Environmental Pressures on Top-Down and Bottom-Up Forces in Coastal Ecosystems
Abstract
1. Introduction
2. Materials and Methods
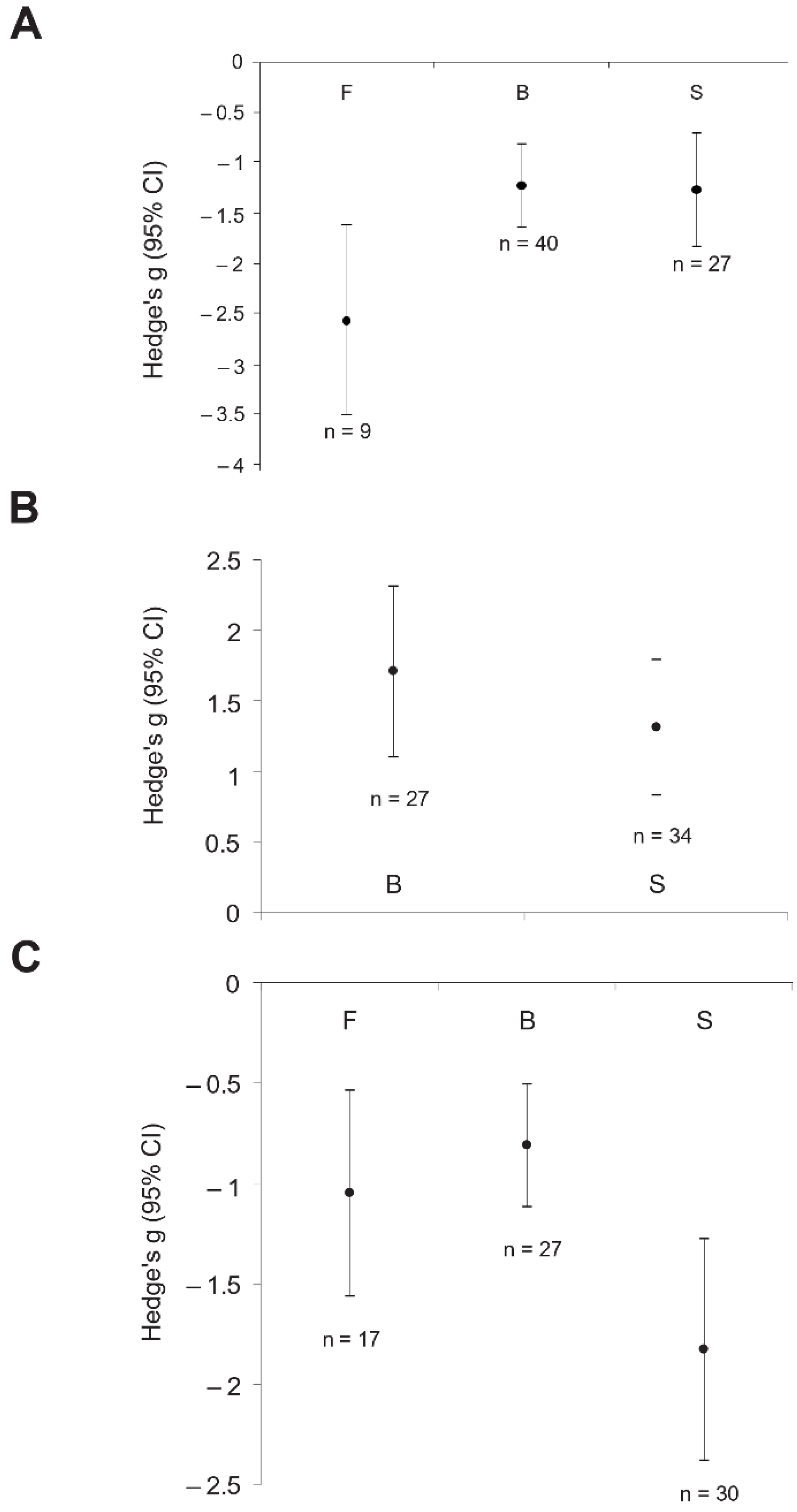
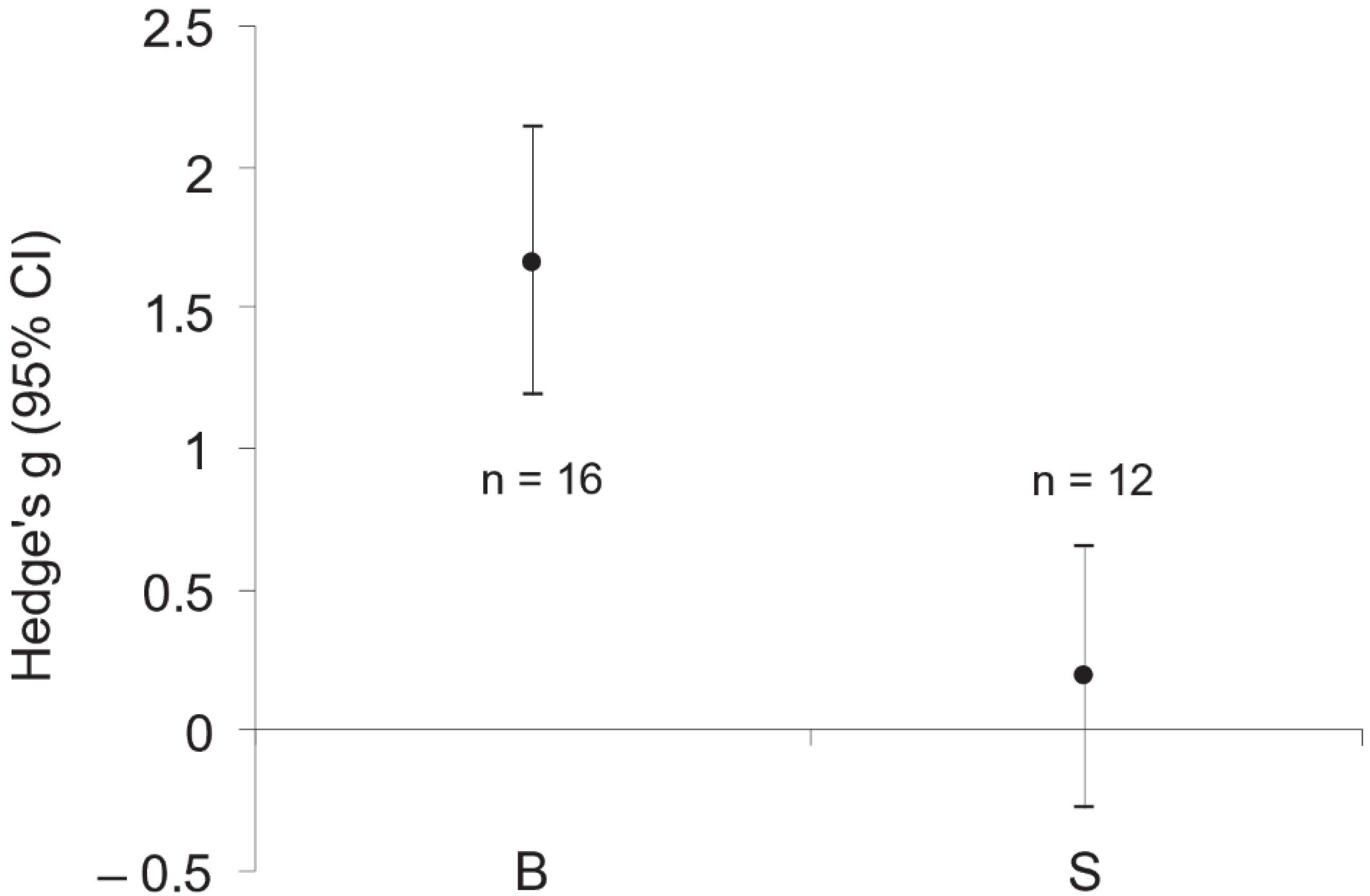
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curtis, P.S.; Drake, B.G.; Leadley, P.W.; Arp, W.J.; Whigham, D.F. Growth and senescence in plant communities exposed to elevated CO2 concentrations on an estuarine marsh. Oecologia 1989, 78, 20–26. [Google Scholar] [CrossRef]
- Arp, W.J.; Drake, B.G. Increased photosynthetic capacity of Scirpus olneyi after 4 years of exposure to elevated CO2. Plant Cell Environ. 1991, 14, 1003–1006. [Google Scholar] [CrossRef]
- Jacob, J.; Greitner, C.; Drake, B.G. Acclimation of photosynthesis in relation to Rubisco and non-structural carbohydrate contents and in situ carboxylase activity in Scirpus olneyi grown at elevated CO2 in the field. Plant Cell Environ. 1995, 18, 875–884. [Google Scholar] [CrossRef]
- Blum, M.J.; Saunders, C.J.; McLachlan, J.S.; Summers, J.; Craft, C.; Herrick, J.D. A century-long record of plant evolution reconstructed from a coastal marsh seed bank. Evol. Lett. 2021, 5, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Bertness, M.D. Rapid shoreward encroachment of salt marsh cordgrass in response to accelerated sea-level rise. Proc. Nat. Acad. Sci. USA 2001, 98, 14218–14223. [Google Scholar] [CrossRef]
- Jarrell, E.R.; Kolker, A.S.; Campbell, C.; Blum, M.J. Brackish marsh plant community responses to regional precipitation and relative sea-level rise. Wetlands 2016, 36, 607–619. [Google Scholar] [CrossRef]
- Bernik, B.M.; Pardue, J.H.; Blum, M.J. Soil erodibility differs according to heritable trait variation and nutrient-induced plasticity in the salt marsh engineer Spartina alterniflora. Mar. Ecol. Prog. Ser. 2018, 601, 1–14. [Google Scholar] [CrossRef]
- Bernik, B.M.; Lumibao, C.Y.; Zengel, S.; Pardue, J.H.; Blum, M.J. Intraspecific variation in landform engineering across a restored salt marsh shoreline. Evol. Appl. 2021, 14, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Crosby, S.C.; Angermeyer, A.; Adler, J.M.; Bertness, M.D.; Deegan, L.A.; Sibinga, N.; Leslie, H.M. Spartina alterniflora biomass allocation and temperature: Implications for salt marsh persistence with sea-level rise. Estuaries Coasts 2017, 40, 213–223. [Google Scholar] [CrossRef]
- Arp, W.J.; Drake, B.G.; Pockman, W.T.; Curtis, P.S.; Whigham, D.F. Interactions between C3 and C4 salt marsh plant species during four years of exposure to elevated atmospheric CO2. In CO2 and Biosphere; Springer: Dordrecht, The Netherlands, 1993; pp. 133–143. [Google Scholar]
- Rasse, D.P.; Peresta, G.; Drake, B.G. Seventeen years of elevated CO2 exposure in a Chesapeake Bay wetland: Sustained but contrasting responses of plant growth and CO2 uptake. Glob. Chang. Biol. 2005, 11, 369–377. [Google Scholar] [CrossRef]
- Drake, B.G. Rising sea level, temperature, and precipitation impact plant and ecosystem responses to elevated CO2 on a Chesapeake Bay wetland: Review of a 28-year study. Glob. Chang. Biol. 2014, 20, 3329–3343. [Google Scholar] [CrossRef]
- Cherry, J.A.; McKee, K.; Grace, J.B. Elevated CO2 enhances biological contributions to elevation change in coastal wetlands by offsetting stressors associated with sea level rise. J. Ecol. 2009, 97, 67–77. [Google Scholar] [CrossRef]
- Langley, J.A.; McKee, K.L.; Cahoon, D.R.; Cherry, J.A.; Megonigal, J.P. Elevated CO2 stimulates marsh elevation gain, counterbalancing sea-level rise. Proc. Nat. Acad. Sci. USA 2009, 106, 6182–6186. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.A.; Megonigal, J.P. Ecosystem response to elevated CO2 levels limited by nitrogen-induced plant species shift. Nature 2010, 466, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.A.; Mozdzer, T.J.; Shepard, K.A.; Hagerty, S.B.; Megonigal, J.P. Tidal marsh plant responses to elevated CO2, nitrogen fertilization, and sea level rise. Glob. Chang. Biol. 2013, 19, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Power, M.E. Top-down and bottom-up forces in food webs—Do plants have primacy. Ecology 1992, 73, 733–746. [Google Scholar] [CrossRef]
- Polis, G.A. Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos 1999, 86, 3–15. [Google Scholar] [CrossRef]
- Hunter, M.D.; Price, P.W. Playing chutes and ladders—Heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 1992, 73, 724–732. [Google Scholar]
- Gratton, C.; Denno, R.F. Seasonal shift from bottom-up to top-down impact in phytophagous insect populations. Oecologia 2003, 134, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, M.T.; Stiling, P. Bottom-up and top-down effects on insect herbivores do not vary among sites of different salinity. Ecology 2006, 87, 2673–2679. [Google Scholar]
- Stiling, P.; Rossi, A.M. Experimental manipulations of top-down and bottom-up factors in a tri-trophic system. Ecology 1997, 78, 1602–1606. [Google Scholar] [CrossRef]
- Bertness, M.D.; Crain, C.; Holdredge, C.; Sala, N. Eutrophication and consumer control of New England salt marsh primary productivity. Conserv. Biol. 2008, 22, 131–139. [Google Scholar] [CrossRef] [PubMed]
- McFarlin, C.R.; Brewer, J.S.; Buck, T.L.; Pennings, S.C. Impact of fertilization on a salt marsh food web in Georgia. Estuaries Coasts 2008, 31, 313–325. [Google Scholar] [CrossRef]
- Stiling, P.; Moon, D.C. Quality or quantity: The direct and indirect effects of host plants on herbivores and their natural enemies. Oecologia 2005, 142, 413–420. [Google Scholar] [CrossRef]
- Crain, C.M. Shifting nutrient limitation and eutrophication effects in marsh vegetation across estuarine salinity gradients. Estuaries Coasts 2007, 30, 26–34. [Google Scholar] [CrossRef]
- Rand, T.A. Variation in insect herbivory across a salt marsh tidal gradient influences plant survival and distribution. Oecologia 2002, 132, 549–558. [Google Scholar] [CrossRef]
- Crain, C.M.; Silliman, B.R.; Bertness, S.L.; Bertness, M.D. Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology 2004, 85, 2539–2549. [Google Scholar] [CrossRef]
- Fleeger, J.W.; Johnson, D.S.; Galvan, K.A.; Deegan, L.A. Top-down and bottom-up control of infauna varies across the saltmarsh landscape. J. Exp. Mar. Biol. Ecol. 2008, 357, 20–34. [Google Scholar] [CrossRef][Green Version]
- Silliman, B.R.; Bertness, M.D.; Thomsen, M.S. Top-down control and human intensification of consumer pressure in southern U.S. salt marshes. In Human Impacts in Salt Marshes: A Global Perspective; University of California Press: Berkeley, CA, USA, 2009; pp. 103–114. [Google Scholar]
- Bertness, M.D. Zonation of Spartina-patens and Spartina-alterniflora in a New-England saltmarsh. Ecology 1991, 72, 138–148. [Google Scholar] [CrossRef]
- Moon, D.C.; Stiling, P. Relative importance of abiotically induced direct and indirect effects on a salt-marsh herbivore. Ecology 2000, 81, 470–481. [Google Scholar] [CrossRef]
- Tanner, B.R.; Uhle, M.E.; Kelley, J.T.; Mora, C.I. C3/C4 variations in salt-marsh sediments: An application of compound specific isotopic analysis of lipid biomarkers to late Holocene paleoenvironmental research. Org. Geochem. 2007, 38, 474–484. [Google Scholar] [CrossRef]
- Bertness, M.D.; Ewanchuk, P.J.; Silliman, B.R. Anthropogenic modification of New England salt marsh landscapes. Proc. Nat. Acad. Sci. USA 2002, 99, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Deegan, L.A.; Bowen, J.L.; Drake, D.; Fleeger, J.W.; Friedrichs, C.T.; Galvan, K.A.; Hobble, J.E.; Hopkinson, C.; Johnson, D.S.; Johnson, J.M.; et al. Susceptibility of salt marshes to nutrient enrichment and predator removal. Ecol. Appl. 2007, 17, S42–S63. [Google Scholar] [CrossRef]
- Deegan, L.A.; Johnson, D.S.; Warren, R.S.; Peterson, B.J.; Fleeger, J.W.; Fagherazzi, S.; Wollheim, W.M. Coastal eutrophication as a driver of salt marsh loss. Nature 2012, 490, 388–392. [Google Scholar] [CrossRef]
- Ford, M.A.; Grace, J.B. Effects of vertebrate herbivores on soil processes, plant biomass, litter accumulation and soil elevation changes in a coastal marsh. J. Ecol. 1998, 86, 974–982. [Google Scholar] [CrossRef]
- Silliman, B.R.; Van De Koppel, J.; Bertness, M.D.; Stanton, L.E.; Mendelssohn, I.A. Drought, snails, and large-scale die-off of southern US salt marshes. Science 2005, 310, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.J.; Kilheffer, J.; Silliman, B.R. Relative effects of Littoraria irrorata and Prokolesia marginata on Spartina alterniflora. Estuaries Coasts 2006, 29, 639–644. [Google Scholar] [CrossRef]
- Alberti, J.; Montemayor, D.; Alvarez, F.; Agustina, C.; Luppi, T.; Canepuccia, A.; Isacch, J.P.; Iribarne, O. Changes in rainfall pattern affect crab herbivory rates in a SW Atlantic salt marsh. J. Exp. Mar. Biol. Ecol. 2007, 353, 126–133. [Google Scholar] [CrossRef]
- Menge, B.A.; Sutherland, J.P. Community regulation—variation in disturbance, competition, and predation in relation to environmental-stress and recruitment. Am. Nat. 1987, 130, 730–757. [Google Scholar] [CrossRef]
- Menge, B.A.; Sutherland, J.P. Species-diversity gradients—synthesis of roles of predation, competition, and temporal heterogeneity. Am. Nat. 1976, 110, 351–369. [Google Scholar] [CrossRef]
- White, T.C.R. A hypothesis to explain outbreaks of looper caterpillars, with special reference to populations of Selidosema suavis in a plantation of Pinus radiata in New Zealand. Oecologia 1974, 16, 279–301. [Google Scholar] [CrossRef]
- Rhoades, D.F. Evolution of plant chemical defense against herbivores. In Herbivores: Their Interaction with Secondary Plant Metabolites; Academic Press: New York, NY, USA, 1979; pp. 3–54. [Google Scholar]
- Mattson, W.J.; Haack, R.A. The role of drought stress in provoking outbreaks of phytophagous insects. In Insect Outbreaks; Academic Press: San Diego, CA, USA, 1987; pp. 365–407. [Google Scholar]
- Larsson, S. Stressful times for the plant stress—insect performance hypothesis. Oikos 1989, 56, 277–283. [Google Scholar] [CrossRef]
- Locke, A. Applications of the Menge-Sutherland model to acid-stressed lake communities. Ecol. Appl. 1996, 6, 797–805. [Google Scholar] [CrossRef]
- Gurevitch, J.; Hedges, L.V. Meta-analyses: Combining the results of independent experiments. In Analysis of Ecological Experiments; Oxford University Press: New York, NY, USA, 2001; pp. 347–369. [Google Scholar]
- Koricheva, J.; Larsson, S.; Haukioja, E. Insect performance on experimentally stressed woody plants: A meta-analysis. Annu. Rev. Entomol. 1998, 43, 195–216. [Google Scholar] [CrossRef]
- Preisser, E.L.; Strong, D.R. Climate affects predator control of an herbivore outbreak. Am. Nat. 2004, 163, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Huberty, A.F.; Denno, R.F. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Schile, L.; Mopper, S. The deleterious effects of salinity stress on leafminers and their freshwater host. Ecol. Entomol. 2006, 31, 345–351. [Google Scholar] [CrossRef]
- Gough, L.; Grace, J.B. Effects of flooding, salinity and herbivory on coastal plant communities, Louisiana, United States. Oecologia 1998, 117, 527–535. [Google Scholar] [CrossRef]
- Goranson, C.E.; Ho, C.K.; Pennings, S.C. Environmental gradients and herbivore feeding preferences in coastal salt marshes. Oecologia 2004, 140, 591–600. [Google Scholar] [CrossRef]
- Geddes, N.A.; Mopper, S. Effects of environmental salinity on vertebrate florivory and wetland communities. Nat. Areas J. 2006, 26, 31–37. [Google Scholar] [CrossRef]
- Price, P.W. The plant vigor hypothesis and herbivore attack. Oikos 1991, 62, 244–251. [Google Scholar] [CrossRef]
- Jeffries, R.L.; Perkins, N. The effects on the vegetation of the additions of the inorganic nutrients to salt marsh soils at Stiffkey, Norfolk. J. Ecol. 1977, 65, 867–882. [Google Scholar] [CrossRef]
- Buchsbaum, R.; Valiela, I.; Swain, T. The role of phenolic-compounds and other plant constituents in feeding by canada geese in a coastal marsh. Oecologia 1984, 63, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Silliman, B.R.; Bertness, M.D. A trophic cascade regulates salt marsh primary production. Proc. Nat. Acad. Sci. USA 2002, 99, 10500–10505. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.S.; Warren, R.S.; Deegan, L.A.; Mozdzer, T.J. Saltmarsh plant responses to eutrophication. Ecol. Appl. 2016, 26, 2649–2661. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.M.; Morris, J.T. The influence of salinity on the kinetics of NH+4 uptake in Spartina-alterniflora. Oecologia 1991, 85, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Paludan, C.; Morris, J.T. Distribution and speciation of phosphorus along a salinity gradient in intertidal marsh sediments. Biogeochemistry 1999, 45, 197–221. [Google Scholar] [CrossRef]
- Callaway, J.C.; Parker, V.T.; Vasey, M.C.; Schile, L.M. Emerging issues for the restoration of tidal marsh ecosystems in the context of predicted climate change. Madrono 2007, 54, 234–248. [Google Scholar] [CrossRef]
- Touchette, B.W.; Smith, G.A.; Rhodes, K.L.; Poole, M. Tolerance and avoidance: Two contrasting physiological responses to salt stress in mature marsh halophytes Juncus roemerianus Scheele and Spartina alterniflora Loisel. J. Exp. Mar. Biol. Ecol. 2009, 380, 106–112. [Google Scholar] [CrossRef]
- Massad, T.J.; Dyer, L.A. A meta-analysis of the effects of global environmental change on plant-herbivore interactions. Arthropod-Plant Interact. 2010, 4, 181–188. [Google Scholar] [CrossRef]
- Stiling, P.; Cornelissen, T. How does elevated carbon dioxide (CO2) affect plant-herbivore interactions? A field experiment and meta-analysis of CO2-mediated changes on plant chemistry and herbivore performance. Glob. Chang. Biol. 2007, 13, 1823–1842. [Google Scholar] [CrossRef]
- Pennings, S.C.; Callaway, R.M. Salt-marsh plant zonation—the relative importance of competition and physical factors. Ecology 1992, 73, 681–690. [Google Scholar] [CrossRef]
- Iverson, L.R.; Ketzner, D.; Karnes, J. Illinois Plant Information Network. Illinois Natural History Survey and USDA Forest Service. 1999. Available online: https://www.nrs.fs.fed.us/data/il/ilpin/ (accessed on 30 July 2021).
- Scheiner, S.M.; Gurevitch, J. Design and Analysis of Ecological Experiments, 2nd ed.; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis; Version 2; Biostat: Englewood, NJ, USA, 2005. [Google Scholar]
- Alcoverro, T.; Mariani, S. Shoot growth and nitrogen responses to simulated herbivory in Kenyan seagrasses. Bot. Mar. 2005, 48, 1–7. [Google Scholar] [CrossRef]
- Baustian, J.J.; Mendelssohn, I.A.; Hester, M.W. Vegetation’s importance in regulating surface elevation in a coastal salt marsh facing elevated rates of sea level rise. Glob. Chang. Biol. 2012, 18, 3377–3382. [Google Scholar] [CrossRef]
- Bernik, B.M.; Eppinga, M.B.; Kolker, A.S.; Blum, M.J. Clonal vegetation patterns mediate shoreline erosion. Geophys. Res. Lett. 2018, 45, 6476–6484. [Google Scholar] [CrossRef]
- Gallego-Tévar, B.; Grewell, B.J.; Futrell, C.J.; Drenovsky, R.E.; Castillo, J.M. Interactive effects of salinity and inundation on native Spartina foliosa, invasive S. densiflora and their hybrid from San Francisco Estuary, California. Ann. Bot. 2020, 125, 377–389. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Silliman, B.R. Consumer control as a common driver of coastal vegetation worldwide. Ecol. Monogr. 2016, 86, 278–294. [Google Scholar] [CrossRef]
- Silvestri, S.; Marani, M. Salt-marsh vegetation and morphology: Basic physiology, modeling and remote sensing observations. In Ecogeomorphology of Tidal Marshes; American Geophysical Union: Washington, DC, USA, 2004; pp. 5–26. [Google Scholar]
- Maricle, B.R.; Cobos, D.R.; Campbell, C.S. Biophysical and morphological leaf adaptations to drought and salinity in salt marsh grasses. Environ. Exp. Bot. 2007, 60, 458–467. [Google Scholar] [CrossRef]
- Phleger, C.F. Effect of salinity on growth of a salt marsh grass. Ecology 1971, 52, 908–911. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte, Atriplex griffithii var. stocksii. Ann. Bot. 2000, 85, 225–232. [Google Scholar] [CrossRef]
- Alberti, J.; Escapa, M.; Iribarne, O.; Silliman, B.; Bertness, M. Crab herbivory regulates plant facilitative and competitive processes in Argentinean marshes. Ecology 2008, 89, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Linthurst, R.A.; Seneca, E.D. Aeration, nitrogen and salinity as determinants of Spartina alterniflora Loisel. Growth response. Estuaries 1981, 4, 53–63. [Google Scholar] [CrossRef]
- Darby, F.A.; Turner, R.E. Effects of eutrophication on salt marsh root and rhizome biomass accumulation. Mar. Ecol. Prog. Ser. 2008, 363, 63–70. [Google Scholar] [CrossRef]
- Turner, R.E. Beneath the salt marsh canopy: Loss of soil strength with increasing nutrient loads. Estuaries Coasts 2011, 34, 1084–1093. [Google Scholar] [CrossRef]
- Morris, J.T.; Shaffer, G.P.; Nyman, J.A. Brinson review: Perspectives on the influence of nutrients on the sustainability of coastal wetlands. Wetlands 2013, 33, 975–988. [Google Scholar] [CrossRef]
- Engels, J.G.; Jensen, K. Role of biotic interactions and physical factors in determining the distribution of marsh species along an estuarine salinity gradient. Oikos 2010, 119, 679–685. [Google Scholar] [CrossRef]
- Long, J.D.; Porturas, L.D. Herbivore impacts on marsh production depend upon a compensatory continuum mediated by salinity stress. PLoS ONE 2014, 9, e110419. [Google Scholar] [CrossRef] [PubMed]
- Houle, G.; Morel, L.; Reynolds, C.E.; Siegel, J. The effect of salinity on different developmental stages of an endemic annual plant, Aster laurentianus (Asteraceae). Am. J. Bot. 2001, 88, 62–67. [Google Scholar] [CrossRef]
- Ungar, I.A. Effect of salinity on seed germination, growth, and ion accumulation of Atriplex patula (Chenopodiaceae). Am. J. Bot. 1996, 83, 604–607. [Google Scholar] [CrossRef]
- Hemminga, M.A.; Vansoelen, J. The performance of the leaf mining microlepidopteran Bucculatrix-maritima (STT) on the salt-marsh halophyte, aster-tripolium (l), exposed to different salinity conditions. Oecologia 1992, 89, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Etherington, J.R. Relationship between morphological adaptation to grazing, carbon balance and waterlogging tolerance in clones of Dactylis-glomerata L. New Phytol. 1984, 98, 647–658. [Google Scholar] [CrossRef]
- Wieski, K.; Guo, H.Y.; Craft, C.B.; Pennings, S.C. Ecosystem functions of tidal fresh, brackish, and salt marshes on the Georgia coast. Estuaries Coasts 2010, 33, 161–169. [Google Scholar] [CrossRef]
- Pennings, S.C.; Carefoot, T.H.; Siska, E.L.; Chase, M.E.; Page, T.A. Feeding preferences of a generalist salt-marsh crab: Relative importance of multiple plant traits. Ecology 1998, 79, 1968–1979. [Google Scholar] [CrossRef]
- Bowdish, T.I.; Stiling, P. The influence of salt and nitrogen on herbivore abundance: Direct and indirect effects. Oecologia 1998, 113, 400–405. [Google Scholar] [CrossRef]
- Mattson, W.J. Herbivory in relation to plant nitrogen-content. Ann. Rev. Ecol. Syst. 1980, 11, 119–161. [Google Scholar] [CrossRef]
- Waring, G.L.; Cobb, N.S. The impact of plant stress on herbivore population dynamics. In Insect-Plant Interactions; CRC Press: Boca Raton, FL, USA, 1992; pp. 168–226. [Google Scholar]
- Moon, D.C.; Stiling, P. The effects of salinity and nutrients on a tritrophic salt-marsh system. Ecology 2002, 83, 2465–2476. [Google Scholar] [CrossRef]
- Moon, D.C.; Stiling, P. The influence of a salinity and nutrient gradient on coastal vs. upland tritrophic complexes. Ecology 2004, 85, 2709–2716. [Google Scholar] [CrossRef]
- Sage, R.F.; Pearcy, R.W. The nitrogen use efficiency of c-3 and c-4 plants.1. leaf nitrogen, growth, and biomass partitioning in Chenopodium album (l) and Amaranthus retroflexus (L). Plant Physiol. 1987, 84, 954–958. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, S.F., III; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 1998. [Google Scholar]
- Saunders, C.J.; Megonigal, J.P.; Reynolds, J.F. Comparison of belowground biomass in C 3-and C 4-dominated mixed communities in a Chesapeake Bay brackish marsh. Plant Soil 2006, 280, 305–322. [Google Scholar] [CrossRef]
- Caswell, H.; Reed, F.C. Plant-herbivore interactions—indigestibility of C4 bundle sheath-cells by grasshoppers. Oecologia 1976, 26, 151–156. [Google Scholar] [CrossRef]
- Pinder, J.E.; Kroh, G.C. Insect herbivory and photosynthetic pathways in old-field ecosystems. Ecology 1987, 68, 254–259. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Cerling, T.E.; Dearing, M.D. A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems. In Ecological Studies 177; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Cleland, E.E.; Chiariello, N.R.; Loarie, S.R.; Mooney, H.A.; Field, C.B. Diverse responses of phenology to global changes in a grassland ecosystem. Proc. Nat. Acad. Sci. USA 2006, 103, 13740–13744. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blum, M.J. Environmental Pressures on Top-Down and Bottom-Up Forces in Coastal Ecosystems. Diversity 2021, 13, 444. https://doi.org/10.3390/d13090444
Blum MJ. Environmental Pressures on Top-Down and Bottom-Up Forces in Coastal Ecosystems. Diversity. 2021; 13(9):444. https://doi.org/10.3390/d13090444
Chicago/Turabian StyleBlum, Michael J. 2021. "Environmental Pressures on Top-Down and Bottom-Up Forces in Coastal Ecosystems" Diversity 13, no. 9: 444. https://doi.org/10.3390/d13090444
APA StyleBlum, M. J. (2021). Environmental Pressures on Top-Down and Bottom-Up Forces in Coastal Ecosystems. Diversity, 13(9), 444. https://doi.org/10.3390/d13090444





