Positive Interactions Drive Bat Distribution in a Remote Oceanic Archipelago (Azores, Portugal)
Abstract
1. Introduction
2. Materials and Methods
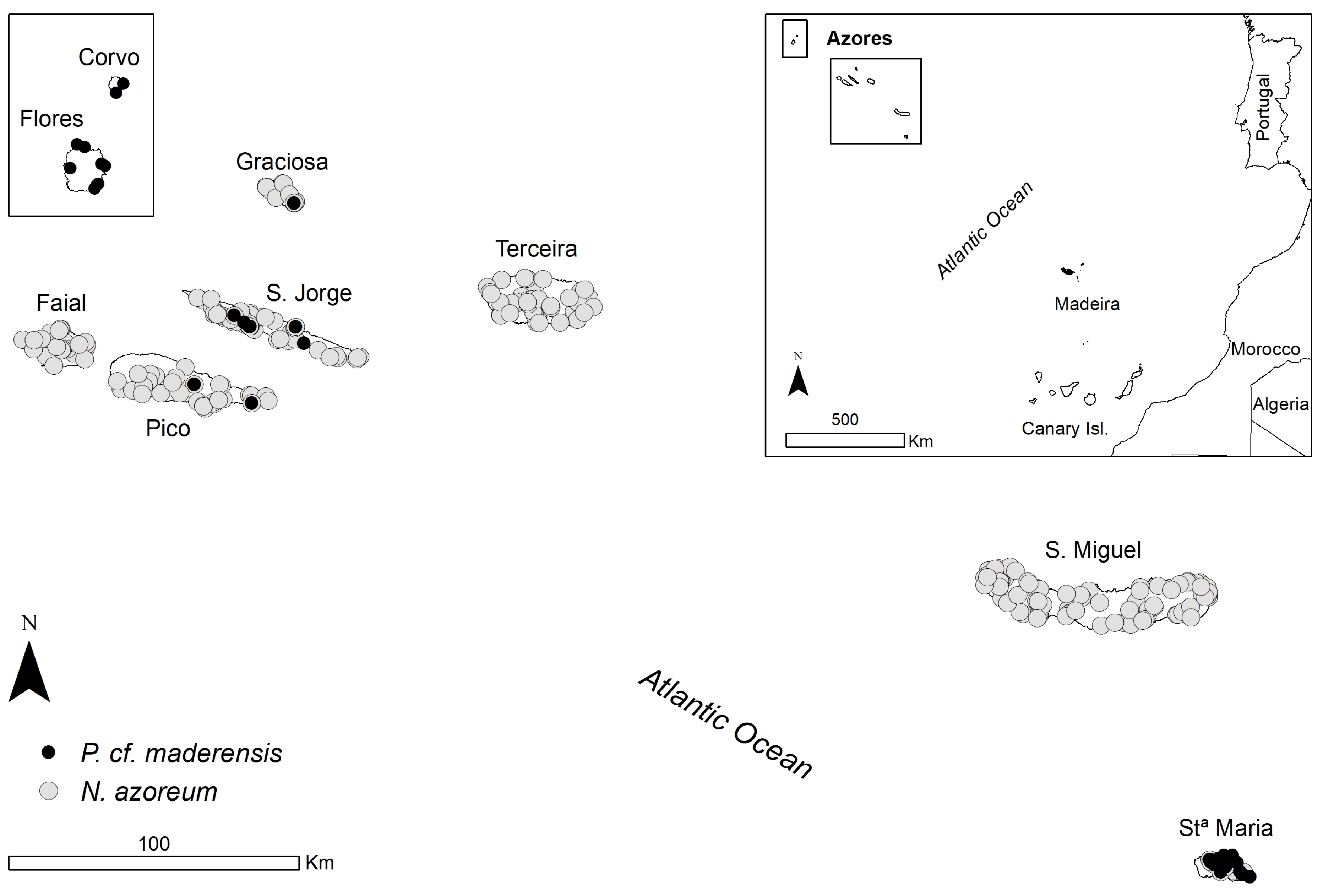
2.1. Study Area
2.2. Bat Sampling
2.3. Data Analysis
3. Results
3.1. Drivers of Occurrence at the Archipelago Scale
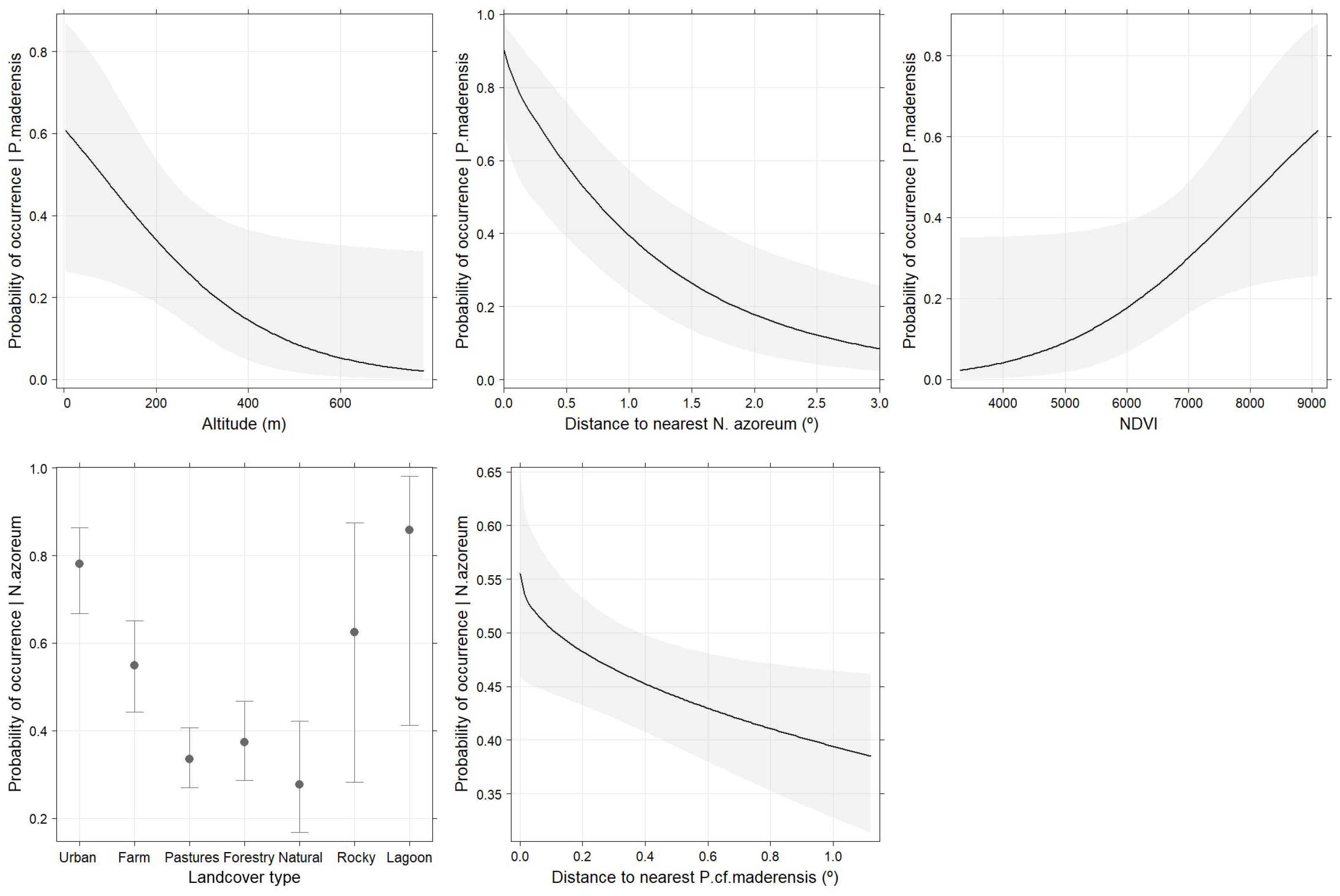
3.2. Drivers of Occurrence at the Island Scale
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Descriptor (Acronym) | Type, Units, and Classes | Data Source and Scale |
|---|---|---|
| Archipelago-scale descriptors | ||
| Island size (Size) | Terrestrial area of each island. Continuous, ranging from 17 to 745 km | Derived from CAOP 2016–Official Administrative Divisions of Portugal [80]. 2016 Vectorial 1:25,000. |
| Distance to the mainland (DistPT) | The minimum distance between the coastline of each island and the coastline of the Portuguese mainland. Continuous, ranging from 1367 to 1865 km | Ditto [80] |
| Distance to the nearest island (DistISL) | The minimum distance between the coastline of each island and the coastline of the nearest island. Continuous, ranging from 5.9 to 80.1 km | Ditto [80] |
| Distance to Madeira (DistMAD) | The minimum distance between each island’s coastline and the coastline of Madeira island (see Figure 1). Continuous, ranging from 840 to 1439 km. | Ditto [80] |
| UNEP isolation index (UNEP) | A measure of the isolation of the island from potential sources of colonisation. Continuous, ranging from 75 to 96. | Retrieved from Island Directory-UNEP/WCMC. Index per island. [81] |
| Island age | The maximum geological age of each island. Continuous, ranging from 0.27 to 8.12 Ma. | Value per island [82]. |
| Species’ occurrence (Nazor or Pmade) | Information on if each species was detected or not on each island. Binomial [0–1] | This study and Rainho et al. [14] |
| Island-scale descriptors | ||
| Altitude | Altitude a.s.l. Continuous, ranging from 0 to 1150 m | Derived from SRTM 90 m DEM [83] and validated using official cartography [84]. Raster 90 m. |
| Land-cover (LCo) | Includes all key land-cover types of the archipelago. Categorical: Urban, farm, pastures, forestry, natural, rocky and lagoon | Retrieved from [85] and validated in the field. 2007 Vectorial 1:15,000. |
| NDVI | 16-days Normalised Difference vegetation Index–a measure of green biomass. Continuous, ranging from 0 to 9201 (valid data ranging from −2000 to 10,000) | Derived from Modis 500 m 16-days imagery [86]. June 2015 Raster 500 m. |
| Distance to the coast (DistCoast) | Distance between the site sampled and the nearest coastline of the island. Continuous, ranging from 0 to 7500 m | Derived from CAOP2016 [80]. 2016 Vectorial 1:25,000. |
| Distance to P made/Nazor (DistPmade/DistNazor) | Distance to the nearest site where the presence of the co-occurring species was detected. Continuous, ranging from 0 to 2.58616º | This study and Rainho et al. [14]. |
| Estimate | Std. Error | z Value | Pr(>|z|) | |
|---|---|---|---|---|
| Pipistrellus cf. maderensis | ||||
| 0.6931 | 1.2247 | 0.566 | 0.571 | |
| Nazor(1) | −0.4055 | 1.4434 | −0.281 | 0.779 |
| 2.6240 | 1.6163 | 1.623 | 0.104 | |
| Size | −0.0070 | 0.0048 | −1.422 | 0.155 |
| −13.4913 | 18.9379 | −0.712 | 0.476 | |
| UNEP | 0.1781 | 0.2404 | 0.741 | 0.459 |
| −8.2110 | 8.3459 | −0.984 | 0.325 | |
| DistPT | 0.0056 | 0.0053 | 1.056 | 0.291 |
| 0.85945 | 1.0432 | 0.824 | 0.410 | |
| Island age | −0.0649 | 0.2928 | −0.222 | 0.825 |
| Nyctalus azoreum | ||||
| 1.0986 | 1.1547 | 0.951 | 0.341 | |
| Pmade(1) | −0.4055 | 1.4434 | −0.281 | 0.779 |
| −0.8788 | 1.6688 | −0.527 | 0.598 | |
| Size | 0.0145 | 0.0136 | 1.064 | 0.287 |
| 0.5186 | 1.1932 | 0.435 | 0.664 | |
| Island age | 0.3675 | 0.5396 | 0.681 | 0.496 |
References
- Fletcher, R.; Fortin, M.J. Introduction to Spatial Ecology and Its Relevance for Conservation. In Spatial Ecology and Conservation Modeling: Applications with R; Fletcher, R., Fortin, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Chapter 1; pp. 1–13. [Google Scholar]
- Hirzel, A.H.; Le Lay, G. Habitat suitability modelling and niche theory. J. Appl. Ecol. 2008, 45, 1372–1381. [Google Scholar] [CrossRef]
- Salinas-Ramos, V.; Ancillotto, L.; Cistrone, L.; Nastasi, C.; Bosso, L.; Smeraldo, S.; Cordero, V.; Russo, D. Artificial illumination influences niche segregation in bats. Environ. Pollut. 2021, 284, 117187. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Melton, A.E.; Soltis, D.E.; Soltis, P.S. Potential distributional shifts in North America of allelopathic invasive plant species under climate change models. Plant Divers. 2021. [Google Scholar] [CrossRef]
- Santos, H.; Juste, J.; Ibáñez, C.; Palmeirim, J.M.; Godinho, R.; Amorim, F.; Alves, P.; Costa, H.; de Paz, O.; Pérez-Suarez, G.; et al. Influences of ecology and biogeography on shaping the distributions of cryptic species: Three bat tales in Iberia. Biol. J. Linn. Soc. 2014, 112, 150–162. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.; Guisan, A. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.T.; Ferreira, M.A.R.; Kays, R.W.; Forrester, T.D.; Kalies, E.L.; McShea, W.J.; Parsons, A.W.; Millspaugh, J.J. A multispecies occupancy model for two or more interacting species. Methods Ecol. Evol. 2016, 7, 1164–1173. [Google Scholar] [CrossRef]
- Lewis, J.S.; Farnsworth, M.L.; Burdett, C.L.; Theobald, D.M.; Gray, M.; Miller, R.S. Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci. Rep. 2017, 7, 44152. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Conenna, I.; Rocha, R.; Russo, D.; Cabeza, M. Insular bats and research effort: A review of global patterns and priorities. Mammal Rev. 2017, 47, 169–182. [Google Scholar] [CrossRef]
- Bosso, L.; Mucedda, M.; Fichera, G.; Kiefer, A.; Russo, D. A gap analysis for threatened bat populations on Sardinia. Hystrix Ital. J. Mammal. 2016, 27, 212–214. [Google Scholar]
- Palmeirim, J.M. A morphometric assessment of the systematic position of the Nyctalus from Azores and Madeira (Mammalia:Chiroptera). Mammalia 1991, 55, 381–388. [Google Scholar] [CrossRef]
- Trujillo, D.; Gonzalez, C. Pipistrellus maderensis (Dobson, 1878), (Chiroptera: Vespertilionidae) a new addition to the Azorean fauna (Atlantic Ocean). Vieraea 2011, 39, 215–218. [Google Scholar]
- Rainho, A.; Marques, J.; Palmeirim, J. Os Morcegos dos Arquipélagos dos Açores e da Madeira: Um Contributo Para a Sua Conservação; Instituto da Conservação da Natureza: Lisboa, Portugal, 2002; p. 49. [Google Scholar]
- Hutson, A.; Aulagnier, S.; Rainho, A.; Palmeirim, J. Nyctalus azoreum. In The IUCN Red List of Threatened Species; e.T14922A4475157; 2008; Available online: https://www.researchgate.net/publication/295260102_Nyctalus_azoreum (accessed on 22 January 2016).
- Speakman, J.R.; Webb, P.I. Taxonomy, status and distribution of the Azorean bat (Nyctalus azoreum). J. Zool. 1993, 231, 27–38. [Google Scholar] [CrossRef]
- Irwin, N.R.; Speakman, J.R. Azorean bats Nyctalus azoreum, cluster as they emerge from roosts, despite the lack of avian predators. Acta Chiropterologica 2003, 5, 185–192. [Google Scholar] [CrossRef][Green Version]
- Salgueiro, P.; Coelho, M.M.; Palmeirim, J.M.; Ruedi, M. Mitochondrial DNA variation and population structure of the island endemic Azorean bat (Nyctalus azoreum). Mol. Ecol. 2004, 13, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, P.; Palmeirim, J.M.; Ruedi, M.; Coelho, M.M. Gene flow and population structure of the endemic Azorean bat (Nyctalus azoreum) based on microsatellites: Implications for conservation. Conserv. Genet. 2008, 9, 1163–1171. [Google Scholar] [CrossRef]
- Skiba, R. Nachweis einer Zwergfleder-maus Pipistrellus pipistrellus (Schreber, 1774), auf der Azorinsel Flores (Portugal). Myotis 1996, 34, 81–84. [Google Scholar]
- Cabral, M.J.C.; Almeida, J.; Almeida, P.R.; Dellinger, T.; Ferrand de Almeida, N.; Oliveira, M.E.; Palmeirim, J.M.; Queiroz, A.I.; Rogado, L.; Santos-Reis, M. Livro Vermelho dos Vertebrados de Portugal, 1st ed.; Instituto da Conservação da Natureza: Lisboa, Portugal, 2005; p. 660. [Google Scholar]
- Alcaldé, J.; Juste, J. Pipistrellus maderensis. In The IUCN Red List of Threatened Species 2016; e.T17315A1380378; 2016; Available online: https://www.iucnredlist.org/species/17315/1380378 (accessed on 22 August 2017).
- Piraccini, R. Nyctalus azoreum. In The IUCN Red List of Threatened Species 2016; e.T14922A546843; 2016; Available online: https://www.iucnredlist.org/species/14922/546843 (accessed on 22 August 2017).
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Priceton, NJ, USA, 1967. [Google Scholar]
- Fernández-Palacios, J.M. Shaped by sea-level shifts. Nature 2016, 532, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Speer, K.A.; Petronio, B.J.; Simmons, N.B.; Richey, R.; Magrini, K.; Soto-Centeno, J.A.; Reed, D.L. Population structure of a widespread bat (Tadarida brasiliensis) in an island system. Ecol. Evol. 2017, 7, 7585–7598. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.H. Bat Migration. Encycl. Anim. Behav. 2019, 2019, 605–610. [Google Scholar]
- Whittaker, R.J.; Fernández-Palacios, J.M.; Matthews, T.J.; Borregaard, M.K.; Triantis, K.A. Island biogeography: Taking the long view of nature’s laboratories. Science 2017, 357, eaam8326. [Google Scholar] [CrossRef]
- Ramalho, R.S.; Helffrich, G.; Madeira, J.; Cosca, M.; Thomas, C.; Quartau, R.; Hipólito, A.; Rovere, A.; Hearty, P.J.; Ávila, S.P. Emergence and evolution of Santa Maria Island (Azores)? The conundrum of uplifted islands revisited. Bulletin 2017, 129, 372–390. [Google Scholar] [CrossRef]
- Schoeman, M. Light pollution at stadiums favors urban exploiter bats. Anim. Conserv. 2016, 19, 120–130. [Google Scholar] [CrossRef]
- Spoelstra, K.; van Grunsven, R.H.; Ramakers, J.J.; Ferguson, K.B.; Raap, T.; Donners, M.; Veenendaal, E.M.; Visser, M.E. Response of bats to light with different spectra: Light-shy and agile bat presence is affected by white and green, but not red light. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170075. [Google Scholar] [CrossRef] [PubMed]
- Birkhofer, K.; Wolters, V. The global relationship between climate, net primary production and the diet of spiders. Glob. Ecol. Biogeogr. 2012, 21, 100–108. [Google Scholar] [CrossRef]
- Coelho, R.; Vieira, A. Aves dos Açores. 2020. Available online: https://avesdosazores.wordpress.com (accessed on 22 August 2020).
- Speakman, J.R. The impact of predation by birds on bat populations in the British Isles. Mammal Rev. 1991, 21, 123–142. [Google Scholar] [CrossRef]
- Khayat, R.O.S.; Grant, R.A.; Ryan, H.; Melling, L.M.; Dougill, G.; Killick, D.R.; Shaw, K.J. Investigating cat predation as the cause of bat wing tears using forensic DNA analysis. Ecol. Evol. 2020, 10, 8368–8378. [Google Scholar] [CrossRef]
- Patterson, B.D.; Willig, M.R.; Stevens, R.D. Trophic strategies, niche partitioning, and patterns of ecological organization. In Bat Ecology; Kunz, T.H., Fenton, M.B., Eds.; The University of Chicago Press: Chicago, IL, USA, 2003; Chapter 12; pp. 536–557. [Google Scholar]
- Richmond, O.M.W.; Hines, J.E.; Beissinger, S.R. Two-species occupancy models: A new parameterization applied to co-occurrence of secretive rails. Ecol. Appl. 2010, 20, 2036–2046. [Google Scholar] [CrossRef]
- Razgour, O.; Korine, C.; Saltz, D. Does interspecific competition drive patterns of habitat use in desert bat communities? Oecologia 2011, 167, 493–502. [Google Scholar] [CrossRef]
- Haynes, T.B.; Schmutz, J.A.; Lindberg, M.S.; Wright, K.G.; Uher-Koch, B.D.; Rosenberger, A.E. Occupancy of yellow-billed and Pacific loons: Evidence for interspecific competition and habitat mediated co-occurrence. J. Avian Biol. 2014, 45, 296–304. [Google Scholar] [CrossRef]
- Rainho, A.; Palmeirim, J.M. Understanding the long term consequences of fragmentation: Lessons from the bats of Bijagós (Guinea-Bissau, West Africa). Hystrix 2017, 28, 173–179. [Google Scholar]
- Fernández-Palacios, J.M.; de Nascimento, L.; Otto, R.; Delgado, J.D.; García-del Rey, E.; Arévalo, J.R.; Whittaker, R.J. A reconstruction of Palaeo-Macaronesia, with particular reference to the long-term biogeography of the Atlantic island laurel forests. J. Biogeogr. 2011, 38, 226–246. [Google Scholar] [CrossRef]
- Elias, R.B.; Gil, A.; Silva, L.; Fernández-Palacios, J.M.; Azevedo, E.B.; Reis, F. Natural zonal vegetation of the Azores Islands: Characterization and potential distribution. Phytocoenologia 2016, 46, 107–123. [Google Scholar] [CrossRef]
- Monteiro, R.; Furtado, S.; Rocha, M.; Freitas, M.; Medeiros, R.; Cruz, J. O Ordenamento do Território nos Açores: Política e Instrumentos; Secretaria Regional do Ambiente e do Mar, Direcção Regional do Ordenamento do Território e dos Recursos Hídricos: Ponta Delgada, Portugal, 2008. [Google Scholar]
- Cruz, J.V. Groundwater and volcanoes: Examples from the Azores archipelago. Environ. Geol. 2003, 44, 343–355. [Google Scholar] [CrossRef]
- Fenton, M.B. A technique for monitoring bat activity with results obtained from different environments in southern Ontario. Can. J. Zool. 1970, 48, 847–851. [Google Scholar] [CrossRef]
- Tabachnick, B.; Fidell, L. Using Multivariate Statistics, 3rd ed.; HarperCollins Publishers: New York, NY, USA, 1996; p. 880. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Dobson, A.J. An Introduction to Generalized Linear Models, 2nd ed.; Texts in Statistical Science; Chapman & Hall/CRC: Boca Raton, FL, USA, 2002; p. 221. [Google Scholar]
- Smeraldo, S.; Bosso, L.; Salinas-Ramos, V.B.; Ancillotto, L.; Sánchez-Cordero, V.; Gazaryan, S.; Russo, D. Generalists yet different: Distributional responses to climate change may vary in opportunistic bat species sharing similar ecological traits. Mammal Rev. 2021, 51, 571–584. [Google Scholar] [CrossRef]
- Delgado-Jaramillo, M.; Aguiar, L.M.; Machado, R.B.; Bernard, E. Assessing the distribution of a species-rich group in a continental-sized megadiverse country: Bats in Brazil. Divers. Distrib. 2020, 26, 632–643. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference; R Package Version 1.43.0; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Fox, J.; Weisberg, S. Visualizing fit and lack of fit in complex regression models with predictor effect plots and partial residuals. J. Stat. Softw. 2018, 87, 1–27. [Google Scholar] [CrossRef]
- McCracken, G.F.; Hayes, J.P.; Cevallos, J.; Guffey, S.Z.; Romero, F.C. Observations on the distribution, ecology, and behaviour of bats on the Galapagos Islands. J. Zool. 1997, 243, 757–770. [Google Scholar] [CrossRef]
- Smeraldo, S.; Di Febbraro, M.; Bosso, L.; Flaquer, C.; Guixé, D.; Lisón, F.; Meschede, A.; Juste, J.; Prüger, J.; Puig-Montserrat, X.; et al. Ignoring seasonal changes in the ecological niche of non-migratory species may lead to biases in potential distribution models: Lessons from bats. Biodivers. Conserv. 2018, 27, 2425–2441. [Google Scholar] [CrossRef]
- Castella, V.; Ruedi, M.; Excoffier, L.; Ibáñez, C.; Arlettaz, R.; Hausser, J. Is the Gibraltar Strait a barrier to gene flow for the bat Myotis myotis (Chiroptera: Vespertilionidae)? Mol. Ecol. 2000, 9, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.; Teixeira, S.; Freitas, T.; Teixeira, D.; Brehm, A. Genetic identity of Pipistrellus maderensis from the Madeira archipelago: A first assessment, and implications for conservation. Hystrix 2013, 177–180. [Google Scholar]
- Juste, J.; Paunovic, M. Nyctalus leisleri. In The IUCN Red List of Threatened Species 2016; e.T14919A22016159; 2016; Available online: https://www.iucnredlist.org/species/14919/22016159 (accessed on 22 August 2017).
- Pettorelli, N.; Gaillard, J.M.; Mysterud, A.; Duncan, P.; Chr. Stenseth, N.; Delorme, D.; Van Laere, G.; Toïgo, C.; Klein, F. Using a proxy of plant productivity (NDVI) to find key periods for animal performance: The case of roe deer. Oikos 2006, 112, 565–572. [Google Scholar] [CrossRef]
- Palmeirim, J.M.; Rodrigues, L.; Rainho, A.; Ramos, M.J. Chiroptera. In Guia dos Mamíferos Terrestres de Portugal Continental, Açores e Madeira; Mathias, M.L., Ed.; Instituto da Conservação da Natureza, Centro de Biologia Ambiental da Universidade de Lisboa: Lisboa, Portugal, 1999; Chapter 4; pp. 41–96. [Google Scholar]
- Roeleke, M.; Johannsen, L.; Voigt, C.C. How bats escape the Competitive Exclusion Principle-Seasonal shift from intraspecific to interspecific competition drives space use in a bat ensemble. Front. Ecol. Evol. 2018, 6, 101. [Google Scholar] [CrossRef]
- Schöner, C.R.; Schöner, M.G.; Grafe, T.U.; Clarke, C.M.; Dombrowski, L.; Tan, M.C.; Kerth, G. Ecological outsourcing: A pitcher plant benefits from transferring pre-digestion of prey to a bat mutualist. J. Ecol. 2017, 105, 400–411. [Google Scholar] [CrossRef]
- Moreno, S.A.; Gelambi, M.; Biganzoli, A.; Molinari, J. Small nutrient molecules in fruit fuel efficient digestion and mutualism with plants in frugivorous bats. Sci. Rep. 2019, 9, 19376. [Google Scholar] [CrossRef]
- Wilkinson, G.S.; Carter, G.G.; Bohn, K.M.; Adams, D.M. Non-kin cooperation in bats. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150095. [Google Scholar] [CrossRef]
- Russo, D.; Di Febbraro, M.; Rebelo, H.; Mucedda, M.; Cistrone, L.; Agnelli, P.; De Pasquale, P.P.; Martinoli, A.; Scaravelli, D.; Spilinga, C.; et al. What Story Does Geographic Separation of Insular Bats Tell? A Case Study on Sardinian Rhinolophids. PLoS ONE 2014, 9, e110894. [Google Scholar] [CrossRef] [PubMed]
- Fukui, D.; Okazaki, K.; Maeda, K. Diet of three sympatric insectivorous bat species on Ishigaki Island, Japan. Endanger. Species Res. 2009, 8, 117–128. [Google Scholar] [CrossRef]
- Emrich, M.A.; Clare, E.L.; Symondson, W.O.; Koenig, S.E.; Fenton, M.B. Resource partitioning by insectivorous bats in Jamaica. Mol. Ecol. 2014, 23, 3648–3656. [Google Scholar] [CrossRef] [PubMed]
- Sedlock, J.L.; Krüger, F.; Clare, E.L. Island bat diets: Does it matter more who you are or where you live? Mol. Ecol. 2014, 23, 3684–3694. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.B. Eavesdropping on the echolocation and social calls of bats. Mammal Rev. 2003, 33, 193–204. [Google Scholar] [CrossRef]
- Gillam, E. Eavesdropping by bats on the feeding buzzes of conspecifics. Can. J. Zool. 2007, 85, 795–801. [Google Scholar] [CrossRef]
- Goyert, H.F.; Manne, L.L.; Veit, R.R. Facilitative interactions among the pelagic community of temperate migratory terns, tunas and dolphins. Oikos 2014, 123, 1400–1408. [Google Scholar] [CrossRef]
- Losey, J.E.; Denno, R.F. Positive predator–predator interactions: Enhanced predation rates and synergistic suppression of aphid populations. Ecology 1998, 79, 2143–2152. [Google Scholar]
- Boyd, C.; Grünbaum, D.; Hunt, G.L., Jr.; Punt, A.E.; Weimerskirch, H.; Bertrand, S. Effects of variation in the abundance and distribution of prey on the foraging success of central place foragers. J. Appl. Ecol. 2017, 54, 1362–1372. [Google Scholar] [CrossRef]
- Veit, R.R.; Harrison, N.M. Positive Interactions among Foraging Seabirds, Marine Mammals and Fishes and Implications for Their Conservation. Front. Ecol. Evol. 2017, 5, 121. [Google Scholar] [CrossRef]
- Lett, C.; Semeria, M.; Thiebault, A.; Tremblay, Y. Effects of successive predator attacks on prey aggregations. Theor. Ecol. 2014, 7, 239–252. [Google Scholar] [CrossRef]
- Sinervo, B. Optimal Foraging Theory: Constraints and Cognitive Processes. In Behavioral Ecology; University of California: Santa Cruz, CA, USA, 1997; pp. 105–130. [Google Scholar]
- DGT. CAOP-Carta Administrativa Oficial de Portugal-Açores, Grupos Central. Oriental e Ocidental; 2016. Available online: https://www.dgterritorio.gov.pt/cartografia/cartografia-tematica/caop?language=en (accessed on 28 December 2016).
- Dahl, A.L. Island Directory, UNEP/WCMC. 2010. Available online: http://islands.unep.ch/Tisolat.htm (accessed on 28 December 2016).
- França, Z.; Cruz, J.V.; Nunes, J.C.; Forjaz, V. Geologia dos Açores: Uma perspectiva actual. Açoreana 2003, 10, 11–140. [Google Scholar]
- Jarvis, A.; Reuter, H.I.; Nelson, A.; Guevara, E. Hole-Filled SRTM for the Globe Version 4. CGIAR Consortium for Spatial Information. 2008. Available online: http://srtm.csi.cgiar.org/ (accessed on 28 December 2016).
- IGeoE; DROTRH. Curvas de nível das Ilhas dos Açores. In Cartografia Vectorial Produzida à Escala 1:25,000 Pelo; Instituto Geográfico do Exército (IGeoE) com Actualizações da Direcção Regional do Ordenamento do Território e Recursos Hídricos (DROTRH): Horta, Portugal, 2000. [Google Scholar]
- DROTRH. Carta de Ocupação do solo da Região Autónoma dos Açores. Cartografia Produzida com Base nas Imagens de Satélite-LANDSAT7; Direcção Regional do Ordenamento do Território e Recursos Hídricos (DROTRH): Lisboa, Portugal, 2007. [Google Scholar]
- Didan, K. MOD13A1 MODIS/Terra Vegetation Indices 16-Day L3 Global 500 m SIN Grid V006. 2015. Available online: https://lpdaac.usgs.gov/products/mod13a1v006/ (accessed on 28 December 2016).
| Estimate | St. Error | z Value | p | AUC | |
|---|---|---|---|---|---|
| P. cf. maderensis | |||||
| N.azor | −0.405 | 1.443 | −0.281 | 0.779 | 0.46 |
| Size | −0.007 | 0.005 | −1.422 | 0.155 | 0.83 |
| UNEP | 0.178 | 0.240 | 0.741 | 0.459 | 0.61 |
| DistPT | 0.006 | 0.005 | 1.056 | 0.291 | 0.72 |
| Island age | −0.065 | 0.293 | −0.222 | 0.825 | 0.39 |
| N. azoreum | |||||
| P.made | −0.406 | 1.443 | −0.281 | 0.779 | 0.45 |
| Size | 0.014 | 0.013 | 1.064 | 0.287 | 0.86 |
| Island age | 0.367 | 0.540 | 0.681 | 0.496 | 0.64 |
| Estimate | St. Error | z Value | p | AUC | |
|---|---|---|---|---|---|
| P. cf. maderensis | 0.883 | ||||
| Intercept | −5.589 | 2.656 | −1.314 | 0.189 | |
| Altitude | −0.006 | 0.003 | −2.007 | 0.045 | |
| DistNazor | −2.659 | 0.715 | −3.719 | 0.002 | |
| NDVI | 0.112 | 0.059 | 1.901 | 0.057 | |
| N. azoreum | 0.673 | ||||
| Intercept | 1.723 | 0.360 | 4.800 | 0.000 | |
| LCo: farmland | −1.079 | 0.365 | −2.954 | 0.003 | |
| LCo: pastures | −1.963 | 0.334 | −5.864 | 0.000 | |
| LCo: forestry | −1.795 | 0.356 | −5.063 | 0.000 | |
| LCo: natural | −2.236 | 0.441 | −5.063 | 0.000 | |
| LCo: rocky | −1.043 | 0.736 | −1.417 | 0.156 | |
| LCo: lagoon | 0.525 | 1.137 | 0.462 | 0.644 | |
| DistPmade | −0.650 | 0.284 | −2.290 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rainho, A. Positive Interactions Drive Bat Distribution in a Remote Oceanic Archipelago (Azores, Portugal). Diversity 2022, 14, 17. https://doi.org/10.3390/d14010017
Rainho A. Positive Interactions Drive Bat Distribution in a Remote Oceanic Archipelago (Azores, Portugal). Diversity. 2022; 14(1):17. https://doi.org/10.3390/d14010017
Chicago/Turabian StyleRainho, Ana. 2022. "Positive Interactions Drive Bat Distribution in a Remote Oceanic Archipelago (Azores, Portugal)" Diversity 14, no. 1: 17. https://doi.org/10.3390/d14010017
APA StyleRainho, A. (2022). Positive Interactions Drive Bat Distribution in a Remote Oceanic Archipelago (Azores, Portugal). Diversity, 14(1), 17. https://doi.org/10.3390/d14010017







