Abstract
The persistence and resilience of marginal shallow coral reefs at their limits of environmental tolerance have declined due to chronic environmental degradation and climate change. However, the consequences for the natural recovery ability of reefs of disturbance remain poorly understood. This study considered the potential for natural recovery through coral recruitment on fringing reefs across different geographic regions under contrasting environmental conditions in Puerto Rico. Reefs in areas with significant water quality degradation and more severe physical impacts of hurricanes were expected to have lower coral recruit density and diversity, and therefore less potential for recovery. Sixteen reefs were assessed across three geographic regions. Degraded reefs sustained a lower percentage of live coral cover and had higher macroalgae and turf algae abundance. Locations affected by high PO4, NH3+ and optical brightness concentrations, high turbidity, and high sea surface temperature anomalies, chlorophyll-a concentration and light attenuation Kd490 evidenced significantly lower coral recruit density and diversity. Hurricane-decimated reefs also exhibited impoverished coral recruit assemblages. Low coral recruitment could have important long-term implications under projected climate change and sea level rise, particularly in coastal urban habitats. There is a need to implement effective environmental conservation, ecological restoration and community participation strategies that facilitate enhanced coral recruitment success and assisted recovery processes.
1. Introduction
Coral reefs provide critical resources and ecological services of significant socio-economic value for multiple island nations on a global scale [,,]. Yet in recent decades, coral reefs have been in continuous decline due to a combination of local human factors and climate change [,,,,]. They constitute habitats for multiple species, support important subsistence fisheries and tourism [], buffer wave energy [], and capture carbon dioxide (CO2) from the atmosphere, thus helping to reduce the impact of greenhouse gas emissions []. However, changes in the frequency and severity of disturbance regimes due to combined anthropogenic stressors and climate change are increasingly challenging the natural capacity of coral reefs to absorb impacts and recover from disturbance [,,]. Given the social and ecological importance of coral reef systems, a better understanding of coral reef recovery dynamics following disturbance is required []. This is particularly concerning in shallow urban coastal coral reef ecosystems, which are frequently impacted by turbidity, sedimentation, and a combination of anthropogenic stressors, such as eutrophication, pollution, and fishing [,,], and that are often characterized by the loss of critical reef builders, changes in biodiversity and productivity, and the evolution of novel assemblages []. Therefore, an improved understanding of factors influencing the success of coral recruitment is necessary. This is vital to make long-term projections of reef health, persistence and resilience under projected climate change and sea level rise impacts through the Anthropocene.
Coral recruitment is the process in which small coral larvae undergo settlement and become members of the adult coral community []. Settlement is the transformation between the planktonic larval stage (planulae) and the sessile benthic stage physically attached to the reef bottom. A recruit is an already settled larva which has developed into a sessile polyp stage and has survived and grown long enough to be detected by the naked eye. Coral recruit abundance is usually lower than that of settlers, often due to high post-settlement mortality, settling patterns on cryptic habitats difficult to sample, sampling bias or methodological limitations. In addition, the timing between settling and recruitment sampling, as well as variations in reef trophic conditions may also determine recruitment sampling ability. Recruitment is an essential marine ecological process determining ecosystem persistence, resilience and reef framework building, providing habitat for a myriad of species []. It should be noted that coral recruitment is determined not only by larval settlement, but also by their growth and survival after settling. The more successful coral recruitment is, the greater will be the potential for future growth and community recovery following any disturbance []. However, increasing environmental stressors, whether anthropogenic or natural, are concerning as they could have long-term adverse effects on reef accretion rates and persistence []. Many anthropogenic stressors are associated with coastal development, which in turn affects water quality []. Sediment loading can block sunlight, smother corals and suffocate reefs [,,,]. It can also impair successful coral settlement and recruitment. High dissolved nutrient concentrations can lead to an increased incidence of coral diseases and fuel macroalgal growth that might outcompete coral for space and prevent coral larvae from settling [,,]. Shallow coastal coral reefs are constantly exposed to nutrient-loaded, sediment-laden, turbid terrestrial runoff, which can degrade coral reefs at local scales []. Such factors may also result in a significant decline in dissolved oxygen concentration, which under localized hypoxic conditions can be detrimental to corals and to associated fauna []. Further, altered nutrient and carbon cycling may also explain the rapidly declining resilience of Caribbean reefs and their poor ability to recover from disturbance []. Most of these impacts affect marginal, shallow reef habitats adjacent to urban environments. The term “marginal” is used here in a broad sense to describe habitats where coral reefs occur in suboptimal locations under highly fluctuating conditions, often close to environmental thresholds for coral survival and growth, which may include high or low temperature, salinity, nutrient and dissolved oxygen concentration, and water transparency sensu [,], and which are threatened by a wide range of local and global scale disturbances []. The benthic community dynamics and natural recovery potential from disturbance on such shallow marginal habitats remain largely unknown, although these habitats play a paramount role in protecting coastal infrastructure and life.
Other significant stressors to shallow coral reef habitats are the impacts of global warming, mostly as a consequence of elevated sea surface temperatures that can result in recurrent mass coral bleaching, disease outbreaks and mortality events [,,,]. Projections of increasing recurrence and severity of coral bleaching are concerning [,] and could severely impair coral reef recovery ability []. There is already evidence that long-term recovery from mass coral bleaching on Caribbean reefs is severely limited due to impacts on major reef-building species [,,,]. Mass coral bleaching and mortality and coral disease outbreaks have had severe impacts across the wider Caribbean [,,,,]. These factors can lead to concomitant adverse impacts on fish assemblages [,,,,,,,], which may indirectly influence fishery productivity and long-term coral reef recovery through effects of reduced herbivory and altered trophic interactions. Concentrations of pCO2 have increased significantly, which leaves shallow reefs more vulnerable to ocean acidification impacts and carbonate dissolution []. Coral calcification rates have shown a 33% decline across the wider Caribbean since the mid-Holocene due to ocean acidification []. Further, the combined impact of surface warming and ocean acidification can lower the threshold at which declining herbivory (due to fishing impacts) can affect coral reef resilience []. A further disturbing concern regarding natural reef recovery ability has to do with the long-term effects of declining governance effectiveness and socio-political factors. It has been shown that lower social-ecological vulnerability to coral bleaching is more common in colonial states, in comparison to sovereign states [], probably resulting from colonial legacies and neo-colonial politics. Therefore, a complex combination of factors involving local anthropogenic stressors, pressing climate change-related impacts, declining governance, and complicated neo-colonial politics may play synergic roles affecting the ability of coral reefs to recover naturally.
After disturbance events, coral reefs go through transitional phases of ecological succession that can help rebuild a system that resembles the one prior to disturbance [], mostly through coral recruitment [,]. However, the increased recurrence of disturbances has resulted in an accelerated deterioration of coral reefs and in longer recovery time scales, compromising their natural capacity to recover through coral larval recruitment, mainly due to macroalgal overgrowth and sediment deposition [,]. This complex combination of stressors often limits the natural regenerative capacity of corals and impairs settlement and recruitment, with dire long-term consequences for ecosystem persistence and resilience (natural reef ability to recover from disturbance). This may often lead to major living coral losses favoring non-reef builders and macroalgae [,,]. In general, live coral cover has decreased throughout the Caribbean []. Therefore, interest in investigating the potential for natural reef recovery through recruitment processes [], as well as through tissue regeneration, addressing coral species- and genetic-specific susceptibility to disease, restoring fish assemblages and herbivory, recovering trophic conditions, and other important processes, has increased. In spite of this, there is limited information regarding coral recruitment dynamics in Puerto Rico. A cross-shelf study from SW Puerto Rico showed higher abundance of Siderastrea siderea and Porites astreoides recruits on deeper reef zones []. Another study showed that coral recruit communities were dominated by weedy, high recruitment species, such as S. radians and P. astreoides, eight years after mass coral bleaching and mortality in the oceanic island of Mona, off western Puerto Rico, on spur and groove habitats formerly dominated by Orbicella spp. and other large reef builders, suggesting a major shift in coral assemblages [].
In Puerto Rico there is not enough information on the spatial distribution patterns of coral recruitment; therefore, the natural recovery capacity of shallow, nearshore coral reefs remains unknown. Further, there are limited accounts regarding the role of factors such as water quality decline and hurricane mechanical impacts on coral recruitment dynamics (i.e., resulting from effects on adult corals, recruit densities, substrate integrity, stability, or complexity). Hurricanes can severely affect coral demographic dynamics []. Given the projected climate change and sea level rise impacts [], it is vital to understand the natural recovery capacity of shallow coral reefs (i.e., <5 m depth) adjacent to the shoreline. In this way, future coral reef ability to absorb wave energy and protect coastlines against erosion can be modeled []. This study addressed the following question: How do environmental conditions and geographic location impact natural coral reef recovery potential following disturbances? The hypothesis that coral reefs in areas with the greatest water quality degradation and the greatest hurricane physical damage to bottom structural integrity, stability and complexity show a lower coral recruit density and diversity, and, therefore, have lower potential for natural recovery following disturbance, was tested using a combination of multivariate approaches. A rapid characterization of the ecological condition of sixteen coral reefs in different locations under variable water quality conditions on the island of Puerto Rico was conducted. Coral community composition showed significant correlation with water quality condition and with benthic community structure. Coral reefs under chronic environmental degradation, but also those severely impacted by recent strong hurricanes, which caused significant pulverization of the reef framework, had the lowest potential for recovery. The results of this study help to provide basic information on the spatial distribution of coral recruits and on benthic community structure across a wide range of locations and across a water quality stress gradient, thus providing fundamental conservation-oriented information for managers with wider application for other Caribbean coral reefs.
2. Materials and Methods
2.1. Study Sites
The study was carried out between January and March 2020 across sixteen shallow coral reefs (2–4 m) of three geographic locations of Puerto Rico: the west coast, the east coast and the island of Culebra (Figure 1). Three replicate coral reefs were studied on each of the west and east coasts, while ten were assessed on Culebra Island, located 27 km northeast of Puerto Rico. The study reefs on the west coast were La Cacula (CAC) and Bridges (BRI) located in the town of Aguadilla, and the Tres Palmas Marine Reserve (TPA) in Rincón. In the eastern area of the island, study reefs were located in Palmas del Mar (PDM), Humacao, and in Punta Figueras (PFI) and Cayo Cabeza de Perro (CDP) in Ceiba. Finally, reefs evaluated in Culebra included: Playa Carlos Rosario (PCR), Bahía Tamarindo (BTA), Bahía Tarja (TAR), Punta Melones (PME), Bahía Sardinas (BSA), Punta Tampico (TAM), Playa Dátiles (DAT), Playa Cascajo (PCA), Arrecife Dákity (DAK), and Punta Vaca (PVA) (Figure 1).
Figure 1.
Spatial distribution of the sampling locations. Refer to the list of locations in the Methodology for acronyms.
Sampling sites were selected for having presumably different water quality conditions. Previous studies have established that fecal pollution, high nutrient loads and suspended sediments are the main factors affecting coastal water quality in Puerto Rico [,,].
2.2. Sampling Design
Benthic community assessments were conducted once on each location using six replicate transects parallel to the shoreline, with approximately 4–8 m separation from each other at depths ranging from 2 to 4 m. Positioning of the first transect was randomly selected on each location, with other transects following haphazard positioning. Each transect was 10 m long and used a 50 × 50 cm quadrant. Five high definition images were taken at 1, 3, 5, 7 and 9 m intervals along each transect []. Images were used to identify and count all coral recruit colonies (coral colonies > 2.5 cm diameter), though some smaller colonies were identified in the field during sampling. Point counts were also used to characterize benthic community structure by analyzing 20 points per transect (every half meter). Points along each transect were used to determine each benthic component percent cover, including: corals, sponges, zoanthids, macroalgae, filamentous algae, the brown macroalgae Dictyota spp., red encrusting macroalgae Peyssonnellia spp., and Ramicrusta textilis, Halimeda spp., erect calcareous algae (ECA), crustose coralline algae (CCA), cyanobacteria, open reef pavement, rubble, and sand.
A one-time water quality sampling effort was conducted to produce a snapshot view of water quality conditions across coral reef study sites. Triplicate grab water samples were collected using 1 L plastic containers 30 cm below the surface by locality. Turbidity was measured in triplicates in nephelometric turbidity units (NTU) using a portable turbidimeter (LaMotte Co., Chestertown, MD, USA). Chlorophyll-a and optical brightener (OAB) concentrations were determined in triplicates using a portable fluorometer (Turner Designs Co., San Jose, CA, USA). OABs are a fluorescent substance commonly added to detergents in order to produce a whitening effect on laundry and can indicate the presence of gray waters in the environment. Dissolved oxygen, phosphate (PO4), nitrate (NO3−), ammonia (NH3+), and total nitrogen concentration were also evaluated in triplicates from each location using a Smart 2 portable spectrophotometer (Lamotte Co., Chestertown, MD, USA). Ten ml water samples were transferred to clean glass vials in triplicates for dissolved oxygen and nutrient analysis. Sample processing was conducted immediately to avoid the need for preservation.
Remotely sensed data regarding sea surface temperature anomaly, light attenuation coefficient (Kd490), chlorophyll-a concentration, net primary productivity, particulate organic carbon concentration (POC), and particulate inorganic carbon concentration (PIC) were obtained from ERDDAP (https://coastwatch.pfeg.noaa.gov/erddap/index.html (accessed on 1 July 2022)) for the period of 2017 to 2020, using weekly averages (Summarized in Tables S4 and S5 and Figure S1).
2.3. Statistical Analyses
A nested permutational analysis of variance (PERMANOVA) was used to determine if there were significant differences in benthic and in coral recruit community structure among regions and locations (nested in regions) []. Spatial patterns were projected using a multivariate shade plot and principal coordinates ordination (PCO) [] or non-metric multidimensional scaling (nMDS) []. Water quality data were log(x+1)-transformed and normalized prior to analysis through Euclidean distance ordination []. A nested PERMANOVA was also used to test the hypothesis of differences in the multivariate water quality data matrix among the regions and sampling locations (nested in regions) []. Principal components analysis (PCA) was carried out to determine the observed spatial patterns of the multivariate water quality matrix []. A multivariate correlation (RELATE) was used to correlate benthic community structure and water quality parameters, coral recruit density and water quality parameters, and coral recruit density and benthic community structure. The BIOENV multivariate routine was used to determine which variable or combination of environmental variables correlated best with the determined spatial patterns of the benthic community structure and with coral recruit density []. A distance-based linear model analysis (DISTLM), in combination with a distance-based redundant analysis (dbRDA) were used to conduct a combination of marginal and sequential tests to address the impact of individual and combined environmental variables on coral recruit community structure []. Abundance data were log(x+1)-transformed, while percent cover data were √-transformed prior to analysis through Bray–Curtis ordination []. All multivariate analysis were carried out using 9999 permutations in PRIMER v.7.0.13 + PERMANOVA v1.0 (PRIMER-e, Quest Research, Ltd., Auckland, New Zealand). Species acronyms used are as follows: Srad = Siderastrea radians, Ssid = S. siderea, Past = Porites astreoides, Ppor = P. porites, Pfur = P. furcata, Pstr = Pseudodiploria strigosa, Pcli = p. clivosa, Dlab = Diploria labyrinthiformis, Ffra = Favia fragum, Mmea = Meandrina meandrites, Oann = Orbicella annularis, Ofav = Orbicella faveolata, Ofra = O. franksi, Mcav = Montastreaea cavernosa, Isin = Isophyllia sinuosa, Aaga = Agaricia agaricites, Afra = A. fragilis, Ahum = A. humilis, Apal = Acropora palmata, Acer = A. cervicornis, Apro = A. prolifera (hybrid species), Mcom = Millepora complanata, Malc = M. alcicornis, Ecar = Erythropodium caribbaeorum, Eun = Eunice sp., Plexrla = Plexaurella sp., Mur = Muricea sp.
3. Results
3.1. Coral Reef Benthic Community Analysis
A nested PERMANOVA analysis showed no significant differences in the spatial variation in the coral reef benthic community structure among the three different regions (Pseudo-F = 1.12; df = 2, p = 0.3565), but significant differences among sampling locations (Pseudo-F = 50.62, df = 13, p < 0.0001). Variation among locations explained most of the observed differences (√ = 33.4). Pairwise analyses showed significant variation among all possible combinations of study locations (p < 0.0500). Data is summarized in Table S1. The highest mean percent coral cover was observed at CDP with 18.8%, followed by PCR with 18.3%, and TPA with 16.8% (Figure 2a). Dominant adult coral species were Porites astreoides and Siderastrea radians. The highest mean macroalgal cover was documented at DAK, with 73.8%, followed by PFI with 49.2%, and PDM with 35.0% (Figure 2b). Brown macroalgae Dictyota spp. percent cover was highest at TPA with 26.5%, followed by PCR with 16.8%, and PFI with 14.7% (Figure 2c). Mean crustose coralline algae (CCA) was highest at BTA with 62.7%, followed by PME with 46.4%, and CDP with 21.9% (Figure 2d).
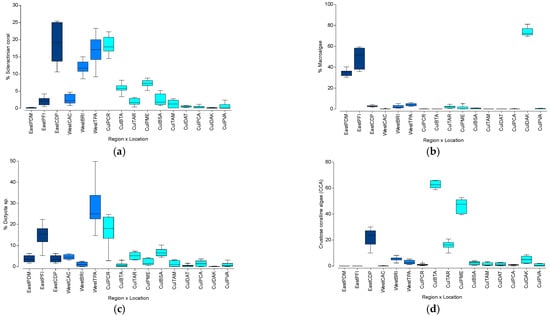
Figure 2.
Spatial variation pattern in the percent cover of: (a) Scleractinian corals; (b) Macroalgae; (c) Dictyota spp., and (d) Crustose coralline algae (CCA). Refer to the Methodology for the description of acronyms.
A PCO analysis based on the spatial variation in coral reef benthic community structure by region and location explained 57% of the observed variation, and showed four distinctive groupings based on community composition (Figure 3). Benthic assemblages at CDP and BRI were mostly dominated by scleractinian corals. Octocorals explained variation at BSA, and brown macroalgae Dictyota spp. at TPA. Sponges did the same at PME, and Palythoa caribbaeorum at BTA. Hydrocorals and CCA dominated the variation observed at TAR, while algal turf was dominant at CAC and PFI. Macroalgae and cyanobacteria explained most of the variation at PDM, while macroalgae and open substrate did at DAK. Peyssonnelia algal crusts (PAC) and encrusting Ramicrusta textilis explained most of the observed variation at TAM and DAT, but also at PCA and PVA. Brown macroalgae Lobophora variegata species complex were dominant at PCR.
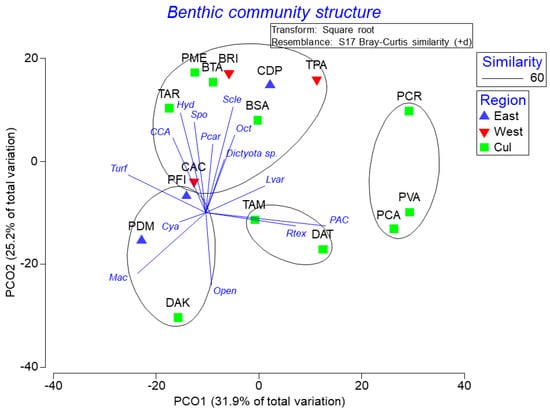
Figure 3.
Spatial variation pattern in coral reef benthic community structure. This solution explained 57.1% of the observed spatial variation. Refer to the Methodology for the description of acronyms.
3.2. Coral Recruit Analysis
A nested PERMANOVA analysis showed no significant differences in the spatial variation in the coral recruit benthic community structure among the three different regions (Pseudo-F = 0.46; df = 2, p = 0.7989), but significant differences among sampling locations (Pseudo-F = 25.06, df = 13, p < 0.0001). Variation among locations explained most of the observed variation (√ = 27.4). Similarly, no significant spatial variation at the regional level was documented for total coral recruit density (Pseudo-F = 0.21; df = 2; p = 0.8445), but highly significant differences were found among sampling locations (Pseudo-F = 40.95; df = 13; p < 0.0001). Variation among locations similarly explained most of the observed differences (√ = 25.1). Data is summarized in Table S2. Coral recruit density was highly variable among regions and locations (Figure 4a). The highest density at the regional scale was observed at Culebra (8.5/m2), followed by the eastern coast (6.2/m2), and the west coast (4.4/m2). The highest densities at the location scale were observed in three locations in Culebra: BTA (42.0/m2), followed by PME (19.4/m2) and TAM (10.7/m2). CDP on the eastern coast had 17.0/m2, while BRI on the western coast had 8.1/m2. Hurricane-impacted reefs in Culebra had a recruit density of 0–4.4/m2. Recruit density on reefs under chronic water quality decline ranged from 0.2 to 1.5/m2 in the eastern coast and averaged 0.7/m2 on the western coast.
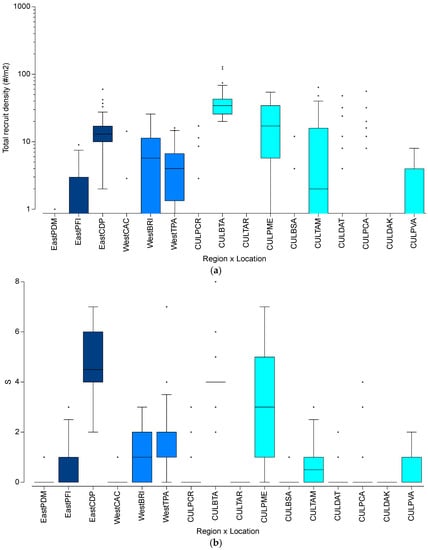
Figure 4.
Spatial distribution pattern of: (a) Coral recruit density (#/m2); and (b) Coral recruit species richness (#/m2) by location. Isolated points show outlier values. Refer to the Methodology for the description of acronyms.
The highest coral recruit species richness at the regional scale was observed at the eastern coast (1.88/m2), followed by the western coast (1.06/m2), and the west coast (0.93/m2). The highest species richness at the location scale were observed at CDP, in the eastern coast (4.73/m2), followed by BTA (4.13/m2) and PME, both in Culebra (2.93/m2) (Figure 4b). TPA on the western coast had 1.77 species/m2. Hurricane-impacted reefs in Culebra had a recruit species richness of 0–0.63/m2. Recruit species richness on reefs under chronic water quality decline ranged from 0.2 to 0.7/m2 in the eastern coast and averaged 0.1/m2 on the western coast.
Overall, a total of 3547 coral recruit colonies were counted. Porites astreoides was the most abundant species (929 counts; 26.2%), followed by S. radians (768 counts, 21.6%), Millepora complanata (498 counts, 14.0%), Agaricia humilis (286 counts, 8.0%), and S. siderea (222 counts, 6.3%) (Table S2). In combination, the five most abundant species accounted for 76.2% of the total recruits documented in this study. Other Porites spp., Pseudodiploria strigosa, Millepora alcicornis, Diploria labyrinthiformis, and Erythropodium caribbaeorum accounted for 5 to 1% of the total recruits. None of the remaining 16 species accounted for 1% of the total recruit abundance. Four coral species showed the highest recruit densities throughout the study locations. The highest mean density of S. radians recruits was documented at BTA with 15.9/m2, followed by PME with 6.5/m2 (Figure 5a). The highest mean density of S. siderea recruits was documented at PME with 3.8/m2, followed by BTA with 1.8/m2 (Figure 5b). Porites astreoides showed a mean recruit density of 10.1/m2 at TAM, followed by CDP with 4.5/m2 (Figure 5c). The highest mean density of A. humilis recruits was documented at CDP with 5.5/m2, followed by BTA with 2.6/m2 (Figure 5d). Spatial patterns in recruit density are also detailed in Figure S2.
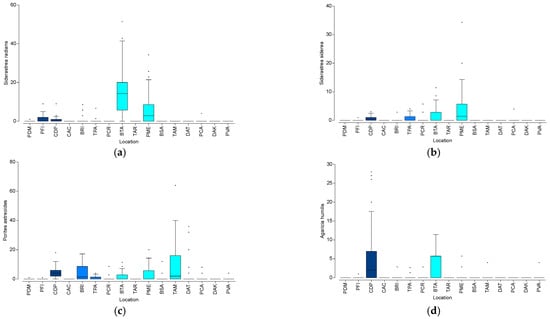
Figure 5.
Variation in the spatial patterns in the average density of coral recruits (#/m2) in the four most abundant species: (a) Siderastrea radians; (b) S. siderea; (c) Porites astreoides; and (d) Agaricia humilis. Isolated points show outlier values. Refer to the Methodology for the description of acronyms.
A PCO analysis yielded five clustering patterns of the spatial organization of coral recruit species richness and described 62% of the observed spatial variation (Figure 6). A first cluster was composed by locations CDP, PME and BTA, which were mostly explained by S. radians, Millepora alcicornis, and Favia fragum recruits. Porites porites, P. astreoides, and A. humilis recruits formed a second cluster composed of TAM and BRI. A third cluster was composed of DAT, PCR, BSA, PCA in Culebra, and by TPA in the western coast, and was dominated by S. siderea, M. complanata, P. furcata, and A. agaricites recruits. A small cluster of PVA and PFI was mostly grouped by A. humilis recruits. A final group formed by CAC, PDM, TAR and DAK showed a very low coral recruit species richness. Spatial patterns in recruit species richness are also detailed in Figure S3.

Figure 6.
PCO analysis based on the √-transformed coral recruit density (#/m2) by region and by location. This solution explains 61.7% of the observed spatial variation. Refer to the Methodology for the description of acronyms.
3.3. Water Quality Analysis
A nested PERMANOVA analysis showed significant differences in the spatial variation in water quality parameters among the three different regions (Pseudo-F = 3.05; df = 2, p = 0.0200) and among sampling locations (Pseudo-F = 8.33, df = 13, p < 0.0001). Variation among locations explained most of the observed variation (√ = 2.45). Pairwise tests showed a significant difference between the western coast and Culebra (t = 2.16; p = 0.0128), and only a marginal difference between the eastern coast and Culebra (t = 1.77; p = 0.0630). No significant difference was observed between the eastern and western coasts (t = 0.95; p = 0.5945). Data is summarized in Table S3. The highest turbidity levels were observed at PFI (3.56 NTU) and PDM (2.52 NTU) along the eastern region (Figure 7a). These also showed the highest concentration of chlorophyll-a with 2.15 μg/L at PDM and 2.46 μg/L at PFI (Figure 7b), and the highest PO4 concentration with 3.5 μM at PDM and PFI (Figure 7c). These localities also showed the lowest dissolved oxygen concentration with 4.0 mg/L at PDM and 2.5 mg/L at PFI (Figure 7d).
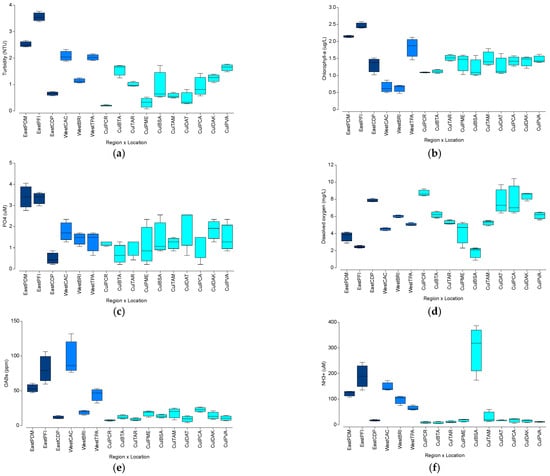
Figure 7.
Spatial patterns in the mean values of six water quality variables by region (East, West, Culebra) and by locality: (a) Turbidity; (b) Chlorophyll-a; (c) Phosphate (PO4); (d) Dissolved oxygen; (e) Optical brighteners (OABs); and (f) Ammonia (NH3+). Refer to the Methodology for the description of acronyms.
Dissolved oxygen concentration showed high spatial variability within the different regions. For instance, it ranged from 4.5 mg/L at CAC to 6.0 mg/L at BRI along the western region, and from 2.0 mg/L at BSA to 6.5 mg/L at PVA along the Culebra Island region. The highest dissolved oxygen concentration was documented at CDP, off the eastern coast, with 9.0 mg/L. Optical brightener (OAB) concentration was also highly variable, with higher values on the Eastern and western regions. CAC showed the highest OAB concentration (98 ppm), followed by PFI in the east (82 ppm) and PDM with 54 ppm (Figure 7e). Ammonia (NH3+) also showed patterns of high spatial variability, with higher mean values within the eastern and western regions. However, BSA at Culebra Island showed the highest mean NH3+ with 293 μM, followed by PDM (130 μM), BRI (100 μM) and TPA (60 μM), along the western region, and by PFI (180 μM) and CAC (150 μM), along the eastern coast (Figure 7f).
PCA analysis showed three clustering patterns (Figure 8). One of the clusters was dominated by PLM, PFI (eastern coast) and TPA (western coast) and was largely explained by the high concentration of Chl-a, by higher turbidity, and to a lesser degree by higher concentrations of OABs and PO4. A second cluster was composed by BSA (Culebra), and CAC and BRI (western coast), and was dominated by total nitrogen, NO2, and NH3+ concentrations. The final cluster was composed of the remaining nine locations in Culebra and CDP (off the eastern coast) and was mostly explained by the higher concentrations of dissolved oxygen. This PCA model explained 74.1% of the observed spatial variation in water quality parameters. The noted differences in water quality among locations could indicate actual long-term differences in water quality and suggest that a potential distinctive spatial gradient of water quality variables may exist, which may influence coral recruit assemblages.
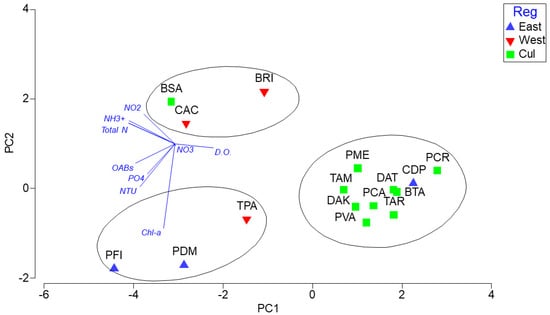
Figure 8.
Principal components analysis (PCA) based on the log(x+1)-transformed and normalized concentration of water quality parameters by region and location. This solution explains 74.1% of the observed spatial variation. Refer to the Methodology for the description of acronyms.
3.4. Relationship between Water Quality and Coral Recruit Community Structure
A multivariate correlation analysis (RELATE) resulted in a highly significant correlation between the coral recruit community matrix and the global water quality matrix obtained during the single sampling effort (Rho = 0.292; p = 0.0273). The BEST-BIOENV multivariate routine showed that the best combination of environmental parameters that explained the spatial variation observed in coral recruit community structure was the combination of low concentration of OABs, PO4 and total N (correlation = 0.448). However, these analyses only indicate potential trends, which require long-term quantification and validation.
Figure S4 shows spatial patterns in remotely sensed water quality parameters across study locations during the period 2017–2020 and evidence significant pulse events in most parameters, particularly along the eastern and western coasts. A DISTILM marginal test of the remotely sensed water quality data matrix based on maximum values of each parameter showed a significant effect (p = 0.0349; proportion = 0.2649) of chlorophyll-a concentration on total coral recruit density (Table S5). This analysis excluded data from TAR and DAK, which had no recruits. The sequential test showed a highly significant combined effect of SST anomaly + Kd490 + chlorophyll-a concentration (p = 0.0083; proportion = 0.4400) on coral recruit assemblages. A dbRDA analysis showed four clustering patterns of the coral recruit community structure based on the water quality impacts (Figure S5). Extremely low coral recruit density at PDM was mostly explained by higher chlorophyll-a concentration. Kd490 was also highest at PDM, PFI, and CAC, which also exhibited low coral recruit densities. The dbRDA model explained 98% of the observed fitted variation and 58% of the total variation in coral recruit density.
3.5. Relationship between Coral Recruit and Benthic Communities
A multivariate correlational analysis (RELATE) showed a highly significant correlation between the coral recruit community matrix and the coral reef benthic community global matrix (Rho = 0.304; p = 0.0029). The BEST-BIOENV multivariate routine showed that the best combination of coral reef benthic community parameters explaining the spatial variation observed in the coral recruit community structure was the combination of high percent cover of adult scleractinian corals and CCA, and the low percent cover of sponges, R. textilis and PAC (correlation = 0.404).
3.6. Relationship between Water Quality and the Benthic Community Structure
A multivariate RELATE showed no significant relationship between the benthic community structure matrix and the water quality matrix obtained during the single sampling event (Rho = −0.063, p = 0.6663). The BEST-BIOENV multivariate routine showed that the best combination of environmental parameters that explained the spatial variation observed in coral reef benthic community structure was the combination of high OABs and PO4 concentrations, however, with a very weak correlation (0.084). The spatial pattern of variation in shallow coral reef benthic community structure is influenced by a highly variable combination of factors beyond water quality variables.
4. Discussion
Coral reef benthic community structure across shallow fringing marginal reefs in Puerto Rico showed non-significant variation at regional scales (10–100 km), but significant spatial variation at the location scale (1–10 km). Higher percent live coral cover was documented at CDP and PCR, areas presumably characterized by better water quality. Higher percent macroalgal cover was documented at DAK, followed by PFI and PDM, locations known to be impacted by runoff pulses. Similarly, coral recruit community structure across shallow fringing marginal reefs in Puerto Rico showed non-significant variation at regional scales, but significant spatial variation at the location scale as a combined function of a presumed water quality stress gradient, hurricane mechanical impacts, and benthic assemblage composition. In general, higher coral recruit density and species richness was observed across locations with apparently better water quality, limited hurricane physical impacts, higher percent live coral cover and species diversity, in comparison to locations showing signs of apparently chronic water quality stress, severe hurricane-driven framework pulverization, instability, highly fragmented bottom, and/or limited percent coral cover and adult coral species diversity. In general, this supported the hypothesis that coral reefs in geographic areas with the greatest water quality degradation or the greatest hurricane physical damage to the shallow reef framework showed lower coral recruit density and diversity, therefore lower potential for natural recovery following disturbance.
Water quality sampling in this project was significantly limited to a single sampling event, which provided limited evidence to reach any robust conclusion with respect to water quality influences over coral recruitment dynamics. However, used in combination with an analysis of the water quality dynamics between 2017 and 2020 obtained from remotely sensed sources (Supplementary Data), it suggested apparently important trends. The observed spatial variation pattern of water quality conditions suggested that coral reef distance from known pollution sources and the strength and direction of surface water circulation (i.e., surface current and wave exposure) were important in affecting coral recruit assemblages. Coral reefs showing apparently higher dissolved nutrient and OAB concentrations and high turbidity evidenced significantly lower coral recruit density and species richness. Such impoverished reefs also sustained low percent live coral cover and high macroalgal and turf cover. In particular, locations such as CAC along the western coast, PDM and PFI along the eastern coast, and BSA at Culebra, are known to be significantly influenced by frequent runoff pulses (Hernández-Delgado, personal observations). In addition, locations such as DAK, TAR, TAM, DAT and PVA at Culebra were decimated by recent hurricane impacts (Hernández-Delgado, unpublished data), and exhibited impoverished coral recruit assemblages. The category five Hurricanes Irma and María inflicted severe damage to the abovementioned coral reefs. Irma’s eyewall passed just 18 km north of Culebra Island on 6 September 2017, with 290 km/h winds and a sea level pressure of 914 mb []. Hurricane María passed just 30 km south of Culebra Island with 280 km/h winds and a sea level pressure of 908 mb, and made landfall directly over PDM with 260 km/h winds, and a sea level pressure of 913 mb []. Wave action, in combination with storm swells, caused extensive coral colony dislodgment and fragmentation, as well as localized reef framework pulverization. There was also extensive filling of reef grooves and pavement channels with hurricane-generated debris and sediment shifts, which buried an undetermined number of coral colonies, octocorals, sponges, and other benthic fauna. Therefore, we argue that the combination of environmental history, hurricane disturbance regimes, as well as recent impacts by mass coral bleaching and disease outbreaks, could have played important roles in shaping coral recruit assemblages.
4.1. The Role of Poor Water Quality
Coastal eutrophication is one of the most critical factors affecting shallow coastal coral reefs [,,,,,,], and, therefore, their natural recovery ability through coral recruitment, due to a combination of mechanisms resulting in coral larval settlement interference and in post-settlement mortality. Water quality sampling in this study provided a limited snapshot view of the environmental conditions across each location that might affect recruitment. However, it provided an important insight into the apparently prevailing conditions across multiple coral reefs that were confirmed using remote sensing sources. The western region showed low dissolved oxygen concentration, high OAB concentration and high turbidity (i.e., TPA) associated with sediment-laden, nutrient-loaded urban runoff and sewage from Rincón [] and influenced by the Rio Grande de Añasco outlet []. In Culebra, the lowest dissolved oxygen concentration was found at BSA, which is located adjacent to known sources of urban pollution [,]. The highest concentration of OABs, although with high spatial variability, was found at CAC in the western coast. CAC is a shallow reef located in front of the La Cacula urban creek outlet at Aguadilla, which empties gray waters, raw sewage, septic tank leaks and stormwater runoff directly towards adjacent marginal reefs. PDM and PFI in the eastern coast also presented high OAB concentrations, and they are frequently impacted by recurrent runoff pulses from adjacent streams and rivers. In the case of PDM, two important rivers, the Candelero and Humacao, as well as a regional wastewater treatment plant with an ocean outfall close to shore, are factors that can significantly influence local water quality. Our data suggest that locations adjacent to urban areas showed the highest concentrations of OABs. OABs have been suggested as chemical, organic and fecal pollution indicators on septic systems and surface and estuarine waters [,,,,,]. A high PO4 concentration was also observed at BSA in Culebra, probably influenced by sewage-polluted drainage of the adjacent Canal de Lobina during ebbing tidal flows [,]. High NH3+ concentrations were documented at PDM and PFI in the eastern region and at CAC, BRI and TPA in the western region due to suspected urban sources of fecal pollution.
Locations most affected by runoff, such as PDM and PFI, as well as locations severely impacted by hurricane mechanical impacts, such as DAK and TAR in Culebra, showed the lowest density and diversity of coral recruits, and had an impoverished coral reef benthic community structure. Locations with the strongest water circulation and presumed better water quality in Culebra, BTA and PME, as well as CDP off the eastern coast, presented the highest coral recruit density and diversity among surveyed locations. Findings in this study are consistent with a previous study of coral recruitment spatial patterns in Puerto Rico that suggested that low dissolved oxygen concentration, in combination with high turbidity and OAB concentration were significantly correlated with low coral recruit density and diversity, particularly on urban shallow coral reefs [].
Increasing water quality stress gradients (i.e., sedimentation, high nutrient and chlorophyll-a concentrations, turbidity, and strong light attenuation coefficient Kd490) may often result in localized eutrophication impacts and may potentially have an effect on structuring coral recruit assemblages. DISTLM analysis based on remotely sensed parameters for the period of 2017 to 2020 showed that SST anomaly + Kd490 + chlorophyll-a concentration significantly explained depauperate coral recruit assemblages in some locations across the eastern and western coast of Puerto Rico. This could affect marginal coral reef natural recovery ability through three mechanisms: (a) nutrient enrichment causing trophic alterations; (b) turbidity causing light reduction, which affects photosynthesis; and (c) sedimentation causing reduced larval settlement and mortality [,,,]. The long-term impact of sedimentation and pollution pulses from coastal development or urban stormwater runoff is considered one of the main stressors affecting natural coral recovery [,]. Sediments can accumulate on reefs and can smother or suffocate corals and interfere with their ability to feed, grow and reproduce [,]. Runoff can result in excess inputs of dissolved nutrients (i.e., nitrogen and phosphate) through fertilizers, stormwater or sewage discharges. It can lead to the proliferation of phytoplankton and macroalgae that obstruct sunlight, as well as contribute to microbial growth []. Such potential mechanisms can interfere with coral larval settlement, increasing mortality at this early stage. This may also lead to altered carbon and nutrient dynamics [], and to enhanced dissolved oxygen consumption, fostering the proliferation of opportunist microbes, further increasing stress to corals and other benthic fauna []. Macroalgal overgrowth can trap sediments and further enhance interference mechanisms of larval settlement []. Opportunist microbes and any potential physiological stress on settled coral larvae can enhance post-recruitment mortality. Sewage and sewage-borne microbes have already been implicated in enhanced coral disease prevalence and in coral reef degradation [,,,,,,,,,,,,,]. Further, a combination of other synergic factors associated with sewage, such as freshwater, inorganic nutrients, pathogens, endocrine disrupters, suspended solids, sediments, and heavy metals, can severely impair coral growth and/or reproduction through a combination of potential mechanisms yet unknown [].
In addition, the nonlinear trajectories of coral reef benthic community succession across sediment stress gradients, in combination with strong fishing impacts, have shown significant interactions between sediment and herbivory that can have cascading effects on coral recruitment [], resulting in further post-settlement mortality. There is further concern that the compounding effects of changing climate (i.e., extreme rainfall events, hurricanes) may result in localized enhanced environmental stress pulses to coral reefs [,,]. Increasing trends in coral reef decline, combined with water quality degradation, have already been described in various studies in Puerto Rico, highlighting the interaction between water quality and coral reef benthic assemblages [,,,,,,,,,,,,]. This points to the critical importance of incorporating watershed-scale conservation measures, the protection of coastal water quality, and enhancing successful coral settlement and recruitment as part of sustainable coral reef conservation-oriented management practices.
4.2. The Role of Strong Hurricanes
Shallow coral reefs that showed significant mechanical impacts by hurricanes were characterized by very poor coral recruit assemblages. Strong hurricanes can severely affect coral reef architecture [,,,], especially in shallow zones [,,]. The stochastic physical impact of the category five Hurricanes Irma and María in 2017 were important factors influencing benthic community trajectories across some locations, and thus shallow reef natural recovery ability. This study is one of the first accounts of post-hurricane mechanical impacts on natural coral reef recovery ability and suggests that the combination of presumed chronic water quality degradation and strong hurricane physical impacts appear to operate in a similar way on coral recruit assemblages across larger (regional) spatial scales, but in significantly different ways at the location scale. There was high variability among locations within each studied region. Many of Culebra’s shallow reefs, as well as in CAC in northwestern Puerto Rico, were among the most damaged by hurricanes. Impacted reefs showed flattened bottoms significantly dominated by unstable coral rubble and algal turf. Coral recruit density and species diversity were very low on these locations and very similar to that of locations chronically affected by environmental degradation. Hurricanes can have a wide range of social-ecological impacts, which can affect shallow coastal ecosystems []. They can also affect coral recruits as they can transport a large quantity of sediments that can suffocate shallow marginal reefs adjacent to the shoreline [], and nutrient-loaded runoff that can foster algal growth increasing post-settlement mortality. Further, strong hurricane physical impacts can include severe localized colony fragmentation and dislodgment [], localized coral mortality resulting from catastrophic sediment bedload associated with horizontal transport [] or the mechanical destruction of the shallow-water reef framework []. Such physical impacts can result in highly fragmented, unstable benthic surfaces, which can also lead to increased post-settlement mortality.
4.3. Potential Impacts on Coral Larval Dynamics
In the long term, coral reef decline can alter natural coral larval dynamics. Coral larval settlement and recruit survival and growth is fundamental to maintain coral population viability and is necessary for natural coral reef recovery following disturbance []. Its success will depend on: (1) larval availability, which involves successful gamete production, fertilization success, and connectivity to upstream sources of coral larvae not affected by chronic pollution or by hurricane physical impacts; (2) settlement success, which depends on larval and substrate condition, and on substrate selection behavior; and (3) post-settlement ecology, which affects survival and growth. Coral reef trophic state is also an important driver of coral recruitment success. A combination of ecological interactions may influence coral recruitment success [], including settlement behavior and interactions with substrate components [], post-settlement competition with algae and sessile invertebrates [], and incidental and targeted predation from fish and mobile invertebrates [].
Poor water quality, often leading to localized eutrophication, may result in reduced water transparency and in increased turf and macroalgal overgrowth, with potentially enhanced sediment-trapping effects [,] and decreasing coral recruitment success [,,]. Coral recruits are at least one order of magnitude more sensitive to sedimentation than adult corals [,]. Hurricane-associated runoff events may also lead to major macroalgal blooms [,], which may result in significant coral colony smothering and suffocation, leading to post-settlement mortality. In addition, variation in light intensity [,], as well as the spatial distribution of adult corals [], can affect coral recruitment. Any combination of these factors can lead to reduced recruitment success, particularly on severely degraded reefs [], including bomb- or dynamite-blasted coral reefs (with abundant unstable rubble fields, similar to those generated by strong hurricanes, as in this study) [,,]. Depending on the level of storm-induced damage and on the reef’s environmental history, recovery of coral reef communities may take from a few years to centuries []. In some circumstances, a permanent shift to high algal dominance may occur [], such as the dominance documented in this study to occur at several locations (i.e., PFI, PDM, DAK). Further, mass bleaching events can also result in a significant loss of coral recruits, both through direct and indirect coral mortality from post-bleaching macroalgal blooms, particularly under overfished conditions [], and in combination with strong hurricane physical impacts []. This suggests the importance of managing reef trophic conditions to ameliorate adverse impacts on coral assemblages. Increasing herbivory can modify trophic cascades, which can, in turn, increase coral recruitment success [].
Modeling efforts demonstrated that under significant and frequent thermal stress an unprecedented loss of benthic architectural complexity was predicted over the next two or three decades due to major coral loss and net structural decline []. Warming-related recurrent mass coral bleaching, coral disease outbreaks, and mass coral mortality events across the northeastern Caribbean region have further contributed to extensive coral decline and to the net loss of reef accretion by losing major reef builders [,,,,,,]. There was a large-scale loss of live coral following mass coral bleaching across the northeastern Caribbean in 2005–2006 [,,,,,,]. The geographical scale and magnitude of the recent outbreak of stony coral tissue loss disease (SCTLD) across the wider Caribbean has been unprecedented and have resulted in the decimation of local populations of critical reef-building species [,,,,,]. At the time of sampling in this project, SCTLD was still largely limited to coral reefs in Culebra Island and the eastern coast of Puerto Rico []. Thirteen out of the 16 sampling locations in this study were affected by SCTLD (81%), with prevalence in Culebra ranging from 20 to 60% at the time of sampling (Hernández-Delgado, unpublished data). No impacts were documented at that time across western coast locations. Widespread mass coral mortalities can have long-term paramount adverse consequences on the sustainability of reef carbonate budget and on its geo-ecological functions [,,,]. The long-term recurrence of such events might have contributed to the observed low coral recruit density and diversity observed in many locations in this study.
4.4. Long-Term Consequences for Coral Reef Ecological Functions and Services
The findings of this study suggest important long-term consequences for shallow, marginal coral reef benthic community trajectories under projected climate change and sea-level-rise impacts. Overall, coral recruit densities in this study ranged from 4.4 to 8.5/m2 across regional scales, and from 0 to 19.4/m2 across locations, with reefs with extensive hurricane physical destruction showing <2/m2. These values are comparable to previous studies across the Caribbean. Recruit densities averaged 6.2/m2 at Belize, 26.7/m2 at St. Croix, 28.9/m2 at Barbados, 26.6/m2 at Port Antonio, 15.6/m2 at Bonaire, and 33.8/m2 at Grenada []. Mean ranges from 2.2 to 2.8/m2 were documented at Belize [], from 0.8 to 1.3/m2 at Biscayne Bay, Florida [], from 1.0 to 6.4/m2 at the Mesoamerican Barrier Reef System [], and from 1.2 to 9.5/m2 at Mona Island, Puerto Rico []. However, studies prior to the Diadema antillarum mass mortality (1983–1984) across the Caribbean showed coral recruit densities of 76 to 274/m2 at Jamaica [], 13 to 20/m2 at Curaçao [], and 13 to 33/m2 at the Salt River Canyon, St. Croix, USVI []. A decline in coral recruits from 4.8 to 2.8/m2 was observed between 2003 and 2005 at La Parguera, Puerto Rico []. Coral recruit density in Exuma Cays, Bahamas, increased between 2004 and 2007 from 3.8 to 8.4/m2 in P. astreoides, from 1.4 to 2.3/m2 for A. agaricites, and from 2.1 to 3.1/m2 for O. annularis []. Recruit density ranged from 14.0 to 21.6/m2 at St. John, USVI, but increasing temperature resulted in increasing mortality across a 12-year time span []. This study also found that weedy species (i.e., P. astreoides, S. radians) with limited reef-building value were the dominant recruiters across most locations, similar to the findings of previous studies [,,]. Therefore, present day recruit densities and species assemblages may still reflect the long-term ecological consequences of the D. antillarum mass mortality event nearly four decades ago. A recent D. antillarum mass mortality event documented throughout the wider Caribbean region since February 2022 raised a major concern for rapidly declining coral assemblages and recruitment. This suggests that the environmental and ecological history of each location can play important roles affecting coral recruit density and diversity.
Shallow coral reef physical structure imparts friction on waves and currents and is extremely important for determining hydrodynamic environments adjacent to the shoreline, and for accurately modeling wave, current, and surface circulation patterns [,]. Spatio-temporal variation in coral reef benthic roughness and friction [,,,,], substrate porosity [,], and storm surge dynamics [,] will affect long-term hydrodynamics over any shallow coral reef. Altered hydrodynamics may have a long-term influence on coral recruitment dynamics and reef accretion. Therefore, the impoverished coral recruit assemblages on flattened reef bottoms documented in this study at some locations, were dominated by weedy, poor reef-building species, with limited percent live coral cover. Such reefs were dominated by algal turf and macroalgae. This highly prevalent condition could have long-term vital implications for future conservation and the persistence of reef benthic communities under projected climate change and sea level rise scenarios []. Such a declining reef, with limited natural recovery ability from disturbances may face a restricted capacity to cope with recurrent warming events, mass coral mortalities, and from rising sea level, potentially leading to enhanced coastal vulnerability to increasing wave energy, runup and erosion.
Large, fringing coral reefs with higher benthic physical structure have been shown to provide substantial protection against storm hazards by reducing wave energy by an average of 97%, with crests alone dissipating most of this energy (86%) []. Higher bottom roughness can affect water flow dynamics and sediment transport [,], and can result in enhanced benthic habitat structural complexity, leading to enhanced benthic and fish productivity [,,]. However, declining coral reefs, with significant loss of bottom roughness, rugosity index, bottom friction coefficient, and substrate porosity may significantly lose wave attenuation effectiveness, possibly resulting in depleted biodiversity and in major long-term impacts to life and coastal infrastructure due to stronger wave exposure in the future. Therefore, poor water quality, as well as mechanical destruction of the reef framework by strong hurricanes, may become critical drivers of depleted coral assemblages by significantly limiting coral recruitment success, thus affecting natural recovery following disturbances, and reef accretion in the long term.
This study had some important limitations. The most significant one is the limited number of locations assessed across some of the surveyed geographical regions. In addition, this study did not consider the temporal variation of either water quality or coral recruitment dynamics, which we supplemented with a temporal assessment of remotely sensed water quality parameters. Our data only provides a snapshot view of presumably chronically poor environmental conditions at some locations. Future studies should consider assessing seasonal variation across larger scales and across additional locations. Further, it would be of paramount importance to analyze the relative effects of parameters such as long-term temperature trends, coral disease outbreaks, mass coral bleaching, storm disturbances, etc., along with the spatio-temporal variation in water quality to enable determination of effect sizes relative to each variable or combination of variables.
5. Conclusions
This study showed that shallow coral reefs affected by presumed poor water quality, recurrent hurricanes and climate change-related sea surface warming are showing significant signs of degradation which include low coral recruit density and diversity across large spatial scales. This has resulted in a net loss in coral reef recovery potential following disturbances. Declining coral recruitment across shallow marginal reefs could have important implications under projected climate change and sea level rise impacts, particularly for urban coastal locations. This can affect the long-term sustainability of shallow coral reef ecological functions, such as buffering wave energy, runup, and shoreline erosion. It can also compromise net reef accretion, biodiversity, and other services and socio-economic benefits. As the capacity for natural regeneration is lost, in the long-term the resistance of the system to the impact of future environmental disturbances is lost, therefore, reef and coastal resilience in general can become further compromised.
In light of these findings, it is predicted that, in the long-term, coastlines adjacent to degraded shallow reefs will become more vulnerable to future storm and winter swell events, and the potential impacts of climate change and sea level rise will occur sooner due to the gradual loss of natural recovery ability across multiple locations. Therefore, stronger immediate conservation measures are imperative to prevent further water quality decline and coral reef degradation. This includes the need to implement more aggressive and effective best management practices in environmental conservation (i.e., land-based source pollution management), ecological restoration and community participation strategies that facilitate enhanced coral settlement and recruitment success, and assisted recovery processes through coral reef restoration across substantially enhanced spatial scales. Only in this way can time be borrowed for the recovery of coral reefs during the Anthropocene.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d14100804/s1, Table S1: Benthic community structure data; Table S2: Coral recruit abundance data; Table S3: Water quality summary data; Table S4: Remotely sensed water quality data (2017–2020); Table S5: Distance-based linear model analysis (DISTLM) of the impact of the normalized maximum annual values of remotely sensed water quality parameters (2017–2020) on coral recruit abundance; Figure S1: Shade plot based on the √-density (#/m2) of coral recruits by species x region x location; Figure S2: Bubble plot based on a non-metric multidimensional scaling analysis (nMDS) on the √-density (#/m2) of all coral recruits by region and by sampling location; Figure S3: Bubble plot based on a non-metric multidimensional scale analysis (nMDS) on the species richness (S) of all recruits by region and by locality; Figure S4: Running mean, maximum and minimum values of remotely sensed water quality parameters (2017–2020) across study locations: (A) Sea surface temperature anomaly (°C); (B) Light extinction coefficient Log10 Kd490 m−1; (C) Chlorophyll-a concentration (mg m−3); (D) Net primary productivity (mg C m−2 day−1); (E) Particulate organic carbon concentration (mg m−3); and (F) Particulate organic carbon concentration (Log10 mol m−3); Figure S5: Distance-based redundant analysis (dbRDA) of the impact of the normalized maximum annual values of remotely sensed water quality parameters (2017–2020) on coral recruit abundance by region and location.
Author Contributions
Conceptualization, E.A.H.-D.; methodology, E.A.H.-D.; software, E.A.H.-D.; validation, E.A.H.-D.; formal analysis, E.A.H.-D. and M.F.O.-F.; investigation, E.A.H.-D. and M.F.O.-F.; resources, E.A.H.-D.; data curation, E.A.H.-D.; writing—original draft preparation, E.A.H.-D. and M.F.O.-F.; writing—review and editing, E.A.H.-D.; visualization, E.A.H.-D.; supervision, E.A.H.-D.; project administration, E.A.H.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study regarding benthic community structure, coral recruit assemblages, and water quality are presented in the Supplementary Files. Remotely sensed water quality data are openly available on ERDDAP (https://coastwatch.pfeg.noaa.gov/erddap/index.html (accessed on 1 July 2022)).
Acknowledgments
Field work was conducted under Letters of Agreement 2015-000037 and 2019-000006 with the PR Department of Natural and Environmental Resources. The authors wish to thank Jaime S. Fonseca for his support during part of the data collection efforts. Sociedad Ambiente Marino provided in-kind logistical support for developing this project. An earlier version of this study constituted the honors thesis project of M.F.O.F. at the Department of Environmental Sciences of the University of Puerto Rico, Río Piedras Campus. This manuscript is a contribution of the collaborative efforts between Sociedad Ambiente Marino and the Center for Applied Tropical Ecology and Conservation. Comments from two anonymous reviewers greatly improved this manuscript. This work is dedicated to the beloved memory of Sara (grandmother E.A.H.D.) and Sonia (mother E.A.H.D.).
Conflicts of Interest
The authors declare no conflict of interest, no personal circumstances or interest that may be perceived as inappropriately influencing the representation or interpretation of the reported research results. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Cesar, H.S.J. Coral reefs: Their functions, threats and economic value. In Collected Essays on the Economics of Coral Reefs; Cesar, H.S.J., Ed.; CORDIO: Växjö, Sweden, 2007; pp. 14–39. [Google Scholar]
- Leeworthy, V.R.; Schwarzmann, D.; Hughes, S.; Vaughn, J.; Dato, C.; Padilla, G. Economic Contribution of Reef Using Visitor Spending to the Puerto Rican Economy; Office of National Marine Sanctuaries, National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2018; pp. 1–23. [Google Scholar]
- Storlazzi, C.D.; Reguero, B.G.; Cole, A.D.; Lowe, E.; Shope, J.B.; Gibbs, A.E.; Nickel, B.A.; McCall, R.T.; van Dongeren, A.R.; Beck, M.W. Rigorously valuing the role of US coral reefs in coastal hazard risk reduction. In Open-File Report-US Geological Survey; U.S. Geological Survey: Reston, VA, USA, 2019. [Google Scholar]
- Miller, J.; Muller, E.; Rogers, C.; Waara, R.; Atkinson, A.; Whelan, K.R.T.; Patterson, M.; Witcher, B. Coral disease following massive bleaching in 2005 causes 60% decline in coral cover on reefs in the US Virgin Islands. Coral Reefs 2009, 28, 925–937. [Google Scholar] [CrossRef]
- Edmunds, P.J. Decadal-scale changes in the community structure of coral reefs of St. John, US Virgin Islands. Mar. Ecol. Prog. Ser. 2013, 489, 107–123. [Google Scholar] [CrossRef]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.; Kleypas, J.; Van De Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Simpson, T. Large-scale bleaching of corals on the Great Barrier Reef. Ecology 2018, 99, 501. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G.; et al. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration. NOAA Coral Reef Ecosystems. 2019. Available online: https://www.noaa.gov/education/resource-collections/marine-life-education-resources/coral-reef-ecosystems (accessed on 1 May 2021).
- Ferrario, F.; Beck, M.W.; Storlazzi, C.D.; Micheli, F.; Shepard, C.C.; Airoldi, L. The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nat. Commun. 2014, 5, 3794. [Google Scholar] [CrossRef]
- ICRI What Are Corals? International Coral Reef Initiative. 2015. Available online: https://www.icriforum.org/about-coral-reefs/what-are-corals (accessed on 1 May 2021).
- Scheffer, M.; Barrett, S.; Carpenter, S.R.; Folke, C.; Green, A.J.; Holmgren, M.; Hughes, T.P.; Kosten, S.; Van de Leemput, I.A.; Nepstad, D.C.; et al. Creating a safe operating space for iconic ecosystems. Science 2015, 347, 1317–1319. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Chase, T.J.; Dietzel, A.; Hill, T.; Hoey, A.S.; Hoogenboom, M.O.; Jacobson, M.; et al. Global warming impairs stock–recruitment dynamics of corals. Nature 2019, 568, 387–390. [Google Scholar] [CrossRef]
- Hurlbut, C.J. Community recruitment: Settlement and juvenile survival of seven co-occurring species of sessile marine invertebrates. Mar. Biol. 1991, 109, 507–515. [Google Scholar] [CrossRef]
- Heery, E.C.; Hoeksema, B.W.; Browne, N.K.; Reimer, J.D.; Ang, P.O.; Huang, D.; Friess, D.A.; Chou, L.M.; Loke, L.H.; Saksena-Taylor, P.; et al. Urban coral reefs: Degradation and resilience of hard coral assemblages in coastal cities of East and Southeast Asia. Mar. Pollut. Bull. 2018, 135, 654–681. [Google Scholar] [CrossRef]
- Burt, J.A.; Bartholomew, A. Towards more sustainable coastal development in the Arabian Gulf: Opportunities for ecological engineering in an urbanized seascape. Mar. Pollut. Bull. 2019, 142, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Burt, J.A.; Camp, E.F.; Enochs, I.C.; Johansen, J.L.; Morgan, K.M.; Riegl, B.; Hoey, A.S. Insights from extreme coral reefs in a changing world. Coral Reefs 2020, 39, 495–507. [Google Scholar] [CrossRef]
- Todd, P.A.; Heery, E.C.; Loke, L.H.; Thurstan, R.H.; Kotze, D.J.; Swan, C. Towards an urban marine ecology: Characterizing the drivers, patterns and processes of marine ecosystems in coastal cities. Oikos 2019, 128, 1215–1242. [Google Scholar] [CrossRef]
- Mumby, P.; Steneck, R. The resilience of coral reefs and its implications for reef management. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Sambler, N., Eds.; Springer Science: New York, NY, USA, 2011; pp. 509–519. [Google Scholar]
- Birrell, C.L.; McCook, L.J.; Willis, B.L. Effects of algal turfs and sediment on coral Settlement. Mar. Pollut. Bull. 2005, 51, 408–414. [Google Scholar] [CrossRef]
- Hughes, T.P.; Tanner, J.E. Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology 2000, 81, 2250–2263. [Google Scholar] [CrossRef]
- Connell, J.H. Disturbance and recovery of coral assemblages. Coral Reefs 1997, 16, 101–113. [Google Scholar] [CrossRef]
- Rogers, C.S. Responses of coral reefs and reef organisms to sedimentation. Mar. Ecol. Prog. Ser. 1990, 62, 185–202. [Google Scholar] [CrossRef]
- Hughes, T.P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef]
- Delgado, E.A.H.; Rosado-Matías, B.J. Long-lasting impacts of beach renourishment on nearshore urban coral reefs: A glimpse on future impacts of shoreline erosion, sea level rise and climate change. Ann. Mar. Biol. Res. 2017, 4, 1021. [Google Scholar]
- Erftemeijer, P.L.; Riegl, B.; Hoeksema, B.W.; Todd, P.A. Environmental impacts of dredging and other sediment disturbances on corals: A review. Mar. Pollut. Bull. 2012, 64, 1737–1765. [Google Scholar] [CrossRef]
- Cloern, J.E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 2001, 210, 223–253. [Google Scholar] [CrossRef]
- Roth, F.; Saalmann, F.; Thomson, T.; Coker, D.J.; Villalobos, R.; Jones, B.H.; Wild, C.; Carvalho, S. Coral reef degradation affects the potential for reef recovery after disturbance. Mar. Env. Res. 2018, 142, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, B.E. Chasing nutrients and algal blooms in Gulf and Caribbean waters: A personal story. Gulf Caribb. Res. 2019, 30, xvi-xxx. [Google Scholar] [CrossRef]
- Fabricius, K.E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Poll. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef]
- Nelson, H.R.; Altieri, A.H. Oxygen: The universal currency on coral reefs. Coral Reefs 2019, 38, 177–198. [Google Scholar] [CrossRef]
- Pawlik, J.R.; Burkepile, D.E.; Thurber, R.V. A vicious circle? Altered carbon and nutrient cycling may explain the low resilience of Caribbean coral reefs. BioScience 2016, 66, 470–476. [Google Scholar] [CrossRef]
- Kleypas, J.A.; McManus, J.W.; Meñez, L.A.B. Environmental limits to coral reef development: Where do we draw the line? Am. Zool. 1999, 39, 146–159. [Google Scholar] [CrossRef]
- Guinotte, J.M.; Buddemeier, R.W.; Kleypas, J.A. Future coral reef habitat marginality: Temporal and spatial effects of climate change in the Pacific basin. Coral Reefs 2003, 22, 551–558. [Google Scholar] [CrossRef]
- De Oliveira Soares, M. Marginal reef paradox: A possible refuge from environmental changes? Ocean. Coast. Mgmt. 2020, 185, 105063. [Google Scholar] [CrossRef]
- Skirving, W.J.; Heron, S.F.; Marsh, B.L.; Liu, G.; De La Cour, J.L.; Geiger, E.F.; Eakin, C.M. The relentless march of mass coral bleaching: A global perspective of changing heat stress. Coral Reefs 2019, 38, 547–557. [Google Scholar] [CrossRef]
- UNEP Projections of Future Coral Bleaching Conditions Using IPCC CMIP6 Models: Climate Policy Implications, Management Applica-tions, and Regional Seas Summaries; United Nations Environment Programme: Nairobi, Kenya, 2020; pp. 1–102.
- UNEP Coral Bleaching Futures—Downscaled Projections of Bleaching Conditions for the World’s Coral Reefs, Implications of Climate Policy and Management Responses; United Nations Environment Programme: Nairobi, Kenya, 2017; pp. 1–69.
- Hernández-Pacheco, R.; Hernández-Delgado, E.A.; Sabat, A.M. Demographics of bleaching in the Caribbean reef-building coral Montastraea annularis. Ecosphere 2011, 2, 1–13. [Google Scholar] [CrossRef]
- Levas, S.; Schoepf, V.; Warner, M.E.; Aschaffenburg, M.; Baumann, J.; Grottoli, A.G. Long-term recovery of Caribbean corals from bleaching. J. Exp. Mar. Biol. Ecol. 2018, 506, 124–134. [Google Scholar] [CrossRef]
- Edmunds, P.J. Recruitment hotspots and bottlenecks mediate the distribution of corals on a Caribbean reef. Biol. Lett. 2021, 17, 20210149. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, P.J.; Didden, C.; Frank, K. Over three decades, a classic winner starts to lose in a Caribbean coral community. Ecosphere 2021, 12, e03517. [Google Scholar] [CrossRef]
- Weil, E.; Urreiztieta, I.; Garzón-Ferreira, J. Geographic variability in the incidence of coral and octocoral diseases in the wider Caribbean. Proc. 9th Int. Coral Reef Symp. 2000, 2, 1231–1237. [Google Scholar]
- Cróquer, A.; Weil, E. Changes in Caribbean coral disease prevalence after the 2005 bleaching event. Dis. Aquat. Orgs. 2009, 87, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Weil, E.; Cróquer, A. Spatial variability in distribution and prevalence of Caribbean scleractinian coral and octocoral diseases. I. Community-level analysis. Dis. Aquat. Orgs. 2009, 83, 195–208. [Google Scholar] [CrossRef]
- Weil, E.; Rogers, C.S. Coral reef diseases in the Atlantic-Caribbean. In Coral Reefs: An Ecosystem in Transition; Springer: Dordrecht, The Netherlands, 2011; pp. 465–491. [Google Scholar]
- Weil, E.; Rogers, C.S.; Croquer, A. Octocoral diseases in a changing ocean. In Marine Animal Forests; Springer: Cham, Germany, 2016; pp. 1–55. [Google Scholar]
- Pratchett, M.S.; Wilson, S.K.; Baird, A.H. Declines in the abundance of Chaetodon butterflyfishes following extensive coral depletion. J. Fish. Biol. 2006, 69, 1269–1280. [Google Scholar] [CrossRef]
- Graham, N.A.; Wilson, S.K.; Jennings, S.; Polunin, N.V.; Robinson, J.A.N.; Bijoux, J.P.; Daw, T.M. Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 2007, 21, 1291–1300. [Google Scholar] [CrossRef]
- Munday, P.L.; Jones, G.P.; Sheaves, M.; Williams, A.J.; Goby, G. Vulnerability of fishes of the Great Barrier Reef to climate change. In Climate Change and the Great Barrier Reef; Johnson, J.E., Marshall, P.A., Eds.; Great Barrier Reef Marine Park Authority and Australian Greenhouse Office: Townsville, Australia, 2007; pp. 357–391. [Google Scholar]
- Munday, P.L.; Jones, G.P.; Pratchett, M.S.; Williams, A.J. Climate change and the future for coral reef fishes. Fish Fish. 2008, 9, 261–285. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Munday, P.L.; Wilson, S.K.; Graham, N.A.; Cinner, J.E.; Bellwood, D.R.; Jones, G.P.; Polunin, N.V.; McClanahan, T.R. Effects of climate-induced coral bleaching on coral-reef fishes—ecological and economic consequences. Oceanogr. Mar. Biol. Ann. Rev. 2008, 46, 251–296. [Google Scholar]
- Pratchett, M.S.; Wilson, S.K.; Graham, N.A.J.; Munday, P.L.; Jones, G.P.; Polunin, N.V. Coral bleaching and consequences for motile reef organisms: Past, present and uncertain future effects. In Coral Bleaching, Ecological Studies 205; van Oppen, M.J.H., Lough, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 139–158. [Google Scholar]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K.; Messmer, V.; Graham, N.A. Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity 2011, 3, 424–452. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Paddack, M.J.; Collen, B.; Robertson, D.R.; Côté, I.M. Simplification of Caribbean reef-fish assemblages over decades of coral reef degradation. PLoS ONE 2015, 10, e0126004. [Google Scholar]
- Uthicke, S.; Furnas, M.; Lønborg, C. Coral reefs on the edge? Carbon chemistry on inshore reefs of the Great Barrier Reef. PLoS ONE 2014, 9, e109092. [Google Scholar] [CrossRef]
- Perry, C.T.; Murphy, G.N.; Kench, P.S.; Smithers, S.G.; Edinger, E.N.; Steneck, R.S.; Mumby, P.J. Caribbean-wide decline in carbonate production threatens coral reef growth. Nat. Commun. 2013, 4, 1402–1407. [Google Scholar] [CrossRef]
- Anthony, K.R.; Maynard, J.A.; Díaz-Pulido, G.; Mumby, P.J.; Marshall, P.A.; Cao, L.; Hoegh-Guldberg, O. Ocean acidification and warming will lower coral reef resilience. Glob. Chang. Biol. 2011, 17, 1798–1808. [Google Scholar] [CrossRef]
- Siegel, K.J.; Cabral, R.B.; McHenry, J.; Ojea, E.; Owashi, B.; Lester, S.E. Sovereign states in the Caribbean have lower social-ecological vulnerability to coral bleaching than overseas territories. Proc. R Soc. B 2019, 286, 20182365. [Google Scholar] [CrossRef]
- Hughes, T.P.; Rodrigues, M.J.; Bellwood, D.R.; Ceccarelli, D.; Hoegh-Guldberg, O.; McCook, L.; Moltschaniwskyj, N.; Pratchett, M.S.; Steneck, R.S.; Willis, B. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 2007, 17, 360–365. [Google Scholar] [CrossRef]
- Rogers, C.R.; Fitz, C.; Gilnack, M.; Beets, J.; Hardin, J. Scleractinian coral recruitment patterns at Salt River Submarine Canyon, St. Croix, U.S. Virgin Islands. Coral Reefs 1984, 3, 69–76. [Google Scholar] [CrossRef]
- Connell, J.H.; Hughes, T.P.; Wallace, C.C. A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol. Monogr. 1997, 67, 461–488. [Google Scholar] [CrossRef]
- Carlon, D.B. Depth-related patterns of coral recruitment and cryptic suspension-feeding invertebrates on Guan Island, British Virgin Islands. Bull. Mar. Sci. 2001, 68, 525–541. [Google Scholar]
- Darling, E.S.; Côté, I. Seeking resilience in marine ecosystems. Science 2018, 359, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime shifts, resilience, and biodiversity in ecosystem management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Donovan, M.K.; Cramer, K.L.; Lam, V.V. Status and Trends of Caribbean Coral Reefs: 1970–2012; Global Coral Reef Monitoring Network, IUCN: Gland, Switzerland, 2014; pp. 1–304. [Google Scholar]
- O’Cain, E.D.; Frischer, M.E.; Harrison, J.S.; Walters, T.L.; Thompson, M.E.; Fogarty, N.D.; Ruzicka, R.; Gleason, D.F. Identification of newly settled Caribbean coral recruits by ITS-targeted single-step nested multiplex PCR. Coral Reefs 2019, 38, 79–92. [Google Scholar] [CrossRef]
- Irizarry-Soto, E.; Weil, E. Spatial and temporal variability in juvenile coral densities, survivorship and recruitment in La Parguera, southwestern Puerto Rico. Caribb. J. Sci. 2009, 45, 269–281. [Google Scholar] [CrossRef]
- Hernández-Delgado, E.A.; González-Ramos, C.M.; Alejandro-Camis, P.J. Large-scale coral recruitment patterns on Mona Island, Puerto Rico: Evidence of a transitional community trajectory after massive coral bleaching and mortality. Rev. Biol. Trop 2014, 62, 283–298. [Google Scholar]
- Edmunds, P.J. The demography of hurricane effects on two coral populations differing in dynamics. Ecosphere 2019, 10, e02836. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 3–32. [Google Scholar] [CrossRef]
- Fong, P.; Glynn, P.W. A dynamic size-structure population model: Does disturbance control size structure of a population of the massive coral Gardineroseris planulata in the Eastern Pacific? Mar. Biol. 1998, 130, 663–674. [Google Scholar] [CrossRef]
- Bonkosky, M.; Hernández-Delgado, E.A.; Sandoz, B.; Robledo, I.E.; Norat-Ramírez, J.; Mattei, H. Detection of spatial fluctuations of non-point source fecal pollution in coral reef surrounding waters in southwestern Puerto Rico using PCR-based assays. Mar. Poll. Bull. 2009, 58, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Delgado, E.A.; Sandoz, B.; Bonkosky, M.; Mattei, H.; Norat, J. Impacts of non-point source sewage pollution in Elkhorn coral, Acropora palmata (Lamarck), assemblages of the southwestern Puerto Rico shelf. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, FL, USA, 7–11 July 2008; pp. 747–751. [Google Scholar]
- Díaz-Ortega, G.; Hernández-Delgado, E.A. Land-based source pollution in a climate of change: A roadblock to the conservation and recovery of Elkhorn coral Acropora palmata (Lamarck 1816). Nat. Resour. 2014, 5, 561–581. [Google Scholar]
- Rogers, C.S.; Garrison, G.; Gtober, R.; Hillis, Z.M.; Franke, M.A. Coral Reef Monitoring Manual for the Caribbean and Western Atlantic; National Park Service: St. John, NY, USA, 1994. [Google Scholar]
- Anderson, M.; Gorley, R.; Clarke, K. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.; Gorley, R.; Somerfield, P.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E: Plymouth, UK, 2014. [Google Scholar]
- Cangialosi, J.P.; Latto, A.S.; Berg, R. Hurricane Irma (AL112017) 30 August–12 September 2017; National Hurricane Center Tropical Cyclone Report; NOAA: Washington, DC, USA, 2018; pp. 1–111. [Google Scholar]
- Pasch, R.J.; Penny, A.B.; Berg, R. Hurricane María (AL152017) 16–30 September 2017; National Hurricane Center Tropical Cyclone Report; NOAA: Washington, DC, USA, 2018; pp. 1–48. [Google Scholar]
- Lapointe, B.E.; Clark, M.W. Nutrient inputs from the watershed and coastal eutrophication in the Florida Keys. Estuaries 1992, 15, 465–476. [Google Scholar] [CrossRef]
- Szmant, A.M. Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline? Estuaries 2002, 25, 743–766. [Google Scholar] [CrossRef]
- Norat-Ramírez, J.; Méndez-Lázaro, P.; Hernández-Delgado, E.A.; Mattei-Torres, H.; Cordero-Rivera, L. A septic weight index model to measure the impact of septic tanks on coastal water quality and coral reef communities in Rincón, Puerto Rico. Ocean Coast Manag. 2019, 169, 201–213. [Google Scholar] [CrossRef]
- Díaz, J.R.; Jordan, D.G. Water Resources of the Río Grande de Añasco-Lower Valley, Puerto Rico. U.S Geological Survey Water Resources Investigation Report 85-4237. 1987. Available online: https://pubs.er.usgs.gov/publication/wri854237 (accessed on 20 November 2021).
- Hernández-Delgado, E.A.; Rosado-Matías, B.J.; Sabat, A.M. Management failures and coral decline threatens fish functional groups recovery patterns in the Luis Peña Channel No-Take Natural Reserve, Culebra Island, PR. Proc. Gulf Caribb. Fish. Inst. 2006, 57, 577–605. [Google Scholar]
- Hernández-Delgado, E.A.; Medina-Muñiz, J.L.; Mattei, H.; Norat-Ramírez, J. Unsustainable land use, sediment-laden runoff, and chronic raw sewage offset the benefits of coral reef ecosystems in a no-take marine protected area. Env. Mgmt. Sust. Dev. 2017, 6, 292–333. [Google Scholar] [CrossRef]
- Alhajjar, B.J.; Chesters, G.; Harkin, J.M. Indicators of chemical pollution from septic systems. Groundwater 1990, 28, 559–568. [Google Scholar] [CrossRef]
- Fay, S.R.; Spong, R.C.; Alexander, S.C.; Alexander, E.C., Jr. Optical brighteners: Sorption behavior, detection, septic system tracer applications. In Proceedings of the International Association of Hydrogeologists XXVI International Congress, Edmonton, AB, Canada, June 1995. [Google Scholar]
- Tavares, M.E.; Spivey, M.I.; McIver, M.R.; Mallin, M.A. Testing for optical brighteners and fecal bacteria to detect sewage leaks in tidal creeks. J. North Car. Acad. Sci. 2008, 124, 91–97. [Google Scholar]
- Cao, Y.; Griffith, J.F.; Weisberg, S.B. Evaluation of optical brightener photodecay characteristics for detection of human fecal contamination. Water Res. 2009, 43, 2273–2279. [Google Scholar] [CrossRef]
- Hagedorn, C.; Weisberg, S.B. Chemical-based fecal source tracking methods: Current status and guidelines for evaluation. Rev. Env. Sci. Bio. Technol. 2009, 8, 275–287. [Google Scholar] [CrossRef]
- Chandler, D.M.; Lerner, D.N. A low cost method to detect polluted surface water outfalls and misconnected drainage. Water Environ. J. 2015, 29, 202–206. [Google Scholar] [CrossRef]
- González-Figueroa, M.C.; Hernández-Delgado, E.A. Variación espacial en los patrones de recuperación natural de los arrecifes de coral someros urbanos en Puerto Rico. Perspect. Asun. Ambient. 2021, 9, 90–111. Available online: https://uagm.edu/es/v9-perspectivas (accessed on 1 July 2022).
- Fabricius, K.E. Factors determining the resilience of coral reefs to eutrophication: A review and conceptual model. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Sambler, N., Eds.; Springer Science: New York, NY, USA, 2011; pp. 493–505. [Google Scholar]
- Babcock, R.D.P. Effects of sedimentation on settlement of Acropora millepora. Coral Reefs 1991, 9, 205–208. [Google Scholar] [CrossRef]
- Kuffner, I.R.; Walters, L.J.; Becerro, M.A.; Paul, V.J.; Williams, R.R.; Beach, K.S. Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar. Ecol. Prog. Ser. 2006, 323, 107–117. [Google Scholar] [CrossRef]
- Hoey, A.S.; Pratchett, M.S.; Cvitanovic, C. High macroalgal cover and low coral recruitment undermines the potential resilience of the world’s southernmost coral reef assemblages. PLoS ONE 2011, 6, e25824. [Google Scholar] [CrossRef]
- Patterson, K.L.; Porter, J.W.; Ritchie, K.B.; Polson, S.W.; Mueller, E.; Peters, E.C.; Santavy, D.L.; Smith, G.W. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc. Natl. Acad. Sci. USA 2002, 99, 8725–8730. [Google Scholar] [CrossRef]
- Sutherland, K.P.; Porter, J.W.; Torres, C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Progr. Ser. 2004, 266, 273–302. [Google Scholar] [CrossRef]
- Kaczmarsky, L.T.; Draud, M.; Williams, E.H. Is there a relationship between proximity to sewage effluent and the prevalence of coral disease. Caribb. J. Sci. 2005, 41, 124–137. [Google Scholar]
- Voss, J.D.; Richardson, L.L. Nutrient enrichment enhances black band disease progression in corals. Coral Reefs 2006, 25, 569–576. [Google Scholar] [CrossRef]
- Sutherland, K.P.; Porter, J.W.; Turner, J.W.; Thomas, B.J.; Looney, E.E.; Luna, T.P.; Meyers, M.K.; Futch, J.C.; Lipp, E.K. Human sewage identified as likely source of white pox disease of the threatened Caribbean elkhorn coral, Acropora palmata. Environ. Microbiol. 2010, 12, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Delgado, E.A.; Hutchinson-Delgado, Y.M.; Laureano, R.; Hernández-Pacheco, R.; Ruiz-Maldonado, T.M.; Oms, J.; Díaz, P.L. Sediment stress, water turbidity and sewage impacts on threatened Elkhorn coral (Acropora palmata) stands at Vega Baja, Puerto Rico. Proc. Gulf Caribb. Fish. Inst. 2011, 63, 83–92. [Google Scholar]
- Kaczmarsky, L.; Richardson, L.L. Do elevated nutrients and organic carbon on Philippine reefs increase the prevalence of coral disease? Coral Reefs 2011, 30, 253–257. [Google Scholar] [CrossRef]
- Sutherland, K.P.; Shaban, S.; Joyner, J.L.; Porter, J.W.; Lipp, E.K. Human Pathogen Shown to Cause Disease in the Threatened Eklhorn Coral Acropora palmata. PLoS ONE 2011, 6, e23468. [Google Scholar] [CrossRef] [PubMed]
- Redding, J.E.; Myers-Miller, R.L.; Baker, D.M.; Fogel, M.; Raymundo, L.J.; Kim, K. Link between sewage-derived nitrogen pollution and coral disease severity in Guam. Mar. Poll. Bull. 2013, 73, 57–63. [Google Scholar] [CrossRef]
- Yoshioka, R.M.; Kim, C.J.; Tracy, A.M.; Most, R.; Harvell, C.D. Linking sewage pollution and water quality to spatial patterns of Porites lobata growth anomalies in Puako, Hawaii. Mar. Poll. Bull. 2016, 104, 313–321. [Google Scholar] [CrossRef]
- Wear, S.L.; Thurber, R.V. Sewage pollution: Mitigation is key for coral reef stewardship. Ann. N. Y. Acad. Sci. 2015, 1355, 15–30. [Google Scholar] [CrossRef]
- Wakwella, A.; Mumby, P.J.; Roff, G. Sedimentation and overfishing drive changes in early succession and coral recruitment. Proc. R. Soc. B 2020, 287, 20202575. [Google Scholar] [CrossRef]
- Kennedy, E.V.; Perry, C.T.; Halloran, P.R.; Iglesias-Prieto, R.; Schönberg, C.H.; Wisshak, M.; Form, A.U.; Carricart-Gavinet, J.P.; Fine, M.; Eakin, C.M.; et al. Avoiding coral reef functional collapse requires local and global action. Curr. Biol. 2013, 23, 912–918. [Google Scholar] [CrossRef]
- Hernández-Delgado, E.A. The Emerging Threats of Climate Change on Tropical Coastal Ecosystem Services, Public Health, Local Economies and Livelihood Sustainability of Small Islands: Cumulative Impacts and Synergies. Mar. Poll. Bull. 2015, 101, 5–28. Available online: http://www.sciencedirect.com/science/article/pii/S0025326X15300357 (accessed on 20 November 2021). [CrossRef]
- Rogers, C.S.; Ramos-Scharrón, C.E. Assessing effects of sediment delivery to coral reefs: A Caribbean watershed perspective. Front. Mar. Sci. 2022, 8, 773968. [Google Scholar] [CrossRef]
- Otaño-Cruz, A.; Montañez-Acuña, A.A.; Torres-López, V.I.; Hernández-Figueroa, E.M.; Hernández-Delgado, E.A. Effects of changing weather, oceanographic conditions, and land uses on spatio-temporal variation of sedimentation dynamics along near-shore coral reefs. Front. Mar. Sci 2017, 4, 249. [Google Scholar] [CrossRef]
- Otaño-Cruz, A.; Montañez-Acuña, A.A.; Benson, E.; Cuevas, E.P.; Ortiz, J.; Hernández-Delgado, E.A. Response of near-shore coral reefs benthic communities to changes of sedimentation dynamics and environmental conditions. Front. Mar. Sci 2019, 6, 551. [Google Scholar] [CrossRef]
- Gómez Andújar, N.X.; Hernández-Delgado, E.A. Spatial benthic community analysis of shallow coral reefs to support coastal management in Culebra Island, Puerto Rico. PeerJ 2020, 8, 10080. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.D.; Chornesky, E.A.; Clifford, P.A.; Jackson, J.B.C.; Kaufman, L.S.; Knowlton, N.; Lang, J.C.; Pearson, M.P.; Porter, J.W.; Rooney, M.C.; et al. Hurricane Allen’s impact on Jamaican coral reefs. Science 1981, 214, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Harmelin-Vivien, M.L.; Laboute, P. Catastrophic impact of hurricanes on atoll outer reef slopes in the Tuamotu (French Polynesia). Coral Reefs 1986, 5, 55–62. [Google Scholar] [CrossRef]
- Fenner, D.P. Effects of Hurricane Gilbert on coral reefs, fishes and sponges at Cozumel, Mexico. Bull. Mar. Sci. 1991, 48, 719–730. [Google Scholar]
- Lugo, A.E.; Rogers, C.S.; Nixon, S.W. Hurricanes, coral reefs and rainforests: Resistance, ruin and recovery in the Caribbean. AMBIO 2000, 29, 106–114. [Google Scholar] [CrossRef]
- Stoddart, D.R. Three Caribbean atolls: Turneffe Islands, Lighthouse Reef, and Glover’s Reef, British Honduras. Atoll Res. Bull. 1962, 87, 1–161. [Google Scholar] [CrossRef]
- Stoddart, D.R. Effects of Hurricane Hattie on the British Honduras reefs and cays, 30–31 October 1961. Atoll Res. Bull. 1963, 95, 1–142. [Google Scholar] [CrossRef]
- Rogers, C.S. A matter of scale: Damage from Hurricane Hugo (1989) to US Virgin Islands reefs at the colony, community and whole reef level. In Proceedings of the 7th International Coral Reef Symposium, Guam, Micronesia, 22 June 1992; Volume 1, pp. 127–133. [Google Scholar]
- Zimmerman, J.K.; Willig, M.X.; Hernández-Delgado, E.A. Resistance, resilience and vulnerability of socio-ecological systems to hurricanes in Puerto Rico. Ecosphere 2020, 11, e03159. [Google Scholar] [CrossRef]
- Nieto, R.R.; Carrara, X.C.; Sheppard, C. Efectos de los huracanes sobre la estabilidad de paisajes asociados con arrecifes coralinos. Cien. Mar. 2012, 38, 47–55. [Google Scholar]
- Lirman, D.; Fong, P. Patterns of damage to the branching coral Acropora palmata following Hurricane Andrew: Damage and survivorship ofhurricane-generated asexual recruits. J. Coast. Res. 1997, 13, 67–72. [Google Scholar]
- Hubbard, D.K. Sedimentation as a control of reef development: St. Croix. USVI. Coral Reefs 1986, 5, 117–125. [Google Scholar] [CrossRef]
- Ritson-Williams, R.; Arnold, S.N.; Fogarty, N.D.; Steneck, R.S.; Vermeij, M.J.; Paul, V.J. New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smith Contrib. Mar. Sci. 2009, 38, 437–457. [Google Scholar] [CrossRef]
- Doropoulos, C.; Ward, S.; Díaz-Pulido, G.; Hoegh-Guldberg, O.; Mumby, P.J. Ocean acidification reduces coral recruitment by disrupting intimate larval-algal settlement interactions. Ecol. Lett. 2012, 15, 338–346. [Google Scholar] [CrossRef]
- Harrington, L.; Fabricius, K.; De’ath, G.; Negri, A. Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology 2004, 85, 3428–3437. [Google Scholar] [CrossRef]
- Day, R.W. Effects of benthic algae on sessile animals: Observational evidence from coral reef habitats. Bull. Mar. Sci. 1983, 33, 597–605. [Google Scholar]
- Wolf, A.T.; Nugues, M.M. Predation on coral settlers by the corallivorous fireworm Hermodice carunculata. Coral Reefs 2013, 32, 227–231. [Google Scholar] [CrossRef]
- Arnold, S.N.; Steneck, R.S.; Mumby, P.J. Running the gauntlet: Inhibitory effects of algal turfs on the processes of coral recruitment. Mar. Ecol. Prog. Ser. 2010, 414, 91–105. [Google Scholar] [CrossRef]
- Roff, G.; Chollett, I.; Doropoulos, C.; Golbuu, Y.; Steneck, R.S.; Isechal, A.L.; van Woesik, R.; Mumby, P.J. Exposure-driven macroalgal phase shift following catastrophic disturbance on coral reefs. Coral Reefs 2015, 34, 715–725. [Google Scholar] [CrossRef]
- Maida, M.; Coll, J.C.; Sammarco, P.W. Shedding new light on scleractinian coral recruitment. J. Exp. Mar. Biol. Ecol. 1994, 180, 189–202. [Google Scholar] [CrossRef]
- Baird, A.H.; Babcock, R.C.; Mundy, C.P. Habitat selection by larvae influences the depth distribution of six common coral species. Mar. Ecol. Progr. Ser. 2003, 252, 289–293. [Google Scholar] [CrossRef]
- Edmunds, P.J.; Leichter, J.J.; Adjeroud, M. Landscape-scale variation in coral recruitment in Moorea, French Polynesia. Mar. Ecol. Prog. Ser. 2010, 414, 75–89. [Google Scholar] [CrossRef][Green Version]
- Salinas-de-León, P.; Dryden, C.; Smith, D.J.; Bell, J.J. Temporal and spatial variability in coral recruitment on two Indonesian coral reefs: Consistently lower recruitment to a degraded reef. Mar. Biol. 2013, 160, 97–105. [Google Scholar] [CrossRef]
- Fox, H.E. Coral recruitment in blasted and unblasted sites in Indonesia: Assessing rehabilitation potential. Mar. Ecol. Progr. Ser. 2004, 269, 131–139. [Google Scholar] [CrossRef]
- Fox, H.E.; Mous, P.J.; Pet, J.S.; Muljadi, A.H.; Caldwell, R.L. Experimental assessment of coral reef rehabilitation following blast fishing. Conserv. Biol. 2005, 19, 98–107. [Google Scholar] [CrossRef]
- Raymundo, L.J.; Maypa, A.P.; Gomez, E.D.; Cadiz, P. Can dynamite-blasted reefs recover? A novel, low-tech approach to stimulating natural recovery in fish and coral populations. Mar. Poll. Bull. 2007, 54, 1009–1019. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.L. The effects of storms and cyclones on coral reefs: A review. J. Coast. Res. 1994, 211–231. [Google Scholar]
- Dajka, J.C.; Wilson, S.K.; Robinson, J.P.; Chong-Seng, K.M.; Harris, A.; Graham, N.A. Uncovering drivers of juvenile coral density following mass bleaching. Coral Reefs 2019, 38, 637–649. [Google Scholar] [CrossRef]
- Mallela, J.; Crabbe, M.J.C. Hurricanes and coral bleaching linked to changes in coral recruitment in Tobago. Mar. Environ. Res. 2009, 68, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Mumby, P.J.; Harborne, A.R.; Williams, J.; Kappel, C.V.; Brumbaugh, D.R.; Micheli, F.; Holmes, K.E.; Dahlgren, C.P.; Paris, C.B.; Blackwell, P.G. Trophic cascade facilitates coral recruitment in a marine reserve. Proc. Natl. Acad. Sci. USA 2007, 104, 8362–8367. [Google Scholar] [CrossRef] [PubMed]
- Bozec, Y.M.; Alvarez-Filip, L.; Mumby, P.J. The dynamics of architectural complexity on coral reefs under climate change. Glob. Chang. Biol. 2014, 21, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, P.J.; Elahi, R. The demographics of a 15-year decline in cover of the Caribbean reef coral Montastraea annularis. Ecol. Monogr. 2007, 77, 3–18. [Google Scholar] [CrossRef]
- Weil, E.; Hernández-Delgado, E.A.; González, M.; Williams, S.; Suleimán-Ramos, S.; Figuerola, M.; Metz-Estrella, T. Spread of the new coral disease “SCTLD” into the Caribbean: Implications for Puerto Rico. Reef Encount. 2009, 34, 38–43. [Google Scholar]
- Meiling, S.; Smith, T.B.; Muller, E.; Brandt, M.E. Stony coral tissue loss disease (SCTLD) lesion progression slows in association with thermal stress. Front. Mar. Sci. 2020, 7, 1128. [Google Scholar] [CrossRef]
- Miller, J.; Waara, R.; Muller, E.; Rogers, C. Coral bleaching and disease combine to cause extensive mortality on reefs in US Virgin Islands. Coral Reefs 2006, 25, 418. [Google Scholar] [CrossRef]
- Rogers, C.S.; Muller, E.; Spitzack, T.; Miller, J. Extensive coral mortality in the US Virgin Islands in 2005/2006: A review of the evidence for synergy among thermal stress, coral bleaching and disease. Caribb. J. Sci. 2009, 45, 204–214. [Google Scholar] [CrossRef]
- Rogers, C. Coral bleaching and disease should not be underestimated as causes of Caribbean coral reef decline. Proc. R. Soc. B Biol. Sci. 2009, 276, 197–198. [Google Scholar] [CrossRef]
- Aeby, G.; Ushijima, B.; Bartels, E.; Walter, C.; Kuehl, J.; Jones, S.; Paul, V.J. Changing stony coral tissue loss disease dynamics through time in Montastraea cavernosa. Front. Mar. Sci. 2021, 8, 699075. [Google Scholar] [CrossRef]
- Brandt, M.E.; Ennis, R.S.; Meiling, S.S.; Townsend, J.; Cobleigh, K.; Glahn, A.; Quetel, J.; Brandtneris, V.; Henderson, L.M.; Smith, T.B. The emergence and initial impact of stony coral tissue loss disease (SCTLD) in the United States Virgin Islands. Front. Mar. Sci. 2021, 8, 715329. [Google Scholar] [CrossRef]
- Estrada-Saldívar, N.; Quiroga-García, B.A.; Pérez-Cervantes, E.; Rivera-Garibay, O.O.; Alvarez-Filip, L. Effects of the stony coral tissue loss disease outbreak on coral communities and the benthic composition of Cozumel Reefs. Front. Mar. Sci. 2021, 8, 632777. [Google Scholar] [CrossRef]
- Neely, K.L.; Lewis, C.L.; Lunz, K.S.; Kabay, L. Rapid population decline of the pillar coral Dendrogyra cylindrus along the Florida Reef Tract. Front. Mar. Sci. 2021, 8, 656515. [Google Scholar] [CrossRef]
- Spadafore, R.; Fura, R.; Precht, W.F.; Vollmer, S.V. Multi-variate analyses of coral mortality from the 2014–2015 stony coral tissue loss disease outbreak off Miami-Dade County, Florida. Front. Mar. Sci. 2021, 8, 723998. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; González-Barrios, F.; Pérez-Cervantes, E.; Molina-Hernandez, A.; Estrada-Saldívar, N. An emerging coral disease outbreak decimated Caribbean coral populations and reshaped reef functionality. Res. Sq. 2021, 1–17. [Google Scholar] [CrossRef]
- Perry, C.T.; Murphy, G.N.; Kench, P.S.; Edinger, E.N.; Smithers, S.G.; Steneck, R.S.; Mumby, P.J. Changing dynamics of Caribbean reef carbonate budgets: Emergence of reef bioeroders as critical controls on present and future reef growth potential. Proc. R. Soc. B Biol. Sci. 2014, 281, 20142018. [Google Scholar] [CrossRef]
- Perry, C.T.; Alvarez-Filip, L. Changing geo-ecological functions of coral reefs in the Anthropocene. Funct. Ecol. 2019, 33, 976–988. [Google Scholar] [CrossRef]
- González-Barrios, F.J.; Cabral-Tena, R.A.; Alvarez-Filip, L. Recovery disparity between coral cover and the physical functionality of reefs with impaired coral assemblages. Glob. Chang. Biol. 2021, 27, 640–651. [Google Scholar] [CrossRef]
- Carpenter, R.C.; Edmunds, P.J. Local and regional scale recovery of Diadema promotes recruitment of scleractinian corals. Ecol. Lett. 2006, 9, 271–280. [Google Scholar] [CrossRef]
- Mumby, P.J. Bleaching and hurricane disturbances to populations of coral recruits in Belize. Mar. Ecol. Prog. Ser. 1999, 190, 27–35. [Google Scholar] [CrossRef]
- Miller, M.W.; Weil, E.; Szmant, A.M. Coral recruitment and juvenile mortality as structuring factors for reef benthic communities in Biscayne National Park, USA. Coral Reefs 2000, 19, 115–123. [Google Scholar] [CrossRef]
- Ruiz-Zárate, M.A.; Arias-González, J.E. Spatial study of juvenile corals in the Northern region of the Mesoamerican Barrier Reef System (MBRS). Coral Reefs 2004, 23, 584–594. [Google Scholar]
- Rylaarsdam, K.W. Life histories and abundance patterns of colonial corals on Jamaican reefs. Mar. Ecol. Prog. Ser. 1983, 13, 249–260. [Google Scholar] [CrossRef]
- Bak, R.P.M.; Engel, M.S. Distribution, abundance and survival of juvenile hermatypic corals (Scleractinia) and the importance of life history strategies in the parent coral community. Mar. Biol. 1979, 54, 341–352. [Google Scholar] [CrossRef]
- Mumby, P.J.; Harborne, A.R. Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS ONE 2010, 5, e8657. [Google Scholar] [CrossRef]
- Edmunds, P.J. Juvenile coral population dynamics track rising seawater temperature on a Caribbean reef. Mar. Ecol. Prog. Ser. 2004, 269, 111–119. [Google Scholar] [CrossRef]
- Green, D.H.; Edmunds, P.J.; Carpenter, R.C. Increasing relative abundance of Porites astreoides on Caribbean reefs mediated by an overall decline in coral cover. Mar. Ecol. Progr. Ser. 2008, 359, 1–10. [Google Scholar] [CrossRef]
- Lowe, R.J.; Falter, J.L. Oceanic forcing of coral reefs. Annu. Rev. Mar. Sci. 2015, 7, 43–66. [Google Scholar] [CrossRef]
- Storlazzi, C.; Reguero, B.; Lowe, E.; Shope, J.; Gibbs, A.; Beck, M.; Nickel, B. Rigorously valuing the role of coral reefs in coastal protection: An example from Maui, Hawaii, USA. Coast. Dynam. 2017, 35, 665–674. [Google Scholar]
- Rogers, A.; Blanchard, J.L.; Mumby, P.J. Fisheries productivity under progressive coral reef degradation. J. Appl. Ecol. 2017, 55, 1041–1049. [Google Scholar] [CrossRef]
- Rogers, A.; Blanchard, J.L.; Newman, S.P.; Dryden, C.S.; Mumby, P.J. High refuge availability on coral reefs increases the vulnerability of reef-associated predators to overexploitation. Ecology 2018, 99, 450–463. [Google Scholar] [CrossRef]
- Osorio-Cano, J.D.; Osorio, A.F.; Alcérreca-Huerta, J.C.; Oumeraci, H. Drag and inertia forces on a branched coral colony of Acropora palmata. J. Fluids Struct. 2019, 88, 31–47. [Google Scholar] [CrossRef]
- Reguero, B.G.; Secaira, F.; Toimil, A.; Escudero, M.; Díaz-Simal, P.; Beck, M.W.; Silva, R.; Storlazzi, C.; Losada, I.J. The risk reduction benefits of the Mesoamerican reef in Mexico. Front. Earth Sci. 2019, 7, 125. [Google Scholar] [CrossRef]
- Asher, S.; Shavit, U. The effect of water depth and internal geometry on the turbulent flow inside a coral reef. J. Geophys. Res. Ocean. 2019, 124, 3508–3522. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Q.; Ding, Y.; Jafari, N.; Rosati, J.D. Semianalytical model of depth-integrated vegetal drag force based on Stokes second-order wave theory. J. Waterw. Port Coast. Ocean. Eng. 2019, 145, 04018041. [Google Scholar] [CrossRef]
- Smithers, S.G.; Hoeke, R.K. Geomorphological impacts of high-latitude storm waves on low-latitude reef islands—Observations of the December 2008 event on Nukutoa, Takuu, Papua New Guinea. Geomorphology 2014, 222, 106–121. [Google Scholar] [CrossRef]
- Tajima, Y.; Shimozono, T.; Gunasekara, K.H.; Cruz, E.C. Study on locally varying inundation characteristics induced by Super Typhoon Haiyan. Part 2: Deformation of storm waves on the beach with fringing reef along the East Coast of Eastern Samar. Coast. Eng. J. 2016, 58, 1640003-1–1640003-24. [Google Scholar] [CrossRef]
- Pomeroy, A.W.; Lowe, R.J.; Ghisalberti, M.; Storlazzi, C.; Symonds, G.; Roelvink, D. Sediment transport in the presence of large reef bottom roughness. J. Geophys. Res. Ocean. 2017, 122, 1347–1368. [Google Scholar] [CrossRef]
- Pomeroy, A.W.; Lowe, R.J.; Ghisalberti, M.; Winter, G.; Storlazzi, C.; Cuttler, M. Spatial variability of sediment transport processes over intratidal and subtidal timescales within a fringing coral reef system. J. Geophys. Res. Earth Surf. 2018, 123, 1013–1034. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Nash, K.L. The importance of structural complexity in coral reef ecosystems. Coral Reefs 2013, 32, 315–326. [Google Scholar] [CrossRef]
- Rogers, A.; Blanchard, J.L.; Mumby, P.J. Vulnerability of coral reef fisheries to a loss of structural complexity. Curr. Biol. 2014, 24, 1000–1005. [Google Scholar] [CrossRef]
- Rogers, A.; Harborne, A.R.; Brown, C.J.; Bozec, Y.M.; Castro, C.; Chollett, I.; Hock, K.; Knowland, C.A.; Marshell, A.; Ortiz, J.C.; et al. Anticipative management for coral reef ecosystem services in the 21st century. Glob. Chang. Biol. 2015, 21, 504–514. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).