Indication of Possible Shifts in Copepod Species Composition in St. Pauls Inlet, a Fjordal Estuary Connected to the Gulf of St. Lawrence
Abstract
1. Introduction
2. Materials and Methods
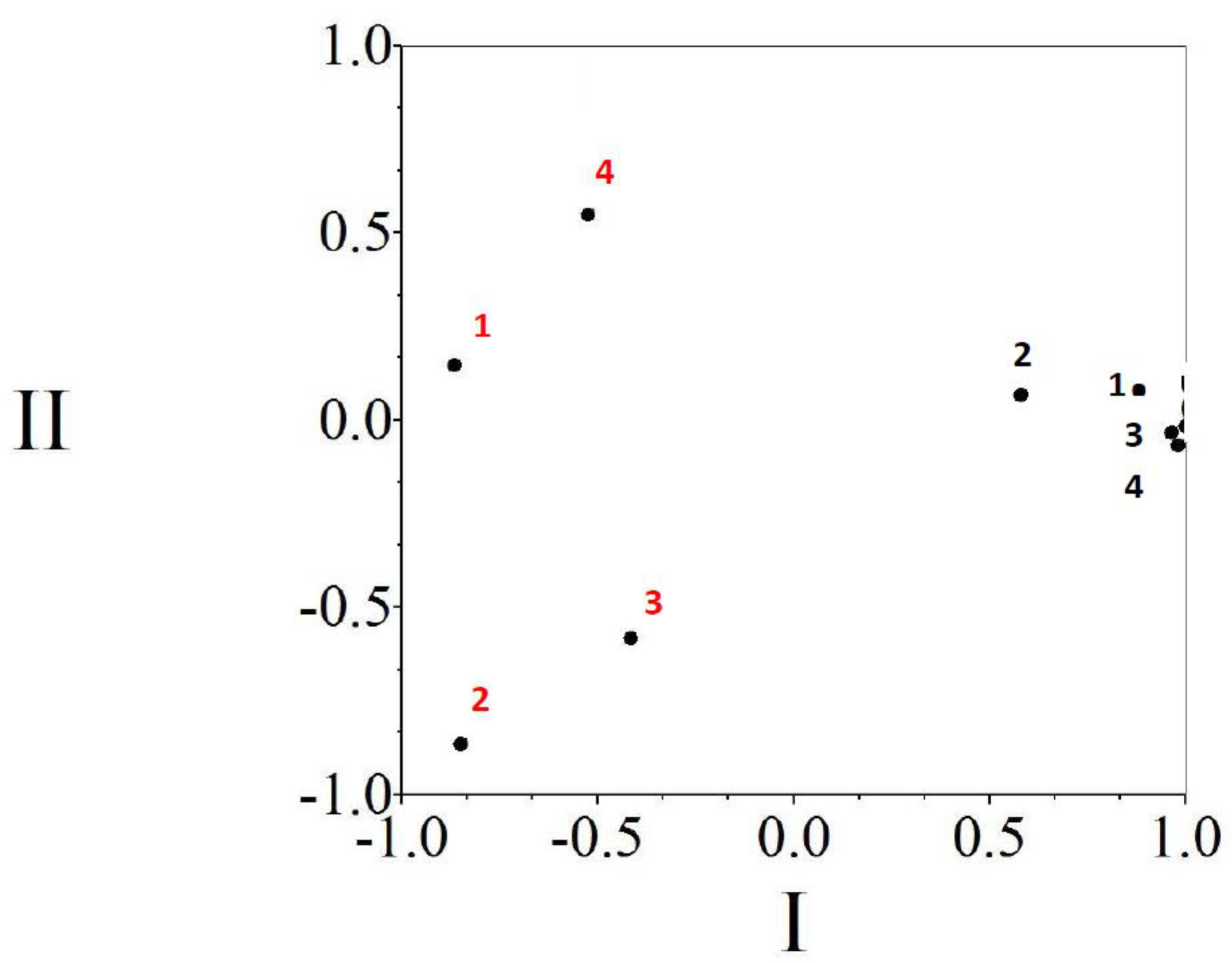
3. Results
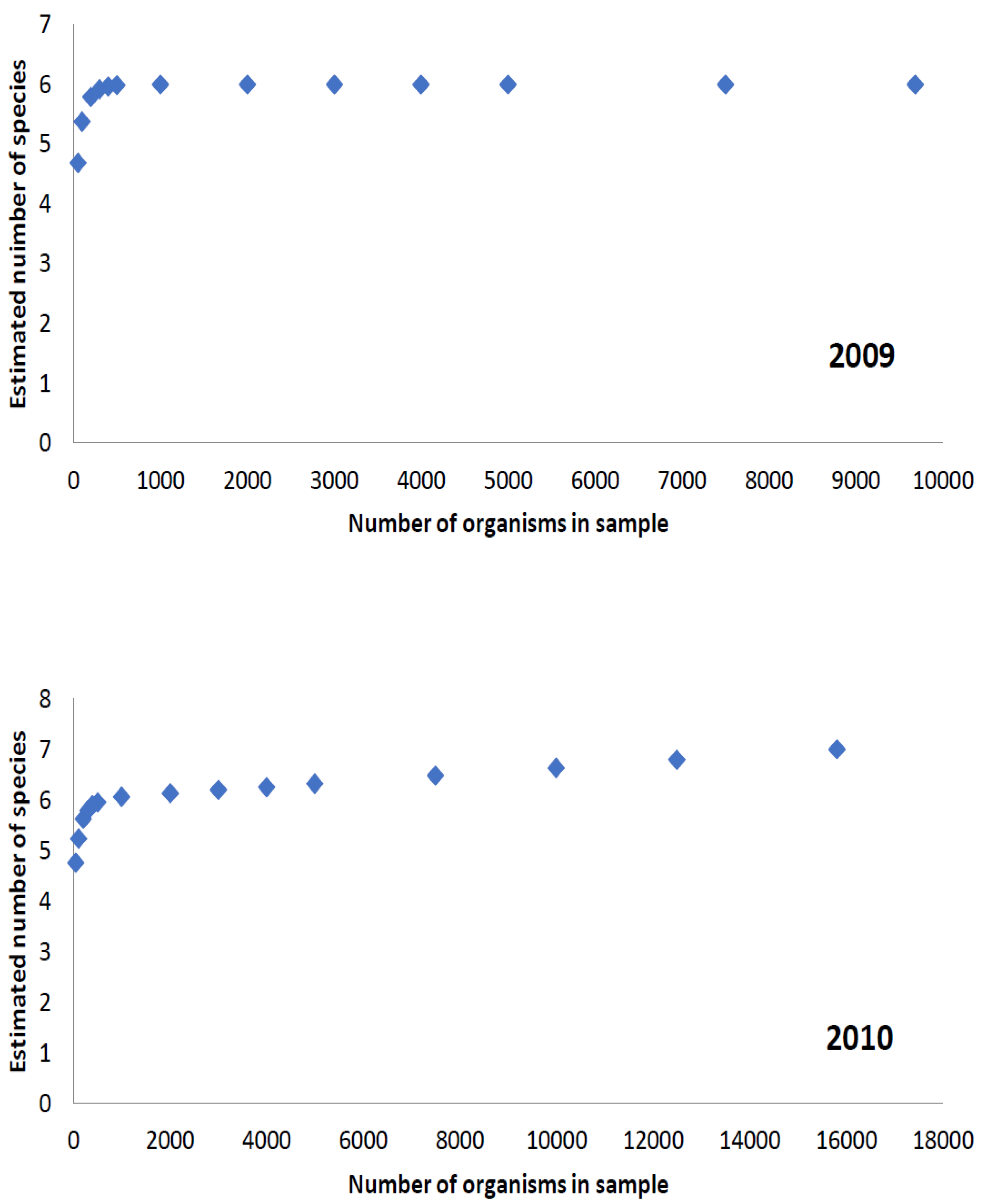
3.1. Species Richness
3.2. Copepods
3.3. Temperature
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Day, J.H. What is an estuary? S. Afr. J. Sci. 1980, 76, 198. [Google Scholar]
- Knox, G.A. Estuarine Ecosystems: A Systems Approach; CRC Press Inc.: Boca Raton, FL, USA, 1986; Volume I. [Google Scholar]
- Marques, S.C.; Pardal, M.A.; Pereira, M.J.; Goncalves, F.; Marques, J.C.; Azeiteiro, U.M. Zooplankton distribution and dynamics in a temperate shallow estuary. Hydrobiologia 2007, 587, 213–223. [Google Scholar] [CrossRef]
- Barnes, R.S.K. The Brackish-Water Fauna of Northwestern Europe; Cambridge University Press: New York, NY, USA, 1994. [Google Scholar]
- Pritchard, D.W. What is an estuary: Physical viewpoint. Am. Assoc. Advan. Sci. 1967, 85, 3–5. [Google Scholar]
- O’Sullivan, W. Hydrological Inventory of Gros Morne National Park, Newfoundland Canada; Inland Waters Directorate, Water Resources Branch: Halifax, NS, Canada, 1976. [Google Scholar]
- Galbraith, P.S. Winter water masses in the Gulf of St. Lawrence. J. Geophys. Res. 2006, 111, 1–23. [Google Scholar] [CrossRef]
- Savenkoff, C.; Vezina, A.F.; Smith, P.C.; Han, G. Summer transports of nutrients in the Gulf of St. Lawrence estimated by inverse modeling. Estuar. Coast. Shelf Sci. 2001, 52, 565–587. [Google Scholar] [CrossRef]
- Kim, H.; Lee, C.-R.; Lee, S.-k.; Oh, S.-Y.; Kim, W. Biodiversity and community structure of mesozooplankton in the marine and coastal national park areas of Korea. Diversity 2020, 12, 233. [Google Scholar] [CrossRef]
- Carter, J.A.; MacGregor, C.D. Marine Inventory of St. Paul’s Inlet for Gros Morne National Park; Report for Parks Canada; Martec Limited: Halifax, NS, Canada, 1979. [Google Scholar]
- Stevens, E.N. A Biological Assessment of Zooplankton in St. Pauls Inlet: An Estuarine Environment in Gros Morne National Park, Newfoundland. Master’s Thesis, Dept. of Biology, Memorial University of Newfoundland, St. Johns, NL, Canada, 2017. [Google Scholar]
- Sella, G.F.; Stein, S.; Dixon, T.H.; Craymer, M.; James, T.S.; Mazzotti, S.; Dokka, R.K. Observation of glacial isostatic adjustment in “stable” North America with GPS. Geophys. Res. Lett. 2007, 34, 1–6. [Google Scholar] [CrossRef]
- ESRI. ArcGIS Desktop: Release 10; ArcMap 10.7.1; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- Black, A.R.; Dodson, S.I. Ethanol: A better preservation technique for Daphnia. Limnol. Oceanogr. Methods. 2003, 1, 45–50. [Google Scholar] [CrossRef]
- Della Croce, N. Cladocera; ICES ID Leaflet No. 143: Copenhagen, Denmark, 1974. [Google Scholar]
- Shih, C.; Marhue, L.; Barrett, N.; Munro, R. Planktonic copepods of the Bras d’Or Lakes system, Nova Scotia, Canada. Hydrobiologia 1988, 167, 319–324. [Google Scholar] [CrossRef]
- Frost, B.W. A taxonomy of the marine calanoid copepod genus Pseudocalanus. Can. J. Zool. 1989, 6, 525–551. [Google Scholar] [CrossRef]
- Todd, C.D.; Laverack, M.S.; Boxshall, G.A. Coastal Marine Zooplankton: A Practical Manual for Students, 2nd ed.; Cambridge University Press: New York, NY, USA, 1996. [Google Scholar]
- Bradford-Grieve, J.M. Copepoda: Calanoida: Acartiidae; ICES ID Leaflet No. 181: Copenhagen, Denmark, 1999. [Google Scholar]
- Gerber, R.P. An Identification Manual to the Coastal and Estuarine Zooplankton from Passamaquoddy Bay to Long Island Sound; Acadia Productions: Brunswick, ME, USA, 2000. [Google Scholar]
- Campbell, C.E.; Knoechel, R. Species occurrences of microcrustacean zooplankton in Labrador, Canada. Verh. Int. Ver. Limnol. 2008, 30, 77–81. [Google Scholar] [CrossRef]
- Johnson, W.S.; Allen, D.M. Zooplankton of the Atlantic and Gulf Coasts: A Guide to their Identifications and Ecology; Johns Hopkins University Press: Baltimore, MD, USA, 2005. [Google Scholar]
- Walter, T.C.; Boxshall, G. World of Copepods Database. 2014. Available online: http://www.marinespecies.org/copepoda (accessed on 12 June 2014).
- Holland, S.M. Non-Metric Multidimensional Scaling (MDS); Dept. of Geology, University of Georgia: Athens, GA, USA, 2008; Available online: https://strata.uga.edu/software/pdf/mdsTutorial.pdf (accessed on 3 June 2016).
- Rohlf, F.J. NTSYSpc Numerical Taxonomy and Multivariate Analysis System; Version 2.21L; Exeter Software, Setauket: New York, NY, USA, 2009. [Google Scholar]
- Winkler, G.; Dodson, J.J.; Bertrand, N.; Thivierge, D.; Vincent, W. Trophic coupling across the St. Lawrence River estuarine transition zone. Mar. Ecol. Prog. Ser. 2003, 251, 59–73. [Google Scholar] [CrossRef]
- Debertin, A.J.; Hanson, J.M.; Courtenay, S.C. Linking zooplankton assemblages with oceanographic zones in an Atlantic coastal system. Can. J. Fish. Aquat. Sci. 2018, 75, 868–882. [Google Scholar] [CrossRef]
- Bernier, R.Y.; Jamieson, R.E.; Moore, A.M. (Eds.) State of the Atlantic Ocean Synthesis Report. Can. Tech. Rep. Fish. Aquat. Sci. 2018, 3167, iii + 149p. [Google Scholar]
- Moore, M.; Folt, C. Zooplankton body size and community structure: Affects of thermal and toxicant stress. Trends Ecol. Evol. 1993, 8, 178–183. [Google Scholar] [CrossRef]
- Preziosi, B.M.; Runge, J.A. The effect of warm temperatures on hatching success of the marine planktonic copepod, Calanus finmarchicus. J. Plankton Res. 2014, 36, 1381–1384. [Google Scholar] [CrossRef][Green Version]
- Favier, J.-B.; Winkler, G. Coexistence, distribution patterns and habitat utilization of the sibling species complex Eurytemora affinis in the St. Lawrence estuarine transition zone. J. Plankton Res. 2014, 36, 1247–1261. [Google Scholar] [CrossRef]
- Hinder, S.L.; Gravenor, M.B.; Edwards, M.; Ostle, C.; Bodger, O.G.; Lee, P.L.; Walne, A.W.; Hays, G.C. Multi-decadal range changes vs. thermal adaptation for north east Atlantic oceanic copepods in the face of climate change. Glob. Chang. Biol. 2014, 20, 140–146. [Google Scholar] [CrossRef]
- Sorochan, K.A.; Plourde, S.; Morse, R.; Pepin, P.; Runge, J.; Thompson, C.; Johnson, C.L. North Atlantic right whale (Eubalaena glacialis) and its food: (II) interannual variations in biomass of Calanus spp. on western North Atlantic shelves. J. Plankton Res. 2019, 41, 687–708. [Google Scholar] [CrossRef]
- Galbraith, P.S.; Chassé, J.; Shaw, J.-L.; Dumas, J.; Caverhill, C.; Lefaivre, D.; Lafleur, C. Physical oceanographic conditions in the Gulf of St. Lawrence during 2019. DFO Can. Sci. Advis. Sec. Res. Doc. 2020, 2020/030, iv + 84p. [Google Scholar]
- Bordages, H.; Brassard, C.; Desgagnés, M.; Galbraith, P.; Gauthier, J.; Nozères, C.; Scallon-Chouinard, P.-M.; Senay, C. Preliminary results from the ecosystemic survey in August 2019 in the estuary and northern Gulf of St. Lawrence. DFO Can. Sci. Advis. Sec. Res. Doc. 2020, 2020/009, iv + 93p. [Google Scholar]
- Melanson, R.; Campbell, C.E. Near-shore fish populations within St. Pauls Inlet, an estuarine system in western Newfoundland. Northeast. Nat. 2012, 19, 487–500. [Google Scholar] [CrossRef][Green Version]
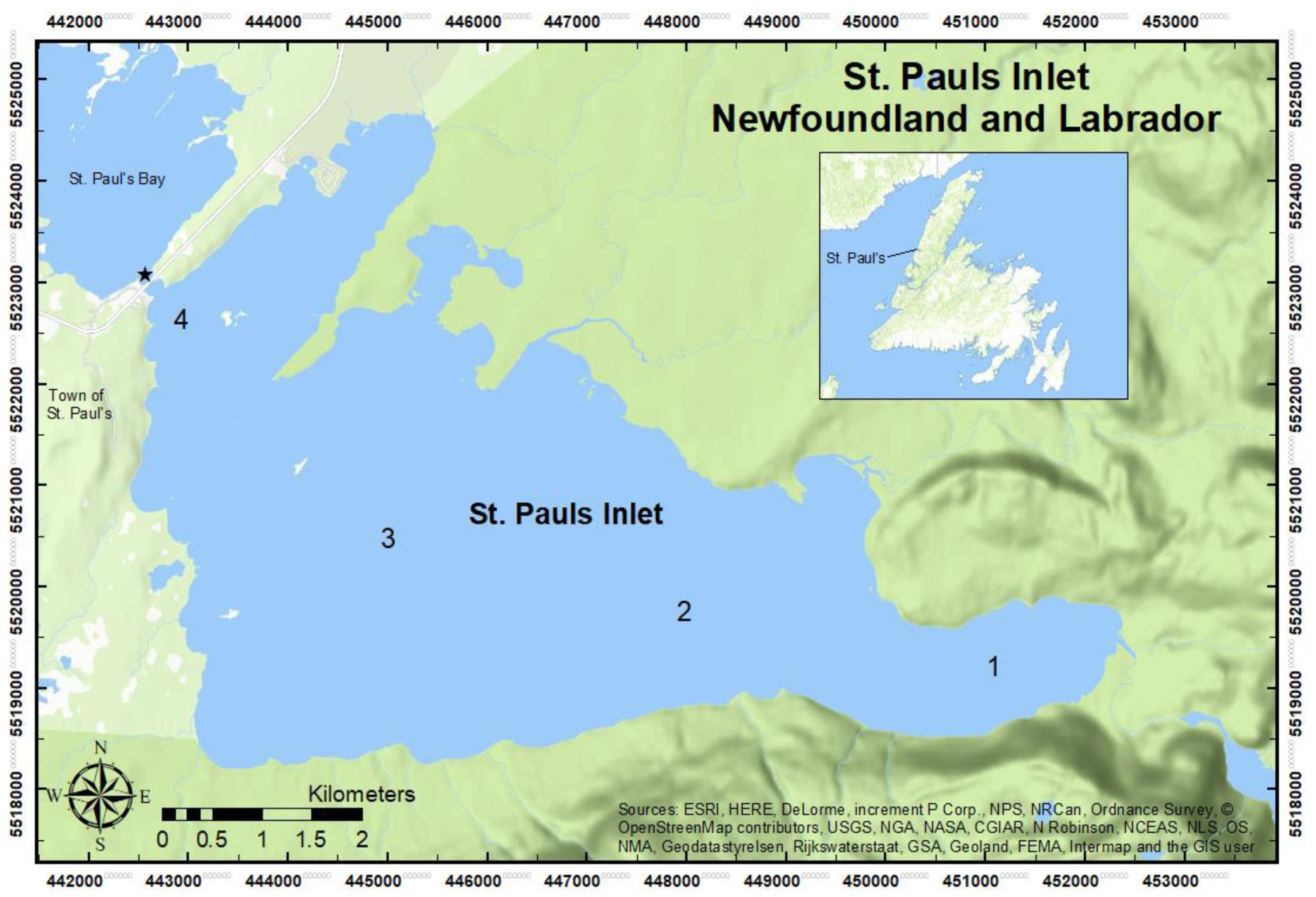
| Survey | Sampling Sites | Sampling Type | Mesh Size |
|---|---|---|---|
| 1977 (July) and 1978 (May) | 4 open-water sites, 1 near shore | Vertical tows (discrete 5 m depth intervals from approx. 15–20 m to the surface). Surface 5 min horizontal tows (vertical + horizontal in summer, horizontal only in spring). | birge-style closing net (17 cm diameter and 108 cm long; mesh size n/a), Niskin bottle. plankton net of 80 μm mesh. |
| 2009 and 2010 (biweekly June–August both years) | 4 open-water sites | Two 2 min oblique horizontal tows per site, either small or large-mesh conical net, with calibrated General Oceanics® flow meter attached for estimation of volume of water filtered. Vertical tows at each site. Two depths of tows were taken per site, one close to 1 m above the bottom and one down to half of the maximum depth of the sampling site, with two tows per depth range. | small-mesh conical net (63 µm mesh, 300 mm mouth diameter, 1.0 m length). large-mesh conical net (500 µm mesh, 300 mm mouth diameter, 1.0 m length). 80 µm mesh conical net, 200 mm mouth diameter, 0.5 m length. |
| 2019 (July) | 4 open-water sites | Two vertical tows at each site. | 80 µm mesh conical net, 200 mm mouth diameter, 0.5 m length. |
| Microcrustacean Species | Habitat Range | SPI C and M | SPI S and C |
|---|---|---|---|
| Cladocera | |||
| Evadne nordmanni Lovén, 1836 | Brackish | ✓ | ✓ |
| Podon leuckarti (Sars, 1862) | Brackish | ✓ | |
| Copepoda | |||
| Acartia hudsonica Pinhey, 1926 * | Marine | ✓ | ✓ |
| Calanus finmarchicus (Gunnerus, 1770) * | Marine | ✓ | ✓ |
| Centropages typicus (Kröyer, 1849) | Marine | ✓ | ✓ |
| Eurytemora affinis (Poppe, 1880) | Brackish | ✓ | |
| Harpacticus chelifer (Müller O.F., 1776) | Marine | ✓ | |
| Macrosetella gracilis (Dana, 1847) | Marine | ✓ | |
| Metridia longa (Lubbock, 1854) | Marine | ✓ | |
| Metridia lucens Boeck, 1865 | Marine | ✓ | |
| Microsetella norvegica (Boeck, 1865) | Marine | ✓ | |
| Oithona similis Claus, 1866 * | Brackish | ✓ | ✓ |
| Oncaea venusta Philippi, 1843 | Marine | ✓ | |
| Pseudocalanus elongatus (Boeck, 1865) | Marine | ✓ | |
| Temora longicornis (Müller O.F., 1785) * | Marine | ✓ | ✓ |
| Mean Density (inds/L) | Head of Inlet | Mid-Inlet I | Mid-Inlet II | Near Mouth | ||
|---|---|---|---|---|---|---|
| Calanus | 1977 | -- | A | C | A | C |
| 2009 | 0.00 | 0 | 0 | 0 | 0 | |
| 2010 | 0.00 | 0 | R (0.04%) | 0 | 0 | |
| 2019 | 0.00 | 0 | 0 | 0 | 0 | |
| Acartia | 1977 | -- | C | I | C | C |
| 2009 | 3.73 ± 2.11 | C | C | C | C | |
| 2010 | 4.02 ± 2.87 | A | A | A | A | |
| 2019 | 2.14 ± 1.35 | C | I | C | I | |
| Oithona | 1977 | -- | C | C | C | C |
| 2009 | 0.42 ± 0.68 | R | I | I | I | |
| 2010 | 0.52 ± 0.79 | I | I | I | R | |
| 2019 | 3.84 ± 2.52 | C | A | C | C | |
| Temora | 1977 | -- | C | I | C | C |
| 2009 | 3.65 ± 2.35 | C | C | C | C | |
| 2010 | 0.70 ± 0.40 | I | I | I | I | |
| 2019 | 0.33 ± 0.16 | I | I | I | I | |
| Density of adult microcrustaceans | 2009 | 11.3 ± 0.00 | 5.14 ± 3.00 | 12.5 ± 8.53 | 2.88 ± 1.59 | |
| 2010 | 9.08 ± 7.38 | 6.02 ± 3.98 | 3.40 ± 3.90 | 1.99 ± 1.82 | ||
| 2019 | 7.99 ± 2.48 | 9.44 ± 3.65 | 7.11 ± 1.77 | 1.34 ± 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevens, E.N.; Campbell, C.E. Indication of Possible Shifts in Copepod Species Composition in St. Pauls Inlet, a Fjordal Estuary Connected to the Gulf of St. Lawrence. Diversity 2022, 14, 59. https://doi.org/10.3390/d14010059
Stevens EN, Campbell CE. Indication of Possible Shifts in Copepod Species Composition in St. Pauls Inlet, a Fjordal Estuary Connected to the Gulf of St. Lawrence. Diversity. 2022; 14(1):59. https://doi.org/10.3390/d14010059
Chicago/Turabian StyleStevens, Erin N., and Christine E. Campbell. 2022. "Indication of Possible Shifts in Copepod Species Composition in St. Pauls Inlet, a Fjordal Estuary Connected to the Gulf of St. Lawrence" Diversity 14, no. 1: 59. https://doi.org/10.3390/d14010059
APA StyleStevens, E. N., & Campbell, C. E. (2022). Indication of Possible Shifts in Copepod Species Composition in St. Pauls Inlet, a Fjordal Estuary Connected to the Gulf of St. Lawrence. Diversity, 14(1), 59. https://doi.org/10.3390/d14010059





