Invasive Water Hyacinth (Eichhornia crassipes) Increases Methane Emissions from a Subtropical Lake in the Yangtze River in China
Abstract
1. Introduction
2. Materials and Methods
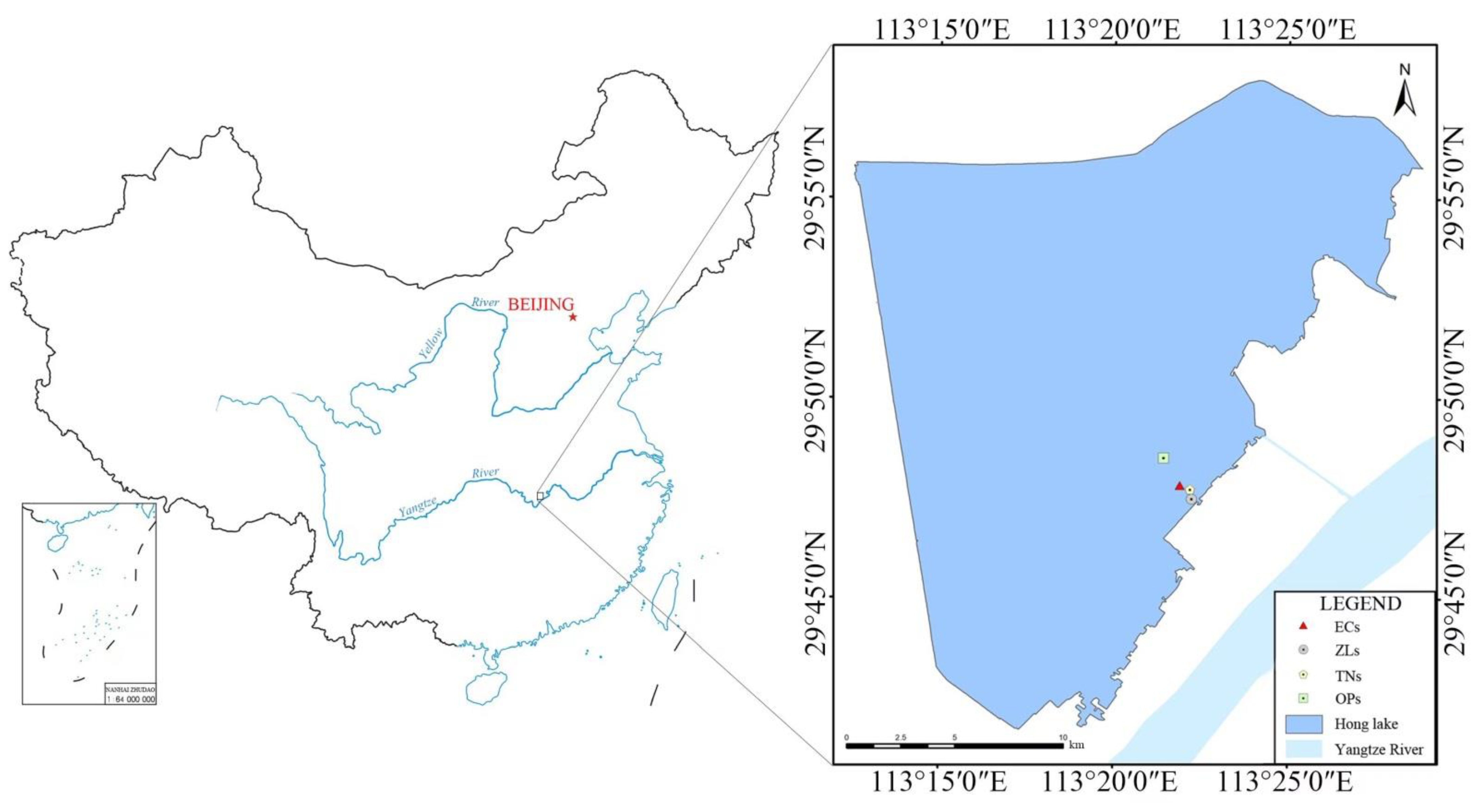
2.1. Study area Description
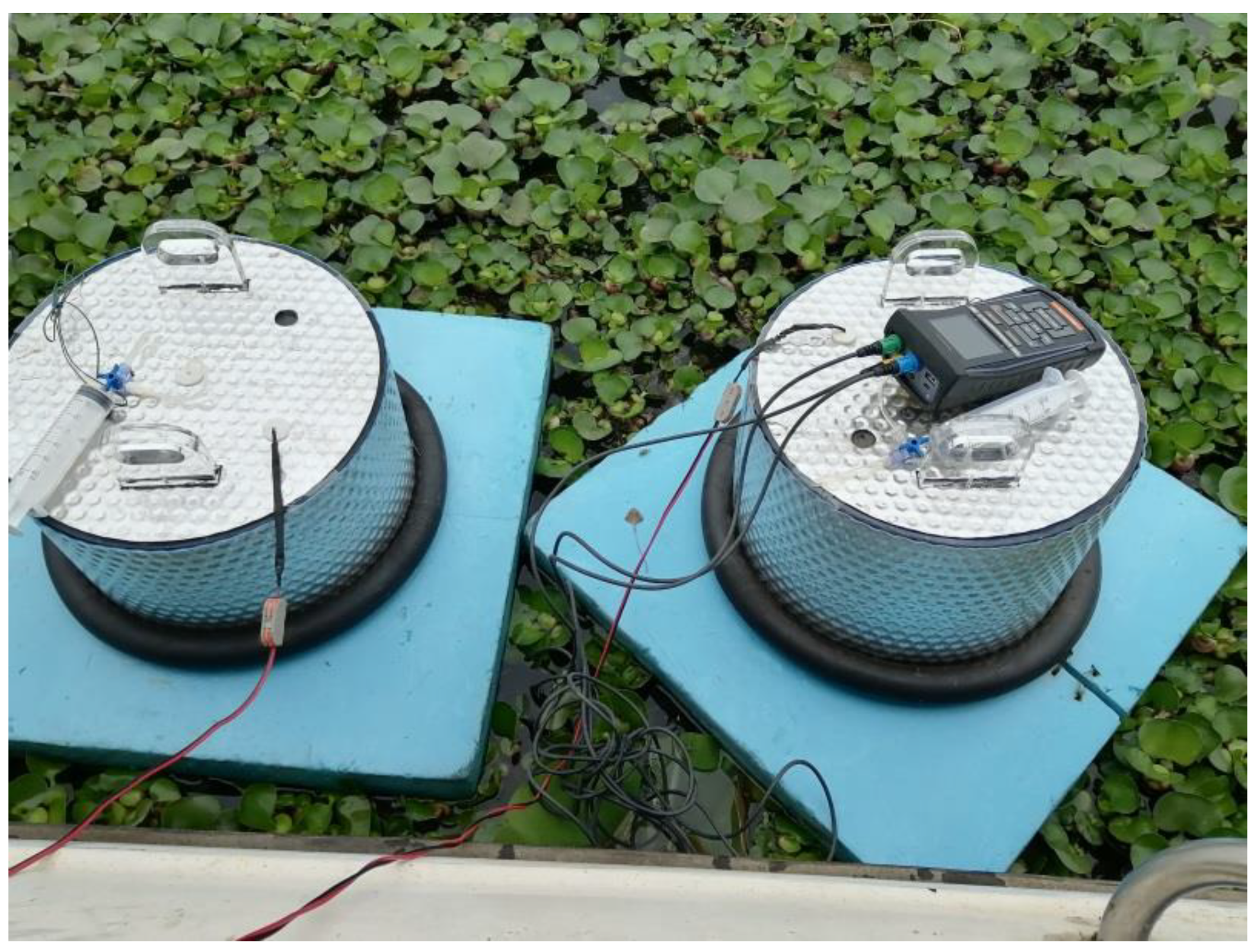
2.2. CH4 Measurements
2.3. Measurement of Environmental Factors
2.4. Statistical Analysis
3. Results
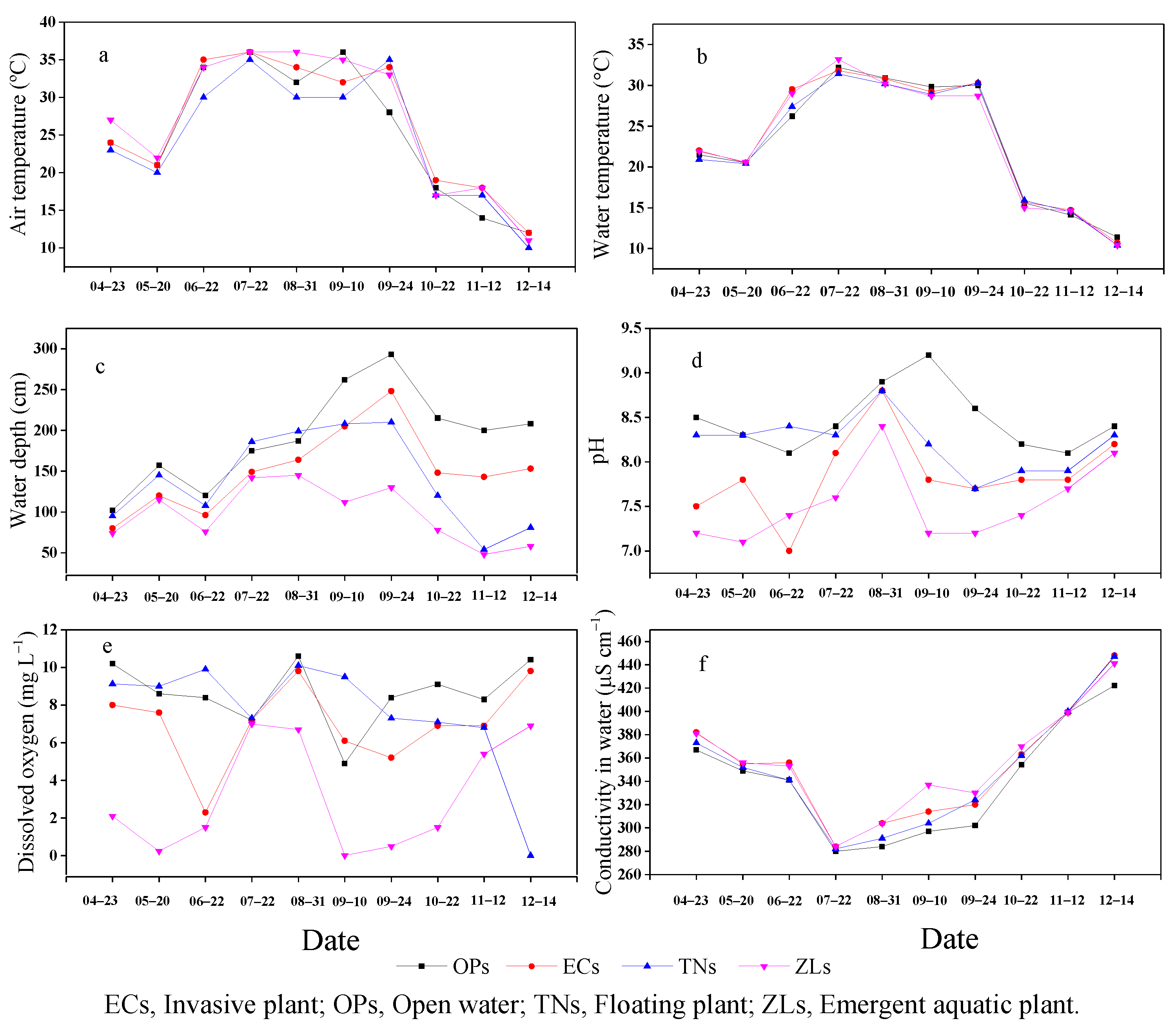
3.1. Environmental Factors
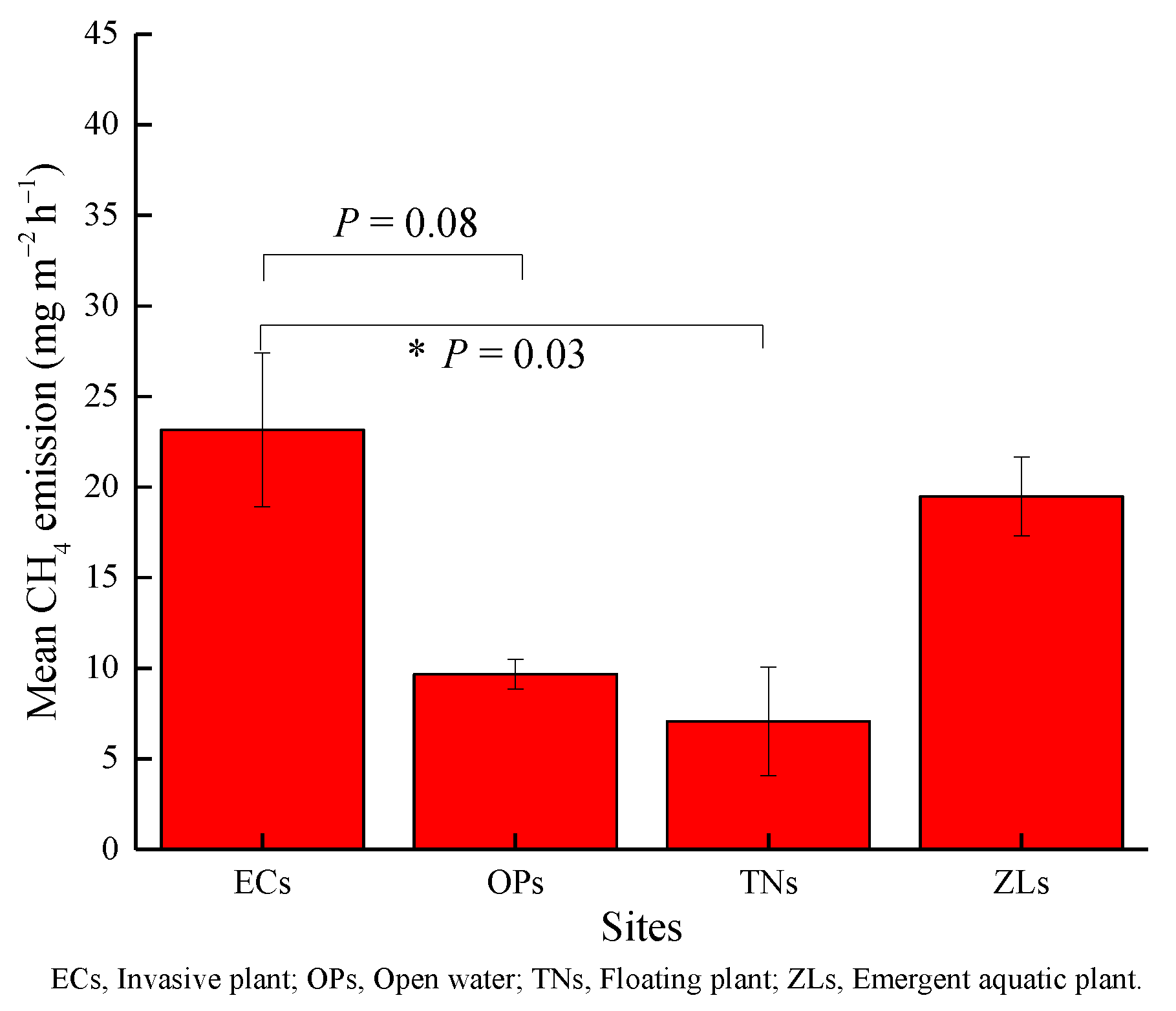
3.2. CH4 Emission Fluxes
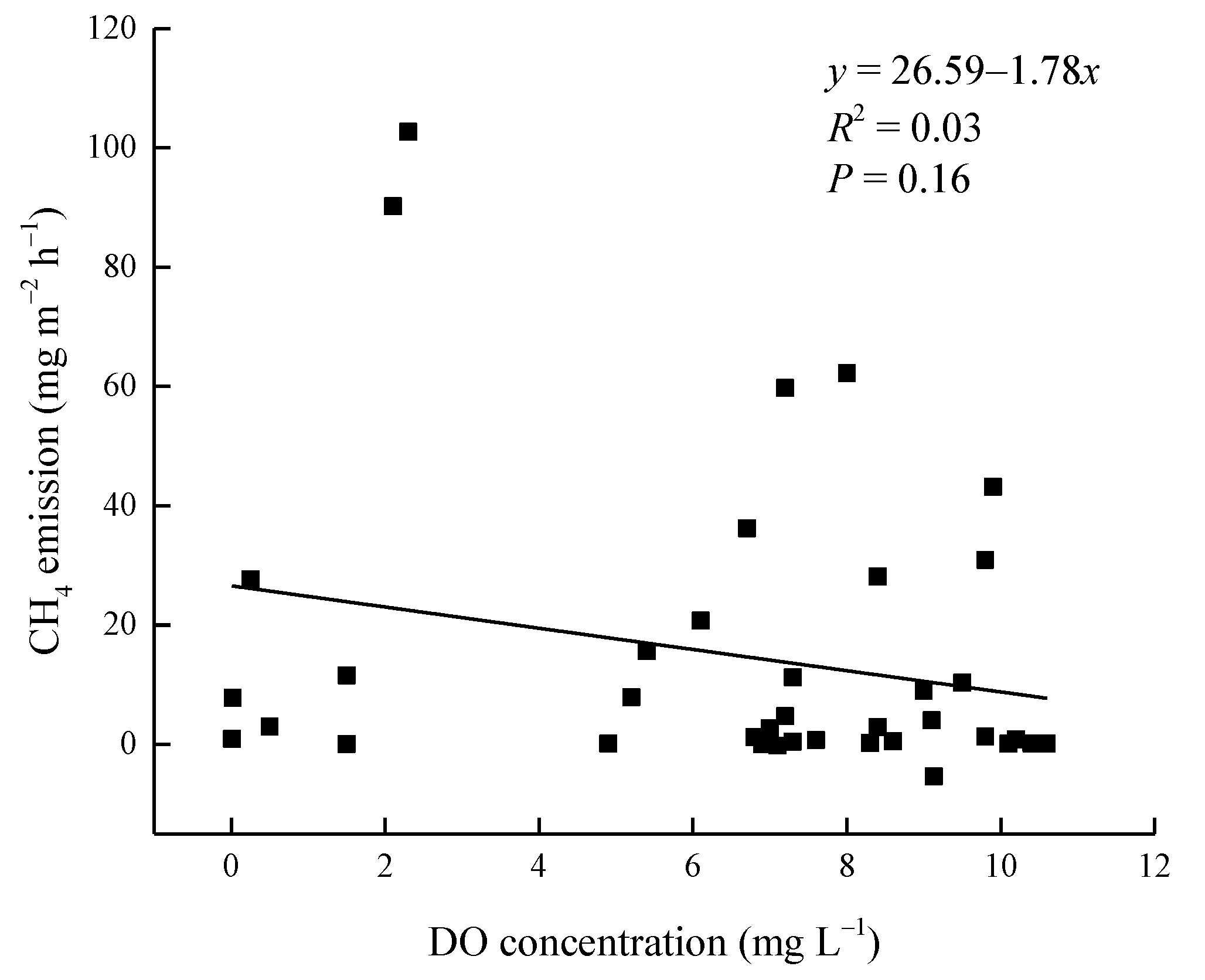
3.3. Dependence of CH4 Fluxes on Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Downing, J.A.; Prairie, Y.T.; Cole, J.J.; Duarte, C.M.; Tranvik, L.J.; Striegl, R.G.; McDowell, W.H.; Kortelainen, P.; Caraco, N.F.; Melack, J.M.; et al. The global abundance and size distribution of lakes, ponds, and impoundments. Limnol. Oceanogr. 2006, 51, 2388–2397. [Google Scholar] [CrossRef]
- Verpoorter, C.; Kutser, T.; Seekell, D.A.; Tranvik, L.J. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 2014, 41, 6396–6402. [Google Scholar] [CrossRef]
- Bastviken, D.; Cole, J.J.; Pace, M.L.; Tranvik, L.J. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Glob. Biogeochem. Cycl. 2004, 18, 1–12. [Google Scholar] [CrossRef]
- Bastviken, D.; Tranvik, L.J.; Downing, J.A.; Crill, P.M.; Enrich-Prast, A. Freshwater Methane Emissions Offset the Continental Carbon Sink. Science 2011, 331, 50. [Google Scholar] [CrossRef]
- IPCC. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 1–2391. [Google Scholar]
- Aben, R.C.H.; Barros, N.; van Donk, E.; Frenken, T.; Hilt, S.; Kazanjian, G.; Lamers, L.P.M.; Peeters, E.T.H.M.; Roelofs, J.G.M.; Domis, L.N.D.S.; et al. Cross continental increase in methane ebullition under climate change. Nat. Commun. 2017, 8, 1682. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xie, P.; Ni, L.; Flower, R.J. Underestimation of CH4 Emission from Freshwater Lakes in China. Environ. Sci. Technol. 2011, 45, 4203–4204. [Google Scholar] [CrossRef] [PubMed]
- Natchimuthu, S.; Sundgren, I.; Gålfalk, M.; Klemedtsson, L.; Crill, P.; Danielsson, Å.; Bastviken, D. Spatio-temporal variability of lake CH4 fluxes and its influence on annual whole lake emission estimates. Limnol. Oceanogr. 2015, 61, S13–S26. [Google Scholar] [CrossRef]
- Johnson, M.S.; Matthews, E.; Du, J.; Genovese, V.; Bastviken, D. Methane Emission from Global Lakes: New Spatiotemporal Data and Observation-Driven Modeling of Methane Dynamics Indicates Lower Emissions. J. Geophys. Res. Biogeosci. 2022, 127, e2022JG006793. [Google Scholar] [CrossRef] [PubMed]
- Attermeyer, K.; Flury, S.; Jayakumar, R.; Fiener, P.; Steger, K.; Arya, V.; Wilken, F.; van Geldern, R.; Premke, K. Invasive floating macrophytes reduce greenhouse gas emissions from a small tropical lake. Sci. Rep. 2016, 6, 20424. [Google Scholar] [CrossRef] [PubMed]
- Marotta, H.; Pinho, L.; Gudasz, C.; Bastviken, D.; Tranvik, L.J.; Prast, A.E. Greenhouse gas production in low-latitude lake sediments responds strongly to warming. Nat. Clim. Change 2014, 4, 467–470. [Google Scholar] [CrossRef]
- Pickard, A.; White, S.; Bhattacharyya, S.; Carvalho, L.; Dobel, A.; Drewer, J.; Jamwal, P.; Helfter, C. Greenhouse gas budgets of severely polluted urban lakes in India. Sci. Total Environ. 2021, 798, 149019. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Zhang, M.; Jia, L.; Zhang, Z.; Xiao, W.; Liu, S.; Zhao, J.; Xie, Y.; Lee, X. Methane emission of a lake aquaculture farm and its response to ecological restoration. Agric. Ecosyst. Environ. 2022, 330, 107883. [Google Scholar] [CrossRef]
- Palma-Silva, C.; Marinho, C.C.; Albertoni, E.F.; Giacomini, I.B.; Barros, M.P.F.; Furlanetto, L.M.; Trindade, C.R.T.; Esteves, F.D.A. Methane emissions in two small shallow neotropical lakes: The role of temperature and trophic level. Atmos. Environ. 2013, 81, 373–379. [Google Scholar] [CrossRef]
- Villamagna, A.M.; Murphy, B.R. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): A review. Freshw. Biol. 2010, 55, 282–298. [Google Scholar] [CrossRef]
- Ding, J.; Ren, W.; Fu, W.; Zhang, G. Water hyacinth in China: Its Distribution, Problems and Control Status. In Biological and Integrated Control of Water Hyacinth, Eichhornia Crassipes; Julien, M.H., Hill, M.P., Center, T.D., Jianqing, D., Eds.; Australian Centre for International Agricultural Research: Canberra, ACT, Australia, 2001; pp. 29–32. [Google Scholar]
- Owens, C.S.; Madsen, J.D. Low temperature limits of water hyacinth. J. Aquat. Plant Manag. 1995, 33, 63–68. [Google Scholar]
- Hu, W.; Salomonsen, J.; Xu, F.-L.; Pu, P. A model for the effects of water hyacinths on water quality in an experiment of physico-biological engineering in Lake Taihu, China. Ecol. Model 1998, 107, 171–188. [Google Scholar] [CrossRef]
- Rai, D.N.; Munshi, J.D. The influence of thick floating vegetation (Water hyacinth: Eichhornia crassipes) on the physic-chemical environment of a fresh water wetland. Hydrobiologia 1979, 62, 65–69. [Google Scholar] [CrossRef]
- Wilson, J.R.; Holst, N.; Rees, M. Determinants and patterns of population growth in water hyacinth. Aquat. Bot. 2005, 81, 51–67. [Google Scholar] [CrossRef]
- Zhang, T.; Ban, X.; Wang, X.; Cai, X.; Li, E.; Wang, Z.; Yang, C.; Lu, X. Analysis of nutrient transport and ecological response in Honghu Lake, China by using a mathematical model. Sci. Total Environ. 2017, 575, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.C.; Shi, Y.H.; Pan, L. Current status and controlling strategies of water pollution in Honghu Lake wetland in Jianghan plain of Middle Reaches of Yangtze River. Wetl. Sci. Manag. 2019, 15, 31–34. (In Chinese) [Google Scholar]
- Han, M.; Dsouza, M.; Zhou, C.; Li, H.; Zhang, J.; Chen, C.; Yao, Q.; Zhong, C.; Zhou, H.; A Gilbert, J.; et al. Agricultural Risk Factors Influence Microbial Ecology in Honghu Lake. Genom. Proteom. Bioinform. 2019, 17, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Morris, J.T.; Huang, J.; Xu, H.; Wan, S. Changes in pore-water chemistry and methane emission following the invasion of Spartina alterniflora into an oliogohaline marsh. Limnol. Oceanogr. 2017, 63, 384–396. [Google Scholar] [CrossRef]
- Yin, S.; An, S.; Deng, Q.; Zhang, J.; Ji, H.; Cheng, X. Spartina alterniflora invasions impact CH4 and N2O fluxes from a salt marsh in eastern China. Ecol. Eng. 2015, 81, 192–199. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, W.; Cai, Z.; Valerie, P.; Han, F. Response of methane emission to invasion of spartina alterniflora and exogenous n deposition in the coastal salt marsh. Atmos. Environ. 2010, 44, 4588–4594. [Google Scholar] [CrossRef]
- Banik, A.; Sen, M.; Sen, S.P. Methane emissions from water hyacinth-infested freshwater ecosystems. Chemosphere 1993, 27, 1539–1552. [Google Scholar] [CrossRef]
- Wang, Z.; Du, Y.; Yang, C.; Liu, X.; Zhang, J.; Li, E.; Zhang, Q.; Wang, X. Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci. Total Environ. 2017, 609, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Bellido, J.L.; Tulonen, T.; Kankaala, P.; Ojala, A. CO2 and CH4 fluxes during spring and autumn mixing periods in a boreal lake (Pääjärvi, southern Finland). J. Geophy. Res. 2009, 114, G04007. [Google Scholar]
- Wang, H.; Lu, J.; Wang, W.; Yang, L.; Yin, C. Methane fluxes from the littoral zone of hypereutrophic Taihu Lake, China. J. Geophys. Res. Earth Surf. 2006, 111, D17. [Google Scholar] [CrossRef]
- Gondwe, M.J.; Masamba, W.R.L. Spatial and temporal dynamics of diffusive methane emissions in the Okavango Delta, northern Botswana, Africa. Wetl. Eco. Manag. 2014, 22, 63–78. [Google Scholar] [CrossRef]
- Xing, Y.; Xie, P.; Yang, H.; Ni, L.; Wang, Y.; Rong, K. Methane and carbon dioxide fluxes from a shallow hypereutrophic subtropical Lake in China. Atmos. Environ. 2005, 39, 5532–5540. [Google Scholar] [CrossRef]
- Fernandez, J.M.; Townsend-Small, A.; Zastepa, A.; Watson, S.B.; Brandes, J.A. Methane and nitrous oxide measured throughout Lake Erie over all seasons indicate highest emissions from the eutrophic Western Basin. J. Great Lakes Res. 2020, 46, 1604–1614. [Google Scholar] [CrossRef]
- Eugster, W.; DelSontro, T.; Sobek, S. Eddy covariance flux measurements confirm extreme CH4 emission from a Swiss hydropower reservoir and resolve their short-term variability. Biogeosciences 2011, 8, 2815–2831. [Google Scholar] [CrossRef]
- Abril, G.; Guérin, F.; Richard, S.; Delmas, R.; Galy-Lacaux, C.; Gosse, P.; Tremblay, A.; Varfalvy, L.; Dos Santos, M.A.; Matvienko, B. Carbon dioxide and methane emissions and the carbon budget of a 10-year old tropical reservoir (Petit Saut, French Guiana). Glob. Biogeochem. Cycl. 2005, 19, GB4007. [Google Scholar] [CrossRef]
- Lu, S.; Wang, S.H.; Yuan, W.B.; Wen, Y.L. Lake environment evolution of Honghu and consideration on development of wetland ecological industry. Ecol. Econ. 2009, 218, 157–159. (In Chinese) [Google Scholar]
- Zhou, Y.; Song, K.; Han, R.; Riya, S.; Xu, X.; Yeerken, S.; Geng, S.; Ma, Y.; Terada, A. Nonlinear response of methane release to increased trophic state levels coupled with microbial processes in shallow lakes. Environ. Pollut. 2020, 265, 114919. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yu, R.; Liu, X.; Cao, Z.; Li, X.; Zhang, Z.; Wang, J.; Zhuang, S.; Ge, Z.; Zhang, L.; et al. Drivers of spatial and seasonal variations of CO2 and CH4 fluxes at the sediment water interface in a shallow eutrophic lake. Water Res. 2022, 222, 118916. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.B.; Marotta, H.; Bastviken, D.; Enrich-Prast, A. Floating Aquatic Macrophytes Can Substantially Offset Open Water CO2 Emissions from Tropical Floodplain Lake Ecosystems. Ecosystems 2016, 19, 724–736. [Google Scholar] [CrossRef]
- Mwamburi, J. Spatial variations in sedimentary organic matter in surficial lake sediments of Nyanza Gulf (Lake Victoria, Kenya) after invasion of water hyacinth. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2016, 21, 94–113. [Google Scholar] [CrossRef]
- Whiting, G.J.; Chanton, J.P. Primary production control of methane emission from wetlands. Nature 1993, 364, 794–795. [Google Scholar] [CrossRef]
- Juutinen, S.; Alm, J.; Larmola, T.; Huttunen, J.T.; Morero, M.; Martikainen, P.J.; Silvola, J. Major implication of the littoral zone for methane release from boreal lakes. Global Biogeochem. Cycl. 2003, 28, 1–11. [Google Scholar] [CrossRef]
- Furlanetto, L.M.; Marinho, C.C.; Palma-Silva, C.; Albertoni, E.F.; Figueiredo-Barros, M.P.; de Assis Estevesb, F. Methane levels in shallow subtropical lake sediments: Dependence on the trophic status of the lake and allochthonous input. Limnologica 2012, 42, 151–155. [Google Scholar] [CrossRef]
- Capone, D.G.; Kiene, R.P. Comparison of microbial dynamics in marine and freshwater sediments: Contrasts in anaerobic carbon catabolism. Limnol. Oceanogr. 1998, 33, 725–749. [Google Scholar] [CrossRef]
- Ding, W.; Cai, Z.; Tsuruta, H.; Li, X. Key factors affecting spatial variation of methane emissions from freshwater marshes. Chemosphere 2003, 51, 167–173. [Google Scholar] [CrossRef]
- Flury, S.; McGinnis, D.F.; Gessner, M.O. Methane emissions from a freshwater marsh in response to experimentally simulated global warming and nitrogen enrichment. J. Geophys. Res. Earth Surf. 2010, 115, G1. [Google Scholar] [CrossRef]
- Maruya, Y.; Nakayama, K.; Sasaki, M.; Komai, K. Effect of dissolved oxygen on methane production from bottom sediment in a eutrophic stratified lake. J. Environ. Sci. 2023, 125, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cui, L.; Wang, Y.; Li, W. Methane emissions from natural and drained peatlands in the Zoigê, eastern Qinghai-Tibet Plateau. J. Forestry Res. 2017, 28, 539–547. [Google Scholar] [CrossRef]
- Bolpagni, R.; Pierobon, E.; Longhi, D.; Nizzoli, D.; Bartoli, M.; Tomasellli, M.; Viaroli, P. Diurnal exchanges of CO2 and CH4 across the water–atmosphere interface in a water chestnut meadow (Trapa natans L.). Aquat. Bot. 2007, 87, 43–48. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, M.; Hu, Z.; Gao, Y.; Hu, C.; Liu, C.; Liu, S.; Zhang, Z.; Zhao, J.; Xiao, W.; et al. Spatial variations of methane emission in a large shallow eutrophic lake in subtropical climate. J. Geophys. Res. Biogeosci. 2017, 122, 1597–1614. [Google Scholar] [CrossRef]
- Zeikus, J.G.; Winfrey, M.R. Temperature limitation of methanogenesis in aquatic sediments. Appl. Environ. Microb. 1976, 31, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Duc, N.T.; Crill, P.; Bastviken, D. Implications of temperature and sediment characteristics on methane formation and oxidation in lake sediments. Biogeochemistry 2010, 100, 185–196. [Google Scholar] [CrossRef]
- Hirota, M.; Tang, Y.; Hu, Q.; Hirata, S.; Kato, T.; Mo, W.; Cao, G.; Mariko, S. Methane emissions from different vegetation zones in a Qinghai-Tibetan Plateau wetland. Soil Biol. Biochem. 2004, 36, 737–748. [Google Scholar] [CrossRef]



| Sites | Vegetation | Soil | |||||
|---|---|---|---|---|---|---|---|
| Types | Biomass (g m−2) | pH | SOC/g kg−1 | TN/g kg−1 | C:N Ratio | TP/g kg−1 | |
| Open water (OPs) | — | No grown vegetations | 8.12 ± 0.05a | 16.63 ± 1.54a | 1.33 ± 0.14a | 12.54 ± 0.21a | 0.64 ± 0.01a |
| Invasive plant (ECs) | E. crassipes | 270.02 ± 20.64a | 7.96 ± 0.04ab | 29.10 ± 1.71a | 2.42 ± 0.05a | 12.01 ± 0.65a | 0.63 ± 0.01a |
| Floating plant (TNs) | T. natans | 211.08 ± 17.63a | 7.57 ± 0.29ab | 46.47 ± 11.31ab | 3.44 ± 0.78ab | 13.37 ± 0.49a | 0.61 ± 0.01a |
| Emergent aquatic plant (ZLs) | Z. latifolia, N. nucifera, T. natans | 618.30 ± 187.50a | 7.03 ± 0.07b | 56.63 ± 2.84b | 4.49 ± 0.30b | 12.61 ± 0.17a | 0.67 ± 0.01a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Xiang, S.; Shi, Y.; Xu, X.; Lu, H.; Ou, W.; Yang, J. Invasive Water Hyacinth (Eichhornia crassipes) Increases Methane Emissions from a Subtropical Lake in the Yangtze River in China. Diversity 2022, 14, 1036. https://doi.org/10.3390/d14121036
Zhou W, Xiang S, Shi Y, Xu X, Lu H, Ou W, Yang J. Invasive Water Hyacinth (Eichhornia crassipes) Increases Methane Emissions from a Subtropical Lake in the Yangtze River in China. Diversity. 2022; 14(12):1036. https://doi.org/10.3390/d14121036
Chicago/Turabian StyleZhou, Wenchang, Shanshan Xiang, Yuhu Shi, Xiuhuan Xu, Huicui Lu, Wenhui Ou, and Jiawei Yang. 2022. "Invasive Water Hyacinth (Eichhornia crassipes) Increases Methane Emissions from a Subtropical Lake in the Yangtze River in China" Diversity 14, no. 12: 1036. https://doi.org/10.3390/d14121036
APA StyleZhou, W., Xiang, S., Shi, Y., Xu, X., Lu, H., Ou, W., & Yang, J. (2022). Invasive Water Hyacinth (Eichhornia crassipes) Increases Methane Emissions from a Subtropical Lake in the Yangtze River in China. Diversity, 14(12), 1036. https://doi.org/10.3390/d14121036










