Mechanistic Thermal Modeling of Late Triassic Terrestrial Amniotes Predicts Biogeographic Distribution
Abstract
1. Introduction
2. Materials and Methods
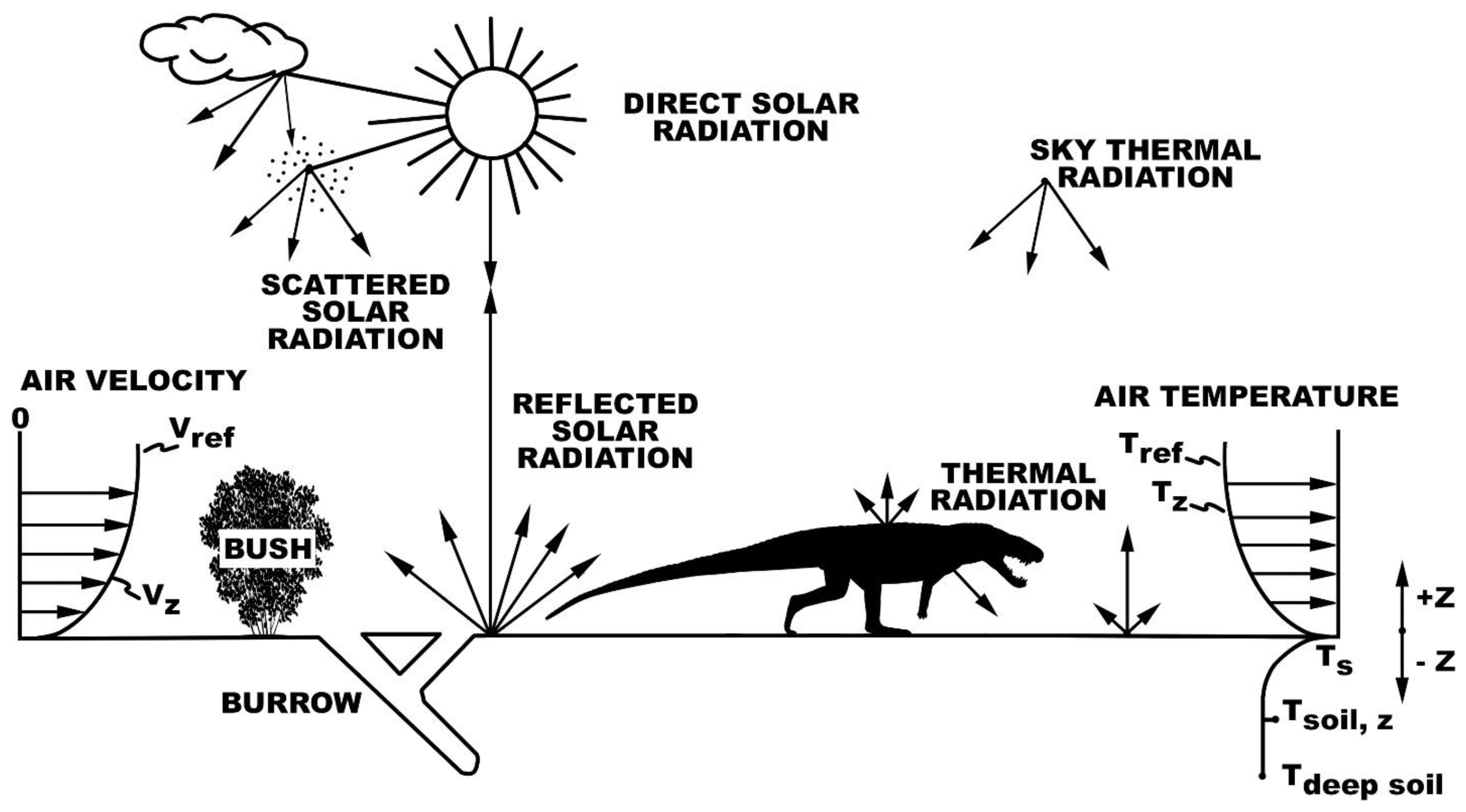
2.1. Microclimate Models
| Parameter | Model | Source | Input Range |
|---|---|---|---|
| Air Temperature | Microclimate | [14,36] | 21–31 °C LowLat; 14–24 °C HiLat |
| Relative Humidity | Microclimate | [14,36,42] | Dry: 13–65%; Monsoon: 48–96% |
| Cloud Cover | Microclimate | [14,36] | 50–90% |
| Wind Speeds | Microclimate | [14,43,44] | 1–4 m/s |
| Atmospheric % O2 | Biophysical | [14,45] | 18% |
| Atmospheric % CO2 | Biophysical | [14,46,47] | 0.13% |
2.2. Biophysical Models
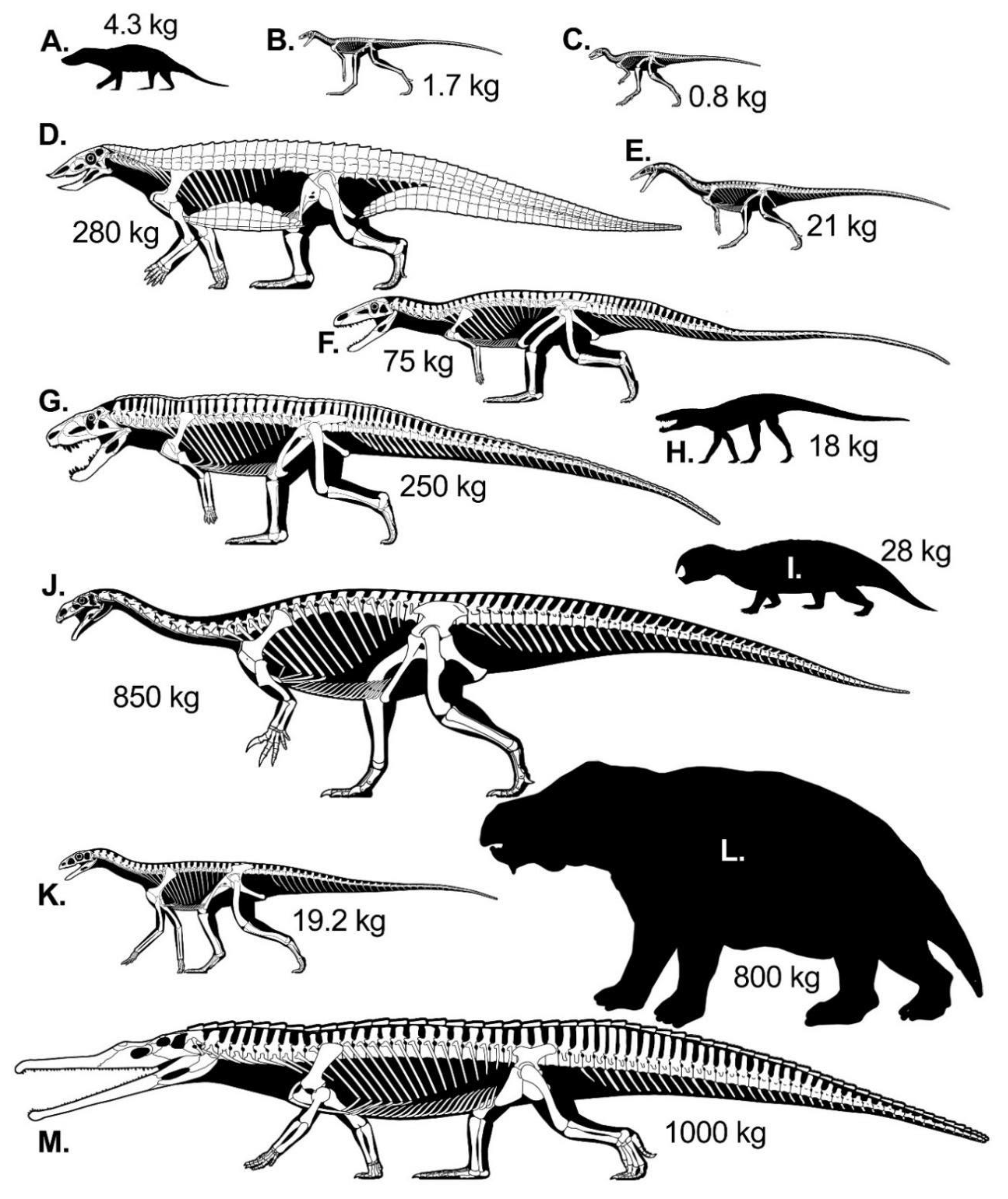
2.3. Modeled Late Triassic Taxa
2.3.1. Synapsids
2.3.2. Archosaurormorpha
2.3.3. Croc-Line Archosaurs
2.3.4. Bird-Line Archosaurs
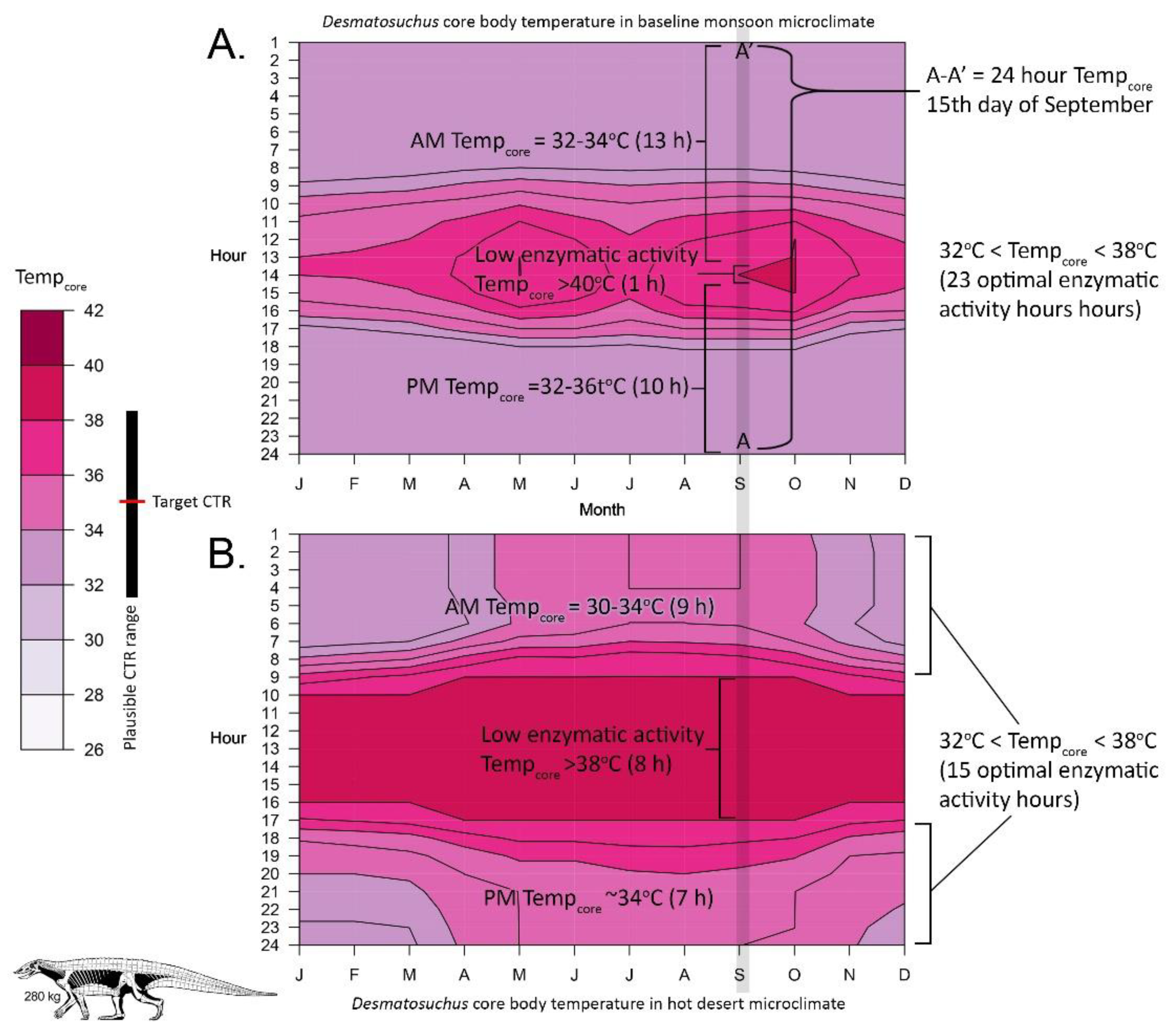
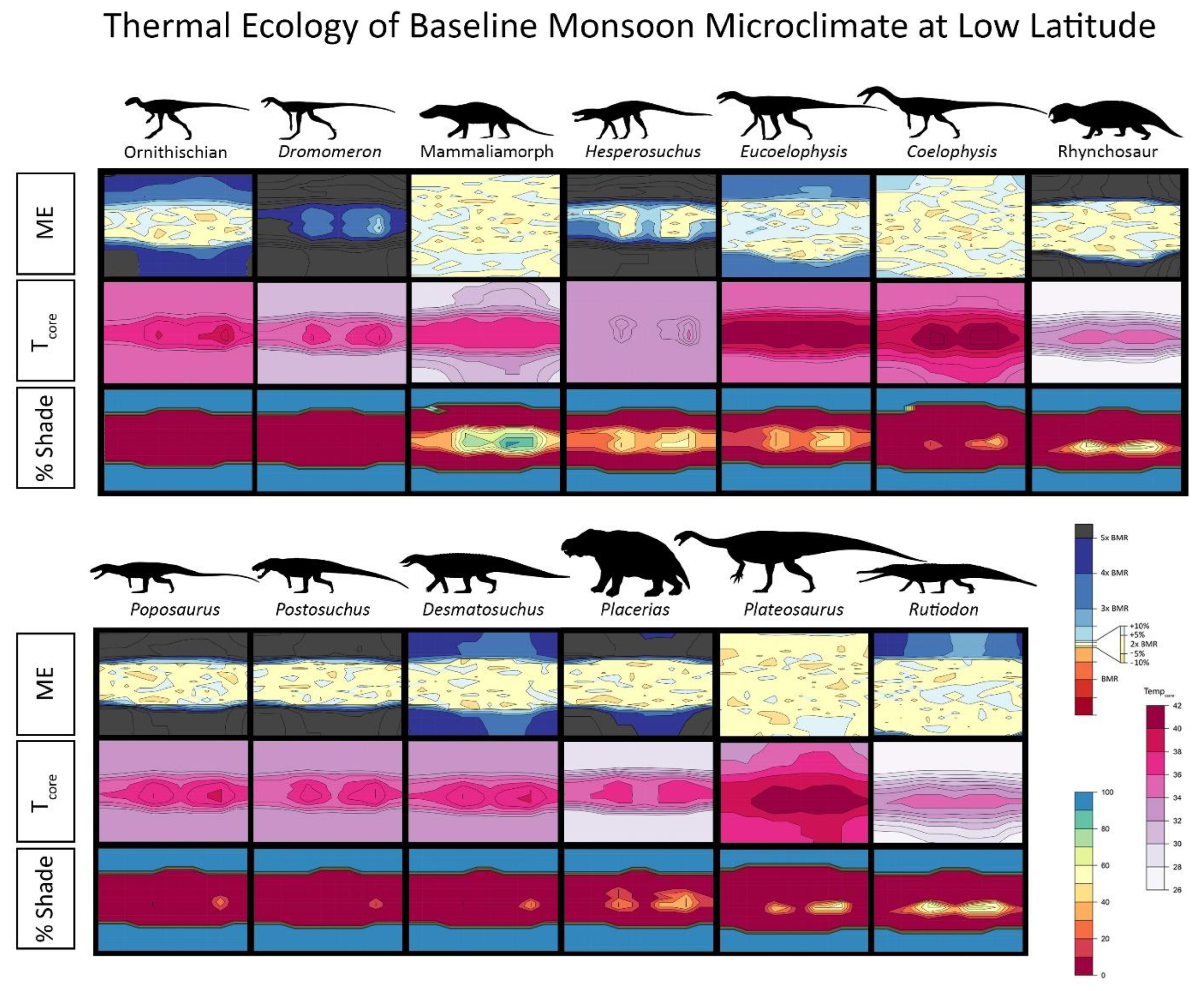
2.4. Niche Mapper Plots
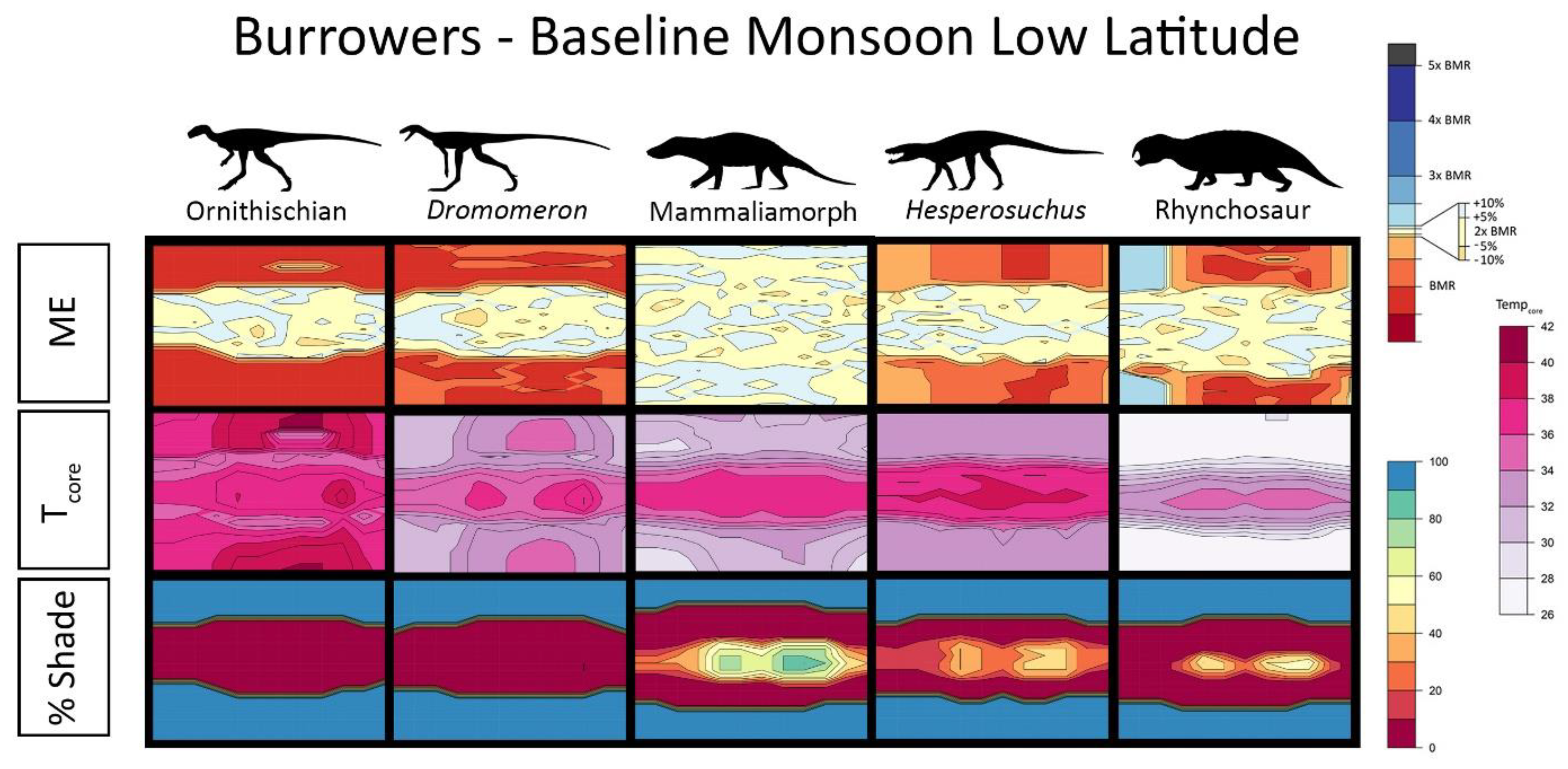
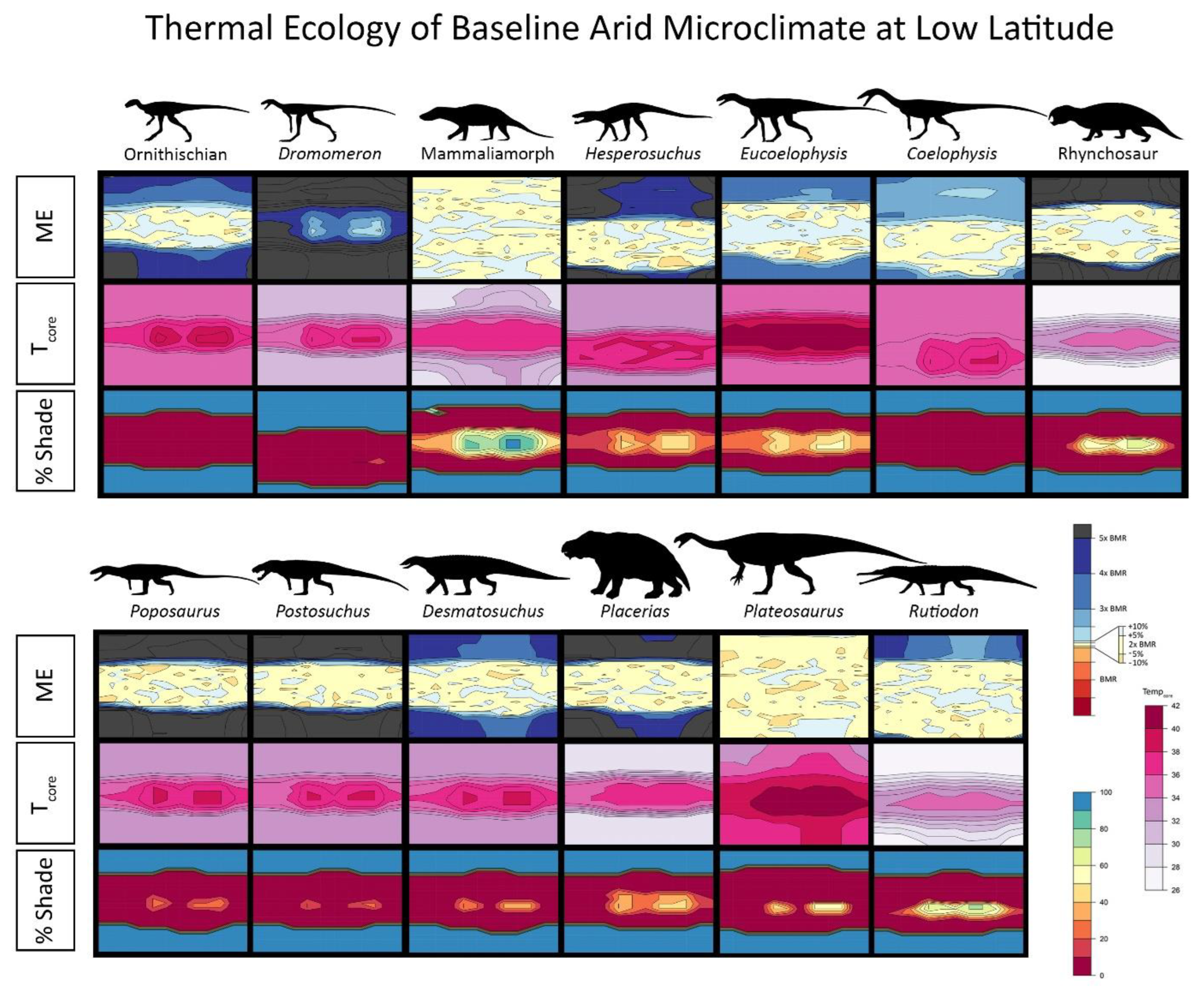
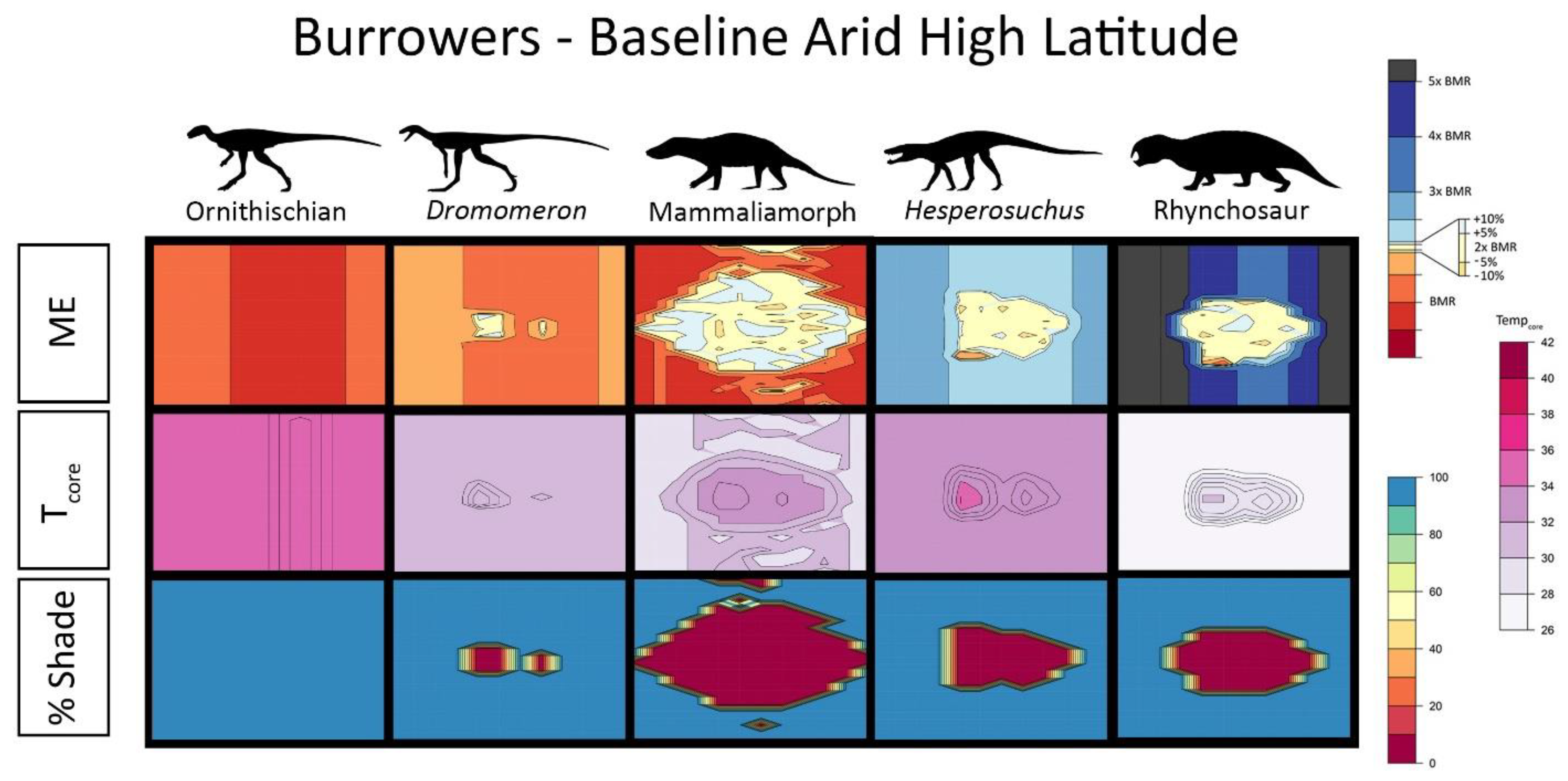
2.5. Interpreting Burrowing Results
2.6. Results for Environmental Simulations
3. Results
4. Discussion
4.1. Late Triassic Microclimate Results Are Congruous with Previously Inferred Activity Patterns and Fossil Paleobiogeography
4.2. The Importance of Burrowing and Dormancy Are Often Overlooked in Vertebrate Paleoecology
4.3. Limitations and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barlett, P.N.; Gates, D.M. The Energy Budget of a Lizard on a Tree Trunk. Ecology 1967, 48, 315–322. [Google Scholar] [CrossRef]
- Levy, O.; Dayan, T.; Kronfeld-Schor, N.; Porter, W.P. Biophysical Modeling of the Temporal Niche: From First Principles to the Evolution of Activity Patterns. Am. Nat. 2012, 179, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Dudley, P.; Bonazza, R.; Porter, W. Consider a Non-Spherical Elephant: Computational Fluid Dynamics Simulations of Heat Transfer Coefficients and Drag Verified Using Wind Tunnel Experiments. J. Exp. Zool. Part Ecol. Genet. Physiol. 2013, 319, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Porter, W. Mechanistic Niche Modelling: Combining Physiological and Spatial Data to Predict Species’ Ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, P.D.; Porter, W.P. Simulating Polar Bear Energetics during a Seasonal Fast Using a Mechanistic Model. PLoS ONE 2013, 8, e72863. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, M.J.; Mathewson, P.D.; Porter, W.P. Validation of a Mechanistic Model for Non-Invasive Study of Ecological Energetics in an Endangered Wading Bird with Counter-Current Heat Exchange in Its Legs. PLoS ONE 2015, 10, e0136677. [Google Scholar] [CrossRef]
- Dudley, P.; Bonazza, R.; Porter, W. Climate Change Impacts on Nesting and Internesting Leatherback Sea Turtles Using 3D Animated Computational Fluid Dynamics and Finite Volume Heat Transfer. Ecol. Model. 2016, 320, 231–240. [Google Scholar] [CrossRef]
- Porter, W.P.; Gates, D.M. Thermodynamic Equilibria of Animals with Environment. Ecol. Monogr. 1969, 39, 227–244. [Google Scholar] [CrossRef]
- Spotila, J.R.; Lommen, P.W.; Bakken, G.S.; Gates, D.M. A Mathematical Model for Body Temperatures of Large Reptiles: Implications for Dinosaur Ecology. Am. Nat. 1973, 107, 391–404. [Google Scholar] [CrossRef]
- Skoczylas, R. Thermoregulation in Dinosaurs. In Contributions to Thermal Physiology; Elsevier: Amsterdam, The Netherlands, 1981; pp. 249–251. [Google Scholar]
- Dunham, A.; Overall, K.; Porter, W.; Forster, C. Implications of Ecological Energetic and Biophysical and Developmental Constraints for Life-History Variation in Dinosaurs. In Paleobiology of the Dinosaurs; Geological Society of America Special: Boulder, CO, USA, 1989; Volume 238, pp. 1–21. ISBN 978-0-8137-2238-2. [Google Scholar]
- Long, R.A.; Bowyer, R.T.; Porter, W.P.; Mathewson, P.; Monteith, K.L.; Findholt, S.L.; Dick, B.L.; Kie, J.G. Linking Habitat Selection to Fitness-Related Traits in Herbivores: The Role of the Energy Landscape. Oecologia 2016, 181, 709–720. [Google Scholar] [CrossRef]
- Lin, T.-E.; Chen, T.-Y.; Wei, H.-L.; Richard, R.; Huang, S.-P. Low Cold Tolerance of the Invasive Lizard Eutropis Multifasciata Constrains Its Potential Elevation Distribution in Taiwan. J. Therm. Biol. 2019, 82, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, D.M.; Hartman, S.A.; Mathewson, P.D.; Linzmeier, B.J.; Porter, W.P. Modeling Dragons: Using Linked Mechanistic Physiological and Microclimate Models to Explore Environmental, Physiological, and Morphological Constraints on the Early Evolution of Dinosaurs. PLoS ONE 2020, 15, e0223872. [Google Scholar] [CrossRef] [PubMed]
- Hartman, S.; Lovelace, D.; Linzmeier, B.; Porter, W. Using Ecological Modelling to Quantify Thermal Constraints on Two Late Triassic Dinosaurs. J. Vertebr. Paleontol. 2015, 36, 139. [Google Scholar]
- Hartman, S.; Lovelace, D.; Linzmeier, B.; Mathewson, P.; Porter, W. Mechanistic Physiological Modelling Predicts Geographic Distribution of Late Triassic Tetrapods. J. Vertebr. Paleontol. 2016, 35. [Google Scholar]
- Mathewson, P.D.; Moyer-Horner, L.; Beever, E.A.; Briscoe, N.J.; Kearney, M.; Yahn, J.M.; Porter, W.P. Mechanistic Variables Can Enhance Predictive Models of Endotherm Distributions: The American Pika under Current, Past, and Future Climates. Glob. Chang. Biol. 2017, 23, 1048–1064. [Google Scholar] [CrossRef]
- Wang, Y.; Porter, W.; Mathewson, P.D.; Miller, P.A.; Graham, R.W.; Williams, J.W. Mechanistic Modeling of Environmental Drivers of Woolly Mammoth Carrying Capacity Declines on St. Paul Island. Ecology 2018, 99, 2721–2730. [Google Scholar] [CrossRef]
- Wang, Y.; Widga, C.; Graham, R.W.; McGuire, J.L.; Porter, W.; Wårlind, D.; Williams, J.W. Caught in a Bottleneck: Habitat Loss for Woolly Mammoths in Central North America and the Ice-Free Corridor during the Last Deglaciation. Glob. Ecol. Biogeogr. 2021, 30, 527–542. [Google Scholar] [CrossRef]
- Porter, W.; Mitchell, J. Method and System for Calculating the Spatial-Temporal Effects of Climate and Other Environmental Conditions on Animals. In Office Up, Editor; 11-0E Wisconsin Alumni Research Foundation: Madison, WI, USA, 2006. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Urbanek, S. Png: Read and Write PNG Images, 2013.
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Liaw, W.H.A.; Lumley, T. Package ‘Gplots’: Various r Programming Tools for Plotting Data, R Package Version 3.0.1. 2016.
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Neuwirth, E.; Neuwirth, M.E. Package ‘RColorBrewer’. Color. Palettes 2014. [Google Scholar]
- Lemon, J. Plotrix: A Package in the Red Light District of R. R-News 2006, 6, 8–12. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System, 2019.
- Laskar, J.; Robutel, P.; Joutel, F.; Gastineau, M.; Correia, A.C.M.; Levrard, B. A Long-Term Numerical Solution for the Insolation Quantities of the Earth. Astron. Astrophys. 2004, 428, 261–285. [Google Scholar] [CrossRef]
- Crucifix, M. Palinsol: Insolation for Palaeoclimate Studies, 2016.
- Geiger, R.; Aron, R.H.; Todhunter, P. The Climate near the Ground; Rowman & Littlefield: Lanham, MD, USA, 2009. [Google Scholar]
- McCullough, E.C.; Porter, W.P. Computing Clear Day Solar Radiation Spectra for the Terrestrial Ecological Environment. Ecology 1971, 52, 1008–1015. [Google Scholar] [CrossRef]
- Porter, W.P.; Mitchell, J.W.; Beckman, W.A.; DeWitt, C.B. Behavioral Implications of Mechanistic Ecology. Oecologia 1973, 13, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.R.; Shamakhy, A.; Tingley, R.; Karoly, D.J.; Hoffmann, A.A.; Briggs, P.R.; Porter, W.P. Microclimate Modelling at Macro Scales: A Test of a General Microclimate Model Integrated with Gridded Continental-Scale Soil and Weather Data. Methods Ecol. Evol. 2014, 5, 273–286. [Google Scholar] [CrossRef]
- Porter, W.P.; Vakharia, N.; Klousie, W.D.; Duffy, D. Po’ouli Landscape Bioinformatics Models Predict Energetics, Behavior, Diets, and Distribution on Maui. Integr. Comp. Biol. 2006, 46, 1143–1158. [Google Scholar] [CrossRef]
- Long, R.A.; Bowyer, R.T.; Porter, W.P.; Mathewson, P.; Monteith, K.L.; Kie, J.G. Behavior and Nutritional Condition Buffer a Large-Bodied Endotherm against Direct and Indirect Effects of Climate. Ecol. Monogr. 2014, 84, 513–532. [Google Scholar] [CrossRef]
- Sellwood, B.W.; Valdes, P.J. Mesozoic Climates: General Circulation Models and the Rock Record. Sediment. Geol. 2006, 190, 269–287. [Google Scholar] [CrossRef]
- Landwehrs, J.P.; Feulner, G.; Hofmann, M.; Petri, S. Climatic Fluctuations Modeled for Carbon and Sulfur Emissions from End-Triassic Volcanism. Earth Planet. Sci. Lett. 2020, 537, 116174. [Google Scholar] [CrossRef]
- Kutzbach, J. Idealized Pangean Climates: Sensitivity to Orbital Change. In Pangea: Paleoclimate, Tectonics, and Sedimentation During Accretion, Zenith, and Breakup of a Supercontinent; Klein, G., Ed.; Geological Society of America: Boulder, CO, USA, 1994; ISBN 978-0-8137-2288-7. [Google Scholar]
- Kustatscher, E.; Ash, S.R.; Karasev, E.; Pott, C.; Vajda, V.; Yu, J.; McLoughlin, S. Flora of the Late Triassic. In The Late Triassic World: Earth in a Time of Transition; Tanner, L.H., Ed.; Topics in Geobiology; Springer International Publishing: Cham, Switzerland, 2018; pp. 545–622. ISBN 978-3-319-68009-5. [Google Scholar]
- Mancuso, A.C.; Horn, B.L.D.; Benavente, C.A.; Schultz, C.L.; Irmis, R.B. The Paleoclimatic Context for South American Triassic Vertebrate Evolution. J. S. Am. Earth Sci. 2021, 110, 103321. [Google Scholar] [CrossRef]
- Olsen, P.; Sha, J.; Fang, Y.; Chang, C.; Whiteside, J.H.; Kinney, S.; Sues, H.-D.; Kent, D.; Schaller, M.; Vajda, V. Arctic Ice and the Ecological Rise of the Dinosaurs. Sci. Adv. 2022, 8, eabo6342. [Google Scholar] [CrossRef]
- Parrish, J.T. Climate of the Supercontinent Pangea. J. Geol. 1993, 101, 215–233. [Google Scholar] [CrossRef]
- Timbuktu Climate, Weather by Month, Average Temperature (Mali)—Weather Spark. Available online: https://weatherspark.com/y/36520/Average-Weather-in-Timbuktu-Mali-Year-Round (accessed on 1 April 2022).
- Tamale Climate, Weather by Month, Average Temperature (Ghana)—Weather Spark. Available online: https://weatherspark.com/y/42343/Average-Weather-in-Tamale-Ghana-Year-Round (accessed on 1 April 2022).
- Berner, R.A.; Beerling, D.J.; Dudley, R.; Robinson, J.M.; Wildman, R.A. Phanerozoic Atmospheric Oxygen. Annu. Rev. Earth Planet. Sci. 2003, 31, 105–134. [Google Scholar] [CrossRef]
- Cleveland, D.M.; Nordt, L.C.; Atchley, S.C. Paleosols, Trace Fossils, and Precipitation Estimates of the Uppermost Triassic Strata in Northern New Mexico. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 257, 421–444. [Google Scholar] [CrossRef]
- Berner, R.A.; Kothavala, Z. Geocarb III: A Revised Model of Atmospheric CO2 over Phanerozoic Time. Am. J. Sci. 2001, 301, 182–204. [Google Scholar] [CrossRef]
- Peczkis, J. Implications of Body-Mass Estimates for Dinosaurs. J. Vertebr. Paleontol. 1995, 14, 520–533. [Google Scholar] [CrossRef]
- Paul, G.S. Dinosaur Models: The Good, the Bad, and Using Them to Estimate the Mass of Dinosaurs. In DinoFest International Proceedings; Academy of Natural Science: Philadelphia, PA, USA, 1997; pp. 129–154. [Google Scholar]
- Seebacher, F. A New Method to Calculate Allometric Length-Mass Relationships of Dinosaurs. J. Vertebr. Paleontol. 2001, 21, 51–60. [Google Scholar] [CrossRef]
- Farlow, J.O.; Holtz, T.R. The Fossil Record of Predation in Dinosaurs. Paleontol. Soc. Pap. 2002, 8, 251–266. [Google Scholar] [CrossRef]
- Melstrom, K.M.; Irmis, R.B. Repeated Evolution of Herbivorous Crocodyliforms during the Age of Dinosaurs. Curr. Biol. 2019, 29, 2389–2395. [Google Scholar] [CrossRef]
- Porter, W.P.; Kearney, M. Size, Shape, and the Thermal Niche of Endotherms. Proc. Natl. Acad. Sci. USA 2009, 106, 19666–19672. [Google Scholar] [CrossRef]
- Kearney, M.R.; Briscoe, N.J.; Mathewson, P.D.; Porter, W.P. NicheMapR—An R Package for Biophysical Modelling: The Endotherm Model. Ecography 2021, 44, 1595–1605. [Google Scholar] [CrossRef]
- Bennett, A.F. Activity Metabolism of the Lower Vertebrates. Annu. Rev. Physiol. 1978, 40, 447–469. [Google Scholar] [CrossRef]
- McKechnie, A.E.; Wolf, B.O. The Allometry of Avian Basal Metabolic Rate: Good Predictions Need Good Data. Physiol. Biochem. Zool. 2004, 77, 502–521. [Google Scholar] [CrossRef] [PubMed]
- Sulej, T.; Niedźwiedzki, G. An Elephant-Sized Late Triassic Synapsid with Erect Limbs. Science 2019, 363, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chi, H.; Li, L.; Rossiter, S.J.; Zhang, S. Molecular Data Support an Early Shift to an Intermediate-Light Niche in the Evolution of Mammals. Mol. Biol. Evol. 2018, 35, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-X.; Meng, Q.-J.; Ji, Q.; Liu, D.; Zhang, Y.-G.; Neander, A.I. Evolutionary Development in Basal Mammaliaforms as Revealed by a Docodontan. Science 2015, 347, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Luo, Z.-X.; Yuan, C.-X.; Tabrum, A.R. A Swimming Mammaliaform from the Middle Jurassic and Ecomorphological Diversification of Early Mammals. Science 2006, 311, 1123–1127. [Google Scholar] [CrossRef]
- Fiorillo, A.R.; Padian, K.; Musikasinthorn, C. Taphonomy and Depositional Setting of the Placerias Quarry (Chinle Formation: Late Triassic, Arizona). Palaios 2000, 15, 373–386. [Google Scholar] [CrossRef]
- Pond, C.M. An Evolutionary and Functional View of Mammalian Adipose Tissue. Proc. Nutr. Soc. 1992, 51, 367–377. [Google Scholar] [CrossRef]
- Botha-Brink, J.; Angielczyk, K.D. Do Extraordinarily High Growth Rates in Permo-Triassic Dicynodonts (Therapsida, Anomodontia) Explain Their Success before and after the End-Permian Extinction? Zool. J. Linn. Soc. 2010, 160, 341–365. [Google Scholar] [CrossRef]
- Rey, K.; Amiot, R.; Fourel, F.; Abdala, F.; Fluteau, F.; Jalil, N.-E.; Liu, J.; Rubidge, B.S.; Smith, R.M.; Steyer, J.S.; et al. Oxygen Isotopes Suggest Elevated Thermometabolism within Multiple Permo-Triassic Therapsid Clades. eLife 2017, 6, e28589. [Google Scholar] [CrossRef]
- Bajdek, P.; Owocki, K.; Niedźwiedzki, G. Putative Dicynodont Coprolites from the Upper Triassic of Poland. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 411, 1–17. [Google Scholar] [CrossRef]
- McNab, B.K. An Analysis of the Factors That Influence the Level and Scaling of Mammalian BMR. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2008, 151, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Bajdek, P.; Qvarnström, M.; Owocki, K.; Sulej, T.; Sennikov, A.G.; Golubev, V.K.; Niedźwiedzki, G. Microbiota and Food Residues Including Possible Evidence of Pre-Mammalian Hair in Upper Permian Coprolites from Russia. Lethaia 2016, 49, 455–477. [Google Scholar] [CrossRef]
- Rowe, T. Definition, Diagnosis, and Origin of Mammalia. J. Vertebr. Paleontol. 1988, 8, 241–264. [Google Scholar] [CrossRef]
- Luo, Z.-X. Transformation and Diversification in Early Mammal Evolution. Nature 2007, 450, 1011–1019. [Google Scholar] [CrossRef]
- Datta, P.M. Earliest Mammal with Transversely Expanded Upper Molar from the Late Triassic (Carnian) Tiki Formation, South Rewa Gondwana Basin, India. J. Vertebr. Paleontol. 2005, 25, 200–207. [Google Scholar] [CrossRef]
- Kielan-Jaworowska, Z.; Cifelli, R.L.; Luo, Z.-X. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure; Columbia University Press: New York, NY, USA, 2005; ISBN 978-0-231-11918-4. [Google Scholar]
- Hammer, W.R.; Smith, N.D. A Tritylodont Postcanine from the Hanson Formation of Antarctica. J. Vertebr. Paleontol. 2008, 28, 269–273. [Google Scholar] [CrossRef]
- Kemp, T.S. The Origin and Evolution of Mammals; Oxford University Press on Demand: Oxford, UK, 2005. [Google Scholar]
- Matsuoka, H.; Kusuhashi, N.; Corfe, I.J. A New Early Cretaceous Tritylodontid (Synapsida, Cynodontia, Mammaliamorpha) from the Kuwajima Formation (Tetori Group) of Central Japan. J. Vertebr. Paleontol. 2016, 36, e1112289. [Google Scholar] [CrossRef]
- Araújo, R.; David, R.; Benoit, J.; Lungmus, J.K.; Stoessel, A.; Barrett, P.M.; Maisano, J.A.; Ekdale, E.; Orliac, M.; Luo, Z.-X.; et al. Inner Ear Biomechanics Reveals a Late Triassic Origin for Mammalian Endothermy. Nature 2022, 607, 726–731. [Google Scholar] [CrossRef]
- Newham, E.; Gill, P.G.; Brewer, P.; Benton, M.J.; Fernandez, V.; Gostling, N.J.; Haberthür, D.; Jernvall, J.; Kankaanpää, T.; Kallonen, A.; et al. Reptile-like Physiology in Early Jurassic Stem-Mammals. Nat. Commun. 2020, 11, 5121. [Google Scholar] [CrossRef]
- Sues, H.-D. Skull and Dentition of Two Tritylodontid Synapsids from the Lower Jurassic of Western North America; Harvard University: Cambridge, MA, USA, 1986. [Google Scholar]
- Hu, Y.; Meng, J.; Clark, J.M. A New Tritylodontid from the Upper Jurassic of Xinjiang, China. Acta Palaeontol. Pol. 2009, 54, 385–391. [Google Scholar] [CrossRef][Green Version]
- Nowak, R.M.; Walker, E.P. Walker’s Mammals of the World; JHU Press: Baltimore, MD, USA, 1999; Volume 1. [Google Scholar]
- Ezcurra, M.D. The Phylogenetic Relationships of Basal Archosauromorphs, with an Emphasis on the Systematics of Proterosuchian Archosauriforms. PeerJ 2016, 4, e1778. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Ray, S. A New Hyperodapedon (Archosauromorpha, Rhynchosauria) from the Upper Triassic of India: Implications for Rhynchosaur Phylogeny. Palaeontology 2014, 57, 1241–1276. [Google Scholar] [CrossRef]
- de Ricqlès, A.; Padian, K.; Knoll, F.; Horner, J.R. On the Origin of High Growth Rates in Archosaurs and Their Ancient Relatives: Complementary Histological Studies on Triassic Archosauriforms and the Problem of a “Phylogenetic Signal” in Bone Histology. In Annales de Paléontologie; Elsevier: Amsterdam, The Netherlands, 2008; Volume 94, pp. 57–76. [Google Scholar]
- Botha-Brink, J.; Smith, R.M. Osteohistology of the Triassic Archosauromorphs Prolacerta, Proterosuchus, Euparkeria, and Erythrosuchus from the Karoo Basin of South Africa. J. Vertebr. Paleontol. 2011, 31, 1238–1254. [Google Scholar] [CrossRef]
- Veiga, F.H.; Soares, M.B.; Sayão, J.M. Osteohistology of Hyperodapedontine Rhynchosaurs from the Upper Triassic of Southern Brazil. Acta Palaeontol. Pol. 2014, 60, 829–836. [Google Scholar]
- Traeholt, C. Notes on the Burrows of the Water Monitor Lizard, Varanus salvator. Malay. Nat. J. 1995, 49, 103–112. [Google Scholar]
- Lutz, R.L.; Lutz, J.M. Komodo, the Living Dragon; Dimi Press: Portland, OR, USA, 1997; ISBN 978-0-931625-27-5. [Google Scholar]
- Stevenson, R.D. The Relative Importance of Behavioral and Physiological Adjustments Controlling Body Temperature in Terrestrial Ectotherms. Am. Nat. 1985, 126, 362–386. [Google Scholar] [CrossRef]
- Nesbitt, S.J.; Desojo, J.B.; Irmis, R.B. Anatomy, Phylogeny and Palaeobiology of Early Archosaurs and Their Kin. Geol. Soc. Lond. Spec. Publ. 2013, 379, 1–7. [Google Scholar] [CrossRef]
- Brusatte, S.L.; Benton, M.J.; Desojo, J.B.; Langer, M.C. The Higher-Level Phylogeny of Archosauria (Tetrapoda: Diapsida). J. Syst. Palaeontol. 2010, 8, 3–47. [Google Scholar] [CrossRef]
- Padian, K.; Li, C.; Pchelnikova, J. The Trackmaker of Apatopus (Late Triassic, North America): Implications for the Evolution of Archosaur Stance and Gait. Palaeontology 2010, 53, 175–189. [Google Scholar] [CrossRef]
- de Ricqlès, A.J.; Padian, K.; Horner, J.R. On the Bone Histology of Some Triassic Pseudosuchian Archosaurs and Related Taxa. In Annales de Paléontologie; Elsevier: Amsterdam, The Netherlands, 2003; Volume 89, pp. 67–101. [Google Scholar]
- Scheyer, T.M.; Desojo, J.B.; Cerda, I.A. Bone Histology of Phytosaur, Aetosaur, and Other Archosauriform Osteoderms (Eureptilia, Archosauromorpha). Anat. Rec. 2014, 297, 240–260. [Google Scholar] [CrossRef]
- Huene, F.R. A New Phytosaur from the Palisades near New York; Bulletin of the AMNH, American Museum of Natural History: New York, NY, USA, 1913; Volume 32, p. 15. [Google Scholar]
- Hurlburt, G.R.; Heckert, A.B.; Farlow, J.O. Body Mass Estimates of Phytosaurs (Archosauria: Parasuchidae) from the Petrified Forest Formation (Chinle Group: Revueltian) Based on Skull and Limb Bone Measurements. New Mex. Mus. Nat. Hist. Sci. Bull. 2003, 24, 105–113. [Google Scholar]
- Desojo, J.; Ezcurra, M.; Kischlat, E.E. A New Aetosaur Genus (Archosauria: Pseudosuchia) from the Early Late Triassic of Southern Brazil. Zootaxa 2012, 3166, 1–33. [Google Scholar] [CrossRef]
- Small, B.J. The Triassic Thecodontian Reptile Desmatosuchus: Osteology and Relationships. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 1985. [Google Scholar]
- Walker, A.D. Triassic Reptiles from the Elgin Area: Stagonolepis, Dasygnathus and Their Allies. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1961, 244, 103–204. [Google Scholar]
- Gauthier, J.A.; Nesbitt, S.J.; Schachner, E.R.; Bever, G.S.; Joyce, W.G. The Bipedal Stem Crocodilian Poposaurus Gracilis: Inferring Function in Fossils and Innovation in Archosaur Locomotion. Bull. Peabody Mus. Nat. Hist. 2011, 52, 107–126. [Google Scholar] [CrossRef]
- Schachner, E.R.; Irmis, R.B.; Huttenlocker, A.K.; Sanders, K.; Cieri, R.L.; Nesbitt, S.J. Osteology of the Late Triassic Bipedal Archosaur Poposaurus Gracilis (Archosauria: Pseudosuchia) from Western North America. Anat. Rec. 2020, 303, 874–917. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Pörtner, H.-O. Temperature, Metabolic Power and the Evolution of Endothermy. Biol. Rev. 2010, 85, 703–727. [Google Scholar] [CrossRef]
- Nesbitt, S. The Anatomy of Effigia Okeeffeae (Archosauria, Suchia), Theropod-like Convergence, and the Distribution of Related Taxa. Bull. Am. Mus. Nat. Hist. 2007, 2007, 1–84. [Google Scholar] [CrossRef]
- Chatterjee, S. Postosuchus, a New Thecodontian Reptile from the Triassic of Texas and the Origin of Tyrannosaurs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 309, 395–460. [Google Scholar]
- Weinbaum, J.C. Postcranial Skeleton of Postosuchus Kirkpatricki (Archosauria: Paracrocodylomorpha), from the Upper Triassic of the United States. Geol. Soc. Lond. Spec. Publ. 2013, 379, 525–553. [Google Scholar] [CrossRef]
- Legendre, L.J.; Segalen, L.; Cubo, J. Evidence for High Bone Growth Rate in Euparkeria Obtained Using a New Paleohistological Inference Model for the Humerus. J. Vertebr. Paleontol. 2013, 33, 1343–1350. [Google Scholar] [CrossRef]
- Klein, N.; Foth, C.; Schoch, R.R. Preliminary Observations on the Bone Histology of the Middle Triassic Pseudosuchian Archosaur Batrachotomus Kupferzellensis Reveal Fast Growth with Laminar Fibrolamellar Bone Tissue. J. Vertebr. Paleontol. 2017, 37, e1333121. [Google Scholar] [CrossRef]
- Clark, J.M.; Sues, H.-D.; Berman, D.S. A New Specimen of Hesperosuchus Agilis from the Upper Triassic of New Mexico and the Interrelationships of Basal Crocodylomorph Archosaurs. J. Vertebr. Paleontol. 2001, 20, 683–704. [Google Scholar] [CrossRef]
- Lecuona, A.; Desojo, J.B. Hind Limb Osteology of Gracilisuchus Stipanicicorum (Archosauria: Pseudosuchia). Earth Environ. Sci. Trans. R. Soc. Edinb. 2012, 102, 105–128. [Google Scholar] [CrossRef]
- Colbert, E.H. A Pseudosuchian Reptile from Arizona; American Museum of Natural History: New York, NY, USA, 1952; Volume 99. [Google Scholar]
- Myhrvold, N.P. Dinosaur Metabolism and the Allometry of Maximum Growth Rate. PLoS ONE 2016, 11, e0163205. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.R. Evolution of Human Growth. Evol. Anthropol. Issues News Rev. Issues News Rev. 2001, 10, 223–236. [Google Scholar] [CrossRef]
- Seymour, R.S.; Bennett-Stamper, C.L.; Johnston, S.D.; Carrier, D.R.; Grigg, G.C. Evidence for Endothermic Ancestors of Crocodiles at the Stem of Archosaur Evolution. Physiol. Biochem. Zool. 2004, 77, 1051–1067. [Google Scholar] [CrossRef]
- Legendre, L. Did Crocodiles Become Secondarily Ectothermic? A Paleohistological Approach. Ph.D. Thesis, Université Pierre et Marie Curie-Paris VI, Paris, France, 2014. [Google Scholar]
- Molnar, J.L.; Pierce, S.E.; Bhullar, B.-A.S.; Turner, A.H.; Hutchinson, J.R. Morphological and Functional Changes in the Vertebral Column with Increasing Aquatic Adaptation in Crocodylomorphs. R. Soc. Open Sci. 2015, 2, 150439. [Google Scholar] [CrossRef]
- Gauthier, J. Saurischian Monophyly and the Origin of Birds. Mem. Calif. Acad. Sci. 1986, 8, 1–55. [Google Scholar]
- Brusatte, S.L.; Niedźwiedzki, G.; Butler, R.J. Footprints Pull Origin and Diversification of Dinosaur Stem Lineage Deep into Early Triassic. Proc. R. Soc. B Biol. Sci. 2011, 278, 1107–1113. [Google Scholar] [CrossRef]
- Ezcurra, M.D.; Nesbitt, S.J.; Bronzati, M.; Dalla Vecchia, F.M.; Agnolin, F.L.; Benson, R.B.J.; Brissón Egli, F.; Cabreira, S.F.; Evers, S.W.; Gentil, A.R.; et al. Enigmatic Dinosaur Precursors Bridge the Gap to the Origin of Pterosauria. Nature 2020, 588, 445–449. [Google Scholar] [CrossRef]
- Sereno, P.C.; Arcucci, A.B. Dinosaurian Precursors from the Middle Triassic of Argentina: Lagerpeton Chanarensis. J. Vertebr. Paleontol. 1994, 13, 385–399. [Google Scholar] [CrossRef]
- Martínez, R.N.; Apaldetti, C.; Correa, G.A.; Abelín, D. A Norian Lagerpetid Dinosauromorph from the Quebrada Del Barro Formation, Northwestern Argentina. Ameghiniana 2016, 53, 1–13. [Google Scholar] [CrossRef]
- Mallison, H. The Digital Plateosaurus I: Body Mass, Mass Distribution, and Posture Assessed Using CAD and CAE on a Digitally Mounted Complete Skeleton. Palaeontol. Electron. 2010, 13, 8A. [Google Scholar]
- Pontzer, H.; Allen, V.; Hutchinson, J.R. Biomechanics of Running Indicates Endothermy in Bipedal Dinosaurs. PLoS ONE 2009, 4, e7783. [Google Scholar] [CrossRef]
- Eagle, R.A.; Tütken, T.; Martin, T.S.; Tripati, A.K.; Fricke, H.C.; Connely, M.; Cifelli, R.L.; Eiler, J.M. Dinosaur Body Temperatures Determined from Isotopic (13C-18O) Ordering in Fossil Biominerals. Science 2011, 333, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Griebeler, E.M. Allometries of Maximum Growth Rate versus Body Mass at Maximum Growth Indicate That Non-Avian Dinosaurs Had Growth Rates Typical of Fast Growing Ectothermic Sauropsids. PLoS ONE 2014, 9, e88834. [Google Scholar] [CrossRef] [PubMed]
- Irmis, R.; Nesbitt, S.; Padian, K.; Smith, N.; Turner, A. A Late Triassic Dinosauromorph Assemblage from New Mexico and the Rise of Dinosaurs. Science 2007, 317, 358–361. [Google Scholar] [CrossRef]
- Griffin, C.T.; Bano, L.S.; Turner, A.H.; Smith, N.D.; Irmis, R.B.; Nesbitt, S.J. Integrating Gross Morphology and Bone Histology to Assess Skeletal Maturity in Early Dinosauromorphs: New Insights from Dromomeron (Archosauria: Dinosauromorpha). PeerJ 2019, 7, e6331. [Google Scholar] [CrossRef]
- Cabreira, S.F.; Kellner, A.W.A.; Dias-da-Silva, S.; da Silva, L.R.; Bronzati, M.; de Almeida Marsola, J.C.; Müller, R.T.; de Souza Bittencourt, J.; Batista, B.J.; Raugust, T. A Unique Late Triassic Dinosauromorph Assemblage Reveals Dinosaur Ancestral Anatomy and Diet. Curr. Biol. 2016, 26, 3090–3095. [Google Scholar] [CrossRef]
- Sharov, A.G. New Flying Reptiles from the Mesozoic of Kazakhstan and Kyrgyzstan. Tr. Paleontol. Instituta SSSR 1971, 130, 104–113. [Google Scholar]
- Witton, M.P. Pterosaurs. In Pterosaurs; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Kellner, A.W.A.; Wang, X.; Tischlinger, H.; de Almeida Campos, D.; Hone, D.W.E.; Meng, X. The Soft Tissue of Jeholopterus (Pterosauria, Anurognathidae, Batrachognathinae) and the Structure of the Pterosaur Wing Membrane. Proc. R. Soc. B Biol. Sci. 2010, 277, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, X.; You, H. A New Feather Type in a Nonavian Theropod and the Early Evolution of Feathers. Proc. Natl. Acad. Sci. USA 2009, 106, 832–834. [Google Scholar] [CrossRef] [PubMed]
- McKellar, R.C.; Chatterton, B.D.E.; Wolfe, A.P.; Currie, P.J. A Diverse Assemblage of Late Cretaceous Dinosaur and Bird Feathers from Canadian Amber. Science 2011, 333, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; McKellar, R.C.; Xu, X.; Li, G.; Bai, M.; Persons IV, W.S.; Miyashita, T.; Benton, M.J.; Zhang, J.; Wolfe, A.P. A Feathered Dinosaur Tail with Primitive Plumage Trapped in Mid-Cretaceous Amber. Curr. Biol. 2016, 26, 3352–3360. [Google Scholar] [CrossRef]
- Zheng, X.-T.; You, H.-L.; Xu, X.; Dong, Z.-M. An Early Cretaceous Heterodontosaurid Dinosaur with Filamentous Integumentary Structures. Nature 2009, 458, 333–336. [Google Scholar] [CrossRef]
- Godefroit, P.; Sinitsa, S.M.; Dhouailly, D.; Bolotsky, Y.L.; Sizov, A.V.; McNamara, M.E.; Benton, M.J.; Spagna, P. A Jurassic Ornithischian Dinosaur from Siberia with Both Feathers and Scales. Science 2014, 345, 451–455. [Google Scholar] [CrossRef]
- Kundrát, M. When Did Theropods Become Feathered?—Evidence for Pre-Archaeopteryx Feathery Appendages. J. Exp. Zoolog. B Mol. Dev. Evol. 2004, 302, 355–364. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, B.; McNamara, M.E.; Kearns, S.L.; Pittman, M.; Kaye, T.G.; Orr, P.J.; Xu, X.; Benton, M.J. Pterosaur Integumentary Structures with Complex Feather-like Branching. Nat. Ecol. Evol. 2019, 3, 24–30. [Google Scholar] [CrossRef]
- Campione, N.E.; Barrett, P.M.; Evans, D.C. On the Ancestry of Feathers in Mesozoic Dinosaurs. In The Evolution of Feathers; Springer: Berlin/Heidelberg, Germany, 2020; pp. 213–243. [Google Scholar]
- Heath, T.A.; Hedtke, S.M.; Hillis, D.M. Taxon Sampling and the Accuracy of Phylogenetic Analyses. J. Syst. Evol. 2008, 46, 239. [Google Scholar]
- Müller, R.T.; Dias-da-Silva, S. Taxon Sample and Character Coding Deeply Impact Unstable Branches in Phylogenetic Trees of Dinosaurs. Hist. Biol. 2019, 31, 1089–1092. [Google Scholar] [CrossRef]
- Thorington, R.W., Jr.; Hoffmann, R.S. Family Sciuridae. In Mammal Species of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2005; Volume 2, pp. 753–819. [Google Scholar]
- Martin, A.J. The Evolution Underground: Burrows, Bunkers, and the Marvelous Subterranean World Beneath Our Feet; Reprint ed.; Pegasus Books: Berkeley, CA, USA, 2017. [Google Scholar]
- Kammerer, C.F.; Nesbitt, S.J.; Shubin, N.H. The First Silesaurid Dinosauriform from the Late Triassic of Morocco. Acta Palaeontol. Pol. 2011, 57, 277–284. [Google Scholar] [CrossRef]
- Qvarnström, M.; Wernström, J.V.; Piechowski, R.; Tałanda, M.; Ahlberg, P.E.; Niedźwiedzki, G. Beetle-Bearing Coprolites Possibly Reveal the Diet of a Late Triassic Dinosauriform. R. Soc. Open Sci. 2019, 6, 181042. [Google Scholar] [CrossRef]
- Ezcurra, M.D. A Review of the Systematic Position of the Dinosauriform Archosaur Eucoelophysis Baldwini Sullivan & Lucas, 1999 from the Upper Triassic of New Mexico, USA. Geodiversitas 2006, 28, 649–684. [Google Scholar]
- Irmis, R.B.; Parker, W.G.; Nesbitt, S.J.; Liu, J. Early Ornithischian Dinosaurs: The Triassic Record. Hist. Biol. 2007, 19, 3–22. [Google Scholar] [CrossRef]
- Agnolín, F.L.; Rozadilla, S. Phylogenetic Reassessment of Pisanosaurus Mertii Casamiquela, 1967, a Basal Dinosauriform from the Late Triassic of Argentina. J. Syst. Palaeontol. 2018, 16, 853–879. [Google Scholar] [CrossRef]
- BUTLER, R.J. The Anatomy of the Basal Ornithischian Dinosaur Eocursor Parvus from the Lower Elliot Formation (Late Triassic) of South Africa. Zool. J. Linn. Soc. 2010, 160, 648–684. [Google Scholar] [CrossRef]
- McPhee, B.; Bordy, E.; Sciscio, L.; Choiniere, J. The Sauropodomorph Biostratigraphy of the Elliot Formation of Southern Africa: Tracking the Evolution Of Sauropodomorpha across the Triassic-Jurassic Boundary. Acta Palaeontol. Pol. 2017, 3, 441–465. [Google Scholar] [CrossRef]
- Seymour, R.S.; Lillywhite, H.B. Hearts, Neck Posture and Metabolic Intensity of Sauropod Dinosaurs. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 1883–1887. [Google Scholar] [CrossRef]
- Wedel, M.J. Evidence for Bird-like Air Sacs in Saurischian Dinosaurs. J. Exp. Zool. Part Ecol. Genet. Physiol. 2009, 311, 611–628. [Google Scholar] [CrossRef]
- Seymour, R. Were Dinosaurs Warm-Blooded? Australas. Sci. 2013, 34, 16–19. [Google Scholar]
- McNab, B.K. Ecological Factors Affect the Level and Scaling of Avian BMR. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2009, 152, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Maloney, S.K. Thermoregulation in Ratites: A Review. Aust. J. Exp. Agric. 2008, 48, 1293–1301. [Google Scholar] [CrossRef]
- Varricchio, D.J.; Martin, A.J.; Katsura, Y. First Trace and Body Fossil Evidence of a Burrowing, Denning Dinosaur. Proc. R. Soc. B Biol. Sci. 2007, 274, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.N.; Alcober, O.A. A Basal Sauropodomorph (Dinosauria: Saurischia) from the Ischigualasto Formation (Triassic, Carnian) and the Early Evolution of Sauropodomorpha. PLoS ONE 2009, 4, e4397. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.; Cuff, A.R.; Allen, V.; Sumner-Rooney, L.; Pol, D.; Hutchinson, J.R. Ontogenetic Changes in the Body Plan of the Sauropodomorph Dinosaur Mussaurus Patagonicus Reveal Shifts of Locomotor Stance during Growth. Sci. Rep. 2019, 9, 7614. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, C.; Hartman, S.A.; Mateus, O. An Overview Of Non-Avian Theropod Discoveries And Classification. PalArchs J. Vertebr. Palaeontol. 2015, 12, 1–73. [Google Scholar]
- Prochnow, S.J.; Nordt, L.C.; Atchley, S.C.; Hudec, M.R. Multi-Proxy Paleosol Evidence for Middle and Late Triassic Climate Trends in Eastern Utah. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 232, 53–72. [Google Scholar] [CrossRef]
- Peterson, M.E.; Daniel, R.M.; Danson, M.J.; Eisenthal, R. The Dependence of Enzyme Activity on Temperature: Determination and Validation of Parameters. Biochem. J. 2007, 402, 331–337. [Google Scholar] [CrossRef]
- Angilletta, M.J.; Cooper, B.S.; Schuler, M.S.; Boyles, J.G. The Evolution of Thermal Physiology in Endotherms. Front. Biosci. E 2010, 2, 861–881. [Google Scholar]
- Koteja, P. On the Relation between Basal and Field Metabolic Rates in Birds and Mammals. Funct. Ecol. 1991, 5, 56–64. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Konarzewski, M.; Daan, S. The Relationship between Basal Metabolic Rate and Daily Energy Expenditure in Birds and Mammals. Am. Nat. 1996, 147, 1047–1071. [Google Scholar] [CrossRef]
- Mole, M.A.; Rodrigues DÁraujo, S.; van Aarde, R.J.; Mitchell, D.; Fuller, A. Coping with Heat: Behavioural and Physiological Responses of Savanna Elephants in Their Natural Habitat. Conserv. Physiol. 2016, 4, cow044. [Google Scholar] [CrossRef] [PubMed]
- Dubiel, R.; Parrish, J.; Parrish, J.; Good, S. The Pangaean Megamonsoon-Evidence from the Upper Triassic Chinle Formation, Colorado Plateau. Palaios 1991, 6, 347–370. [Google Scholar] [CrossRef]
- Whiteside, J.H.; Grogan, D.S.; Olsen, P.E.; Kent, D.V. Climatically Driven Biogeographic Provinces of Late Triassic Tropical Pangea. Proc. Natl. Acad. Sci. USA 2011, 108, 8972–8977. [Google Scholar] [CrossRef] [PubMed]
- Owen-Smith, N. Assessing the Foraging Effeciency of a Large Herbivore, the Kudu. South Afr. J. Wildl. Res.-24-Mon. Delayed Open Access 1979, 9, 102–110. [Google Scholar] [CrossRef]
- Plumb, G.E.; Dodd, J.L. Foraging Ecology of Bison and Cattle on a Mixed Prairie: Implications for Natural Area Management. Ecol. Appl. 1993, 3, 631–643. [Google Scholar] [CrossRef]
- Bahr, A.; Kolber, G.; Kaboth-Bahr, S.; Reinhardt, L.; Friedrich, O.; Pross, J. Mega-Monsoon Variability during the Late Triassic: Re-Assessing the Role of Orbital Forcing in the Deposition of Playa Sediments in the Germanic Basin. Sedimentology 2020, 67, 951–970. [Google Scholar] [CrossRef]
- Noy-Meir, I. Desert Ecosystems: Higher Trophic Levels. Annu. Rev. Ecol. Syst. 1974, 5, 195–214. [Google Scholar] [CrossRef]
- Preto, N.; Kustatscher, E.; Wignall, P.B. Triassic Climates—State of the Art and Perspectives. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 290, 1–10. [Google Scholar] [CrossRef]
- Crompton, A.; Taylor, C.R.; Jagger, J.A. Evolution of Homeothermy in Mammals. Nature 1978, 272, 333–336. [Google Scholar] [CrossRef]
- Schmitz, L.; Motani, R. Nocturnality in Dinosaurs Inferred from Scleral Ring and Orbit Morphology. Science 2011, 332, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.L.; Bradshaw, D.K.; Chabreck, R.H. Alligator Feeding Habits: New Data and a Review. Gulf Mex. Sci. 1987, 9, 1. [Google Scholar] [CrossRef]
- Ramezani, J.; Fastovsky, D.E.; Bowring, S.A. Revised Chronostratigraphy of the Lower Chinle Formation Strata in Arizona and New Mexico (USA): High-Precision U-Pb Geochronological Constraints on the Late Triassic Evolution of Dinosaurs. Am. J. Sci. 2014, 314, 981–1008. [Google Scholar] [CrossRef]
- Kriloff, A.; Germain, D.; Canoville, A.; Vincent, P.; Sache, M.; Laurin, M. Evolution of Bone Microanatomy of the Tetrapod Tibia and Its Use in Palaeobiological Inference. J. Evol. Biol. 2008, 21, 807–826. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, G.D.; Müller, R.T.; de Araújo-Júnior, H.I.; Dias-da-Silva, S.; Pinheiro, F.L. A Peculiar Bonebed Reinforces Gregarious Behaviour for the Triassic Dicynodont Dinodontosaurus. Hist. Biol. 2020, 32, 764–772. [Google Scholar] [CrossRef]
- Fiorelli, L.E.; Ezcurra, M.D.; Hechenleitner, E.M.; Argañaraz, E.; Taborda, J.R.A.; Trotteyn, M.J.; von Baczko, M.B.; Desojo, J.B. The Oldest Known Communal Latrines Provide Evidence of Gregarism in Triassic Megaherbivores. Sci. Rep. 2013, 3, 3348. [Google Scholar] [CrossRef] [PubMed]
- Green, J.L.; Schweitzer, M.H.; Lamm, E.-T. Limb Bone Histology and Growth in Placerias Hesternus (Therapsida: Anomodontia) from the Upper Triassic of North America. Palaeontology 2010, 53, 347–364. [Google Scholar] [CrossRef]
- Viglietti, P.A.; Smith, R.M.H.; Compton, J.S. Origin and Palaeoenvironmental Significance of Lystrosaurus Bonebeds in the Earliest Triassic Karoo Basin, South Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 392, 9–21. [Google Scholar] [CrossRef]
- Mathewson, P.D.; Porter, W.P.; Barrett, L.; Fuller, A.; Henzi, S.P.; Hetem, R.S.; Young, C.; McFarland, R. Field Data Confirm the Ability of a Biophysical Model to Predict Wild Primate Body Temperature. J. Therm. Biol. 2020, 94, 102754. [Google Scholar] [CrossRef]
- Huynh, T.T.; Poulsen, C.J. Rising Atmospheric CO2 as a Possible Trigger for the End-Triassic Mass Extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 217, 223–242. [Google Scholar] [CrossRef]
- Hale, A.; Merchant, M.; White, M. Detection and Analysis of Autophagy in the American Alligator (Alligator Mississippiensis). J. Exp. Zoolog. B Mol. Dev. Evol. 2020, 334, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Grigg, G.; Beard, L. Hibernation by Echidnas in Mild Climates: Hints about the Evolution of Endothermy? In Life in the Cold; Springer: Berlin/Heidelberg, Germany, 2000; pp. 5–19. [Google Scholar]
- Rial, R.V.; Akaârir, M.; Gamundí, A.; Nicolau, C.; Garau, C.; Aparicio, S.; Tejada, S.; Gené, L.; González, J.; De Vera, L.M.; et al. Evolution of Wakefulness, Sleep and Hibernation: From Reptiles to Mammals. Neurosci. Biobehav. Rev. 2010, 34, 1144–1160. [Google Scholar] [CrossRef] [PubMed]
- Arad, Z. Physiological Responses to Increasing Ambient Temperature in Three Ecologically Different, Congeneric Lizards (Gekkoninae: Ptyodactylus). Comp. Biochem. Physiol. A Physiol. 1995, 112, 305–311. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Cheng, Y.; Sato, T.; Wang, L. An Unusual Archosaurian from the Marine Triassic of China. Naturwissenschaften 2006, 93, 200–206. [Google Scholar] [CrossRef]
- Butler, R.J.; Brusatte, S.L.; Reich, M.; Nesbitt, S.J.; Schoch, R.R.; Hornung, J.J. The Sail-Backed Reptile Ctenosauriscus from the Latest Early Triassic of Germany and the Timing and Biogeography of the Early Archosaur Radiation. PLoS ONE 2011, 6, e25693. [Google Scholar] [CrossRef]
- Romano, M.; Manucci, F. Resizing Lisowicia Bojani: Volumetric Body Mass Estimate and 3D Reconstruction of the Giant Late Triassic Dicynodont. Hist. Biol. 2021, 33, 474–479. [Google Scholar] [CrossRef]
- Ray, S.; Chinsamy, A. Functional Aspects of the Postcranial Anatomy of the Permian Dicynodont Diictodon and Their Ecological Implications. Palaeontology 2003, 46, 151–183. [Google Scholar] [CrossRef]
- Botha-Brink, J. Burrowing in Lystrosaurus: Preadaptation to a Postextinction Environment? J. Vertebr. Paleontol. 2017, 37, e1365080. [Google Scholar] [CrossRef]
- Angielczyk, K.D.; Steyer, J.-S.; Sidor, C.A.; Smith, R.M.H.; Whatley, R.L.; Tolan, S. Permian and Triassic Dicynodont (Therapsida: Anomodontia) Faunas of the Luangwa Basin, Zambia: Taxonomic Update and Implications for Dicynodont Biogeography and Biostratigraphy. In Early Evolutionary History of the Synapsida; Kammerer, C.F., Angielczyk, K.D., Fröbisch, J., Eds.; Vertebrate Paleobiology and Paleoanthropology; Springer Netherlands: Dordrecht, The Netherlands, 2014; pp. 93–138. ISBN 978-94-007-6841-3. [Google Scholar]
- Kammerer, C.F.; Angielczyk, K.D. A Proposed Higher Taxonomy of Anomodont Therapsids. Zootaxa 2009, 2018, 1–24. [Google Scholar] [CrossRef]
- Angielczyk, K.D.; Schmitz, L. Nocturnality in Synapsids Predates the Origin of Mammals by over 100 Million Years. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141642. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Hadly, E.A. Invasion of Ancestral Mammals into Dim-Light Environments Inferred from Adaptive Evolution of the Phototransduction Genes. Sci. Rep. 2017, 7, 46542. [Google Scholar] [CrossRef] [PubMed]
- de Souza Carvalho, I.; Campos, A.D.C.A.; Nobre, P.H. Baurusuchus Salgadoensis, a New Crocodylomorpha from the Bauru Basin (Cretaceous), Brazil. Gondwana Res. 2005, 8, 11–30. [Google Scholar] [CrossRef]
- Guppy, M.; Fuery, C.J.; Flanigan, J.E. Biochemical Principles of Metabolic Depression. Comp. Biochem. Physiol. Part B Comp. Biochem. 1994, 109, 175–189. [Google Scholar] [CrossRef]
- Withers, P.C.; Cooper, C.E. Metabolic Depression: A Historical Perspective. In Aestivation; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–23. [Google Scholar]
- Mitchell, N.J.; Kearney, M.R.; Nelson, N.J.; Porter, W.P. Predicting the Fate of a Living Fossil: How Will Global Warming Affect Sex Determination and Hatching Phenology in Tuatara? Proc. R. Soc. B Biol. Sci. 2008, 275, 2185–2193. [Google Scholar] [CrossRef]
- Mitchell, N.; Hipsey, M.R.; Arnall, S.; McGrath, G.; Tareque, H.B.; Kuchling, G.; Vogwill, R.; Sivapalan, M.; Porter, W.P.; Kearney, M.R. Linking Eco-Energetics and Eco-Hydrology to Select Sites for the Assisted Colonization of Australia’s Rarest Reptile. Biology 2012, 2, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Raup, D.M.; Sepkoski, J.J. Mass Extinctions in the Marine Fossil Record. Science 1982, 215, 1501–1503. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartman, S.A.; Lovelace, D.M.; Linzmeier, B.J.; Mathewson, P.D.; Porter, W.P. Mechanistic Thermal Modeling of Late Triassic Terrestrial Amniotes Predicts Biogeographic Distribution. Diversity 2022, 14, 973. https://doi.org/10.3390/d14110973
Hartman SA, Lovelace DM, Linzmeier BJ, Mathewson PD, Porter WP. Mechanistic Thermal Modeling of Late Triassic Terrestrial Amniotes Predicts Biogeographic Distribution. Diversity. 2022; 14(11):973. https://doi.org/10.3390/d14110973
Chicago/Turabian StyleHartman, Scott A., David M. Lovelace, Benjamin J. Linzmeier, Paul D. Mathewson, and Warren P. Porter. 2022. "Mechanistic Thermal Modeling of Late Triassic Terrestrial Amniotes Predicts Biogeographic Distribution" Diversity 14, no. 11: 973. https://doi.org/10.3390/d14110973
APA StyleHartman, S. A., Lovelace, D. M., Linzmeier, B. J., Mathewson, P. D., & Porter, W. P. (2022). Mechanistic Thermal Modeling of Late Triassic Terrestrial Amniotes Predicts Biogeographic Distribution. Diversity, 14(11), 973. https://doi.org/10.3390/d14110973






