Home-Range Size and Space Use of Territorial Bonelli’s Eagles (Aquila fasciata) Tracked by High-Resolution GPS/GSM Telemetry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Tracking
2.3. Ethical Statement
2.4. Home-Range Analysis
2.5. Data Modeling
3. Results
3.1. Individual Home-Range Size
3.2. Differences in Home-Range Size
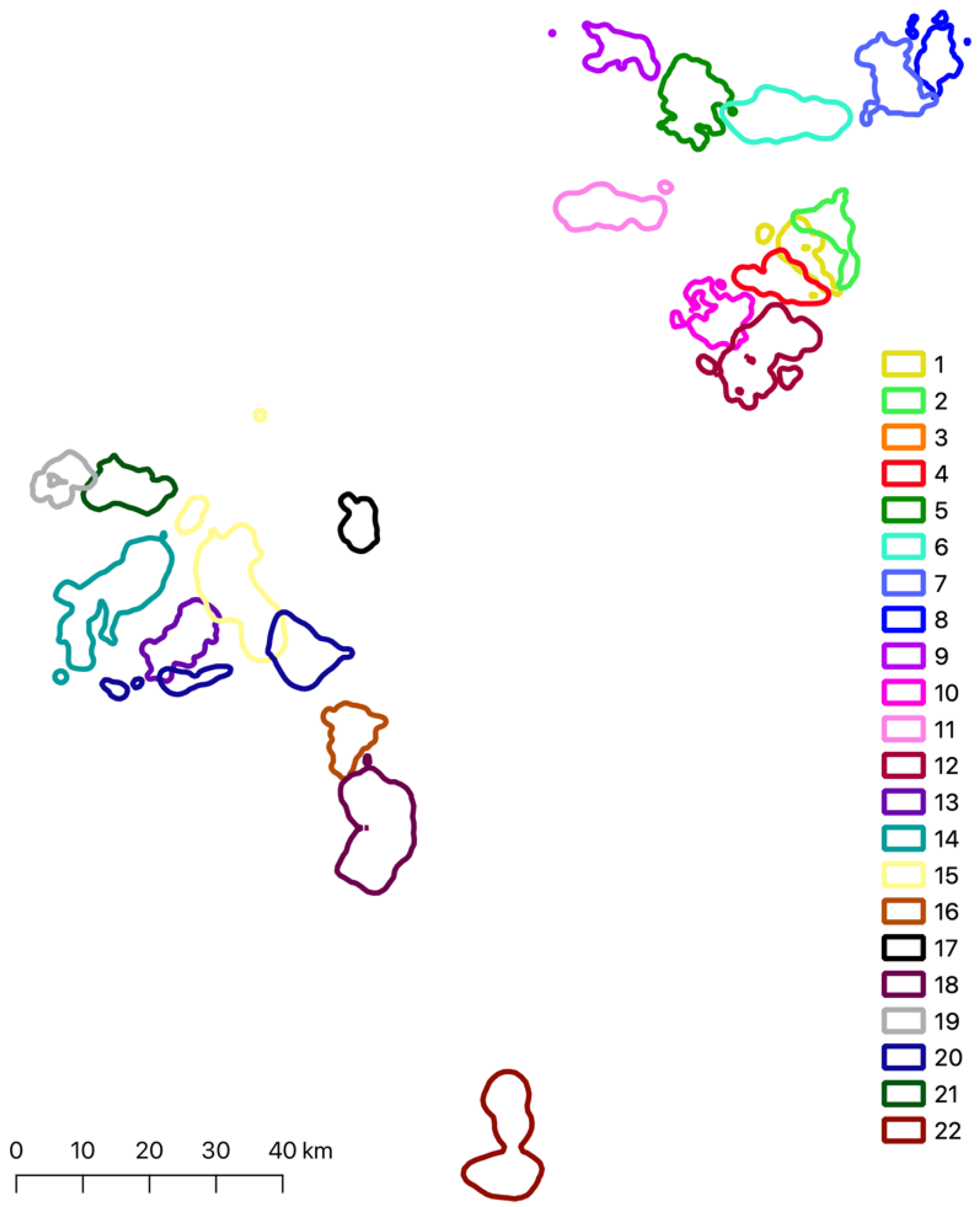
3.3. Overlapping between Neighboring Territories
4. Discussion
4.1. Differences in Home-Range Size between Sexes and Seasons
4.2. Differences in Home-Range Size between Breeding Status and Seasons
4.3. Overlap between Neighboring Territories
4.4. Implications for Conservation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BirdLife International. Species Factsheet: Aquila fasciata. 2022. Available online: http://www.birdlife.org (accessed on 2 July 2022).
- Del Hoyo, J.; Elliot, A.; Sargatal, J. Handbook of the Birds of the World; Linx Edicions: Barcelona, Spain, 1994; Volume II, New world vultures to guineafowl. [Google Scholar]
- López-López, P.; García-Ripollés, C.; Urios, V. Population Size, Breeding Performance and Territory Quality of Bonelli’s Eagle Hieraaetus Fasciatus in Eastern Spain. Bird Study 2007, 54, 335–342. [Google Scholar] [CrossRef]
- López-López, P.; Perona, A.; Egea-Casas, O.; Morant, J.; Urios, V. Tri-Axial Accelerometry Shows Differences in Energy Expenditure and Parental Effort throughout the Breeding Season in Long-Lived Raptors. Curr. Zool. 2022, 68, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Morollón, S.; Urios, V.; López-López, P. Fifteen Days Are Enough to Estimate Home-Range Size in Some Long-Lived Resident Eagles. J. Ornithol. 2022, 163, 849–854. [Google Scholar] [CrossRef]
- Morollón, S.; López-López, P.; Urios, V. A New View of Territoriality in Large Eagles: The Territory Pre-Exists Regardless of Their Occupants. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Bosch, R.; Real, J.; Tinto, A.; Zozaya, E.L.; Castell, C. Home-ranges and Patterns of Spatial Use in Territorial Bonelli’s Eagles Aquila Fasciata. IBIS 2010, 152, 105–117. [Google Scholar] [CrossRef]
- Pérez-García, J.M.; Margalida, A.; Afonso, I.; Ferreiro, E.; Gardiazábal, A.; Botella, F.; Sánchez-Zapata, J.A. Interannual Home Range Variation, Territoriality and Overlap in Breeding Bonelli’s Eagles (Aquila fasciata) Tracked by GPS Satellite Telemetry. J. Ornithol. 2013, 154, 63–71. [Google Scholar] [CrossRef]
- Martínez-Miranzo, B.; Banda, E.; Gardiazábal, A.; Ferreiro, E.; Aguirre, J.I. Differential Spatial Use and Spatial Fidelity by Breeders in Bonelli’s Eagle (Aquila fasciata). J. Ornithol. 2016, 157, 971–979. [Google Scholar] [CrossRef]
- Kenward, R.E. A Manual for Wildlife Radio Tagging; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- García, V.; Iglesias-Lebrija, J.J.; Moreno-Opo, R. Null Effects of the Garcelon Harnessing Method and Transmitter Type on Soaring Raptors. IBIS 2021, 163, 899–912. [Google Scholar] [CrossRef]
- Worton, B.J. Kernel Methods for Estimating the Utilization Distribution in Home-range Studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Signer, J.; Balkenhol, N. Reproducible Home Ranges (Rhr): A New, User-friendly R Package for Analyses of Wildlife Telemetry Data. Wildl. Soc. Bull. 2015, 39, 358–363. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Wells, R.; TinHan, T.C.; Dance, M.A.; Drymon, J.M.; Falterman, B.; Ajemian, M.J.; Stunz, G.W.; Mohan, J.A.; Hoffmayer, E.R.; Driggers, W.B., III. Movement, Behavior, and Habitat Use of a Marine Apex Predator, the Scalloped Hammerhead. Front. Mar. Sci. 2018, 5, 321. [Google Scholar] [CrossRef]
- Miller, K.J.; Erxleben, D.R.; Rains, N.D.; Martin, J.C.; Mathewson, H.A.; Meik, J.M. Spatial Use and Survivorship of Translocated Wild-caught Texas Horned Lizards. J. Wildl. Manag. 2020, 84, 118–126. [Google Scholar] [CrossRef]
- Osipova, L.; Okello, M.; Njumbi, S.; Ngene, S.; Western, D.; Hayward, M.; Balkenhol, N. Using Step-selection Functions to Model Landscape Connectivity for African Elephants: Accounting for Variability across Individuals and Seasons. Anim. Conserv. 2019, 22, 35–48. [Google Scholar] [CrossRef]
- Montalvo, V.H.; Fuller, T.K.; Saénz-Bolaños, C.; Cruz-Díaz, J.C.; Hagnauer, I.; Herrera, H.; Carrillo, E. Influence of Sea Turtle Nesting on Hunting Behavior and Movements of Jaguars in the Dry Forest of Northwest Costa Rica. Biotropica 2020, 52, 1076–1083. [Google Scholar] [CrossRef]
- Van Der Marel, A.; Waterman, J.M.; López-Darias, M. Social Organization in a North African Ground Squirrel. J. Mammal. 2020, 101, 670–683. [Google Scholar] [CrossRef]
- Wysong, M.L.; Hradsky, B.A.; Iacona, G.D.; Valentine, L.E.; Morris, K.; Ritchie, E.G. Space Use and Habitat Selection of an Invasive Mesopredator and Sympatric, Native Apex Predator. Mov. Ecol. 2020, 8, 18. [Google Scholar] [CrossRef]
- Rueda, C.; Jiménez, J.; Palacios, M.J.; Margalida, A. Exploratory and Territorial Behavior in a Reintroduced Population of Iberian Lynx. Sci. Rep. 2021, 11, 14148. [Google Scholar] [CrossRef]
- Shakeri, Y.N.; White, K.S.; Waite, J.N. Staying Close to Home: Ecological Constraints on Space Use and Range Fidelity in a Mountain Ungulate. Ecol. Evol. 2021, 11, 11051–11064. [Google Scholar] [CrossRef]
- Perona, A.M.; Urios, V.; López-López, P. Holidays? Not for All. Eagles Have Larger Home Ranges on Holidays as a Consequence of Human Disturbance. Biol. Conserv. 2019, 231, 59–66. [Google Scholar] [CrossRef]
- Williams, S.M.; Lindell, C.A. The Influence of a Single Species on the Space Use of Mixed-Species Flocks in Amazonian Peru. Mov. Ecol. 2019, 7, 37. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Y.; Mo, B.; Shi, J.; Cheng, Y.; Zhang, W.; Lei, F. Home Range Size and Habitat Use of the Blue-Crowned Laughingthrush during the Breeding Season. PeerJ 2020, 8, e8785. [Google Scholar] [CrossRef]
- Morant, J.; Abad-Gómez, J.M.; Álvarez, T.; Sánchez, Á.; Zuberogoitia, I.; López-López, P. Winter Movement Patterns of a Globally Endangered Avian Scavenger in South-Western Europe. Sci. Rep. 2020, 10, 17690. [Google Scholar] [CrossRef] [PubMed]
- Abril-Colón, I.; Alonso, J.C.; Palacín, C.; Ucero, A.; Álvarez-Martínez, J.M. Factors Modulating Home Range and Resource Use: A Case Study with Canarian Houbara Bustards. Mov. Ecol. 2022, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, X.; Xia, S.; Liu, Y.; Huang, J.; Zhao, W. Potential Habitats and Their Conservation Status for Swan Geese (Anser cygnoides) along the East Asian Flyway. Remote Sens. 2022, 14, 1899. [Google Scholar] [CrossRef]
- Noonan, M.J.; Tucker, M.A.; Fleming, C.H.; Akre, T.S.; Alberts, S.C.; Ali, A.H.; Altmann, J.; Antunes, P.C.; Belant, J.L.; Beyer, D. A Comprehensive Analysis of Autocorrelation and Bias in Home Range Estimation. Ecol. Monogr. 2019, 89, e01344. [Google Scholar] [CrossRef]
- Samuel, M.D.; Pierce, D.J.; Garton, E.O. Identifying Areas of Concentrated Use within the Home Range. J. Anim. Ecol. 1985, 54, 711–719. [Google Scholar] [CrossRef]
- Kie, J.G.; Matthiopoulos, J.; Fieberg, J.; Powell, R.A.; Cagnacci, F.; Mitchell, M.S.; Gaillard, J.-M.; Moorcroft, P.R. The Home-Range Concept: Are Traditional Estimators Still Relevant with Modern Telemetry Technology? Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2221–2231. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: Berlin/Heidelberg, Germany, 2009; Volume 574. [Google Scholar]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A Brief Introduction to Mixed Effects Modelling and Multi-Model Inference in Ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hijmans, R.J.; van Etten, J. Raster: Geographic Data Analysis and Modeling, R Package Version 3.4-5. 2020. Available online: https://cran.r-project.org/web/packages/raster/raster.pdf (accessed on 2 July 2022).
- Sanz, A.; Minguez, E.; Anadon, J.; Hernandez, V. Heterogeneous use of space in three breeding territories of Bonelli’s eagle (Hieraaetus fasciatus). Ardeola 2005, 52, 347–350. [Google Scholar]
- López-López, P. Individual-Based Tracking Systems in Ornithology: Welcome to the Era of Big Data. Ardeola 2016, 63, 5–34. [Google Scholar] [CrossRef]
- Carrascal, L.M.; Seoane, J. Factors Affecting Large-Scale Distribution of the Bonelli’s Eagle Aquila fasciata in Spain. Ecol. Res. 2009, 24, 565–573. [Google Scholar] [CrossRef]
- Ferrer, M.; Morandini, V.; Newton, I. Floater Interference Reflects Territory Quality in the Spanish Imperial Eagle Aquila Adalberti: A Test of a Density-Dependent Mechanism. IBIS 2015, 157, 849–859. [Google Scholar] [CrossRef]
- Martínez-Miranzo, B.; Banda, E.; Aguirre, J.I. Home Range Requirements in Bonelli’s Eagle (Aquila fasciata): Prey Abundance or Trophic Stability? Eur. J. Wildl. Res. 2019, 65, 85. [Google Scholar] [CrossRef]

| Dep. Variable | Indep. Variable | Estimate | Std. Error | t | d.f | p-Value |
|---|---|---|---|---|---|---|
| K95% | (Intercept) | 3.817 | 0.071 | 53.729 | 20.727 | <0.001 |
| Sex (Female) | −0.084 | 0.023 | −3.608 | 24.589 | 0.001 | |
| Breeding (No) | 0.042 | 0.019 | 2.179 | 1234.838 | 0.030 | |
| Season (Breeding) | −0016 | 0.017 | −0.899 | 1221.358 | 0.369 | |
| Sex (Female) × Breeding (No) | 0.017 | 0.018 | 0.948 | 707.351 | 0.343 | |
| Sex (Female) × Season (Breeding) | −0048 | 0.017 | −2.761 | 1222.184 | 0.006 | |
| Breeding (No) × Season (Breeding) | 0.094 | 0.018 | 5.373 | 1232.930 | <0.001 | |
| Sex (Female) × Breeding (No) × Season (Breeding) | 0.011 | 0.017 | 0.616 | 1226.767 | 0.538 | |
| K75% | (Intercept) | 2.974 | 0.076 | 39.063 | 20.855 | <0.001 |
| Sex (Female) | −0.100 | 0.025 | −3.906 | 24.274 | 0.001 | |
| Breeding (No) | 0.057 | 0.020 | 2.851 | 1237.418 | 0.004 | |
| Season (Breeding) | −0.045 | 0.018 | −2.513 | 1220.620 | 0.012 | |
| Sex (Female) × Breeding (No) | 0.026 | 0.019 | 1.349 | 753.480 | 0.178 | |
| Sex (Female) × Season (Breeding) | −0.059 | 0.018 | −3.274 | 1221.557 | 0.001 | |
| Breeding (No) × Season (Breeding) | 0.107 | 0.018 | 5.823 | 1232.456 | <0.001 | |
| Sex (Female) × Breeding (No) × Season (Breeding) | 0.017 | 0.018 | 0.957 | 1227.514 | 0.339 | |
| K50% | (Intercept) | 2.180 | 0.078 | 28.047 | 20.908 | <0.001 |
| Sex (Female) | −0.106 | 0.027 | −4.012 | 24.016 | 0.001 | |
| Breeding (No) | 0.067 | 0.020 | 3.313 | 1238.865 | 0.001 | |
| Season (Breeding) | −0.063 | 0.018 | −3.422 | 1220.088 | 0.001 | |
| Sex (Female) × Breeding (No) | 0.028 | 0.019 | 1.467 | 781.441 | 0.143 | |
| Sex (Female) × Season (Breeding) | −0.063 | 0.018 | −3.440 | 1221.091 | 0.001 | |
| Breeding (No) × Season (Breeding) | 0.116 | 0.019 | 6.252 | 1232.181 | <0.001 | |
| Sex (Female) × Breeding (No) × Season (Breeding) | 0.019 | 0.018 | 1.055 | 1227.970 | 0.292 |
| Season | Sex | Breeding Status | n | K95% | K75% | K50% |
|---|---|---|---|---|---|---|
| Breeding | Females | - | 283 | 48.30 ± 30.60 | 20.60 ± 14.40 | 9.21 ± 6.65 |
| Breeding | Males | - | 319 | 62.80 ± 46.70 | 27.40 ± 22.60 | 12.40 ± 10.80 |
| Non-Breeding | Females | - | 318 | 56.20 ± 35.30 | 25.30 ± 17.30 | 11.70 ± 8.30 |
| Non-Breeding | Males | - | 337 | 57.20 ± 29.80 | 25.60 ± 13.90 | 11.80 ± 6.62 |
| Breeding | - | No | 270 | 61.50 ± 35.60 | 26.90 ± 17.00 | 12.30 ± 8.13 |
| Breeding | - | Yes | 332 | 51.60 ± 43.70 | 22.10 ± 21.10 | 9.77 ± 9.90 |
| Non-Breeding | - | No | 288 | 51.40 ± 32.10 | 23.20 ± 15.30 | 10.70 ± 7.28 |
| Non-Breeding | - | Yes | 367 | 60.80 ± 32.40 | 27.20 ± 15.60 | 12.50 ± 7.54 |
| Territory 1 (T1) | Territory 2 (T2) | Average Overlap % T1-T2 | SD Overlap % T1-T2 | Average Overlap % T2-T1 | SD Overlap % T2-T1 |
|---|---|---|---|---|---|
| 1 | 2 | 3.71 | 3.76 | 3.60 | 3.14 |
| 1 | 3 | 0.15 | NA | 0.07 | NA |
| 1 | 4 | 9.95 | 1.85 | 10.71 | 2.19 |
| 1 | 12 | 0.49 | NA | 0.30 | NA |
| 2 | 3 | 2.67 | 3.21 | 1.66 | 2.30 |
| 6 | 3 | 5.23 | 3.86 | 3.98 | 3.05 |
| 8 | 7 | 0.72 | 0.33 | 0.45 | 0.24 |
| 10 | 12 | 0.72 | 0.33 | 1.96 | 0.85 |
| 15 | 13 | 1.12 | 0.52 | 2.76 | 1.70 |
| 15 | 20 | 19.74 | 12.03 | 9.30 | 3.06 |
| 16 | 18 | 1.09 | 1.24 | 0.55 | 0.61 |
| 20 | 13 | 4.68 | 2.43 | 20.17 | 19.18 |
| 21 | 19 | 1.19 | 0.51 | 1.70 | 0.87 |
| Total | 4.18 | 5.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morollón, S.; Urios, V.; López-López, P. Home-Range Size and Space Use of Territorial Bonelli’s Eagles (Aquila fasciata) Tracked by High-Resolution GPS/GSM Telemetry. Diversity 2022, 14, 1082. https://doi.org/10.3390/d14121082
Morollón S, Urios V, López-López P. Home-Range Size and Space Use of Territorial Bonelli’s Eagles (Aquila fasciata) Tracked by High-Resolution GPS/GSM Telemetry. Diversity. 2022; 14(12):1082. https://doi.org/10.3390/d14121082
Chicago/Turabian StyleMorollón, Sara, Vicente Urios, and Pascual López-López. 2022. "Home-Range Size and Space Use of Territorial Bonelli’s Eagles (Aquila fasciata) Tracked by High-Resolution GPS/GSM Telemetry" Diversity 14, no. 12: 1082. https://doi.org/10.3390/d14121082
APA StyleMorollón, S., Urios, V., & López-López, P. (2022). Home-Range Size and Space Use of Territorial Bonelli’s Eagles (Aquila fasciata) Tracked by High-Resolution GPS/GSM Telemetry. Diversity, 14(12), 1082. https://doi.org/10.3390/d14121082










