, R.; Nel, A.; Day, M.O.; Muir, R.A.; Matiwane, A.; Kirkaldy, A.P.; Moyo, S.; Staniczek, A.; Cariglino, B.; Maseko, Z.; et al. South African Lagerstätte reveals middle Permian Gondwanan lakeshore ecosystem in exquisite detail. Commun. Biol. 2022, 5, 1154. [Google Scholar] [CrossRef]
- Prokoph, A.; Shields, G.; Veizer, J. Compilation and time-series analysis of a marine carbonate δ18O, δ13C, 87Sr/86Sr and δ34S database through Earth history. Earth. Sci. Rev. 2008, 87, 113–133. [Google Scholar] [CrossRef]
- Condamine, F.L.; Silvestro, D.; Koppelhus, E.B.; Antonelli, A. The rise of angiosperms pushed conifers to decline during global cooling. Proc. Natl. Acad. Sci. USA 2020, 117, 28867–28875. [Google Scholar] [CrossRef]
- Condamine, F.; Clapham, M.; Kergoat, G. Global patterns of insect diversification: Towards a reconciliation of fossil and molecular evidence? Sci. Rep. 2016, 6, 19208. [Google Scholar] [CrossRef] [Green Version]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and recent climate change. Proc. Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, H.W.; Thomson, M.C. Changes in dominance patterns in Upper Carboniferous plant fossil assemblages. Geology 1982, 10, 641. [Google Scholar] [CrossRef]
- DiMichele, W.A.; Phillips, T.L. Climate change, plant extinctions and vegetational recovery during the Middle-Late Pennsylvanian transition: The case of tropical peat-forming environments in North America. Geol. Soc. Spec. Publ. 1996, 102, 201–221. [Google Scholar] [CrossRef] [Green Version]
- Davies, N.S.; Gibling, M.R. Evolution of fixed-channel alluvial plains in response to Carboniferous vegetation. Nat. Geosci. 2011, 21, 629–633. [Google Scholar] [CrossRef]
- Lehtonen, S.; Silvestro, D.; Karger, D.N.; Scotese, C.; Tuomisto, H.; Kessler, M.; Peña, C.; Wahlberg, N.; Antonelli, A. Environmentally driven extinction and opportunistic origination explain fern diversification patterns. Sci. Rep. 2017, 7, 4831. [Google Scholar] [CrossRef] [Green Version]
- Jouault, C.; Nel, A.; Perrichot, V.; Legendre, F.; Condamine, F. L Multiple drivers and lineage-specific insect extinctions during the Permo–Triassic. Nat. Commun. 2022, 13, 7512. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.A. The Stephanian A-B flora and stratigraphy of the Sabero Coalfield (León, N.W. Spain). Compte Rendu 7e Congrès Int. Stratigr. Géologie Carbonifère Kref. 1974, 3, 283–315. [Google Scholar]
- Remy, R.; Remy, W. Die Floren des Erdaltertums. Glückauf Essen 1977, 468. [Google Scholar]
- Barthel, M. Pflanzengruppen und Vegetationseinheiten der Manebach-Formation. Beiträge Zur Geol. Thüringen N.F. 2001, 8, 93–123. [Google Scholar]
- Barthel, M. Die Rotliegendflora des Thüringer Waldes. Teil 3: Farne. Veröffentlichungen Naturhist. Mus. Schleus. 2005, 20, 27–56. [Google Scholar]
- Rasnitsyn, A.P. Order Blattinopseida Bolton, 1925. p. 106. In History of Insects; Rasnitsyn, A.P., Quicke, D.L.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2002; p. xi + 517. [Google Scholar]
- Rasnitsyn, A.P. Subclass Scarabaeones Laicharting, 1781. The winged insects (Pterygota Lang, 1888). pp. 75–82. In History of Insects; Rasnitsyn, A.P., Quicke, D.L.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2002; p. xi + 517. [Google Scholar]
- Jouault, C.; Nel, A.; Legendre, F.; Condamine, F.L. Estimating the drivers of diversification of stoneflies through time and the limits of their fossil record. Insect Syst. Diver. 2022, 6, 1–14. [Google Scholar] [CrossRef]


| Allochthonous Flora |
| Order: Equisetales Trevisan, 1876 |
|
| Order: Bowmanitales Meyen, 1978 |
|
| Order: Filicales |
|
| Order: Marattiales Link, 1833 |
|
| Order: Medullosales Corsin, 1960 |
|
| Order: Callistophytales Rothwell, 1981 |
|
| Order: incertae sedis (Filicophyta) |
|
| Parautochthonous flora |
| Order: Filicales |
|
| Order: Marattiales Link, 1833 |
|
| Order: Dicranophyllales Němejc, 1959 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nel, A.; Garrouste, R.; Peñalver, E.; Hernández-Orúe, A.; Jouault, C. Discovery of the First Blattinopsids of the Genus Glaphyrophlebia Handlirsch, 1906 (Paoliida: Blattinopsidae) in the Upper Carboniferous of Southern France and Spain and Hypothesis on the Diversification of the Family. Diversity 2022, 14, 1129. https://doi.org/10.3390/d14121129
Nel A, Garrouste R, Peñalver E, Hernández-Orúe A, Jouault C. Discovery of the First Blattinopsids of the Genus Glaphyrophlebia Handlirsch, 1906 (Paoliida: Blattinopsidae) in the Upper Carboniferous of Southern France and Spain and Hypothesis on the Diversification of the Family. Diversity. 2022; 14(12):1129. https://doi.org/10.3390/d14121129
Chicago/Turabian StyleNel, André, Romain Garrouste, Enrique Peñalver, Antonio Hernández-Orúe, and Corentin Jouault. 2022. "Discovery of the First Blattinopsids of the Genus Glaphyrophlebia Handlirsch, 1906 (Paoliida: Blattinopsidae) in the Upper Carboniferous of Southern France and Spain and Hypothesis on the Diversification of the Family" Diversity 14, no. 12: 1129. https://doi.org/10.3390/d14121129
APA StyleNel, A., Garrouste, R., Peñalver, E., Hernández-Orúe, A., & Jouault, C. (2022). Discovery of the First Blattinopsids of the Genus Glaphyrophlebia Handlirsch, 1906 (Paoliida: Blattinopsidae) in the Upper Carboniferous of Southern France and Spain and Hypothesis on the Diversification of the Family. Diversity, 14(12), 1129. https://doi.org/10.3390/d14121129







