Abstract
Food resources are key limiting factors for migratory waterbirds, and the foraging strategies adopted by herbivorous waterbirds are affected by food availability in wetland habitats. The greater white-fronted goose (Anser albifrons) is mainly dependent on Carex in the lower and middle Yangtze River floodplain. Exploring the relationship between the growth conditions of Carex and the foraging strategies adopted by wintering greater white-fronted geese has important ecological implications for habitat protection and management. In this study, scan sampling and focal animal sampling were used to record the foraging behaviors of greater white-fronted geese wintering at Shengjin Lake, and the plant height and water content of Carex were surveyed simultaneously. The relationship between plant characteristics and foraging behaviors was tested using a linear regression equation. The results showed that Carex had two growth periods at Shengjin Lake, and the pecking rate and foraging time budget of greater white-fronted geese were higher during these two periods. Plant characteristics were positively correlated with goose foraging behaviors. The strategic adjustment of the foraging behaviors adopted by wintering greater white-fronted geese was consistent with the growth stage of Carex, which is the optimal foraging window for greater white-fronted geese. During the foraging windows, geese changed their foraging strategies to obtain more energy in order to guarantee successful wintering and migration.
1. Introduction
Optimal foraging theory (OFT) describes the optimal behavioral strategies adopted by animals to obtain the greatest benefits at the lowest cost [1,2]. Generally, food resources are considered the key limiting factors of migratory waterbird survival and successful migration back to their breeding grounds during resource-poor winters, playing an important role in their annual lifecycle [3]. According to OFT, wintering waterbirds increase their fitness by minimizing the time and effort required to obtain maximum energy from food resources [4]. However, foraging strategies are affected by a variety of factors, especially the abundance and availability of food resources in habitats, which are considered the most important factors [2,5]. Animals affected by changes in food resources maintain their energy acquisition by changing their foraging behaviors [5]; these behavioral changes manifest in many aspects, including foraging habitat selection [2,6], foraging time budgets [7], and pecking rate adjustments [8]. For example, changes in the water level reduce the food availability in foraging patches, and waterbirds make strategic adjustments after weighing their energy budgets and choosing to move to new foraging patches [9]. For migratory waterbirds, insufficient food resources delay their departure from wintering grounds and their arrival at breeding grounds [10], which has important implications for their reproductive performance and population dynamics [11].
Animals ensure the acquisition of energy by adopting different foraging behavioral strategies and changing their foraging activity at a specific time in a certain life history stage called the foraging window [12]. The resource availability hypothesis (RAH) proposes that herbivores have higher foraging levels on fast-growing plants [13]. Therefore, herbivorous waterbirds may choose to feed during the rapid growth stage of plants, because these plants contain more nutrients [2,9]. As a plant grows, its fiber and lignin contents increase [14], which reduces the availability of food resources. Therefore, herbivorous waterbirds must change their foraging patterns according to the plant growth conditions in the foraging window to maximize their fitness.
Seasonal water level fluctuations substantially effect the growth conditions of plants in the wintering grounds of the middle and lower Yangtze River floodplain [3,12,15], which directly affects the foraging strategies of herbivorous waterbirds. Owing to water level reductions and surface exposure during the dry season, food resources in the riparian zone increase, and large areas of mudflats and bare sedge meadows provide foraging habitats for migratory waterbirds [3,15,16]. However, the degradation of lake wetlands in the middle and lower Yangtze River floodplain and the increasing influence of human activities have led to the fragmentation of wetland landscapes [17], and the deterioration of habitat quality has led to the loss of food resources and shelters [18,19]. This has resulted in changes in the distribution of wintering populations of migratory waterbirds and their use of foraging habitats in this region [20]. Wintering greater white-fronted geese (Anser albifrons) in the middle and lower Yangtze River floodplain mainly feed on sedges (Carex) and are highly dependent on them [21,22]; thus, greater white-fronted geese are markedly affected by the growth conditions of sedges. Therefore, it is very important to understand the relationship between the foraging behavioral strategies adopted by wintering greater white-fronted geese and sedge growth. This will help in exploring the ecological adaptability of wintering greater white-fronted geese and in providing scientific and reasonable suggestions for their wintering habitat management and population protection.
Owing to the water level fluctuation, there are two peak periods of Carex, the plant may provide more food than at other times, and the wintering greater white-fronted geese mainly forage sedge; thus, we predict that (1) geese will increase their foraging behaviors during the peak period of Carex, so the growth period of Carex is consistent with the foraging window of wintering greater white-fronted geese; and (2) greater white-fronted geese strategically adjust their foraging behavior according to the growth status of sedges and increase their foraging time budget and pecking rate during the sedge growth period and periods of high nutrient content.
2. Materials and Methods
The study area, Shengjin Lake National Nature Reserve, is located on the right bank of the Yangtze River. It is a Ramsar Site of international importance among the many river-connected lakes in the middle and lower Yangtze River floodplain. It is also an important wintering ground for geese, including the greater white-fronted goose, which is the main goose species at Shengjin Lake [2,23]. The number of wintering waterbird species and populations of some ducks have increased because of wetland restoration activities (planting of aquatic vegetation) [19]. The lakeside and shoals provide foraging patches for wintering waterbirds during the dry season (October–April) [9]. Sedges grow widely in the upper part of Shengjin Lake. Owing to cyclical changes in the hydrology at Shengjin Lake, there are two distinct sedge growing seasons: autumn and spring (October and February, respectively), and the first peak (late October–late January) was much smaller than the second (early February–mid April) in terms of biomass [16,22]. Thus, the upper part of Shengjin Lake (30.25°–30.50° N, 116.92°–117.25° E) is an important foraging region for geese. According to the regular migration of waterbirds at Shengjin Lake, the wintering season is divided into three stages: early winter (October–December), middle winter (December–February), and late winter (February–April) [2]. Sedges grow in meadows and mudflats that are exposed after periodic drawdowns of the upper part of the lake, and greater white-fronted geese occur here during winter. Five survey sites (Yang’etou, She Gan, Lan Daochen, Shen Shanzui, and Lian He) were selected in this area (Figure 1), and four plots (1 km × 1 km) were established within each survey site between October 2017 and April 2018. Foraging behavior and food item data were collected during six surveys in each wintering stage.
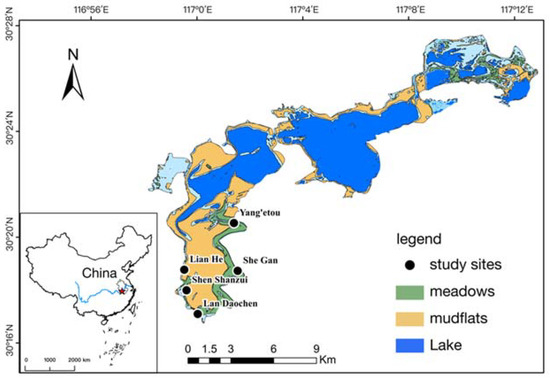
Figure 1.
The study sites in the upper part of Shengjin Lake, China.
Scan sampling was used to observe the foraging behaviors of greater white-fronted geese from October 2017 to April 2018. Binoculars (EL 10 × 42, Swarovski Optik, Absam, Austria) and a spotting scope (ATS 40–60 × 80, Swarovski Optik, Absam, Austria) were used to make observations from 07:00 to 17:00 on survey days [24]. Each observation was at least 20 min in duration (data less than 20 min in duration were discarded), and the time interval between observations was 15 min [25]. The direction of each observation was from left to right, and the behaviors observed included pecking plants (individual diet), resting, being alert, socializing, and moving. Focal animal sampling was used to observe individual foraging times [26], we recorded all the pecks in a whole day’s observation. Each sample was typically videotaped for about 20 min [2]. Video records shorter than 20 min were discarded in order to increase the data reliability and representativeness, as suggested in various studies [20]. The pecking rate was defined as the times of pecks per hour performed by an individual and was expressed as pecks/h [9,27]. When the geese were concerned, the time was recorded, and we recorded the time of each foraging. Each sampling time was 20 min, and the time interval between samplings was 15 min. During a complete foraging cycle, the amount of time that greater white-front geese spent foraging for sedges was recorded.
Nine quadrats (0.5 m × 0.5 m [14,20]) were randomly selected in each plot, and the plant height in each quadrat was recorded only when the geese occurred. All the aboveground parts of sedges including the leaves and stems in each quadrat were collected and stored in ziplock bags. And then returned to the laboratory for measurement. The fresh weight (g) of a sample was measured, and then it was dried in a drying oven (YHG-9050A, Derip, Suzhou, China) at 60 °C for 72 h to a constant weight, after which its dry mass (g) and water content (g) were measured [20].
The Kolmogorov–Smirnov test was used to determine the normality of the plant characteristics and foraging behavior time budgets. The test results showed that our data were in accordance with the normal distribution. Trends in plant height and water content variation were also analyzed, the Pearson method was used to test the correlation between plant characteristics and waterbird behaviors, and a linear regression equation was used to analyze the relationship between them based on the 95% confidence set [2,28]. We calculated the average and standard error of the plant height and water content; all data are presented as the mean ± standard error, with a significance level of p ≤ 0.05. All computations and statistical tests were performed using R software (version 4.0.3).
3. Results
The sedges foraged by greater white-fronted geese were 0.12 ± 0.09 m in height (n = 216) during the wintering season. The sedges in period a2 were the highest (0.19 ± 0.02 m), followed by those in period c2 (0.14 ± 0.07 m) and those in period c1 (0.14 ± 0.06 m). The lowest plant height was observed in period b2 (0.12 ± 0.05 m) (Figure 2a). The water content was the highest in periods a2 (210.55 ± 80.54 g/m2) and c1 (149.64 ± 33.56 g/m2), and it was the lowest in period b2 (53.45 ± 21.25 g/m2) (Figure 2b). During the entire wintering season, the water content and plant height of sedges showed a bimodal distribution trend, and bimodal distributions appeared from 4 to 13 December and 4 to 13 March (Figure 2c).
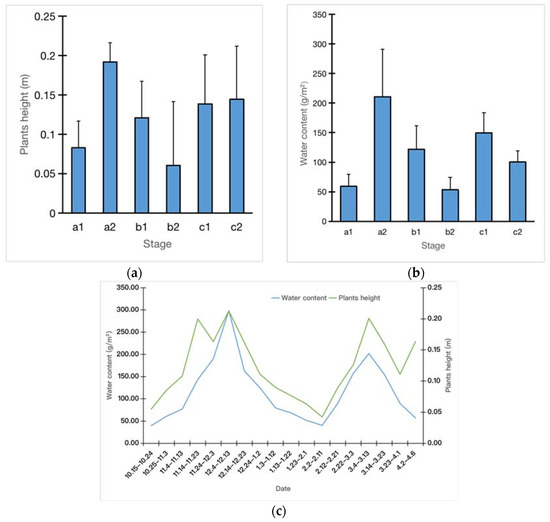
Figure 2.
Dynamic changes in the plant height and water content of sedges at Shengjin Lake. (a) Changes in the height (b) and in the water content. (c) The dynamic changes in plant height and water content showed a bimodal distribution trend during the whole winter. (a1 and a2—Early Winter, b1 and b2—Middle Winter, c1 and c2—Late Winter).
During the wintering season, the foraging time budget and pecking rate showed a trend of first increasing, then decreasing, and finally increasing again. The foraging time budget of period a2 was the highest (57.45% ± 8.62%), followed by that of period c2 (46.39% ± 5.70%). The foraging time budget of period b2 was the lowest (24.70% ± 2.77%) (Figure 3a). Among them, the highest time budgets for foraging behavior were observed from 4 to 13 December (Figure 3b). The Pearson’s correlation test and regression model analysis showed that water content (r = 0.492, p = 0.038) and plant height (r = 0.626, p = 0.004) were positively correlated with foraging rate, and water content (r = 0.622, p = 0.006) and plant height (r = 0.670, p = 0.002) were positively correlated with foraging time budget (Figure 4, Table 1).
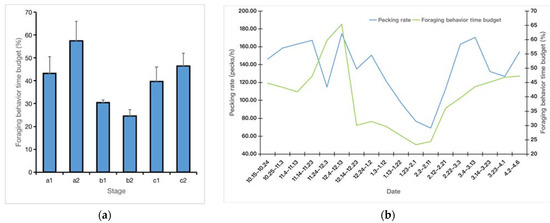
Figure 3.
Dynamic changes in the pecking rate and foraging behavior time budget of the greater white-fronted geese at Shengjin Lake. (a) Changes in the foraging behavior time budget; (b) dynamic changes in the pecking rate and foraging behavior time budget showed a bimodal distribution trend. (a1 and a2—Early Winter, b1 and b2—Middle Winter, c1 and c2—Late Winter).
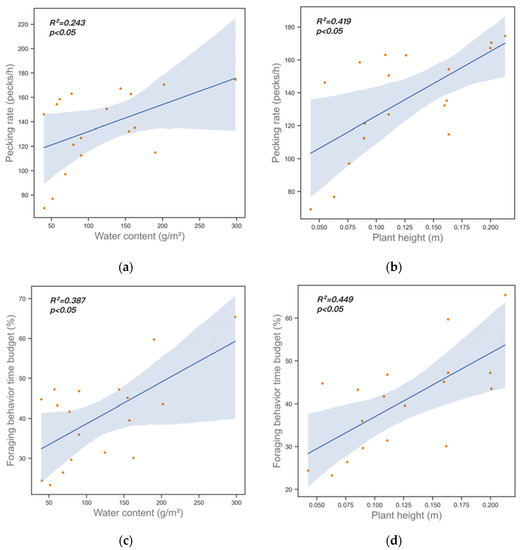
Figure 4.
Results of Linear Regression Tests on plant conditions and foraging behavior. (a) Water content and pecking rate; (b) plant height and pecking rate; (c) water content and foraging behavior time budget; (d) plant height and foraging behavior time budget.

Table 1.
Pearson correlation test results between plant characteristics and foraging behaviors.
4. Discussion
This study comprised two main focuses: (1) the plant height and water content characteristics, and (2) the waterbird pecking rate and foraging time budget. In addition, the relationship between plant characteristics and waterbird behavior was also analyzed. The results confirmed the hypothesis that the foraging behavior of the wintering greater white-fronted geese at Shengjin Lake was affected by the growth conditions of sedges. There was a positive correlation between plant characteristics and geese behaviors. The growth stage of sedges was consistent with the foraging window for overwintering greater white-fronted geese.
The food resources of wintering grounds affect the physical condition of waterbirds. They determine whether migratory waterbirds can survive in the winter and return to their breeding grounds, thereby playing an important role in their lifecycle [3]. The sedges at Shengjin Lake showed a special bimodal pattern with two growth stages during a single winter (Figure 2), which was affected by changes in the water level [9,12,22], leading to a change in food availability. Their foraging behavior followed a similar trend (Figure 4). The pecking rate and foraging time budget were positively correlated with plant height and water content (p < 0.05). Geese strategically changed their foraging behaviors according to the growth conditions of food resources by increasing their pecking rate and foraging time budget during the vigorous growth stage of sedges to increase the degree of foraging, which also supported the RAH [13]. The period when the plant height and water content of sedges are higher (4–13 December and 4–13 March) is the optimal foraging window for greater white-fronted geese; this was when both the pecking rate and the time budget of foraging behavior were the highest.
From September to October, the water level of Shengjin Lake begins to drop [9]. The combination of lower water levels and a warm climate promotes the first rapid growth of the sedges [29,30,31] on which the greater white-fronted geese forage at Shengjin Lake. This was of positive significance for geese during the early winter stage (autumn migration) in supplementing their huge energy consumption requirements; this led to a higher pecking rate and foraging time budget for greater white-fronted geese. When sedges grew again in the spring of the second year, the pecking rate and foraging time budget increased but were lower than those in the autumn of the previous year (Figure 3b). This indicates lower vegetation coverage [2] or an increase in biomass [16] but not an increase in food availability [9]. In the middle winter period, the foraging behaviors decreased; this may be a trade-off between food availability and quantity [32], and greater white-fronted geese may reduce foraging in each surveyed area [2]. The higher pecking rate and foraging time budget in the peak growth periods of Carex indicated that the greater white-fronted geese increased their foraging behaviors to obtain more food quickly during the foraging windows in order to cope with the coming mid-winter, when Carex will be reduced [2].
Owing to the huge energy consumption of migration [33], which is limited by the time-minimization strategy adopted by migratory waterbirds [34], the amount of energy that can be replenished at stopover sites is limited [35]. Influenced by migration strategies, waterbirds must replenish and accumulate energy through additional foraging when they arrive at and leave wintering grounds. The greater white-fronted geese in our study had a relatively longer foraging time budget and a higher pecking rate when sedges had lower water and nutrition contents in the autumn and spring; this is due to migration activities. In addition, predation risk, competition, individual status, and other nutrients may have had an impact on foraging behaviors [4]. This may be the main reason why the linear regression test results for plant characteristics and waterbird behaviors showed weak or moderate relative relationships.
5. Conclusions
Our results showed that Carex at Shengjin Lake presented a bimodal growth trend. The foraging window for wintering greater white-fronted geese feeding on sedges was consistent with the rapid growth stage of these sedges, and foraging behaviors changed with the growth of sedges. High-intensity foraging coincided with the period when sedges maintained a higher water content and a longer plant height, which provide the optimal foraging window for greater white-fronted geese. The bimodal growth pattern of sedges at Shengjin Lake provided important food resources for wintering geese and guaranteed the completion of migration and wintering activities. The availability of food resources in wintering habitats is a key limiting factor of wintering waterbird survival. Our results demonstrate the ecological adaptability of wintering waterbirds in managing their wintering habitats and protecting their populations. The results of this study provide insights that can inform the development of wetland management strategies; for example, maintaining normal water level fluctuation is conducive to the growth of aquatic vegetation. In addition, these waterbirds and the available food resources should be protected during the wintering season. In future studies, consideration should be given to incorporating more factors, such as interactions between waterbirds, to determine the relationship between the vegetation phenology and all wintering herbivorous waterbirds.
Author Contributions
Conceptualization, L.Z. and Y.F.; methodology, Y.F., L.Z., and L.C.; validation, Y.F. and Y.Z.; formal analysis, Y.F. and Y.Z.; investigation, Y.F., Y.S. and L.C.; resources, L.Z.; data curation, Y.F. and Y.Z.; writing—original draft preparation, Y.F. and Y.Z.; writing—review and editing, Y.Z. and L.Z.; visualization, L.C.; supervision, L.Z.; project administration, L.Z.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number No.32171530, 31472020.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Chao Yu, Yiwei Bao, and Shaojun Fan for their assistance with the data collection. We thank Zhenhua Wei, Shanshan Xia, and Xianglin Ji for their assistance with the data analysis. We also thank the staff of Anhui Shengjin Lake National Nature Reserve for their help with the fieldwork.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Charnov, E.L. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 1976, 9, 129–136. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, L.; Cheng, L.; Song, Y.; Xu, W. Foraging behavior of the greater white-fronted goose (Anser albifrons) wintering at Shengjin Lake: Diet shifts and habitat use. Avian Res. 2020, 11, 3. [Google Scholar] [CrossRef]
- Beerens, J.M.; Gawlik, D.E.; Herring, G.; Cook, M.I. Dynamic habitat selection by two wading bird species with divergent foraging strategies in a seasonally fluctuating wetland. The Auk 2011, 128, 651–662. [Google Scholar] [CrossRef]
- Říha, M.; Prchalová, M. Models of animal distributions in inland waters. In Encyclopedia of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2022; pp. 292–301. ISBN 978-0-12-822041-2. [Google Scholar]
- Durant, D.; Fritz, H.; Blais, S.; Duncan, P. The functional response in three species of herbivorous Anatidae: Effects of sward height, body mass and bill size. J. Anim. Ecology 2003, 72, 220–231. [Google Scholar] [CrossRef]
- Cumming, G.S.; Paxton, M.; King, J.; Beuster, H. Foraging guild membership explains variation in waterbird responses to the hydrological regime of an arid-region flood-pulse river in Namibia: Community dynamics of waterbirds in Namibia. Freshw. Biol. 2012, 57, 1202–1213. [Google Scholar] [CrossRef]
- Falk, K.; Benvenuti, S.; Dall’Antonia, L.; Kampp, K.; Ribolini, A. Time allocation and foraging behavior of chick-rearing Brünnich’s Guillemots Uria Lomvia in high-arctic greenland. Ibis 2008, 142, 82–92. [Google Scholar] [CrossRef]
- Durant, D.; Fritz, H. Variation of pecking rate with sward height in wild wigeon Anas Penelope. J. Ornithol. 2006, 147, 367–370. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Cheng, L.; Song, Y. Water level management plan based on the ecological demands of wintering waterbirds at Shengjin Lake. Glob. Ecol. Conserv. 2021, 27, e01567. [Google Scholar] [CrossRef]
- Hazra, P.; Sinha, A.; Mondal, P.; Khan, T.N. Calendar-effects and temperature-impacts in migratory waterbirds at three tropical Indian wetlands. Acta Oecologica 2012, 43, 60–71. [Google Scholar] [CrossRef]
- Saino, N.; Szep, T.; Romano, M.; Rubolini, D.; Spina, F.; Moller, A.P. Ecological conditions during winter predict arrival date at the breeding quarters in a trans-saharan migratory bird. Ecol. Lett. 2004, 7, 21–25. [Google Scholar] [CrossRef]
- Meng, Z.; Xia, S.; Yu, X.; Rao, D.; JIN, B. A study on the suitable time window of feeding vegetation fit for overwintering geese in Poyang Lake. Acta Ecol. Sin. 2018, 38, 7539–7548. [Google Scholar] [CrossRef]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Wilmshurst, J.F.; Fryxell, J.M.; Hudsonb, R.J. Forage quality and patch choice by Wapiti (Cervus elaphus). Behav. Ecol. 1995, 6, 209–217. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Xu, W.; Ye, X. Effects of waterbird herbivory on dominant perennial herb Carex thunbergii in Shengjin Lake. Diversity 2022, 14, 331. [Google Scholar] [CrossRef]
- Guan, L.; Wen, L.; Feng, D.; Zhang, H.; Lei, G. Delayed flood recession in central Yangtze floodplains can cause significant food shortages for wintering geese: Results of inundation experiment. Environ. Manag. 2014, 54, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Y.; Wang, Z.; Yang, L.; Zhang, D.; Zhou, L. The relationship between seasonal water level fluctuation and habitat availability for wintering waterbirds at Shengjin Lake, China. Bird Conserv. Int. 2019, 29, 100–114. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, L.; Mahtab, N.; Fan, S.; Song, Y. The influence of food density, flock size, and disturbance on the functional response of Bewick’s Swans (Cygnus columbianus bewickii) in wintering habitats. Animals 2019, 9, 946. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, L.Z.; Xu, W. Diversity of wintering waterbirds enhanced by restoring aquatic vegetation at Shengjin Lake, China. Sci. Total Environ. 2020, 737, 140190. [Google Scholar] [CrossRef]
- Zheng, M.; Zhou, L.; Zhao, N.; Xu, W. Effects of variation in food resources on foraging habitat use by wintering hooded cranes (Grus monacha). Avian Res. 2015, 6, 11. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Lu, J.-J. Feeding ecology of two wintering geese species at Poyang Lake, China. J. Freshw. Ecol. 1999, 14, 439–445. [Google Scholar] [CrossRef]
- Zhao, M.; Cong, P.; Barter, M.; Fox, A.D.; Cao, L. The changing abundance and distribution of greater white-fronted geese Anser albifrons in the Yangtze River Floodplain: Impacts of recent hydrological changes. Bird Conserv. Int. 2012, 22, 135–143. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Q.; Solovyeva, D.; Lameris, T.; Batbayar, N.; Bysykatova-Harmey, I.; Lee, H.; Emelyanov, V.; Rozenfeld, S.B.; Park, J.; et al. Population trends and migration routes of the east Asian bean goose Anser fabalis middendorffii and A. f. serrirostris. Wildfowl 2020, 6, 124–156. [Google Scholar]
- Lehner, P.N. Sampling methods in behavior research. Poult. Sci. 1992, 71, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, Y.; Guan, L.; Lu, C.; Lei, G.; Wen, L.; Liu, G. Optimising hydrological conditions to sustain wintering waterbird populations in Poyang Lake National Natural Reserve: Implications for dam operations. Freshw. Biol. 2013, 58, 2366–2379. [Google Scholar] [CrossRef]
- Bosholn, M.; Anciães, M. Focal animal sampling. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–3. ISBN 978-3-319-47829-6. [Google Scholar]
- Li, C.; Zhou, L.; Xu, L.; Zhao, N.; Beauchamp, G. Vigilance and activity time-budget adjustments of wintering hooded cranes, Grus monacha, in human-dominated foraging habitats. PLoS ONE 2015, 10, e0118928. [Google Scholar] [CrossRef]
- Wan, W.; Zhou, L.; Song, Y. Shifts in foraging behavior of wintering hooded cranes (Grus monacha) in three different habitats at Shengjin Lake, China. Avian Res. 2016, 7, 13. [Google Scholar] [CrossRef]
- Liang, J.; Meng, Q.; Li, X.; Yuan, Y.; Peng, Y.; Li, X.; Li, S.; Zhu, Z.; Yan, M. The influence of hydrological variables, climatic variables and food availability on Anatidae in interconnected river-lake systems, the middle and lower reaches of the Yangtze River Floodplain. Sci. Total Environ. 2021, 768, 144534. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, Y.; Shao, R.; Yan, C.; Jin, J.; Shan, J.; Li, F.; Ji, W.; Bin, L.; Zhang, X.; et al. Modified hydrological regime from the three gorges dam increases the risk of food shortages for wintering waterbirds in Poyang Lake. Glob. Ecol. Conserv. 2020, 24, e01286. [Google Scholar] [CrossRef]
- Yang, M.; Xia, S.; Liu, G.; Wang, M.; Ding, Z.; Yu, P.; Tang, X. Effect of hydrological variation on vegetation dynamics for wintering waterfowl in China’s Poyang Lake wetland. Glob. Ecol. Conserv. 2020, 22, e01020. [Google Scholar] [CrossRef]
- Durant, D.; Fritz, H.; Duncan, P. Feeding patch selection by herbivorous Anatidae: The influence of body size, and of plant quantity and quality. J. Avian Biol. 2004, 35, 144–152. [Google Scholar] [CrossRef]
- Alerstam, T.; Hedenström, A.; Åkesson, S. Long-distance migration: Evolution and determinants. Oikos 2003, 103, 247–260. [Google Scholar] [CrossRef]
- Bayly, N.J.; Rosenberg, K.V.; Easton, W.E.; Gomez, C.; Carlisle, J.; Ewert, D.N.; Drake, A.; Goodrich, L. Major stopover regions and migratory bottlenecks for Nearctic-Neotropical land birds within the Neotropics: A review. Bird Conserv. Int. 2018, 28, 1–26. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Fink, D. Migration distance, ecological barriers and en-route variation in the migratory behavior of terrestrial bird populations: Migratory behavior of terrestrial bird populations. Glob. Ecol. Biogeogr. 2017, 26, 216–227. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).