A GIS Modeling Study of the Distribution of Viviparous Invasive Alien Fish Species in Eastern Europe in Terms of Global Climate Change, as Exemplified by Poecilia reticulata Peters, 1859 and Gambusia holbrooki Girarg, 1859
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence Data Collection
2.2. Environmental Data
2.3. Model Building
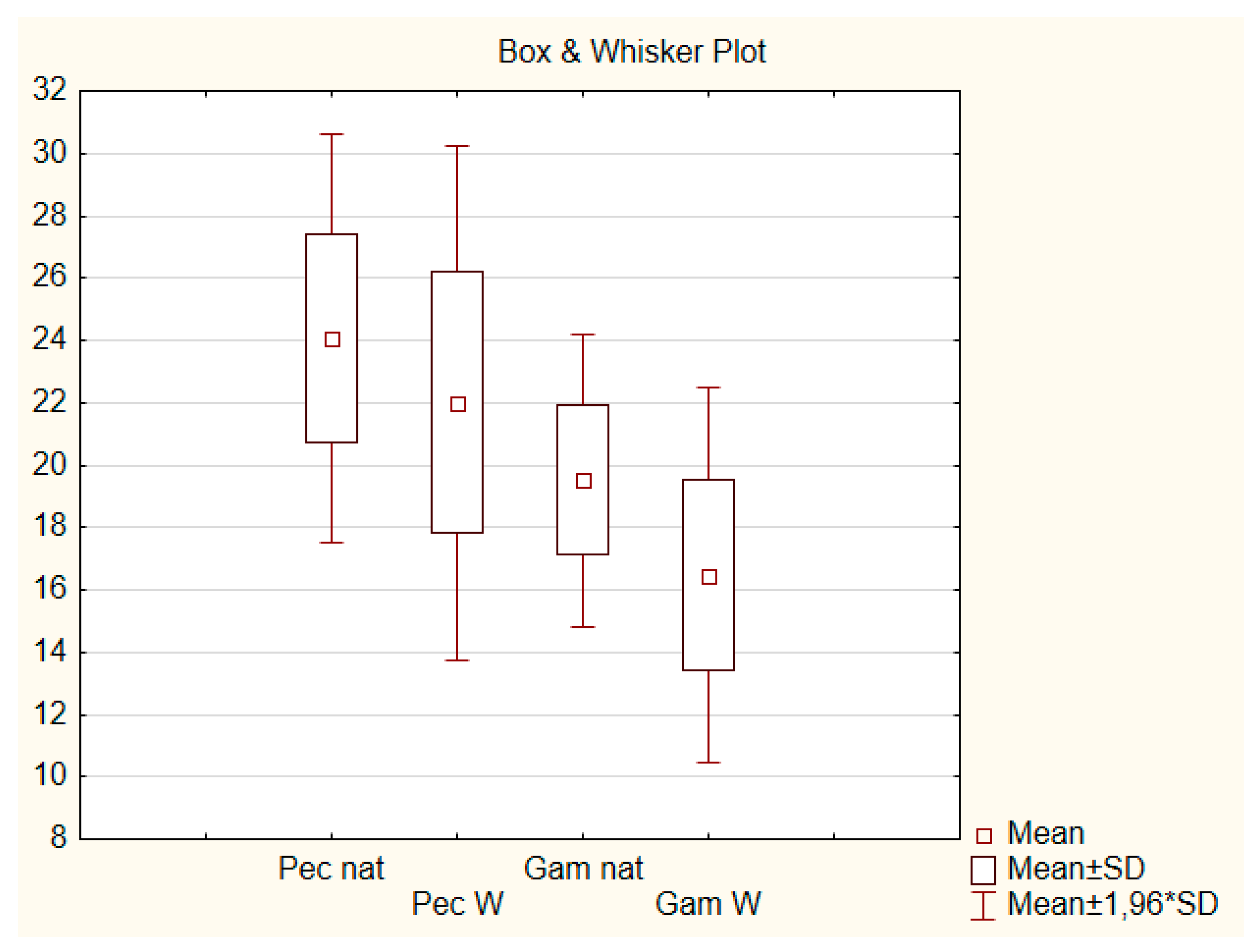
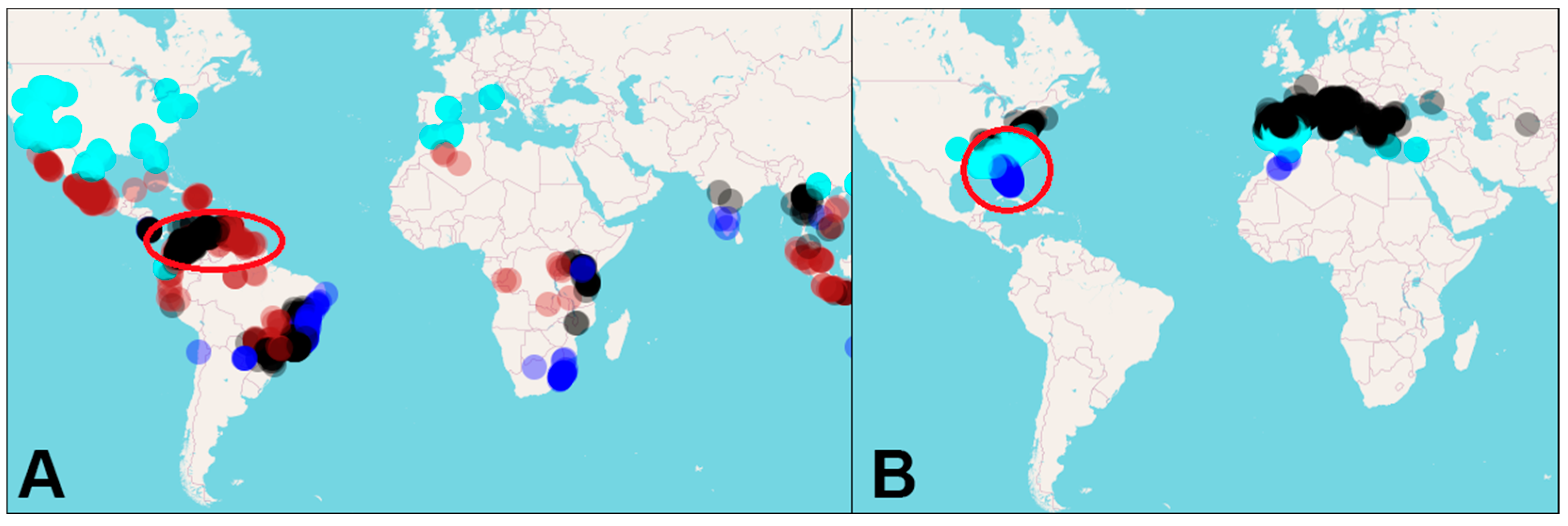
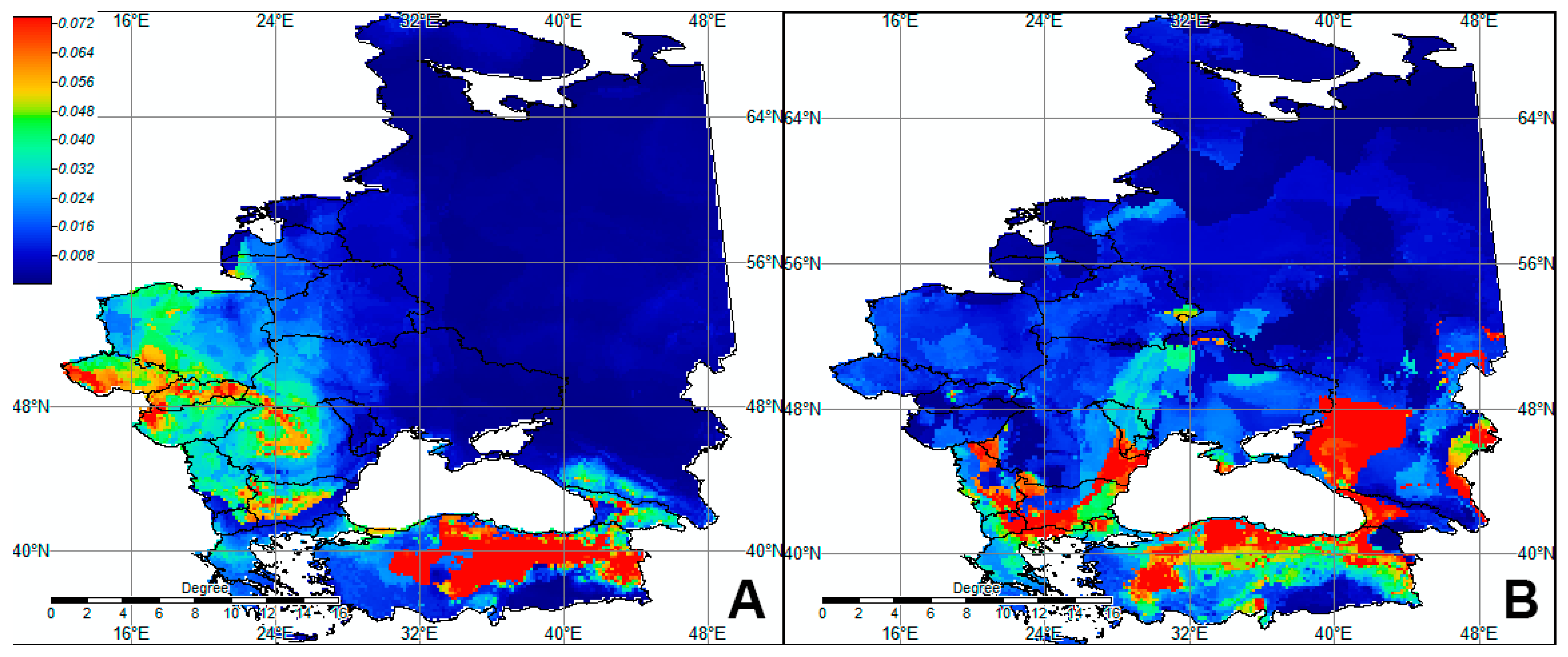
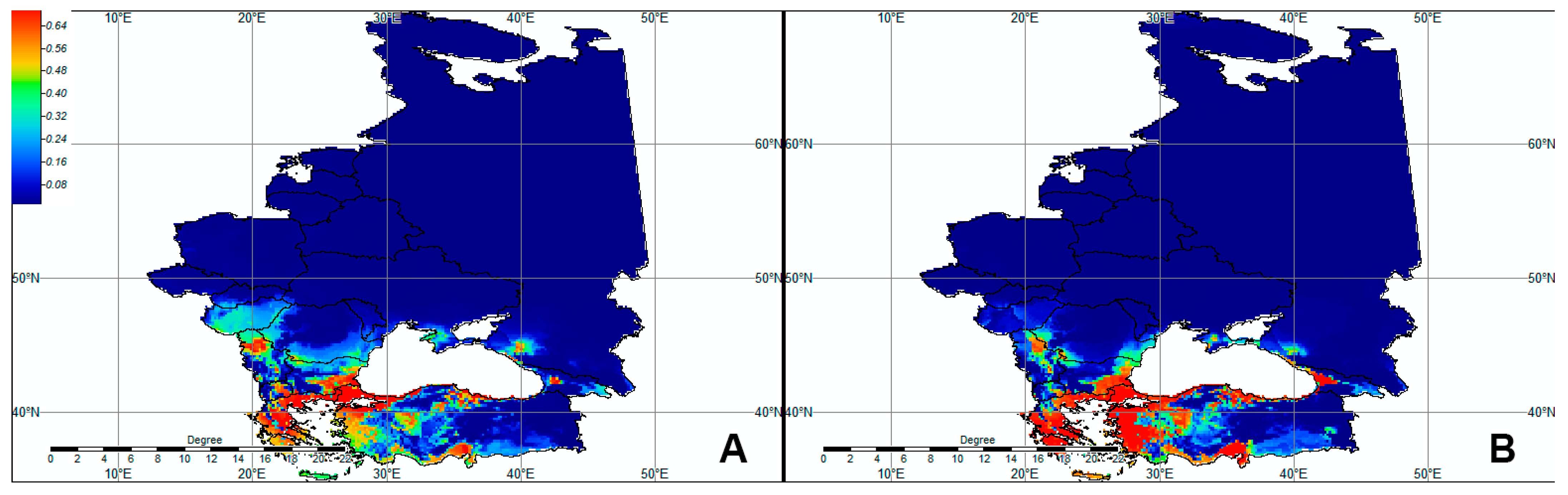
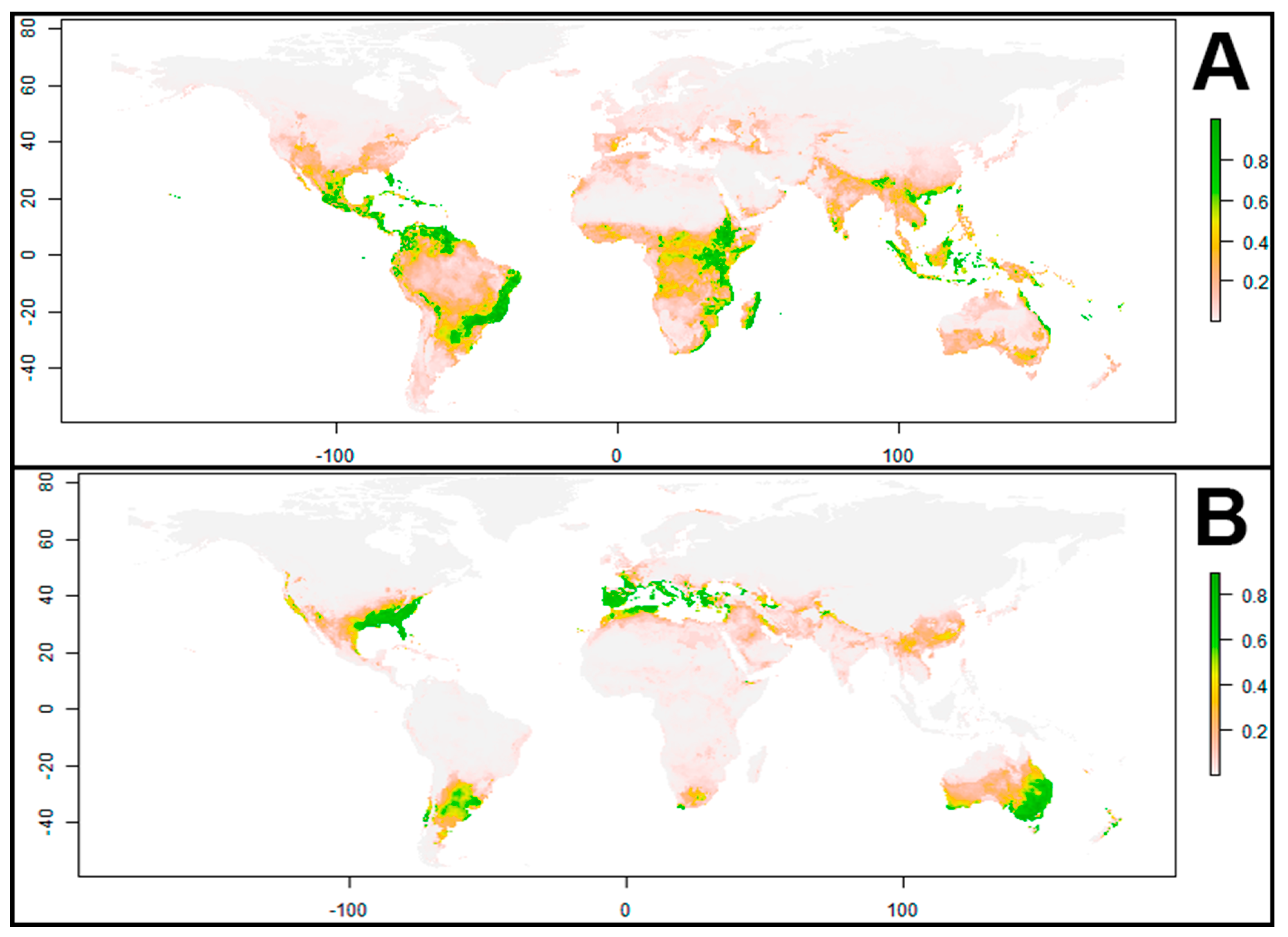
3. Results
3.1. Limiting Factors for P. reticulata Distribution
3.2. Limiting Factors for G. holbrooki Distribution
3.3. Niche Clustering
3.4. Ecological Niche Modeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuybida, V.V.; Nekrasova, O.D.; Kutsokon, Y.K.; Lopatynska, V.V. Summer fish kills in the Kaniv Reservoir. Hydrobiol. J. 2019, 55, 103–106. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Osipov, V.V.; Fayzulin, A.I.; Bakin, O.V.; Tselishcheva, L.G.; Bayanov, N.G. Chinese sleeper (Perccottus glenii Dybowski, 1877) (Pisces, Odontobutidae) in the reserves and National Parks of the middle and lower Volga (Russia): Mini-review. AACL Bioflux 2019, 12, 1114–1124. [Google Scholar]
- Takács, P.; Abonyi, A.; Bánó, B.; Erős, T. Effect of non-native species on taxonomic and functional diversity of fish communities in different river types. Biodivers. Conserv. 2021, 30, 2511–2528. [Google Scholar] [CrossRef]
- Clusa, L.; Garcia-Vazquez, E.; Fernández, S.; Meyer, A.; Machado-Schiaffino, G. Nuisance species in lake constance revealed through eDNA. Biol. Invasions 2021, 23, 1619–1636. [Google Scholar] [CrossRef]
- Radočaj, T.; Špelić, I.; Vilizzi, L.; Povž, M.; Piria, M. Identifying threats from introduced and translocated non-native freshwater fishes in Croatia and Slovenia under current and future climatic conditions. Glob. Ecol. Conserv. 2021, 27, e01520. [Google Scholar] [CrossRef]
- Iftime, A.; Iftime, O. Alien fish, amphibian and reptile species in Romania and their invasive status: A review with new data. Trav. Mus. Natl. d’Hist. Nat. “Grigore Antipa” 2021, 64, 131–186. [Google Scholar] [CrossRef]
- Kestemont, B. The bottom-up assessment of threatened species. Nat. Conserv. Res. Запoведная наука 2019, 4, 93–106. [Google Scholar] [CrossRef]
- Pupina, A.; Pupins, M.; Nekrasova, O.; Tytar, V.; Kozynenko, I.; Marushchak, O. Species distribution modelling: Bombina bombina (Linnaeus, 1761) and its important invasive threat Perccottus glenii (Dybowski, 1877) in Latvia under global climate change. J. Environ. Res. Eng. Manag. 2018, 74, 79–86. [Google Scholar] [CrossRef][Green Version]
- Vidal, O.; García-Berthou, E.; Tedesco, P.A.; Garcia-Marin, J.-L. Origin and genetic diversity of mosquitofish (Gambusia holbrooki) introduced to Europe. Biol. Invasions 2010, 12, 841–851. [Google Scholar] [CrossRef]
- Padilla, D.K.; Williams, S.L. Beyond ballast water: Aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Front. Ecol. Environ. 2004, 2, 131–138. [Google Scholar] [CrossRef]
- Kats, L.B.; Ferrer, R.P. Alien predators and amphibian declines: Review of two decades of science and the transition to conservation. Divers. Distrib. 2003, 9, 99–110. [Google Scholar] [CrossRef]
- Krumholz, L.A. Reproduction in the western mosquitofish, Gambusia affinis affinis (Baird & Girard), and its use in mosquito control. Ecol. Monogr. 1948, 18, 1–43. [Google Scholar]
- Garcı´a-Berthou, E.; Alcaraz, C.; Pou-Rovira, Q.; Zamora, L.; Coenders, G.; Feo, C. Introduction pathways and establishment rates of invasive aquatic species in Europe. Can. J. Fish. Aquat. Sci. 2005, 62, 453–463. [Google Scholar] [CrossRef]
- Alcaraz, C.; Bisazza, A.; García-Berthou, E. Salinity mediates the competitive interactions between invasive mosquitofish and an endangered fish. Oecologia 2008, 155, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Strecker, A.L.; Campbell, P.M.; Olden, J.D. The aquarium trade as an invasion pathway in the Pacific Northwest. Fisheries 2011, 36, 74–85. [Google Scholar] [CrossRef]
- Evans, J.P.; Magurran, A.E. Multiple benefits of multiple mating in guppies. Proc. Nat. Acad. Sci. USA 2000, 97, 10074–10076. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, L.I.; Sokolova, E.L.; Pegasov, V.A. Ichthyofauna of Moskva river within the city of Moscow and some data on its condition. Quest. Ichthyol. 1994, 34, 634–641. [Google Scholar]
- Reshetnikov, Y.S.; Bogutskaya, N.G.; Vasiljeva, E.D. List of fish-like animals and fish of fresh water of Russia. Quest. Ichthyol. 1997, 37, 723–771. [Google Scholar]
- Slynko, Y.V.; Kyiashko, V.N.; Yakovlev, V.N. List of fish-like animals and fish of Volga river basin. In Catalogue of Plants and Animals of Volga River Basin; ИБВВ РАН: Yaroslavl, Russian, 2000; pp. 252–277. [Google Scholar]
- Yakovlev, V.N.; Slynko, Y.V.; Kyiashko, V.N. Annotated List of Cyclostomata and Fish of Water Bodies of Upper Volga. Ecological Problems of Upper Volga; ИБВВ РАН: Yaroslavl, Russian, 2001; pp. 52–69. [Google Scholar]
- Kozynenko, I.I.; Tytar, V.M. Bioclimatic modeling of the European distribution of the invasive Asian tiger mosquito, Aedes (Stegomyia) albopictus (Skuse, 1895), with special reference to Ukraine. Rep. Natl. Acad. Sci. Ukr. 2020, 3, 88–93. [Google Scholar] [CrossRef]
- Kutsokon, Y.; Nekrasova, O.; Shkamerda, V.; Loparev, S. The spread of guppy (Poecilia reticulata Peters, 1859) in the Bortnychi aeration station channel of Kyiv City. In Biodiversity Dynamics 2012: In the Abstract of Scientific Materials; Zagorodniuk, I., Ed.; Lugansk, Ukraine, 2012; pp. 94–95. Available online: http://www.terioshkola.org.ua/library/conf2012-biodiv/DBD2012-Dynamics_of_Biodiversity-all.pdf (accessed on 12 May 2021).
- Gambusia holbrooki Girard, 1859 in GBIF Secretariat (2021). GBIF.org (2 April 2021) GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.d5e6w9 (accessed on 2 April 2021).
- Poecilia reticulata Peters, 1859 in GBIF Secretariat (2021). GBIF.org (16 June 2021) GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.tuwazq (accessed on 16 June 2021).
- Osorio-Olvera, L.; Lira-Noriega, A.; Soberón, J.; Peterson, A.T.; Falconi, M.; Contreras-Díaz, R.G. ntbox: An r package with graphical user interface for modelling and evaluating multidimensional ecological niches. Methods Ecol. Evol. 2020, 11, 1199–1206. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Jarošik, V.; Ota, N. Extending the suite of Bioclim variables: A proposed registry system and case study using principal components analysis. Methods Ecol. Evol. 2014, 5, 956–960. [Google Scholar] [CrossRef]
- Colwell, R.K.; Rangel, T.F. Hutchinson’s duality: The once and future niche. Proc. Natl. Acad. Sci. USA 2009, 106, 19651–19658. [Google Scholar] [CrossRef]
- Phillips, S.J. A brief tutorial on Maxent. AT&T Res. 2005, 190, 231–259. [Google Scholar]
- Peterson, A.T.; Papes, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Anderson, R.P.; Lew, D.; Peterson, A.T. Evaluating predictive models of species’ distributions: Criteria for selecting optimal models. Ecol. Model. 2003, 162, 211–232. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Kvach, Y.; Kutsokon, Y. The non-indigenous fishes in the fauna of Ukraine: A potentia ad actum. BioInvasions Rec. 2017, 6, 269–279. [Google Scholar] [CrossRef]
- National Geographic Russia. Available online: https://nat-geo.ru/nature/v-sochi-ozhidayut-nashestviya-malyarijnyh-komarov-iz-za-massovoj-gibeli-ryby-gambuzii/ (accessed on 11 June 2020).
- Nekrasova, O.; Tytar, V.; Pupins, M.; Čeirāns, A.; Marushchak, O. Distribution of Viviparous American Fish Species in Eastern Europe on the Example of Gambusia holbrooki Girarg, 1859 and Poecilia reticulata Peters, 1859 in the Context of Global Climate Change. In Proceedings of the 1st International Electronic Conference on Biological Diversity, Ecology and Evolution, (E-meeting address: www.sciforum.net), Online, 15–31 March 2021; Volume 68. [Google Scholar] [CrossRef]
- Nekrasova, O.D.; Tytar, V.M.; Kuybida, V.V. GIS Modeling of Climate Change Vulnerability of Amphibians and Reptiles in Ukraine; Shmalgausen Institute of Zoology NAS: Kyiv, Ukraine, 2019; 204p, ISBN 978-966-02-8956-7. [Google Scholar]
- Nekrasova, O.; Marushchak, O.; Pupins, M.; Skute, A.; Tytar, V.; Čeirāns, A. Distribution and Potential Limiting Factors of the European Pond Turtle (Emys orbicularis) in Eastern Europe. Diversity 2021, 13, 280. [Google Scholar] [CrossRef]
- Global Invasive Species Database: Gambusia holbrooki. Available online: http://issg.org/database/species/ecology.asp?si=617&fr=1&sts=sss&lang=EN (accessed on 11 June 2020).
- Dussault, G.V.; Kramer, D.L. Food and feeding behavior of the guppy, Poecilia reticulata (Pisces: Poeciliidae). Can. J. Zool. 1981, 59, 684–701. [Google Scholar] [CrossRef]
- Lawal, M.O.; Edokpayi, C.A.; Osibona, A.O. Food and feeding habits of the guppy, Poecilia reticulata, from drainage canal systems in Lagos, Southwestern Nigeria. West Afr. J. Appl. Ecol. 2018, 20, 1–9. [Google Scholar]
- Gkenas, C.; Oikonomou, A.; Economou, A.; Kiosse, F.; Leonardos, I. Life history pattern and feeding habits of the invasive mosquitofish, Gambusia holbrooki, in Lake Pamvotis (NW Greece). J. Biol. Res. 2012, 17, 121–136. [Google Scholar]
- Blanco, S.; Romo, S.; Villena, M.-J. Experimental study on the diet of mosquitofish (Gambusia holbrooki) under different ecological conditions in a shallow lake. Int. Rev. Hydrobiol. 2004, 89, 250–262. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, P.K. Food and feeding habits of an introduced mosquitofish, Gambusia holbrooki (Girard) (Poeciliidae) in a subtropical lake, lake Nainital, India. Asian Fish. Sci. 2010, 23, 355–366. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekrasova, O.; Tytar, V.; Pupins, M.; Čeirāns, A.; Marushchak, O.; Skute, A. A GIS Modeling Study of the Distribution of Viviparous Invasive Alien Fish Species in Eastern Europe in Terms of Global Climate Change, as Exemplified by Poecilia reticulata Peters, 1859 and Gambusia holbrooki Girarg, 1859. Diversity 2021, 13, 385. https://doi.org/10.3390/d13080385
Nekrasova O, Tytar V, Pupins M, Čeirāns A, Marushchak O, Skute A. A GIS Modeling Study of the Distribution of Viviparous Invasive Alien Fish Species in Eastern Europe in Terms of Global Climate Change, as Exemplified by Poecilia reticulata Peters, 1859 and Gambusia holbrooki Girarg, 1859. Diversity. 2021; 13(8):385. https://doi.org/10.3390/d13080385
Chicago/Turabian StyleNekrasova, Oksana, Volodymyr Tytar, Mihails Pupins, Andris Čeirāns, Oleksii Marushchak, and Arturs Skute. 2021. "A GIS Modeling Study of the Distribution of Viviparous Invasive Alien Fish Species in Eastern Europe in Terms of Global Climate Change, as Exemplified by Poecilia reticulata Peters, 1859 and Gambusia holbrooki Girarg, 1859" Diversity 13, no. 8: 385. https://doi.org/10.3390/d13080385
APA StyleNekrasova, O., Tytar, V., Pupins, M., Čeirāns, A., Marushchak, O., & Skute, A. (2021). A GIS Modeling Study of the Distribution of Viviparous Invasive Alien Fish Species in Eastern Europe in Terms of Global Climate Change, as Exemplified by Poecilia reticulata Peters, 1859 and Gambusia holbrooki Girarg, 1859. Diversity, 13(8), 385. https://doi.org/10.3390/d13080385






