Meet Me Halfway: Will Photoperiodic Responses of Interpopulation Hybrids of the Brown Marmorated Stink Bug Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) Promote or Constrain Subsequent Invasions?
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Experimental Design
2.2.1. Parental Generation
2.2.2. Progeny Generation
2.3. Statistical Analysis
3. Results
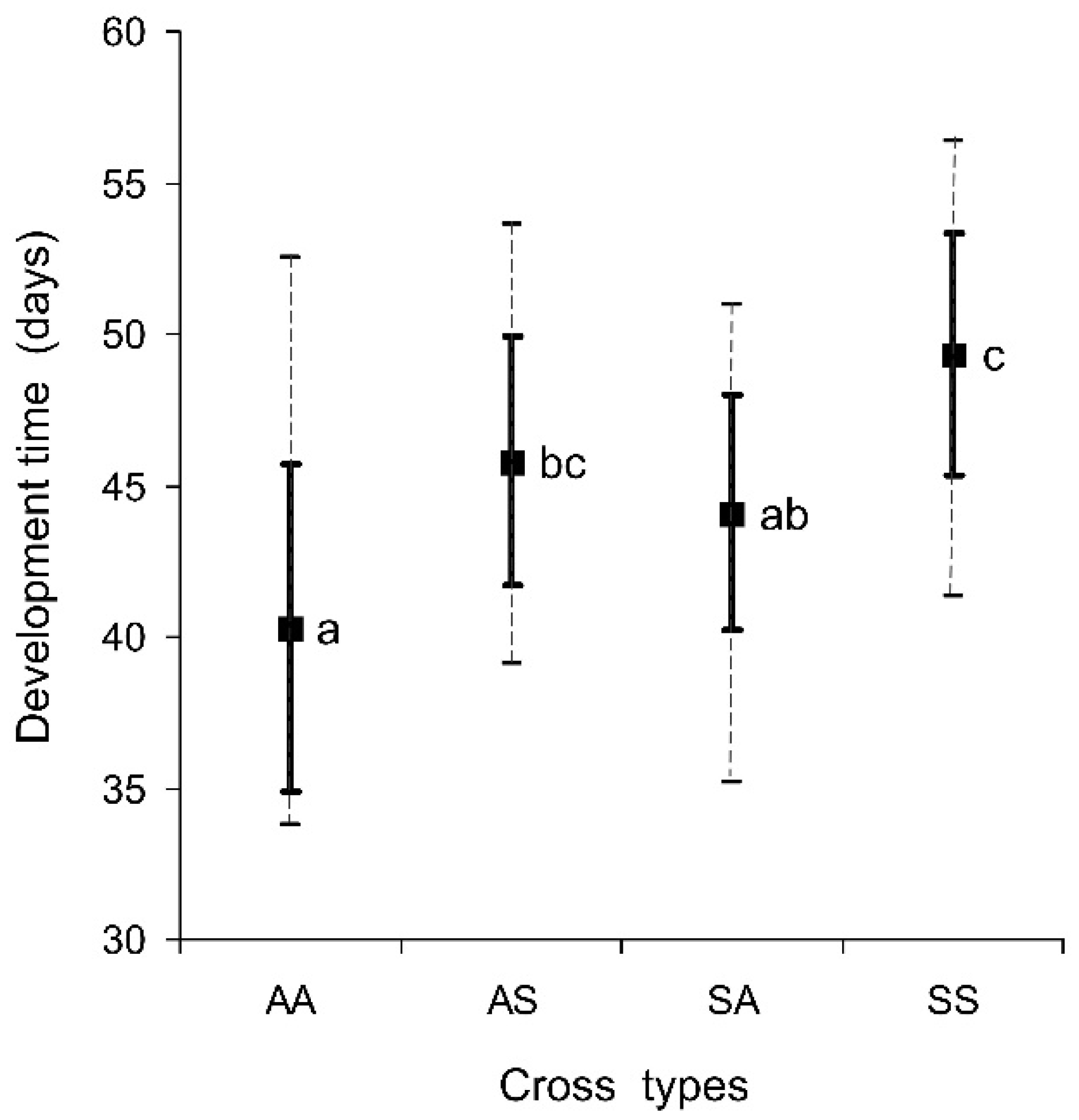
3.1. Pre-Adult Development Time
3.2. Diapause
3.3. Adult Maturation
4. Discussion
5. Conclusions
- (1)
- The pre-adult development time and the incidence of winter adult diapause in the progeny of the interpopulation crosses between the native Korean and the invasive Caucasian populations of H. halys are close to the average of the values recorded in their parents;
- (2)
- Male and female genotypes are equally important in the determination of the pre-adult development time and the incidence of winter adult diapause;
- (3)
- The modes of inheritance of these characters are most probably determined by polygenic control;
- (4)
- The observed modes of inheritance would only partially promote subsequent multiple invasion events from the native South Asian range of H. halys.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brookes, D.R.; Hereward, J.P.; Wilson, L.J.; Walter, G.H. Multiple invasions of a generalist herbivore—Secondary contact between two divergent lineages of Nezara viridula Linnaeus in Australia. Evol. Appl. 2020, 13, 2113–2129. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Guidetti, R.; Cesari, M.; Gariepy, T.D.; Park, Y.L.; Park, C.G. Genetic diversity of Halyomorpha halys (Hemiptera, Pentatomidae) in Korea and comparison with COI sequence datasets from East Asia, Europe, and North America. Fla. Entomol. 2018, 101, 49–54. [Google Scholar] [CrossRef]
- Wongnikong, W.; Hereward, J.P.; van Brunschot, S.L.; Walter, G.H. Multiple invasions of Bemisia argentifolii into Australia and its current genetic connectivity across space. J. Pest Sci. 2021, 94, 1331–1343. [Google Scholar] [CrossRef]
- Britton, J.R.; Gozlan, R.E. How many founders for a biological invasion? Predicting introduction outcomes from propagule pressure. Ecology 2013, 94, 2558–2566. [Google Scholar] [CrossRef]
- Guillemaud, T.; Ciosi, M.; Lombaert, E.; Estoup, A. Biological invasions in agricultural settings: Insights from evolutionary biology and population genetics. Comptes Rendus Biol. 2011, 334, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Lawson Handley, L.J.; Estoup, A.; Evans, D.M.; Thomas, C.E.; Lombaert, E.; Facon, B.; Aebi, A.; Roy, H.E. Ecological genetics of invasive alien species. BioControl 2011, 56, 409–428. Available online: https://core.ac.uk/download/pdf/43658546.pdf (accessed on 31 August 2022). [CrossRef]
- Lockwood, J.L.; Cassey, P.; Blackburn, T. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 2005, 20, 223–228. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Cassey, P.; Blackburn, T.M. The more you introduce the more you get: The role of colonization pressure and propagule pressure in invasion ecology. Divers. Distrib. 2009, 15, 904–910. [Google Scholar] [CrossRef]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Syndallas, B.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. Available online: https://www.jstor.org/stable/2678643 (accessed on 31 August 2022). [CrossRef]
- Simberloff, D. The role of propagule pressure in biological invasions. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 81–102. [Google Scholar] [CrossRef]
- Danilevskii, A.S. Photoperiodism and Seasonal Development of Insects; Oliver & Boyd: Edinburgh, UK, 1965. [Google Scholar]
- Tauber, M.J.; Tauber, C.A.; Masaki, S. Seasonal Adaptations of Insects; Oxford University Press: New York, NY, USA, 1986. [Google Scholar]
- Danks, H.V. Insect Dormancy: An Ecological Perspective; The Biological Survey of Canada: Ottawa, ON, Canada, 1987. [Google Scholar]
- Danks, H.V. The elements of seasonal adaptations in insects. Can. Entomol. 2007, 139, 1–44. [Google Scholar] [CrossRef]
- Musolin, D.L.; Saulich, A.K. Diapause in Pentatomoidea. In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 497–564. [Google Scholar] [CrossRef]
- Numata, H. Environmental factors that determine the seasonal onset and termination of reproduction in seed-sucking bugs (Heteroptera) in Japan. Appl. Entomol. Zool. 2004, 39, 565–573. [Google Scholar] [CrossRef]
- Tougeron, K. Diapause research in insects: Historical review and recent work perspectives. Entomol. Exp. Appl. 2019, 167, 27–36. [Google Scholar] [CrossRef]
- Denlinger, D.L. Insect Diapause; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Saunders, D.S.; Steel, C.G.H.; Vafopoulou, X.; Lewis, R.D. Insect Clocks; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Goto, S.G.; Numata, H. Insect photoperiodism. In Insect Molecular Biology and Ecology; Hoffmann, K.H., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 217–244. [Google Scholar]
- Ragland, G.J.; Armbruster, P.A.; Meuti, M.E. Evolutionary and functional genetics of insect diapause: A call for greater integration. Curr. Opin. Insect Sci. 2019, 36, 74–81. [Google Scholar] [CrossRef]
- Musolin, D.L.; Numata, H. Timing of diapause induction and its life-history consequences in Nezara viridula: Is it costly to expand the distribution range? Ecol. Entomol. 2003, 28, 694–703. [Google Scholar] [CrossRef]
- Urbanski, J.; Mogi, M.; O’Donnell, D.; DeCotiis, M.; Toma, T.; Armbruster, P. Rapid adaptive evolution of photoperiodic response during invasion and range expansion across a climatic gradient. Am. Nat. 2012, 179, 490–500. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Dolgovskaya, M.Y.; Ovchinnikov, A.N.; Belyakova, N.A. Weak photoperiodic response facilitates the biological invasion of the harlequin ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). J. Appl. Entomol. 2015, 139, 241–249. [Google Scholar] [CrossRef]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, A1–A13. [Google Scholar] [CrossRef]
- Haye, T.; Gariepy, T.; Hoelmer, K.; Rossi, J.P.; Streito, J.C.; Tassus, X.; Desneux, N. Range expansion of the invasive brown marmorated stinkbug, Halyomorpha halys: An increasing threat to field, fruit and vegetable crops worldwide. J. Pest Sci. 2015, 88, 665–673. [Google Scholar] [CrossRef]
- Lee, D.H. Current status of research progress on the biology and management of Halyomorpha halys (Hemiptera: Pentatomidae) as an invasive species. Appl. Entomol. Zool. 2015, 50, 277–290. [Google Scholar] [CrossRef]
- Leskey, T.C.; Nielsen, A.L. Impact of the invasive brown marmorated stink bug in North America and Europe: History, biology, ecology, and management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef]
- Hamilton, G.C.; Ahn, J.J.; Bu, W.; Leskey, T.C.; Nielsen, A.L.; Park, Y.L.; Rabitsch, W.; Hoelmer, K.A. Halyomorpha halys (Stål). In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 243–292. [Google Scholar] [CrossRef]
- Musolin, D.L.; Kirichenko, N.I.; Karpun, N.N.; Aksenenko, E.V.; Golub, V.B.; Kerchev, I.A.; Mandelshtam, M.Y.; Vasaitis, R.; Volkovitsh, M.G.; Zhuravleva, E.N.; et al. Invasive insect pests of forests and urban trees in Russia: Origin, pathways, damage, and management. Forests 2022, 13, 521. [Google Scholar] [CrossRef]
- Gapon, D.A. First records of the brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera, Pentatomidae) in Russia, Abkhazia, and Georgia. Entomol. Rev. 2016, 96, 1086–1088. [Google Scholar] [CrossRef]
- Musolin, D.L.; Konjević, A.; Karpun, N.N.; Protsenko, V.Y.; Ayba, L.Y.; Saulich, A.K. Invasive brown marmorated stink bug Halyomorpha halys (Stål) (Heteroptera: Pentatomidae) in Russia, Abkhazia, and Serbia: History of invasion, range expansion, early stages of establishment, and first records of damage to local crops. Arthropod-Plant Interact. 2018, 12, 517–529. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Karpun, N.N.; Zakharchenko, V.Y.; Shoshina, Y.I.; Dolgovskaya, M.Y.; Saulich, A.K.; Musolin, D.L. To every thing there is a season: Phenology and photoperiodic control of seasonal development in the invasive Caucasian population of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae). Insects 2022, 13, 580. [Google Scholar] [CrossRef]
- Musolin, D.L.; Dolgovskaya, M.Y.; Protsenko, V.Y.; Karpun, N.N.; Reznik, S.Y.; Saulich, A.K. Photoperiodic and temperature control of nymphal growth and adult diapause induction in the invasive Caucasian population of the brown marmorated stink bug, Halyomorpha halys. J. Pest Sci. 2019, 92, 621–631. [Google Scholar] [CrossRef]
- Musolin, D.L.; Dolgovskaya, M.Y.; Zakharchenko, V.Y.; Karpun, N.N.; Haye, T.; Saulich, A.K.; Reznik, S.Y. Flying over Eurasia: Geographic variation of photoperiodic control of nymphal development and adult diapause induction in native and invasive populations of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae). Insects 2022, 13, 522. [Google Scholar] [CrossRef]
- Valentin, R.E.; Nielsen, A.L.; Wiman, N.G.; Lee, D.-H.; Fonseca, D.M. Global invasion network of the brown marmorated stink bug, Halyomorpha halys. Sci. Rep. 2017, 7, 9866. [Google Scholar] [CrossRef]
- Gariepy, T.D.; Musolin, D.L.; Konjević, A.; Karpun, N.N.; Zakharchenko, V.Y.; Zhuravleva, E.N.; Tavella, L.; Bruin, A.; Haye, T. Diversity and distribution of cytochrome oxidase I (COI) haplotypes of the brown marmorated stink bug, Halyomorpha halys Stål (Hemiptera, Pentatomidae), along the eastern front of its invasive range in Eurasia. NeoBiota 2021, 68, 53–77. [Google Scholar] [CrossRef]
- Watanabe, M. Ecology and extermination of Halyomorpha halys. 4. The relationship between day length and ovarian development. Ann. Rep. Toyama Inst. Health 1979, 3, 33–37. [Google Scholar]
- Nielsen, A.L.; Hamilton, G.C.; Matadha, D. Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera: Pentatomidae). Environ. Entomol. 2008, 37, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Numata, H. Seasonal life cycle of Aelia fieberi (Hemiptera: Pentatomidae) in relation to the phenology of its host plants. Ann. Entomol. Soc. Am. 1997, 90, 625–630. [Google Scholar] [CrossRef]
- Musolin, D.L.; Numata, H. Photoperiodic and temperature control of diapause induction and colour change in the southern green stink bug Nezara viridula. Physiol. Entomol. 2003, 28, 65–74. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Fleischer, S.; Hamilton, G.C.; Hancock, T.; Krawczyk, G.; Lee, J.C.; Ogburn, E.; Pote, J.M.; Raudenbush, A.; Rucker, A.; et al. Phenology of brown marmorated stink bug described using female reproductive development. Ecol. Evol. 2017, 7, 6680–6690. [Google Scholar] [CrossRef]
- Nakamura, K.; Numata, H. Effect of photoperiod and temperature on the induction of adult diapause in Dolycoris baccarum (L.) (Heteroptera: Pentatomidae) from Osaka and Hokkaido, Japan. Appl. Entomol. Zool. 2006, 41, 105–109. [Google Scholar] [CrossRef][Green Version]
- Doležel, D.; Vaněčková, H.; Šauman, I.; Hodkova, M. Is period gene causally involved in the photoperiodic regulation of reproductive diapause in the linden bug, Pyrrhocoris apterus? J. Insect Physiol. 2005, 51, 655–659. [Google Scholar] [CrossRef]
- Sakakibara, M.; Kawakami, K. Larval diapause inheritance mode in two ecotypes of the yellow-spotted longicorn beetle, Psacothea hilaris (Pascoe) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1992, 27, 47–56. [Google Scholar] [CrossRef]
- Kuang, X.J.; Xu, J.; Xia, Q.W.; He, H.M.; Xue, F.S. Inheritance of the photoperiodic response controlling imaginal summer diapause in the cabbage beetle, Colaphellus bowringi. J. Insect Physiol. 2011, 57, 614–619. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, L.; He, H.M.; Xu, J.; Xue, F.S. A genetic analysis of diapause in crosses of a southern and a northern strain of the cabbage beetle Colaphellus bowringi (Coleoptera: Chrysomelidae). Bull. Entomol. Res. 2014, 104, 586–591. [Google Scholar] [CrossRef]
- French, B.W.; Coates, B.S.; Sappington, T.W. Inheritance of an extended diapause trait in the Northern corn rootworm, Diabrotica barberi (Coleoptera: Chrysomelidae). J. Appl. Entomol. 2014, 138, 213–221. [Google Scholar] [CrossRef]
- Lehmann, P.; Margus, A.; Lindström, L. Inheritance patterns of photoperiodic diapause induction in Leptinotarsa decemlineata. Physiol. Entomol. 2016, 41, 218–223. [Google Scholar] [CrossRef]
- Tanaka, K.; Murata, K. Genetic basis underlying rapid evolution of an introduced insect Ophraella communa (Coleoptera: Chrysomelidae): Heritability of photoperiodic response. Environ. Entomol. 2016, 46, 167–173. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Ovchinnikova, A.A.; Ovchinnikov, A.N.; Barabanova, L.V.; Belyakova, N.A. Inheritance of diapause regulation in the multicoloured Asian ladybird Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 2017, 114, 415–421. [Google Scholar] [CrossRef][Green Version]
- Ikten, C.; Skoda, S.R.; Hunt, T.E.; Molina-Ochoa, J.; Foster, J.E. Genetic variation and inheritance of diapause induction in two distinct voltine ecotypes of Ostrinia nubilalis (Lepidoptera: Crambidae). Ann. Entomol. Soc. Am. 2011, 104, 567–575. [Google Scholar] [CrossRef]
- Söderlind, L.; Nylin, S. Genetics of diapause in the comma butterfly Polygonia c-album. Physiol. Entomol. 2011, 36, 8–13. [Google Scholar] [CrossRef]
- Xia, Q.W.; Chen, C.; Tu, X.Y.; Yang, H.Z.; Xue, F.S. Inheritance of photoperiodic induction of larval diapause in the Asian corn borer Ostrinia furnacalis. Physiol. Entomol. 2012, 37, 185–191. [Google Scholar] [CrossRef]
- Xiao, L.; He, H.M.; Zhong, P.S.; Fu, S.; Chen, C.; Xue, F.S. Inheritance of photoperiodic control of larval diapause in the Asian corn borer Ostrinia furnacalis (Guenée). Bull. Entomol. Res. 2015, 105, 326–334. [Google Scholar] [CrossRef]
- Huang, L.L.; Chen, C.; Xiao, L.; Xia, Q.W.; Hu, L.T.; Xue, F. Geographical variation and inheritance of the photoperiodic response controlling larval diapause in two distinct voltine ecotypes of the Asian corn borer Ostrinia furnacalis. Physiol. Entomol. 2013, 38, 126–132. [Google Scholar] [CrossRef]
- Chen, C.; Xia, Q.W.; Chen, Y.S.; Xiao, H.J.; Xue, F.S. Inheritance of photoperiodic control of pupal diapause in the cotton bollworm, Helicoverpa armigera (Hübner). J. Insect Physiol. 2012, 58, 1582–1588. [Google Scholar] [CrossRef]
- Fu, S.; Chen, C.; Xiao, L.; He, H.; Xue, F. Inheritance of diapause in crosses between the northernmost and the southernmost strains of the Asian corn borer Ostrinia furnacalis. PLoS ONE 2015, 10, e0118186. [Google Scholar] [CrossRef] [PubMed]
- Pruisscher, P.; Larsdotter-Mellström, H.; Stefanescu, C.; Nylin, S.; Wheat, C.W.; Gotthard, K. Sex-linked inheritance of diapause induction in the butterfly Pieris napi. Physiol. Entomol. 2017, 42, 257–265. [Google Scholar] [CrossRef]
- Paolucci, S.; Salis, L.; Vermeulen, C.J.; Beukeboom, L.W.; Zande, L. QTL analysis of the photoperiodic response and clinal distribution of period alleles in Nasonia vitripennis. Mol. Ecol. 2016, 25, 4805–4817. [Google Scholar] [CrossRef] [PubMed]
- Lumme, J.; Lakovaara, S.; Oikarinen, A.; Lokki, J. Genetics of the photoperiodic diapause in Drosophila littoralis. Hereditas 1975, 79, 143–148. [Google Scholar] [CrossRef]
- Henrich, V.C.; Denlinger, D.L. Genetic differences in pupal diapause incidence between two selected strains of the flesh fly. J. Hered. 1983, 74, 371–374. [Google Scholar] [CrossRef]
- Kim, Y.; Krafsur, E.S.; Bailey, T.B.; Zhao, S. Mode of inheritance of face fly diapause and its correlation with other developmental traits. Ecol. Entomol. 1995, 20, 359–366. [Google Scholar] [CrossRef]
- McWatters, H.G.; Saunders, D.S. Inheritance of the photoperiodic response controlling larval diapause in the blow fly, Calliphora vicina. J. Insect Physiol. 1997, 43, 709–717. [Google Scholar] [CrossRef]
- Han, B.; Denlinger, D.L. Mendelian inheritance of pupal diapause in the flesh fly, Sarcophaga bullata. J. Hered. 2009, 100, 251–255. [Google Scholar] [CrossRef][Green Version]
- Goto, S.G. Genetic analysis of diapause capability and association between larval and pupal photoperiodic responses in the flesh fly Sarcophaga similis. Physiol. Entomol. 2009, 34, 46–51. [Google Scholar] [CrossRef]
- Meuti, M.E.; Short, C.A.; Denlinger, D.L. Mom matters: Diapause characteristics of Culex pipiens–Culex quinquefasciatus (Diptera: Culicidae) hybrid mosquitoes. J. Med. Entomol. 2015, 52, 131–137. [Google Scholar] [CrossRef]
- Togashi, K.; Toki, W. Effects of inter-subspecies hybridization of non-native Monochamus alternatus alternatus and native M. a. endai (Coleoptera: Cerambycidae) on the induction of larval diapause and adult body size. Appl. Entomol. Zool. 2018, 53, 29–40. [Google Scholar] [CrossRef]
- Belt, A.L.; Burnet, B. Experimental modification of the dominance relations of a melanotic tumour gene in Drosophila melanogaster. Genet. Res. 1972, 20, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, M.K.; Hasan, F.; Tanwar, A.K.; Jaba, J.; Singh, N.; Sharma, H.C. Genetic regulation of diapause and associated traits in Chilo partellus (Swinhoe). Sci. Rep. 2020, 10, 1793. Available online: https://www.nature.com/articles/s41598-020-58640-0 (accessed on 31 August 2022). [CrossRef] [PubMed]
- Erickson, P.A.; Weller, C.A.; Song, D.Y.; Bangerter, A.S.; Schmidt, P.; Bergland, A.O. Unique genetic signatures of local adaptation over space and time for diapause, an ecologically relevant complex trait, in Drosophila melanogaster. PLoS Genet. 2020, 16, e1009110. [Google Scholar] [CrossRef]
- Lai, X.T.; Yang, D.; Wu, S.H.; Zhu, X.F.; Xue, F.S. Diapause incidence of progeny in relation to parental geographic origin, host plant and rearing density in the cabbage beetle, Colaphellus bowringi. Entomol. Exp. Appl. 2008, 129, 117–123. [Google Scholar] [CrossRef]
- Tauber, C.A.; Tauber, M.J.; Nechols, J.R. Two genes control seasonal isolation in sibling species. Science 1977, 197, 592–593. [Google Scholar] [CrossRef]
- Shroyer, D.A.; Craig, G.B. Egg diapause in Aedes triseriatus (Diptera: Culicidae): Geographic variation in photoperiodic response and factors influencing diapause termination. J. Med. Entomol. 1983, 20, 601–607. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reznik, S.Y.; Karpun, N.N.; Dolgovskaya, M.Y.; Saulich, A.K.; Musolin, D.L. Meet Me Halfway: Will Photoperiodic Responses of Interpopulation Hybrids of the Brown Marmorated Stink Bug Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) Promote or Constrain Subsequent Invasions? Diversity 2022, 14, 878. https://doi.org/10.3390/d14100878
Reznik SY, Karpun NN, Dolgovskaya MY, Saulich AK, Musolin DL. Meet Me Halfway: Will Photoperiodic Responses of Interpopulation Hybrids of the Brown Marmorated Stink Bug Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) Promote or Constrain Subsequent Invasions? Diversity. 2022; 14(10):878. https://doi.org/10.3390/d14100878
Chicago/Turabian StyleReznik, Sergey Ya., Natalia N. Karpun, Margarita Yu. Dolgovskaya, Aida Kh. Saulich, and Dmitry L. Musolin. 2022. "Meet Me Halfway: Will Photoperiodic Responses of Interpopulation Hybrids of the Brown Marmorated Stink Bug Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) Promote or Constrain Subsequent Invasions?" Diversity 14, no. 10: 878. https://doi.org/10.3390/d14100878
APA StyleReznik, S. Y., Karpun, N. N., Dolgovskaya, M. Y., Saulich, A. K., & Musolin, D. L. (2022). Meet Me Halfway: Will Photoperiodic Responses of Interpopulation Hybrids of the Brown Marmorated Stink Bug Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) Promote or Constrain Subsequent Invasions? Diversity, 14(10), 878. https://doi.org/10.3390/d14100878









