1. Introduction
Waterbird assemblages coexist through various interactions determined by factors such as resources (e.g., food) [
1]. Habitat quality, the availability of food resources, interspecific relationships, and anthropogenic disturbances all influence the food and energy requirements of waterbirds and ultimately their population fitness and ability to survive [
2]. Food resources, as the energy and nutritional basis for the formation and maintenance of the waterbird community structure, influence the guild structure, ecological niches, and interspecific relationships of communities and are key to habitat quality [
3]. Shorebirds are small- to medium-sized wading birds that are primary macrobenthic predators [
4]. Shorebird distribution is strongly linked to the availability of their prey, which mainly consists of macrobenthos, especially during wintering and migratory stopovers [
5]. However, aquatic plants are the most critical factor influencing the macrobenthos community [
6]. Therefore, the effects of aquatic plants on macrobenthos indirectly affect shorebird assemblages.
In shallow lakes, aquatic plants play an important role in maintaining macrobenthic diversity [
7]. Many aquatic plants directly change the spatial structures of lake ecosystems and increase spatial heterogeneity, not only providing macrobenthos with habitats and living, feeding, and breeding sites but also shelter from predators [
7,
8]. Macrobenthos in areas with aquatic plants show higher abundance, biomass, and species richness than those in areas characterized by bare sediments [
9]. Post-apoptotic floating-leaved plants, which make water bodies more nutrient-rich, release organic matter directly into water bodies during senescence [
10]. After the degradation of floating-leaved plants, large amounts of organic matter and nutrients are transferred to the sediment [
11], creating an organic environment suitable for the survival of macrobenthos by providing a food source [
12]. Gastropods have a particular affinity for lakes containing high quantities of organic matter [
13], and floating-leaved plants are common plants that are indicators of gastropod populations. The death and decay of plants results in high rates of detritus production, providing favorable conditions for coleopteran growth and abundance [
14]. However, floating-leaved plants create greater shade and reduce the production of surface sediment algae on which many macrobenthic feeders may depend [
15]. Dense floating-leaved plant cover results in hypoxia, which significantly decreases the abundance of oligochaetes in the macrobenthic community [
16].
Macrobenthos are the main prey of shorebirds [
17], the abundance of which is significantly and positively correlated with the number of available macrobenthos [
3,
18,
19,
20]. The distribution of shorebirds also largely depends on macrobenthos, and the capacity to partition available resources varies according to shorebird morphological and behavioral diversity [
21,
22]. The morphological characteristics of shorebirds, such as the lengths of the tarsometatarsus and bill, influence food availability [
23]. The tarsometatarsus length determines the depth of water in which shorebirds can roost [
24]. Bills vary in length and shape, and the depth at which they are inserted into the water or sediment when foraging varies; thus, food available varies [
25]. Moreover, shorebirds discover food through vision and touch, adopt continuous and intermittent movements to find food, and combine foraging mechanisms and movement patterns to form different types of foraging strategies [
23]. There is evidence that different types of shorebird prey, such as surface-dwelling (epifauna) and substrate-inhabiting macrobenthos (infauna), have different functions [
26]. This is because small short-billed species use a visual foraging strategy (superficial pecks), whereas long-billed birds favor tactile foraging (probing deep into the sediment) [
27]. Phenotypic differences between species with similar ecological traits allow them to exploit different resources [
28]. Shorebirds with morphologically distinct bills and different feeding techniques (superficial pecking or deep probing) exploit the various depths of sediment in the same mudflats in different ways, which mitigates interspecific competition between shorebird species for natural trophic resources [
29].
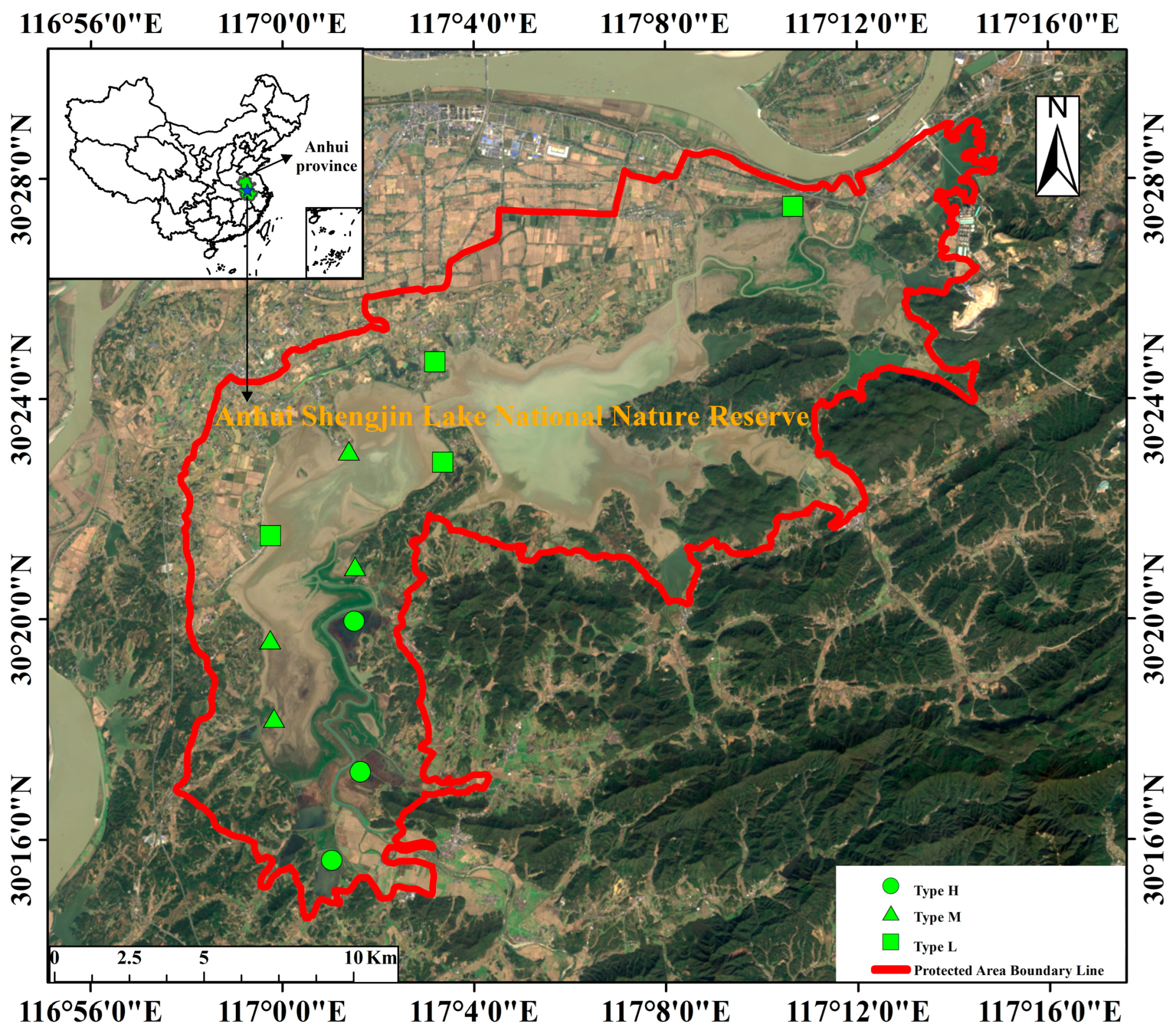
Shengjin Lake, where floating-leaved plants such as
Trapa bispinosa flourish owing to eutrophication in early seine culture waters, provides an important wintering and stopover site for migratory waterbirds on the East Asian–Australasian flyway [
30]. In 2018, the local government began to implement a variety of measures to restore vegetation in this wetland [
31], and many areas were managed uniformly and planted with
Euryale ferox. The return of large areas of floating-leaved plants to the sediment after dieback becomes a natural bait for macrobenthos, which enhances macrobenthic colonization and forms more diverse and abundant macrobenthic communities [
32]. Differences among macrobenthic communities have the most direct impact on shorebird assemblages. Therefore, the natural resource conditions of Shengjin Lake provided a good opportunity to undertake this study.
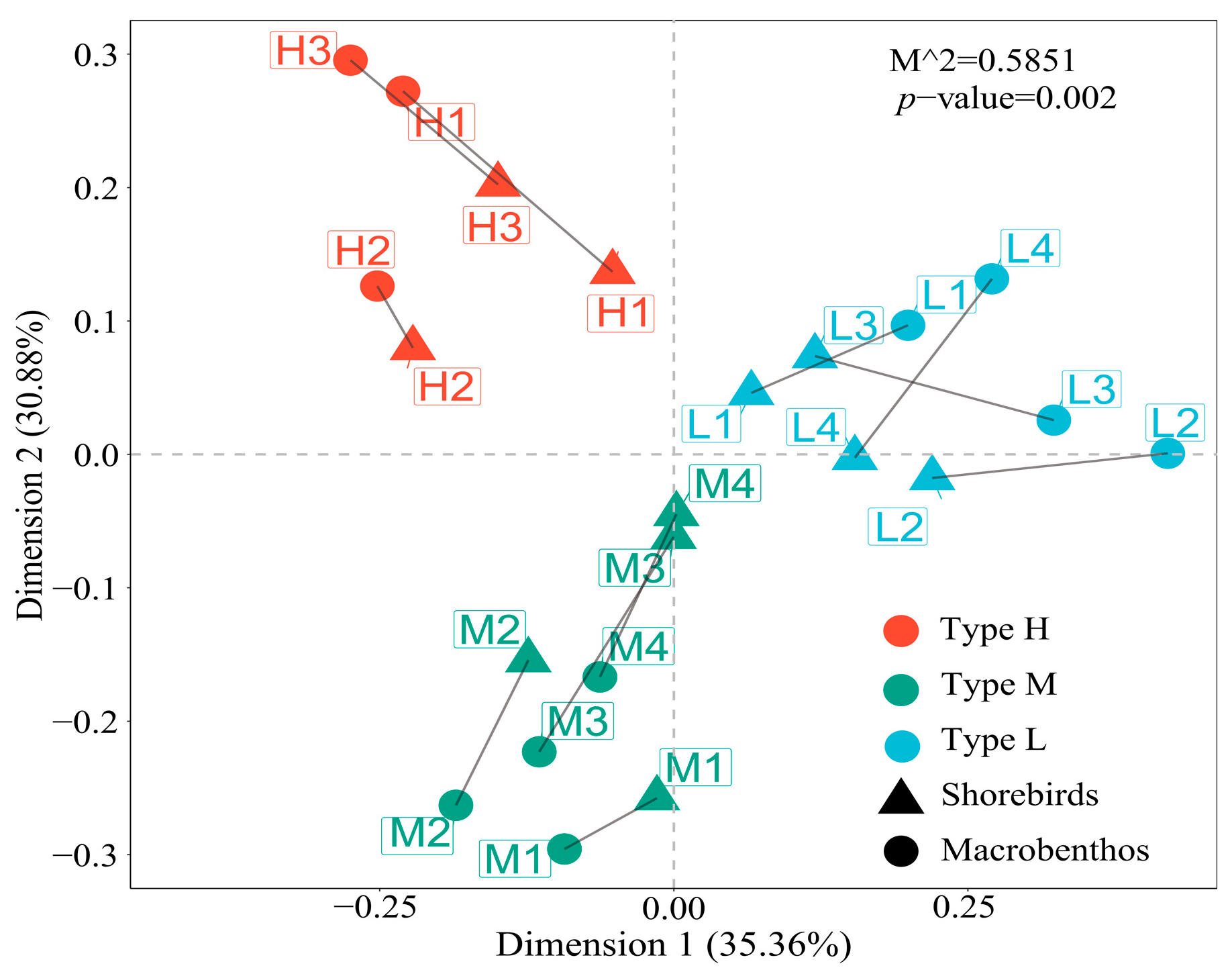
Understanding the effects of macrobenthos relative to floating-leaved plants on wintering shorebird assemblages is crucial for the effective protection of wintering shorebirds at Shengjin Lake. In this study, we hypothesized that the effects of macrobenthos relative to floating-leaved plants on shorebird assemblages were based on food availability. We tested this hypothesis by analyzing the differences in the macrobenthic and shorebird communities in three areas (a Euryale ferox artificial planting area, a Trapa spp. natural growth area, and the control area) of Shengjin Lake and determining the correlations between the two communities. Specifically, (1) we compared the differences among macrobenthic communities in different areas and explored the effects of floating-leaved plants on these communities, and (2) we divided shorebirds into foraging guilds according to their foraging strategies and morphological characteristics, compared the differences among shorebird foraging guilds in different areas, analyzed the correlations between macrobenthos and shorebirds, and explored the responses of different shorebird foraging guilds to differences in food resource availability.
4. Discussion
The focus of our study was the effect of macrobenthos under the influence of floating-leaved plants, which affect the availability of shorebird food owing to differing environmental suitability, resulting in differences in shorebird assemblages. Therefore, whether more refined environmental conditions, such as physicochemical factors, support different faunal assemblages, such as macrobenthos and shorebirds, requires further investigation. Most shorebird studies focus on coastal wetlands, but there are few studies in shallow river-connected lakes. Coastal wetlands may have more abundant food resources and more species of shorebirds than shallow river-connected lakes. However, there are several references of food availability and the foraging behavior of shorebirds [
4,
5,
18,
19,
23,
26], which is the baseline for the study of shorebirds in shallow river-connected lakes. Our results showed that there were significant differences in the composition and abundance of macrobenthic and shorebird communities in the
Euryale ferox artificial planting area,
Trapa spp. natural growth area, and control area. Significant differences among the foraging guilds of shorebirds in different study areas were mainly due to differences in food resources, potential congruence between shorebirds and macrobenthos in the survey locations, and differences in macrobenthic life-forms, which affected food availability.
Macrobenthos in areas with aquatic plants were more abundant than those in areas characterized by bare sediments [
9]. Our study also showed that areas with floating-leaved plants had a higher density of macrobenthos (
Table 1). Correspondingly, shorebirds mainly feed on macrobenthos [
17], and there is a clear positive correlation between their abundance and that of macrobenthos [
3,
18,
19,
44]. Shorebirds also showed a higher abundance in the floating-leaved plant area (
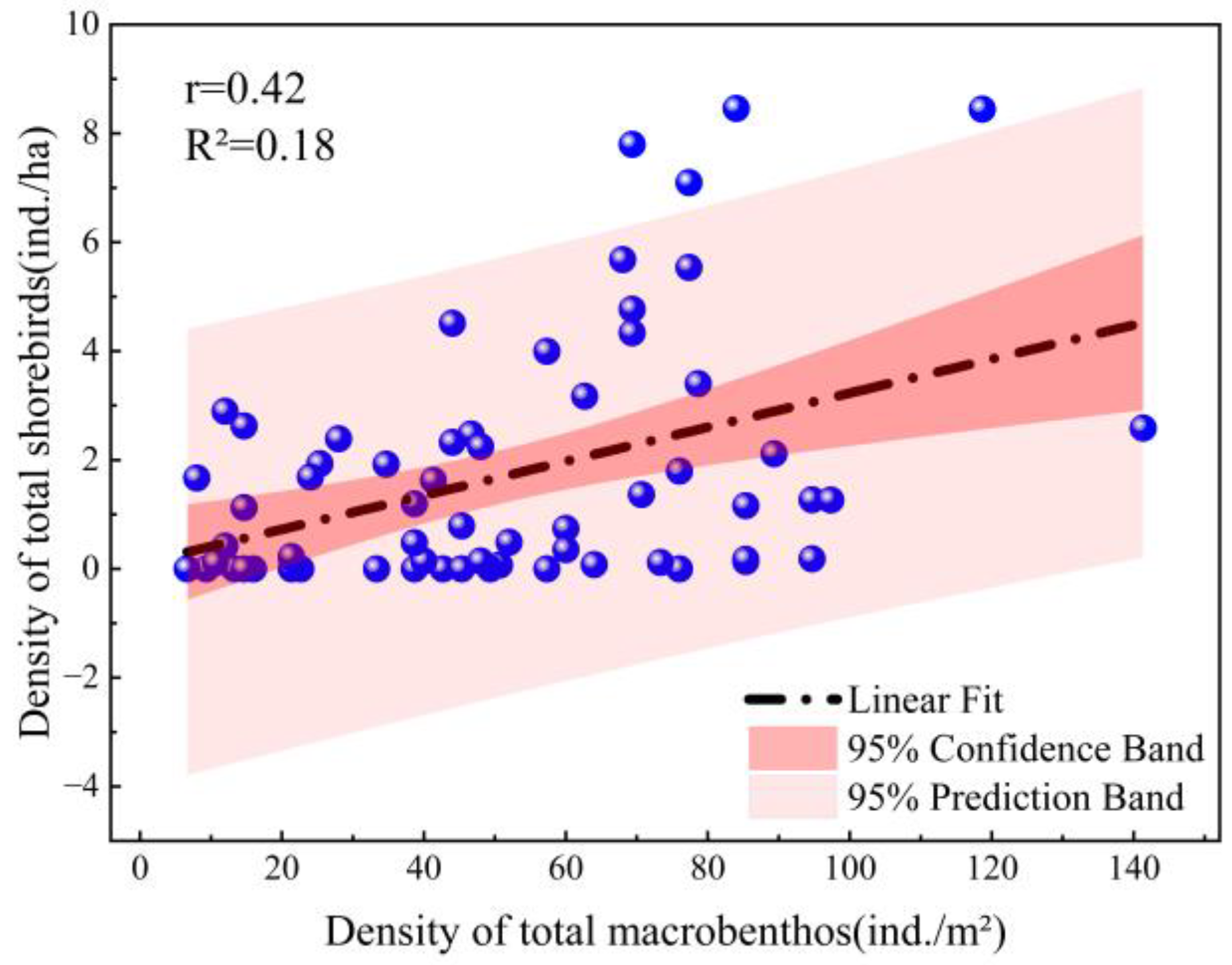
Table 3), and their total density was significantly positively correlated with the total density of macrobenthos (
Figure 6). In the early wintering stage, the shorebirds are migrating, and the total number rises. Without considering the turnover of macrobenthic reproduction, the abundance of macrobenthos may decline. In the later wintering stage, shorebirds gradually move northward. At this time, macrobenthos are less threatened by shorebird predation. Moreover, the air temperature rises, the breeding rate increases, and the abundance may grow. Therefore, their differences over time are not matched when temporal variability is considered. Thus, the linear fit was poor.
Aquatic plants can modify the environment and affect the spatial distribution of macrobenthos and the adaptation of various macrobenthic species to different habitats [
45]. In our study, floating-leaved plants were key to influencing the composition of macrobenthic communities. The close relationship between macrobenthos as prey and shorebirds as predators is predictable [
46]. The prey of shorebirds in different life-forms, such as surface-dwelling (epifauna) and substrate-inhabiting macrobenthos (infauna), have different functions in shorebirds [
26], and prey availability is a key factor in determining the spatial distribution of shorebirds [
47]. Therefore, aquatic plants that affect the composition and abundance of macrobenthos indirectly affect shorebird assemblages.
Studies have shown that large areas of floating-leaved plants can cause water hypoxia, which is not suitable for the survival of oligochaetes [
16,
48]. Our results show that oligochaetes and bivalves have higher densities in the
Trapa spp. natural growth area (
Table 1), which may be because Oligochaeta and Bivalvia belong to classes of grazing and filter-feeding collectors, respectively. Filter feeders are usually found in high-velocity habitats, and their mouthparts have filtering structures to filter and feed on fine-grained organic matter in rivers. Compared with the
Trapa spp. natural growth area, the
Euryale ferox artificial planting area had wider leaves and a stronger shading effect, which is not conducive to the burrowing and habitation of macrobenthos living in the substrate. Further, the slowing of the water flow rate by stems and leaves is not conducive to bivalve filtration of organic matter. Coarse-grained organic matter is processed into fine-grained organic matter under the action of water flow, which is preferred by oligochaetes among the collectors of fine-grained organic matter [
49]. Oligochaetes and bivalves are infaunal macrobenthos; therefore, the
Trapa spp. natural growth area provides a favorable habitat for tactile continuous foragers, which mainly depend on a pressure sensory mechanism for prey detection [
50]. The shorebird species of guild G1 typically adopt a tactile and continuous foraging strategy. In our study, the shorebirds of this foraging guild had a very significant positive correlation with oligochaetes and bivalves as well as a very significant positive correlation with infaunal macrobenthos (
Table 6); therefore, the G1 shorebird densities in the
Trapa spp. natural growth area were significantly higher than those in the other areas (
Table 5).
Insects and gastropods had higher densities in the
Euryale ferox artificial planting area (
Table 1), and they belong to the epifaunal macrobenthos. The rich organic matter environment of the
Euryale ferox artificial planting area provides suitable oviposition places for insects and is more popular with gastropods [
14]. The shorebird density in guild G2 was significantly higher than that in the other areas (
Table 5). This is because the shorebirds of G2 use the visual continuous foraging strategy. A shorter bill length is more suitable for feeding on epifaunal macrobenthos at the surface, and was significantly positively correlated with insects and extremely significantly positively correlated with gastropods and epifaunal macrobenthos. This is consistent with the findings of other studies [
26,
35]. The higher abundance of gastropods and insects created ideal foraging sites for the shorebirds of G2.
For the shorebirds of guilds G5 and G6, except for the shorebirds of G6, which have a longer bill length, the other shorebirds have shorter bill lengths [
41], but they all use the pause–travel foraging strategy characterized by an alternation of very fast steps and abrupt stops to scan the surface of sediment and catch prey [
27]. Pause–travel techniques increase the chances of successful predation attempts by shorebirds, even though it requires more time to scan the prey. The longer the time spent scanning prey, the higher the chance of catching it [
51]. This strategy may be more suitable for foraging for macrobenthos in our study areas; not only does this strategy help shorebirds feed on macrobenthos inhabiting the surface, it may also help them find macrobenthos living in the substrates, so they are distributed in various areas and there is no significant difference in density (
Table 5).
The shorebirds of guild G3 are insectivorous birds with short bills and tarsometatarsi. Except for
Charadrius dubius, all these shorebirds employed the visual continuous foraging strategy. It is conceivable that they are significantly related to epifaunal macrobenthos (
Table 6). Our results showed that they were significantly higher in the
Euryale ferox artificial planting area than in the other areas (
Table 5) because these species lack the tactile sensory cells in their bills that would enable them to detect prey living in the substrates through tactile hunting strategies similar to those of
Calidris alpina [
25]. Furthermore, they do not have long bills to probe deep into the rich infaunal macrobenthos in the
Trapa spp. natural growth area. Therefore, they mainly gathered in the
Euryale ferox artificial planting area because more epifaunal macrobenthos can be harvested as food in this area.
The single shorebird species of guild G4,
Numenius arquata, is insectivorous, with a long bill and a long tarsometatarsus. Although it used a visual continuous foraging strategy, mainly feeding on macrobenthos inhabiting the surface, research has shown that longer-legged and longer-billed shorebirds are frequently observed hunting prey that occurs at deeper levels [
52]. The long bill of
Numenius arquata helps it to find prey buried in deeper substrates; thus, similar to the shorebirds of G5 and G6, the shorebirds of G4 were positively correlated but not significantly correlated with all the macrobenthos and each of the two life-forms. They were also distributed in various areas, and there were no significant differences in density (
Table 5).
The level of competition and predation risk can affect foraging and habitat use decisions [
29,
53]. However, in our study, the level of competition and predation risk had little effect. Due to the small shorebird densities, the competition impact was minimal. In our long-term field observation, we found few predators. In addition, the
Trapa spp. natural growth areas were under the jurisdiction of the Anhui Shengjin Lake National Nature Reserve, and few people entered. From September to October, the
Euryale ferox was artificially harvested before the coming of the wintering shorebirds. Our field observations showed almost no human disturbance in the
Euryale ferox artificial planting areas after the harvest.