Mammoth Cave: A Hotspot of Subterranean Biodiversity in the United States
Abstract
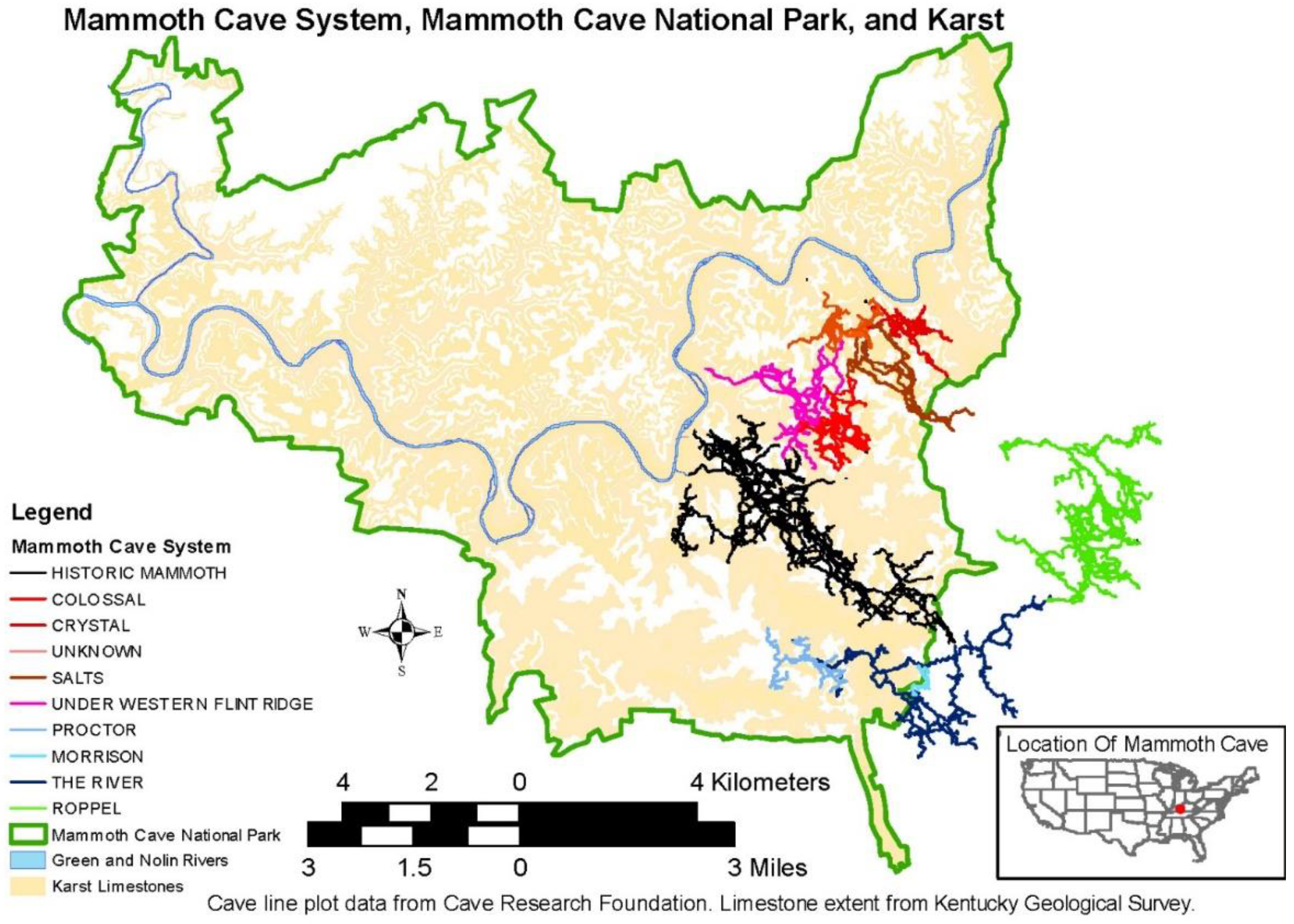
1. Introduction
2. Materials and Methods
3. Results
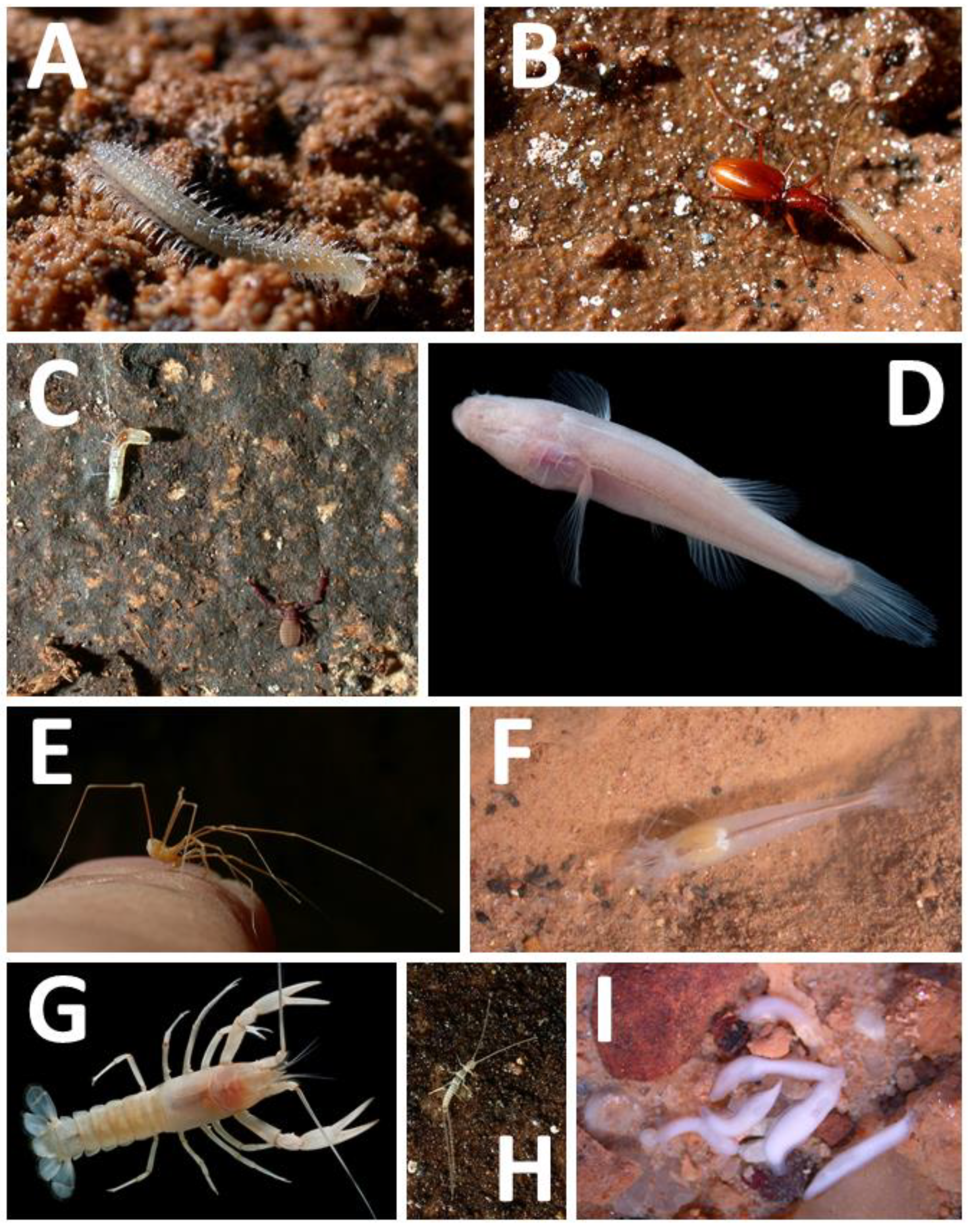
3.1. Terrestrial Fauna
3.2. Aquatic Fauna
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toomey, R.S.; Hobbs, H.H., III; Olson, R.A. An orientation to Mammoth Cave and this volume. In Mammoth Cave: A Human and Natural History, Cave and Karst Systems of the World; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–28. [Google Scholar]
- Miotke, F.D.; Palmer, A.N. Genetic Relationship between Cave Land-Forms in the Mammoth Cave National Park Area; Boehler Verlag: Wuerzburg, Germany, 1972. [Google Scholar]
- Palmer, A.N. A Geological Guide to Mammoth Cave National Park; Zephyrus Press: Teaneck, NJ, USA, 1981. [Google Scholar]
- Palmer, A.N. Cave Geology; Cave Books: Dayton, OH, USA, 2007. [Google Scholar]
- Palmer, A.N. Geology of Mammoth Cave. In Mammoth Cave: A Human and Natural History, Cave and Karst Systems of the World; Springer: Berlin/Heidelberg, Germany, 2017; pp. 97–110. [Google Scholar]
- Palmer, A.N. Geologic history of Mammoth Cave. In Mammoth Cave: A Human and Natural History, Cave and Karst Systems of the World; Springer: Berlin/Heidelberg, Germany, 2017; pp. 111–121. [Google Scholar]
- Quinlan, J.F.; Ewers, R.O. Hydrogeology of the Mammoth Cave region, Kentucky. In GSA Cincinnati 1981 Field Trip Guidebook 3; American Geological Institute: Falls Church, VA, USA, 1981; pp. 457–506. [Google Scholar]
- Glennon, A.; Groves, C. An examination of perennial stream drainage patterns within the Mammoth Cave watershed, Kentucky. J. Cave Karst Stud. 2002, 64, 82–91. [Google Scholar]
- Granger, D.E.; Fabel, D.; Palmer, A.N. Pliocene-Pleistocene incision of the Green River, Kentucky, determined from radioactive decay of cosmogenic 26Al and 10Be in Mammoth Cave sediments. Geo. Soc. Am. Bull. 2001, 113, 825–836. [Google Scholar] [CrossRef]
- Barr, T.C., Jr. Ecological studies in the Mammoth Cave System of Kentucky I: The biota. Int. J. Speleol. 1968, 3, 147–204. [Google Scholar] [CrossRef]
- Rafinesque, C. The caves of Kentucky. Atl. J. 1832, 1, 27–30. [Google Scholar]
- Darwin, C. On the Origin of Species by Means of Natural Slection, or the Preservation of Favoured Races in the Struggle for Life, 1st ed.; John Murray: London, UK, 1859. [Google Scholar]
- DeKay, J.E. Zoology of New York or the New York Fauna; Comprising Detailed Descriptions of All the Animals Hitherto Observed within the State of New York, with Brief Notices of Those Occasionally found Near Its Borders, and Accompanied by Appropriate Illustrations. Part IV, Fishes; W.& A. White & J. Visscher: Albany, NY, USA, 1842. [Google Scholar]
- Wyman, J. Description of a blind-fish from a cave in Kentucky. Am. J. Sci. Arts 1843, 45, 94–96. [Google Scholar]
- Wyman, J. Description of a blind-fish from a cave in Kentucky. Ann. Mag. Nat. Hist. 1843, 12, 298–299. [Google Scholar]
- Wyman, J. Account of dissections of the blind fishes (Amblyopsis spelaeus) from the Mammoth Cave, Kentucky. Proc. Boston Soc. Nat. Hist. 1851, 3, 349–375. [Google Scholar]
- Wyman, J. The eyes and organs of hearing in Amblyopsis spelaeus. Proc. Boston Soc. Nat. Hist. 1854, 4, 149–151. [Google Scholar]
- Wyman, J. On the eye and the organ of hearing in the blind fishes (Amblyopsis spelaeus DeKay) of the Mammoth Cave. Proc. Boston Soc. Nat. Hist. 1854, 4, 395–396. [Google Scholar]
- Wyman, J. Notes and drawings of the rudimentary eyes, brain and tactile organs of Amblyopsis spelaeus, in Putnam (1872). Am. Nat. 1872, 6, 6–30. [Google Scholar]
- Tellkampf, T.G. Ueber den blinden Fisch der Mammuth-Hohle in Kentucky, mit Bemerkungen ueber einige undere in dieser Hohle lebenden Thiere. Arch. Anat. Phys. Wiss. Med. 1844, 4, 381–394. [Google Scholar]
- Tellkampf, T.G. Beschreibung einiger neuer in der Mammuth-Höhle in Kentucky aufgefundener Gattungen von Gliedertieren. Arch. Nat. 1844, 10, 318–322. [Google Scholar] [CrossRef]
- Tellkampf, T.G. Memoirs on the blind-fishes and some other animals living in the Mammoth Cave in Kentucky. N. Y. J. Med. Collat. Sci. 1845, 5, 84–93. [Google Scholar]
- Agassiz, J.L.R. Plan for an investigation of the embryology, anatomy and effect of light on the blind-fish of the Mammoth Cave, Amblyopsis spelaeus. Proc. Am. Acad. Arts Sci. 1847, 1, 1–180. [Google Scholar]
- Agassiz, J.L.R. Observations of the blind fish of the Mammoth Cave. Am. J. Sci. Arts 1851, 11, 127–128. [Google Scholar]
- Agassiz, J.L.R. Recent researches of Prof. Agassiz. Am. J. Sci. Arts 1853, 16, 134. [Google Scholar]
- von Mutschulsky, T.V. Etudes Entomologiques, 3e Annee; Helsingfors, Finland, 1854. [Google Scholar]
- von Mutschulsky, T.V. Etudes Entomologiques, 11e Annee; Dresden, Germany, 1862. [Google Scholar]
- Call, R.E. Some notes on the flora and fauna of Mammoth Cave, Kentucky. Am. Nat. 1897, 31, 377–392. [Google Scholar] [CrossRef]
- Packard, A.S. On the crustaceans and insects of the Mammoth Cave. Am. Nat. 1871, 5, 744–761. [Google Scholar]
- Packard, A.S. Occurrence of Japan in the United States. Am. Nat. 1874, 8, 501–502. [Google Scholar]
- Packard, A.S. Larvae of Anophthalmus and Adelops. Am. Nat. 1874, 8, 562–563. [Google Scholar]
- Packard, A.S. The invertebrate cave fauna of Kentucky and adjoining states, Araneina. Am. Nat. 1875, 9, 271–278. [Google Scholar] [CrossRef]
- Packard, A.S. Cave-inhabiting spiders. Am. Nat. 1875, 9, 663–664. [Google Scholar]
- Packard, A.S. The cave-beetles of Kentucky. Am. Nat. 1876, 10, 282–287. [Google Scholar] [CrossRef]
- Packard, A.S. On the structure of the brain of Asellus and the eyeless form Caecidotea. Am. Nat. 1885, 19, 85–86. [Google Scholar]
- Packard, A.S. The cave fauna of North America, with remarks on the anatomy of the brain and origin of the blind species. Mem. Nat. Acad. Sci. USA 1888, 4, 1–156. [Google Scholar]
- Culver, D.C.; Hobbs, H.H., III. Biodiversity of Mammoth Cave. In Mammoth Cave: A Human and Natural History, Cave and Karst Systems of the World; Hobbs, H.H., III, Olson, R.A., Winkler, E.G., Culver, D.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 227–234. [Google Scholar]
- Putnam, F.W. The blind fishes of the Mammoth Cave and their allies. Am. Nat. 1872, 6, 6–30. [Google Scholar] [CrossRef]
- Eigenmann, C.H. The Amblyopsidae, the blind fish of America. Rep. Br. Assoc. Adv. Sci. 1897, 1897, 685–686. [Google Scholar]
- Eigenmann, C.H. The blind fishes of North America. Pop. Sci. Mon. 1899, 56, 473–486. [Google Scholar]
- Eigenmann, C.H. Divergence and convergence in fishes. Biol. Lect. Mar. Biol. Lab. Woods Hole 1905, 8, 59–66. [Google Scholar] [CrossRef]
- Eigenmann, C.H. Cave Vertebrates of America: A Study in Degenerative Evolution; Carnegie Institution of Washington: Washington, DC, USA, 1909. [Google Scholar]
- Bolivar, C.; Jeannel, R. Campagne Speleologique dans I’Amerique du Nord en 1928. Biospeleologie LVI. Arch. Zool. Exp. Gen. 1931, 71, 293–499. [Google Scholar]
- Bailey, V. Cave life of Kentucky, mainly in the Mammoth Cave region. Am. Midl. Nat. 1933, 14, 385–635. [Google Scholar] [CrossRef]
- Buchanan, J.W. Notes on an American cave flatworm, Sphalloplana percaeca (Packard). Ecology 1936, 17, 194–241. [Google Scholar] [CrossRef]
- Park, O. Key to the more common adult animals of Mammoth and adjacent caves. In A Laboratory Introduction to Animal Ecology and Taxonomy; Park, O., Allee, W.C., Shelford, V.E., Eds.; University of Chicago Press: Chicago, IL, USA, 1939; pp. 118–124. [Google Scholar]
- Dearolf, K. Report of a biological reconnaissance of the New Discovery in Mammoth Cave, Kentucky, January 7–11, 1941. Bul. Nat. Speleol. Soc. 1942, 4, 48–52. [Google Scholar]
- Hubricht, L. Studies of the Nearctic freshwater Amphipoda, III. Notes on the freshwater Amphipoda of eastern United States, with description of ten new species. Am. Midl. Nat. 1943, 29, 683–712. [Google Scholar] [CrossRef]
- Hubricht, L. The cave snail, Carychium stygium Call. Trans. Ky. Acad. Sci. 1960, 21, 35–38. [Google Scholar]
- Hubricht, L. New species of Helicodiscus from the eastern United States. Nautilus 1962, 75, 102–107. [Google Scholar]
- Hubricht, L. New species of Hydrobiidae. Nautilus 1963, 76, 138–140. [Google Scholar]
- Hubricht, L. Land snails from the caves of Kentucky, Tennessee, and Alabama. Nat. Speleol. Soc. Bull. 1964, 26, 33–36. [Google Scholar]
- Hubricht, L. Four new land snails from the southeastern United States. Nautilus 1965, 79, 4–7. [Google Scholar]
- Jeannel, R.; Henrot, H. Les coleopteres cavernicoles de la region des Appalaches. I. La region des Appalaches (Jeannel), and II. Enumeration des grottes explorees (Henrot). Notes Biospel. 1949, 4, 11–36. [Google Scholar]
- Barr, T.C., Jr. New cave beetles (Carabidae) from Tennessee and Kentucky. J. Tenn. Acad. Sci. 1959, 34, 5–30. [Google Scholar]
- Barr, T.C., Jr. The male of Pseudanophthalmus audax (Coleoptera: Carabidae). Trans. Ky. Acad. Sci. 1959, 20, 1–3. [Google Scholar]
- Barr, T.C., Jr. The blind beetles of Mammoth Cave, Kentucky. Am. Midl. Nat. 1962, 68, 278–284. [Google Scholar] [CrossRef]
- Barr, T.C., Jr. Studies on the cavernicole Ptomaphagus of the United States (Coleoptera, Catopidae). Psyche 1963, 70, 50–58. [Google Scholar] [CrossRef][Green Version]
- Barr, T.C., Jr. Cave Carabidae (Coleoptera) of Mammoth Cave. Pysche 1966, 73, 284–287. [Google Scholar]
- Barr, T.C., Jr. Cave Carabidae (Coleoptera) of Mammoth Cave, Part II. Pysche 1967, 74, 24–25. [Google Scholar]
- Poulson, T.L. The Mammoth Cave ecosystem. In The Natural History of Biospeleology; Camacho, A., Ed.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 1992; pp. 569–611. [Google Scholar]
- Poulson, T.L. Terrestrial cave ecology of the Mammoth Cave region. In Mammoth Cave: A Human and Natural History, Cave and Karst Systems of the World; Hobbs, H.H., III, Olson, R.A., Winkler, E.G., Culver, D.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 199–207. [Google Scholar]
- Helf, K.L.; Olson, R.A. Subsurface aquatic ecology of Mammoth Cave. In Mammoth Cave: A Human and Natural History, Cave and Karst Systems of the World; Hobbs, H.H., III, Olson, R.A., Winkler, E.G., Culver, D.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 209–226. [Google Scholar]
- Coney, C.C.; Tarpley, W.A.; Bohannan, R. Ecological studies of land snails in the Hiawassee River Basin of Tennessee, U.S.A. Malacol. Rev. 1982, 15, 69–106. [Google Scholar]
- Bowman, T.E.; Prins, R.; Morris, B.F. Notes on the harpacticoid copepods Attheyella pilosa and A. carolinensis, associates of crayfishes in the eastern United States. Proc. Biol. Soc. Wash. 1968, 81, 571–586. [Google Scholar]
- Lewis, J.J. The Systematics, Zoogeography and Life History of the Troglobitic Isopods of the Interior Plateaus of the Eastern United States. Ph.D. Dissertation, University of Louisville, Louisville, KY, USA, 1988. [Google Scholar]
- Borgmeier, T. Revision of the North American Phorid flies. Part III. The species of the genus Megaselia, subgenus Megaselia (Diptera, Phoridae): Studia Entomologica. Petropolis 1966, 8, 1–160. [Google Scholar]
- Lewis, J.J. Bioinventory of Caves of the Cumberland Escarpment Area of Tennessee, Final Report; Tennessee Chapter of the Nature Conservancy: Nashville, TN, USA, 2005. [Google Scholar]
- Zigler, K.S.; Niemiller, M.L.; Stephen, C.D.R.; Ayala, B.N.; Milne, M.A.; Gladstone, N.S.; Engel, A.S.; Jensen, J.B.; Camp, C.D.; Ozier, J.C.; et al. Biodiversity from caves and other subterranean habitats of Georgia, USA. J. Cave Karst Stud. 2020, 82, 125–167. [Google Scholar] [CrossRef]
- Weigand, A.M.; Jochum, A.; Pfenninger, M.; Steinke, D.; Klussmann-Kolb, A. A new approach to an old conundrum—DNA barcoding sheds new light on phenotypic plasticity and morphological stasis in microsnails (Gastropoda, Pulmonata, Carychiidae). Mol. Ecol. Res. 2011, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.M.; Jochum, A.; Slapnik, R.; Schnitzler, J.; Zarza, E.; Klussmann-Kolb, A. Evolution of microgastropods (Ellobioidea, Carychiidae): Integrating taxonomic, phylogenetic and evolutionary hypotheses. BMC Evol. Biol. 2013, 13, 18. [Google Scholar] [CrossRef]
- Gladstone, N.S.; Carter, E.T.; McKinney, M.L.; Niemiller, M.L. Status and distribution of the cave-obligate land snails in the Appalachians and Interior Low Plateau of the eastern United States. Am. Malacol. Bull. 2018, 36, 62–78. [Google Scholar] [CrossRef]
- Poulson, T.L.; Lavoie, K.H.; Helf, K. Long-term effects of weather on the cricket (Hadenoecus subterraneus, Orthoptera, Rhaphidophoridae) guano community in Mammoth Cave National Park. Am. Midl. Nat. 1995, 134, 226–236. [Google Scholar] [CrossRef]
- Dourson, D.C. Kentucky’s Land Snails and Their Ecological Communities; Goatslug Publications: Bakersville, NC, USA, 2010. [Google Scholar]
- Miller, J.A. A redescription of Porrhomma cavernicola Keyserling (Araneae, Linyphiidae) with notes on Appalachian troglobites. J. Arachnol. 2005, 33, 426–439. [Google Scholar] [CrossRef]
- Holsinger, J.R.; Culver, D.C. The invertebrate cave fauna of Virginia and a part of eastern Tennessee: Zoogeography and ecology. Brimleyana 1988, 14, 1–162. [Google Scholar]
- Paquin, P.; Dupérré, N. Guide d’identifcation des Araignées (Araneae) du Québec. Fabreries Supplément 2003, 11, 1–251. [Google Scholar]
- Holsinger, J.R.; Culver, D.C.; Hubbard, D.A., Jr.; Orndorff, W.D.; Hobson, C.S. The invertebrate cave fauna of Virginia. Banisteria 2013, 42, 9–56. [Google Scholar]
- Peck, S.B. A summary of diversity and distribution of the obligate cave-inhabiting faunas of the United States and Canada. J. Caves Karst Stud. 1998, 60, 18–26. [Google Scholar]
- Keyserling, E.G. Neue Spinnen aus Amerika, III. Verh. zool.-bot. Ges. Wien 1881, 31, 269–314. [Google Scholar]
- Platnick, N.I. A revision of the Appalachian spider genus Liocranoides (Araneae: Tengellidae). Amer. Mus. Nov. 1999, 3285, 1–13. [Google Scholar]
- Emerton, J.H. Notes on spiders from caves in Kentucky and Indiana. Am. Nat. 1875, 9, 278–281. [Google Scholar] [CrossRef]
- Hubbard, H.G. Two days collecting in the Mammoth Cave, with contributions to a study of its fauna. Am. Entomol. 1880, 3, 34–40. [Google Scholar]
- McIndoo, N.E. Notes on some arachnids from Ohio valley caves. The Biol. Bull. 1911, 20, 183–186. [Google Scholar] [CrossRef]
- Berland, L. Arachnides araneides (Biospeologica LVI) . Arch Zool. Exp. Gen. 1931, 71, 383–388. [Google Scholar]
- Poulson, T.L.; Culver, D.C. Diversity in terrestrial cave communities. Ecology 1969, 50, 153–158. [Google Scholar] [CrossRef]
- Poulson, T.L. Variations in life history of Linyphiid cave spiders. In Proceedings of the 8th International Congress of Speleology, Tampa, FL, USA, 18–24 July 1981; Volume 1, pp. 60–62. [Google Scholar]
- Goodnight, C.J.; Goodnight, M.L. Speciation among cave opilionids of the United States. Am. Midl. Nat. 1960, 64, 34–38. [Google Scholar] [CrossRef]
- Hedin, M.; Thomas, S.M. Molecular systematics of eastern North American Phalangodidae (Arachnida: Opiliones: Laniatores), demonstrating convergent morphological evolution in caves. Mol. Phylogenet. Evol. 2010, 54, 107–121. [Google Scholar] [CrossRef]
- Malcolm, D.R.; Chamberlin, J.C. The pseudoscorpion genus Kleptochthonius Chamberlin (Chelonethida, Chthoniidae). Am. Mus. Nov. 1961, 2063, 1–35. [Google Scholar]
- Muchmore, W.B. Redescription of some cavernicolous pseudoscorpions (Arachnida, Chelonethida) in the collection of the Museum of Comparative Zoology. Breviora 1963, 188, 1–16. [Google Scholar]
- Muchmore, W.B. The genus Tyrannochthonius in the eastern United States (Pseudoscorpionida: Chthoniidae). Part II. More recently described species. Insecta Mundi 1996, 10, 153–168. [Google Scholar]
- Banks, N. Notes on the Pseudoscorpionida. J. N. Y. Entomol. Soc. 1895, 3, 1–13. [Google Scholar]
- Vitzthum, H. Die unterirdische Acarofauna. Z. Naturw. 1925, 62, 125–186. [Google Scholar]
- Holsinger, J.R. Redescriptions of two poorly known species of cavernicolous rhagidiid mites (Acarina, Trombidiformes) from Virginia and Kentucky. Acarologia 1965, 7, 654–662. [Google Scholar]
- Zacharda, M. Soil mites of the family Rhagidiidae (Actinedida: Eupodoidea). Morphology, systematics, ecology. Acta Univ. Carol. Biol. 1980, 1978, 489–785. [Google Scholar]
- Cope, E.D. On the Wyandotte Cave and its fauna. Am. Nat. 1872, 6, 406–422. [Google Scholar] [CrossRef]
- Loomis, H.F. New cave and epigean millipedes of the United States, with notes on some established species. Bull. Mus. Comp. Zool. 1943, 92, 373–410. [Google Scholar]
- Shear, W.A. The milliped family Trichopetalidae, Part 2: The genera Trichopetalum, Zygonopus and Scoterpes (Diplopoda: Chordeumatida, Cleidogonoidea). Zootaxa 2010, 2385, 1–62. [Google Scholar] [CrossRef]
- Christiansen, K.A. The genus Arrhopalites (Collembola, Sminthuridae) in the United States and Canada. Int. J. Speleol. 1966, 2, 43–73. [Google Scholar] [CrossRef]
- Christiansen, K.; Bellinger, P. Cave Pseudosinella and Oncopodura new to science. J. Cave Karst Stud. 1996, 58, 38–53. [Google Scholar]
- Harker, D.F.; Barr, T.C. Western Kentucky coal field: Preliminary investigations of natural features and cultural resources. In Caves and Associated Fauna of the Western Kentucky Coal Field; Technical Report; Kentucky Nature Preserves Commission: Frankfort, KY, USA, 1980; Volume 2. [Google Scholar]
- Christiansen, K.A. The genus Pseudosinella (Collembola, Entomobryidae) in caves of the United States. Psyche 1960, 67, 1–25. [Google Scholar] [CrossRef][Green Version]
- Christiansen, K.A. A preliminary survey of the knowledge of North American cave Collembola. Am. Midi. Nat. 1960, 64, 39–44. [Google Scholar] [CrossRef]
- Christiansen, K.A. The genus Sinella Brook (Collembola, Entomobryidae) in Nearctic caves. Ann. Entomol. Soc. Am. 1960, 53, 481–491. [Google Scholar] [CrossRef]
- Ferguson, L.M. Systematics, Evolution, and Zoogeography of the Cavernicolous Campodeids of the Genus Litocampa (Diplura: Campodeidae) in the United States. Ph.D. Dissertation, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 1981. [Google Scholar]
- Silvestri, F. Campodeidae (Biospeologica LVI). Arch. Zool. Expo Gen. 1934, 76, 379–383. [Google Scholar]
- Silvestri, F. On some Tapygidae in the Museum of Comparative Zoology. Psyche 1947, 51, 209–229. [Google Scholar] [CrossRef]
- Conde, B. Campodeides cavernicoles de la region des Appalaches. Notes Biospeol. 1949, 4, 125–139. [Google Scholar]
- Ferguson, L.M. Taxonomy, Distribution, and Evolution of Cavernicolous Campodeids in Virginia (Diplura: Campodeidae). Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 1974. [Google Scholar]
- Barr, T.C., Jr. The taxonomy, distribution, and affinities of Neaphanops, with notes on associated species of Pseudanophthalmus (Coleoptera, Carabidae). Am. Mus. Nov. 1979, 2682, 1–20. [Google Scholar]
- Erichson, W.F. Footnote, p. 384; In Ueber den blinden Fisch der Mammuth-Hohle in Kentucky, mit Bemerkungen uber einige andere in diser Hohle lebenden Thiere; Tellkampf, T. Mullers Arch Anat. Physiol. 1844, 4, 384–394. [Google Scholar]
- Barr, T.C., Jr. Pattern and process in speciation of trechine beetles in eastern North America (Coleoptera: Carabidae: Trechinae). In Taxonomy, Phylogeny, and Biogeography of Beetles and Ants; Ball, G.E., Ed.; Series Entomologia 33; Junk: Dordrecht, The Netherlands, 1985; pp. 350–407. [Google Scholar]
- Friedrich, M. Biological clocks and visual systems in cave-adapted animals at the dawn of speleogenomics. Integr. Comp. Biol. 2013, 53, 50–67. [Google Scholar] [CrossRef]
- Friedrich, M.; Chen, R.; Daines, B.; Bao, R.; Caravas, J.; Rai, P.K.; Zagmajster, M.; Peck, S.B. Phototransduction and clock gene expression in the troglobiont beetle Ptomaphagus hirtus of Mammoth cave. J. Exp. Biol. 2011, 214, 3532–3541. [Google Scholar] [CrossRef]
- Horn, G.H. Catalogue of Coleoptera from south-west Virginia. Trans. Am. Entomol. Soc. 1868, 2, 123–128. [Google Scholar]
- Horn, G.H. Miscellaneous notes and short studies of North American Coleoptera. Trans. Am. Entomol. Soc. 1883, 10, 269–293. [Google Scholar] [CrossRef]
- Jeannel, R. Notes sur les Trechini. Bull. Soc. Entomol. Fr. 1920, 1920, 150–155. [Google Scholar]
- Jeannel, R. Monographie des Trechinae, 3e livraison. L’Abeille 1928, 35, 1–808. [Google Scholar]
- Jeannel, R. Revision des Trechinae de l’Amerique du nord. Arch. Zool. Expo Gen. 1931, 71, 403–499. [Google Scholar]
- Jeannel, R. Les coleopteres cavernicoles de la region des Appalaches. III. Etude systematique. Notes Biospeol. 1949, 4, 37–104. [Google Scholar]
- Valentine, J.M. New cavernicole Carabidae of the subfamily Trechinae Jeannel. J. Elisha Mitchell Sci. Soc. 1931, 46, 247–258. [Google Scholar]
- Valentine, J.M. A classification of the genus Pseudanophthalmus Jeannel (fam. Carabidae) with descriptions of new species and notes on distribution. J. Elisha Mitchell Sci. Soc. 1932, 48, 261–280. [Google Scholar]
- Hatch, M.H. Studies on the Leptodiridae (Catopidae) with descriptions of new species. J. N. Y. Entomol. Soc. 1933, 41, 187–239. [Google Scholar]
- Park, O. New or little-known species of pselaphid beetles from southeastern United States. J. Tenn. Acad. Sci. 1956, 31, 54–100. [Google Scholar]
- Park, O. New or little-known species of pselaphid beetles, chiefly from southeastern United States. J. Tenn. Acad. Sci. 1958, 33, 39–74. [Google Scholar]
- Park, O. Cavernicolous pselaphid beetles of the United States. Amer. Midl. Nat. 1960, 64, 66–104. [Google Scholar] [CrossRef]
- Barr, T.C., Jr. Non-troglobitic Carabidae (Coleoptera) from caves in the United States. Coleopt. Bull. 1964, 18, 1–4. [Google Scholar]
- Barr, T.C., Jr. Cave ecology and the evolution of troglobites. Evol. Biol. 1968, 2, 35–102. [Google Scholar]
- Barr, T.C., Jr. Ecology and evolution of cave faunas. In Proceedings of the Third Annual Symposium on the Natural History of the Lower Tennessee and Cumberland River Valleys; Hamilton, S.W., Finley, M.T., Eds.; Center for Field Biology, Austin Peay State University: Clarksville, TN, USA, 1990; pp. 1–19. [Google Scholar]
- Barr, T.C., Jr. A Classification and Checklist of the Genus Pseudanophthalmus Jeannel (Coleoptera: Carabidae: Trechinae); Special Publication 11; Virginia Museum of Natural History: Martinsville, VA, USA, 2004; p. 52. [Google Scholar]
- Barr, T.C., Jr.; Kuehne, R.A. Ecological studies in the Mammoth Cave System of Kentucky, II: The ecosystem. Ann. Spéléologie 1971, 26, 47–96. [Google Scholar]
- Peck, S.B. A systematic revision and evolutionary biology of the Ptomaphagus adelops. Bull. Mus. Comp. Zool. 1973, 145, 29–162. [Google Scholar]
- Peck, S.B. The life cycle of a Kentucky cave beetle, Ptomaphagus hirtus, (Coleoptera; Leiodidae; Catopinae). Int. J. Speleol. 1975, 7, 7–17. [Google Scholar] [CrossRef]
- Peck, S.B. Experimental hybridizations between populations of cavernicolous Ptomaphagus beetles (Coleoptera: Leiodidae: Cholevinae). Canad. Entomol. 1983, 115, 445–452. [Google Scholar] [CrossRef]
- Peck, S.B. The distribution and evolution of cavernicolous Ptomaphagus beetles in the southeastern United States (Coleoptera; Leiodidae; Cholevinae) with new species and records. Canad. J. Zool. 1984, 62, 730–740. [Google Scholar] [CrossRef]
- Peck, S.B. Evolution of adult morphology and life-history characters in cavernicolous Ptomaphagus beetles. Evolution 1986, 40, 1021–1030. [Google Scholar] [CrossRef]
- Kane, T.C.; Norton, R.M.; Poulson, T.L. The ecology of a predaceous troglobitic beetle, Neaphaenops tellkampfii (Coleoptera: Carabidae, Trechinae). I. Seasonality of food input and early life history stages. Int. J. Speleol. 1975, 7, 45–54. [Google Scholar] [CrossRef]
- Norton, R.M.; Kane, T.C.; Poulson, T.L. The ecology of a predaceous troglobitic beetle, Neaphaenops tellkampfii (Coleoptera: Carabidae, Trechinae). II. Adult seasonality, feeding and recruitment. Int. J. Speleol. 1975, 7, 55–64. [Google Scholar] [CrossRef]
- Kane, T.C.; Poulson, T.L. Foraging by cave beetles: Spatial and temporal heterogeneity of prey. Ecology 1976, 57, 793–800. [Google Scholar] [CrossRef]
- Laing, C.; Carmody, G.R.; Peck, S.B. Population genetics and evolutionary biology of the cave beetle Ptomaphagus hirtus. Evolution 1976, 30, 484–498. [Google Scholar] [CrossRef]
- Giuseffi, S.; Kane, T.C.; Duggleby, W.F. Genetic variability in the Kentucky cave beetle Neaphaenops tellkampfii (Coleoptera: Carabidae). Evolution 1978, 32, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Kane, T.C.; Ryan, T. Population ecology of carabid cave beetles. Oecologia 1983, 60, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.C., Jr.; Holsinger, J.R. Speciation in cave faunas. Ann. Rev. Ecol. Syst. 1985, 16, 313–337. [Google Scholar] [CrossRef]
- Kane, T.C.; Brunner, G.D. Geographic variation in the cave beetle, Neaphaenops tellkampfi (Coleoptera: Carabidae). Psyche 1986, 93, 231–251. [Google Scholar] [CrossRef][Green Version]
- Helf, K.L. Foraging Ecology of the Cave Cricket Hadenoecus subterraneus: Effects of Climate, Ontogeny, and Predation. Ph.D. Dissertation, University of Illinois at Chicago, Chicago, IL, USA, 2003. [Google Scholar]
- Leray, V.L.; Caravas, J.; Friedrich, M.; Zigler, K.S. Mitochondrial sequence data indicate “Vicariance by Erosion” as a mechanism of species diversification in North American Ptomaphagus (Coleoptera, Leiodidae, Cholevinae) cave beetles. Subterr. Biol. 2019, 29, 35–57. [Google Scholar] [CrossRef]
- Marshall, S.A.; Peck, S.B. Distribution of cave-dwelling Sphaeroceridae (Diptera) of eastern North America. Proc. Entomol. Soc. Ontario 1984, 115, 37–41. [Google Scholar]
- De Beachamp, P. Turbellaries triclades (Biospeologica LVI). Arch. Zool. Expo Gen. 1931, 71, 317–331. [Google Scholar]
- Hyman, L.H. Studies on the morphology, taxonomy, and distribution of North American triclad Turbellaria. VIII. Some cave planarians of the United States. Trans. Am. Micr. Soc. 1937, 56, 457–477. [Google Scholar] [CrossRef]
- Carpenter, J.H. Systematics and Ecology of Cave Planarians of the United States. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 1970. [Google Scholar]
- Carpenter, J.H. Observations on the biology of cave planarians of the United States. Int. J. Speleol. 1982, 12, 9–26. [Google Scholar] [CrossRef][Green Version]
- Kenk, R. Freshwater triclads (Turbellaria) of North America, IX. The genus Sphalloplana. Smithson. Contrib. Zool. 1977, 246, 1–38. [Google Scholar] [CrossRef]
- Pearson, W.D.; Boston, C.H. Distribution and Status of the Northern Cavefish; Amblyopsis Spelaea, Final Report; Nongame and Endangered Wildlife Program, Indiana Department of Natural Resources: Indianapolis, IN, USA, 1995.
- Hershler, R.; Hubricht, L. Notes on Antroselates Hubricht, 1963 and Antrobia Hubricht, 1971 (Gastropoda: Hydrobiidae). Proc. Biol. Soc. Wash. 1988, 101, 730–740. [Google Scholar]
- Niemiller, M.L.; Poulson, T.L. Subterranean fishes of North America: Amblyopsidae. In The Biology of Subterranean Fishes; Trajano, E., Bichuette, M.E., Kappor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2010; pp. 169–280. [Google Scholar]
- Kofoid, C.A. The plankton of Echo River, Mammoth Cave. Trans. Am. Micr. Soc. 1899, 21, 113–126. [Google Scholar] [CrossRef]
- Chappuis, P.A. Crustaces copepodes (Biospeologica LVI). Arch. Zool. Expo Gen. 1931, 71, 345–360. [Google Scholar]
- Whitman, R.L. Meiofaunal Sampling at Mammoth Cave National Park. Draft Report; National Park Service, Indiana Dunes National Lakeshore: Chesterton, IN, USA, 1989. [Google Scholar]
- Lewis, J.J. Conservation Assessment for Northern Cavefish Copepod (Cauloxenus stygius). Report; USDA Forest Service: Eastern Region, Ghana, 2002. [Google Scholar]
- Klie, W. Crustaces ostracodes (Biospeologica LVI). Arch. Zool. Expo Gen. 1931, 71, 333–344. [Google Scholar]
- Hart, C.W., Jr.; Hobbs, H.H., Jr. Eight new troglobitic ostracods of the genus Entocythere (Crustacea, Ostracoda) from the eastern United States. Proc. Acad. Nat. Sci. USA 1961, 113, 173–185. [Google Scholar]
- Hart, C.W., Jr.; Hart, D.G. Four new entocytherid ostracods from Kentucky, with notes on the troglobitic Sagittocythere barri. Nolulae Nat. 1966, 388, 1–10. [Google Scholar]
- Hart, D.G.; Hart, C.W., Jr. The ostracod family Entocytheridae. Acad. Nat. Sci. Phila. Monogr. 1974, 18, 1–239. [Google Scholar]
- Garman, H. The origin of the cave fauna of Kentucky, with a description of a new blind beetle. Science 1892, 20, 240–241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hay, W.P. Observations on the crustacean fauna of the region about Mammoth Cave, Kentucky. Proc. U.S. Nat. Mus. 1902, 25, 223–236. [Google Scholar] [CrossRef]
- Giovanolli, L. Invertebrate life of Mammoth and other neighboring caves. Pp. 600–623 In: V. Bailey. Cave Life of Kentucky. Am. Midl. Nat. 1933, 14, 385–635. [Google Scholar]
- Chappuis, P.A. Biospeologica 71: Campagne speologique de C. Bolivar et R. Jeannel dans PAmerique du Nord (1928), 13, Asellides. Arch. Zool. Expo Gen. 1950, 87, 177–182. [Google Scholar]
- Lewis, J.J.; Bowman, T.E. The subterranean asellids (Caecidotea) of Illinois (Crustacea: Isopoda: Asellidae). Smithson. Contrib. Zool. 1981, 335, 1–66. [Google Scholar] [CrossRef]
- Helf, K.L.; Moore, W.; Wells, B. Monitoring Cave Aquatic Biota at Selected Parks in the Cumberland Piedmont Network: Protocol Narrative—Version 1.0. Natural Resource Report NPS/CUPN/NRR—2018/1705; National Park Service: Fort Collins, CO, USA, 2018. [Google Scholar]
- Zhang, J.; Holsinger, J.R. Systematics of the freshwater amphipod genus Crangonyx (Crangonyctidae) in North America. Va. Mus. Nat. Hist. Memoir. 2003, 6, 1–274. [Google Scholar]
- Holsinger, J.R. Freshwater Amphipod Crustaceans (Gammaridae) of North America, Biota of Freshwater Ecosystems, Identification Manual No. 5; U.S. Environmental Protection Agency: Washington, DC, USA, 1972.
- Zhang, J. Systematics of the Freshwater Amphipod Genus Crangonyx (Crangonyctidae) in North America. Ph.D. Dissertation, Old Dominion University, Norfolk, VA, USA, 1997. [Google Scholar]
- United States Fish & Wildlife Service. Kentucky Cave Shrimp (Palaemonias ganteri), 5-Year Review: Summary and Evaluation; Southeast Region, Kentucky Ecological Services Field Office: Frankfort, KY, USA, 2016.
- Fage, L. Crustaces amphipodes et decapodes. In: Biospeologica, LVI: Campagne speologique de C. Bolivar et R. Jeannel dans l’Amerique du Nord (1928). Arch. Zool. Exp. Gen. 1931, 71, 361–374. [Google Scholar]
- Hobbs, H.H., Jr.; Hobbs, H.H., III; Daniel, M.A. A review of the troglobitic decapod crustaceans of the Americas. Smithson. Contrib. Zool. 1977, 244, 1–83. [Google Scholar] [CrossRef][Green Version]
- Holsinger, J.R.; Leitheuser, A.T. Ecological Analysis of the Kentucky Cave Shrimp, Palaemonias ganteri Hay, Mammoth Cave National Park (Phase I), Final Report; Old Dominion Research University Foundation: Norfolk, VA, USA, 1982. [Google Scholar]
- Holsinger, J.R.; Leitheuser, A.T. Ecological Analysis of the Kentucky Cave Shrimp, Palaemonias ganteri Hay, Mammoth Cave National Park (Phase II), Final Report; Old Dominion Research University Foundation: Norfolk, VA, USA, 1982. [Google Scholar]
- Holsinger, J.R.; Leitheuser, A.T. Ecological analysis of the Kentucky Cave Shrimp, Palaemonias ganteri Hay, Mammoth Cave National Park (Phase III), Final Report; Old Dominion Research University Foundation: Norfolk, VA, USA, 1983. [Google Scholar]
- Lisowski, E.A. The endangered Kentucky blind cave shrimp. In Proceedings of the National Cave Management Symposium, Carlsbad, New Mexico and Mammoth Cave National Park, Kentucky; Wilson, R.C., Lewis, J.J., Eds.; Pygmy Dwarf Press: Oregon City, OR, USA, 1982; pp. 138–142. [Google Scholar]
- Lisowski, E.A. Distribution, habitat, and behavior of the Kentucky cave shrimp Palaemonias ganteri Hay. J. Crustacean Biol. 1983, 3, 88–92. [Google Scholar] [CrossRef]
- Lisowski, E.A.; Poulson, T.L. Impacts of Lock and Dam Six on base level ecosystems in Mammoth Cave. In Cave Research Foundation 1979 Annual Report; The Cave Research Foundation: Cave, KY, USA, 1981; pp. 48–54. [Google Scholar]
- Leitheuser, A.T.; Holsinger, J.R. Ecological Analysis of the Kentucky Cave Shrimp, Palaemonias ganteri Hay, Mammoth Cave National Park (Phase IV), Final Report; Old Dominion University Research Foundation: Norfolk, VA, USA, 1983. [Google Scholar]
- Leitheuser, A.T.; Holsinger, J.R.; Olson, R.; Pace, N.R.; Whitman, R.L.; White, T. Ecological Analysis of the Kentucky Cave Shrimp, Palaemonias ganteri Hay, at Mammoth Cave National Park (Phase V), Final Report; Old Dominion University Research Foundation: Norfolk, VA, USA, 1985. [Google Scholar]
- Leitheuser, A.T.; Whitman, R.L.; Gochee, A.V.; Holsinger, J.R. Ecological Analysis of the Kentucky Cave Shrimp, Palaemonias ganteri Hay, at Mammoth Cave National Park (Phase VI), Final Report; Old Dominion University Research Foundation: Norfolk, VA, USA, 1986. [Google Scholar]
- United States Fish & Wildlife Service. Kentucky Cave Shrimp Recovery Plan; United States Fish & Wildlife Service: Atlanta, GA, USA, 1988.
- Pearson, W.D.; Jones, T.G. A Final Report Based on a Faunal Inventory of Subterranean Streams and Development of a Cave Aquatic Biological Monitoring Program Using a Modified Index of Biotic Integrity, Final Report; National Park Service, Mammoth Cave National Park: Brownsville, KY, USA, 1998. [Google Scholar]
- Cooper, J.E.; Cooper, M.R. Observations on the biology of the endangered stygobiotic shrimp Palaemonias alabamae, with notes on P. ganteri (Decapoda: Atyidae). Subterr. Biol. 2011, 8, 9–20. [Google Scholar] [CrossRef]
- Stump, A.J. The Use of Environmental DNA for the Detection of Palaemonias ganteri (Hay, 1901), a Federally Endangered Species. Master’s Thesis, Eastern Kentucky University, Richmond, KY, USA, 2019. [Google Scholar]
- Hagen, H.H. Monograph of the North American Astacidae. Illus. Cat. Mus. Com. Zool. 1870, 3, 1–109. [Google Scholar]
- Hagen, H.H. The blind crayfish. Am. Nat. 1872, 6, 494. [Google Scholar]
- Garman, H. A little-known cave crayfish. Trans. Ky. Acad. Sci. 1924, 1, 87–94. [Google Scholar]
- Park, O.; Roberts, T.W.; Harris, S.J. Preliminary analysis of activity of the cave crayfish, Cambarus pellucidus. Am. Nat. 1941, 75, 154–171. [Google Scholar] [CrossRef]
- Rhoades, R. The crayfishes of Kentucky, with notes on variation, distribution and descriptions of new species and subspecies. Am. Midl. Nat. 1944, 31, 111–149. [Google Scholar] [CrossRef]
- Hobbs, H.H., Jr.; Barr, T.C., Jr. Origins and affinities of the troglobitic crayfishes of North America (Decapoda: Astacidae), I: Genus Cambarus. Am. Midl. Nat. 1960, 64, 12–33. [Google Scholar] [CrossRef]
- Hobbs, H.H., Jr.; Barr, T.C., Jr. Origins and affinities of the troglobitic crayfishes of North America (Decapoda: Astacidae), II: Genus Orconectes. Smithson. Contrib. Zool. 1972, 105, 1–84. [Google Scholar] [CrossRef]
- Brown, F.A. Diurnal rhythm in cave crayfish. Nature 1961, 191, 929–930. [Google Scholar] [CrossRef]
- Wolfe, D.A.; Cornwell, D.G. Carotenoids of cavernicolous crayfish. Science 1964, 144, 1467–1469. [Google Scholar] [CrossRef]
- Compson, Z.G. An Isotopic Examination of Cave, Spring and Epigean Trophic Structures in Mammoth Cave National Park. Master’s Thesis, Western Kentucky University, Bowling Green, KY, USA, 2004. [Google Scholar]
- Taylor, C.A.; Schuster, G.A. The Crayfishes of Kentucky; Illinois Natural History: Champaign, IL, USA, 2004. [Google Scholar]
- Proudlove, G.S. Subterranean Fishes of the World; International Society for Subterranean Biology: Moulis, France, 2006. [Google Scholar]
- Davidson, R. An Excursion to the Mammoth Cave and the Barrens of Kentucky, with Some Notices of the Early Settlement of the State; A.T. Skillman and Son, Lexington; Thomas Cowperthwait and Co.: Philadelphia, PA, USA, 1840. [Google Scholar]
- Thompson, W. Notice of the blindfish, crayfish, and insects from Mammoth Cave, Kentucky. Ann. Mag. Nat. Hist. 1844, 13, 3. [Google Scholar]
- Girard, C.F. Ichthyological notices. Proc. Acad. Nat. Sci. USA 1860, 1859, 56–68. [Google Scholar]
- Woods, L.P.; Inger, R.F. The cave, spring, and swamp fishes of the family Amblyopsidae of central and eastern United States. Am. Midl. Nat. 1957, 58, 232–256. [Google Scholar] [CrossRef]
- Poulson, T.L. Cave Adaptation in Amblyopsid Fishes. Ph.D. Dissertation, Department of Zoology, University of Michigan,, Ann Arbor, MI, USA, 1961. [Google Scholar]
- Poulson, T.L. Cave adaptation in amblyopsid fishes. Am. Midl. Nat. 1963, 70, 257–290. [Google Scholar] [CrossRef]
- Poulson, T.L. Cave Research Foundation Annual Report; Aquatic Cave Communities: Washington, DC, USA, 1968; pp. 16–18. [Google Scholar]
- Barr, T.C., Jr.; Kuehne, R.A. The cavefish, Amblyopsis spelaea, in northern Kentucky. Copeia 1962, 1962, 662. [Google Scholar] [CrossRef]
- Rosen, D.E. Comments on the relationships of the North American cave fishes of the family Amblyopsidae. Am. Mus. Nov. 1962, 2109, 1–35. [Google Scholar]
- Poulson, T.L.; White, W.B. The cave environment. Science 1969, 165, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Clay, W.M. The Fishes of Kentucky; Kentucky Department of Fish and Wildlife Resources: Frankfurt, KY, USA, 1975. [Google Scholar]
- Swofford, D.L.; Branson, B.A.; Sievert, G. Genetic differentiation of cavefish populations (Amblyopsidae). Isozyme Bull. 1980, 13, 109–110. [Google Scholar]
- Swofford, D.L. Genetic Variability, Population Differentiation, and Biochemical Relationships in the Family Amblyopsidae. Master’s Thesis, Eastern Kentucky University, Richmond, KY, USA, 1982. [Google Scholar]
- Burr, B.M.; Warren, M.L., Jr. A Distributional Atlas of Kentucky Fishes, Vol. 4; Kentucky State Nature Preserves Commission Scientific and Technical Series: Frankfort, KY, USA, 1986. [Google Scholar]
- Lewis, J.J. Conservation Assessment for Southern Cavefish (Typhlichthys subterraneus) Report; USDA Forest Service: Eastern Region, Ghana, 2002. [Google Scholar]
- Keith, J.H. Distribution of Northern cavefish, Amblyopsis spelaea DeKay, in Indiana and Kentucky and recommendations for its protection. Nat. Areas J. 1988, 8, 69–79. [Google Scholar]
- Branson, B.A. The Mammoth Cave blindfish. Trop. Fish Hobbyist 1991, 40, 39–40. [Google Scholar]
- Romero, A. Threatened fishes of the world: Typhlichthys subterraneus Girard, 1860 (Amblyopsidae). Environ. Biol. Fishes 1998, 53, 74. [Google Scholar] [CrossRef]
- Romero, A.; Bennis, L. Threatened fishes of the world: Amblyopsis spelaea DeKay, 1842 (Amblyopsidae). Environ. Biol. Fishes 1998, 51, 421–428. [Google Scholar] [CrossRef]
- Niemiller, M.L. Evolution, Speciation, and Conservation of Amblyopsid Cavefishes. Ph.D. Dissertation, University of Tennessee, Knoxville, TN, USA, 2011. [Google Scholar]
- Niemiller, M.L.; Fitzpatrick, B.M. Status and life history of the amblyopsid cavefishes in Kentucky. Ky. Dept. Fish Wildl. Resour. 2012, 5, 9–15. [Google Scholar]
- Niemiller, M.L.; Near, T.J.; Fitzpatrick, B.M. Delimiting species using multilocus data: Diagnosing cryptic diversity in the southern cavefish, Typhlichthys subterraneus (Teleostei: Amblyopsidae). Evolution 2012, 66, 846–866. [Google Scholar] [CrossRef]
- Hart, P.B.; Niemiller, M.L.; Burress, E.D.; Armbruster, J.W.; Ludt, W.B.; Chakrabarty, P. Cave-adapted evolution in the North American amblyopsid fishes inferred using phylogenomics and geometric morphometrics. Evolution 2020, 74, 936–949. [Google Scholar] [CrossRef]
- Culver, D.C.; Sket, B. Hotspots of subterranean biodiversity in caves and wells. J. Cave Karst Stu. 2000, 62, 11–17. [Google Scholar]
- Culver, D.C.; Pipan, T. Subterranean ecosystems. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Hutchins, B.T.; Gibson, J.R.; Diaz, P.H.; Schwartz, B.F. Stygobiont diversity in the San Marcos Artesian Well and Edwards Aquifer groundwater ecosystem, Texas, USA. Diversity 2021, 13, 234. [Google Scholar] [CrossRef]
- Culver, D.C.; Deharveng, L.; Bedos, A.; Lewis, J.J.; Madden, M.; Reddell, J.R.; Sket, B.; Trontelj, P.; White, D. The mid-latitude biodiversity ridge in terrestrial cave fauna. Ecography 2006, 29, 120–128. [Google Scholar] [CrossRef]
- Olson, R. Potential effects of hydrogen sulfide and hydrocarbon seeps on Mammoth Cave ecosystems. In Mammoth Cave National Park’s 10th Research Symposium; Mammoth Cave National Park: Brownsville, KY, USA, 2013; pp. 25–30. [Google Scholar]
- Niemiller, M.L.; Bichuette, E.; Taylor, S.J. Conservation of cave fauna in Europe and the Americas. In Ecological Studies: Cave Ecology; Moldovan, O.T., Kovac, L., Halse, S., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 451–478. [Google Scholar]
- Mammola, S.; Cardoso, P.; Culver, D.C.; Deharveng, L.; Ferreira, R.L.; Fiŝer, C.; Galassi, D.M.P.; Griebler, C.; Halse, S.; Humphreys, W.F.; et al. Scientists’ warning on the conservation of subterranean ecosystems. BioScience 2019, 69, 641–650. [Google Scholar] [CrossRef]
- Brucker, R. Conservation at Mammoth Cave. In Cave Research Foundation 1979 Annual Report; Mammoth Cave National Park: Brownsville, KY, USA, 1979; pp. 40–41. [Google Scholar]
- Pfaff, R.M.; Glennon, J.A.; Groves, C.G.; Anderson, M.; Fry, J.; Meiman, J. Landuse and water quality threats to the Mammoth Cave karst aquifer, Kentucky. In Proceedings of the 12th National Cave and Karst Management Symposium, Chattanooga, TN, USA, 19–22 October 1999. [Google Scholar]
- Meiman, J.; Hopper, H.L.; Brucker, R.W. Management issues and threats to the longest cave. In Proceedings of the 15th National Cave and Karst Management Symposium, Tucson, AZ, USA, 16–19 October 2001. [Google Scholar]
- Toomey, R.; Thomas, S.; Gillespie, J.; Carson, V.; Trimboli, S.R. White-nose Syndrome at Mammoth Cave National Park: Actions before and after its detection. In Proceedings of the 10th Mammoth Cave Research Symposia, Mammoth Cave, KY, USA, 14–15 February 2013; p. 13. [Google Scholar]
- Olson, R.A. Environmental issues relevant to the Mammoth Cave area. In Mammoth Cave: A Human and Natural History, Cave and Karst Systems of the World; Hobbs, H.H., III, Olson, R.A., Winkler, E.G., Culver, D.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 265–275. [Google Scholar]
- Ruhl, M. Flow Reversal Events Increase the Abundance of Nontroglobitic Fish in the Subterranean Rivers of Mammoth Cave National Park. Master’s Thesis, Western Kentucky University, Bowling Green, KY, USA, 2005. [Google Scholar]
- Trimboli, S.R.; Weber, K.; Ryan, S.; Toomey, R.S. An overview of the reverse flow patterns of River Styx in Mammoth Cave, Kentucky: 2009–2012. In Proceedings of the 11th Mammoth Cave Research Symposia, Mammoth Cave, KY, USA, 18–20 April 2016. [Google Scholar]
- Trimboli, S.R.; Toomey, R.S. Temperature and reverse-flow patterns of the River Styx, Mammoth Cave, Kentucky. J. Cave Karst Stud. 2019, 81, 174–187. Available online: https://digitalcommons.wku.edu/mc_reserch_symp/11th_Research_Symposium_2016/Day_three/3 (accessed on 28 June 2021). [CrossRef]
- Niemiller, M.L.; Taylor, S.J.; Slay, M.E.; Hobbs, H.H., III. Biodiversity in the United States and Canada. In Encyclopedia of Caves, 3rd ed.; Culver, D.C., White, W.B., Pipan, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 163–177. [Google Scholar]
- Lewis, J.J.; Lewis, S.L. Cave fauna study for the Interstate 66 EIS (Somerset to London, Kentucky). In Proceedings of the 2005 National Cave and Karst Management Symposium, Albany, NY, USA, 31 October–4 November 2005; pp. 15–20. [Google Scholar]
- Muchmore, W. New terrestrial isopods of the genus Miktoniscus from eastern United States (Crustacea: Isopoda: Oniscoidea). Ohio J. Sci. 1964, 64, 51–57. [Google Scholar]
- Zakšek, V.; Sket, B.; Gottstein, S.; Franjević, D.; Trontelj, P. The limits of cryptic diversity in groundwater: Phylogeography of the cave shrimp Troglocaris anophthalmus (Crustacea: Decapoda: Atyidae). Mol. Ecol. 2009, 18, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Ethridge, J.Z.; Gibson, J.R.; Nice, C.C. Cryptic diversity within and amongst spring-associated Stygobromus amphipods (Amphipoda: Crangonyctidae). Zool. J. Linn. Soc. 2013, 167, 227–242. [Google Scholar] [CrossRef]
- Devitt, T.J.; Wright, A.M.; Cannatella, D.C.; Hillis, D.M. Species delimitation in endangered groundwater salamanders: Implications for aquifer management and biodiversity conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
| Taxon | Authority | Cons. Status | Packard [36] | Barr [10] | Culver and Hobbs [37] | Toomey et al. [1] | This Study |
|---|---|---|---|---|---|---|---|
| TROGLOBIONTS | 13 Species | 28 Species | 32 Species | 32 Species | 32 Species | ||
| Phylum Arthropoda | |||||||
| Class Arachnida | |||||||
| Order Araneae | |||||||
| Family Linyphiidae | |||||||
| Anthrobia monmouthia T | Tellkampf, 1844 | G5 | X | X | X | X | X |
| Bathyphantes weyeri | (Emerton, 1875) | G4 | X | X | X | X | |
| Phanetta subterranea | (Emerton, 1875) | G5 | X | X | X | X | |
| Porhomma cavernicola | (Keyserling, 1886) | G5 | X | X | X | X | |
| Family Zoropsidae | |||||||
| Liocranoides unicolor T | Keyserling, 1881 | GU | X | X | |||
| Order Opiliones | |||||||
| Family Phalangodidae | |||||||
| Phalangodes armata T | Tellkampf, 1844 | G3 | X | X | X | X | X |
| Order Pseudoscorpiones | |||||||
| Family Chernetidae | |||||||
| Hesperochernes mirabilis | (Banks, 1895) | G5 | X | X | X | X | |
| Family Chthoniidae | |||||||
| Kleptochthonius cerberus T,E | Malcolm and Chamberlin, 1961 | G1 | X | X | X | X | |
| Kleptochthonius hageni T | Muchmore, 1963 | G1 | X | X | X | X | X |
| Tyrannochthonius hypogeus T,E | Muchmore, 1996 | G1 | X | X | |||
| Order Acari | |||||||
| Family Belbidae | |||||||
| Dameus bulbipedata T,E | (Packard, 1888) | G1 | X | X | X | X | |
| Family Cocceupodidae | |||||||
| Linopodes mammouthia T | Banks, 1897 | GNR | X | X | X | X | |
| Family Galumnidae | |||||||
| Galumna alata | (Hermann, 1804) | G1 | X | X | X | ||
| Family Laelapidae | |||||||
| Laelaps cavernicola T | Packard, 1888 | GNR | X | X | X | X | X |
| Family Macrochelidae | |||||||
| Macrocheles troglodytes T | (Packard, 1888) | G1 | X | X | X | ||
| Family Rhagidiidae | |||||||
| Traegaardhia holsingeri T | (Zacharda, 1980) | GNR | X | X | X | X | X |
| Class Collembola | |||||||
| Order Entomobryomorpha | |||||||
| Family Entomobryidae | |||||||
| Pseudosinella espanita T,E | Christiansen and Bellinger, 1996 | G1 | X | X | X | ||
| Order Symphypleona | |||||||
| Family Arrhopalitidae | |||||||
| Pygmarrhopalites altus T,E | (Christiansen, 1966) | G2 | X | X | X | X | |
| Class Diplura | |||||||
| Order Rhabdura | |||||||
| Family Campodeidae | |||||||
| Litocampa cookei T | (Packard, 1871) | G5 | X | X | X | X | X |
| Class Diplopoda | |||||||
| Order Chordeumatida | |||||||
| Family Trichopetalidae | |||||||
| Scoterpes copei T | (Packard, 1871) | G3 | X | X | X | X | X |
| Order Polydesmida | |||||||
| Family Macrosternodesmidae | |||||||
| Chaetaspis fragilis T | (Loomis, 1943) | GNR | X | X | X | X | |
| Class Insecta | |||||||
| Order Coleoptera | |||||||
| Family Carabidae | |||||||
| Neaphaenops tellkampfi T | (Erichson, 1844) | G3 | X | X | X | X | X |
| Pseudanophthalmus audax | (Horn, 1883) | G1 | X | X | X | X | |
| Pseudanophthalmus inexpectatus T,E | Barr, 1959 | G1 | X | X | X | X | |
| Pseudanophthalmus menetriesi T | (Motschulsky, 1862) | G3 | X | X | X | X | X |
| Pseudanophthalmus pubescens | (Horn, 1868) | G3 | X | X | X | X | |
| Pseudanophthalmus striatus T | (Motschulsky, 1862) | G2 | X | X | X | X | X |
| Family Leiodidae | |||||||
| Ptomaphagus hirtus T | (Tellkampf, 1844) | G4 | X | X | X | X | X |
| Family Staphylinidae | |||||||
| Batrisodes henroti | Park, 1956 | G2 | X | X | X | X | |
| Order Diptera | |||||||
| Family Phoridae | |||||||
| Megaselia cavernicola | (Brues, 1906) | GNR | X | ||||
| Family Sphaeroceridae | |||||||
| Spelobia tenebrarum | (Aldrich, 1897) | G5 | X | X | X | ||
| Order Psocodea | |||||||
| Family Psyllipsocidae | |||||||
| Psyllipsocus ramburii | Selys-Longchamps, 1872 | GNR | X | ||||
| Phylum Mollusca | |||||||
| Class Gastropoda | |||||||
| Order Basommatophora | |||||||
| Family Carychiidae | |||||||
| Carychium stygium T | Call, 1897 | G3 | X | X | X | X | |
| Order Stylommatophora | |||||||
| Family Helicodiscidae | |||||||
| Helicodiscus hadenoecus | Hubricht, 1962 | G3 | X | ||||
| Helicodiscus punctatellus T | Morrison, 1942 | G1 | X | X | X | ||
| Family Zonitidae | |||||||
| Glyphyalinia specus | Hubricht, 1965 | G4 | X | X | X | X | |
| STYGOBIONTS | 6 species | 16 species | 16 species | 18 species | 17 species | ||
| Phylum Platyhelminthes | |||||||
| Class Turbellaria | |||||||
| Order Tricladida | |||||||
| Family Kenkiidae | |||||||
| Sphalloplana buchanani T | (Hyman, 1937) | G1 | X | X | X | X | |
| Sphalloplana percoeca T | (Packard, 1879) | G5 | X | X | X | X | X |
| Phylum Arthropoda | |||||||
| Class Malacostraca | |||||||
| Order Amphipoda | |||||||
| Crangonyctidae | |||||||
| Crangonyx barri T | Zhang and Holsinger, 2003 | G5 | X | X | X | X | |
| Stygobromus exilis | Hubricht, 1943 | G5 | X | X | X | X | |
| Stygobromus vitreus T | Cope, 1872 | G4; S | X | X | X | X | X |
| Order Decapoda | |||||||
| Family Atyidae | |||||||
| Palaemonias ganteri T | Hay, 1901 | G1; VU; E; LE | X | X | X | X | |
| Family Cambaridae | |||||||
| Orconectes pellucidus T | (Tellkampf, 1844) | G4; LC; S | X | X | X | X | X |
| Order Isopoda | |||||||
| Family Asellidae | |||||||
| Caecidotea bicrenata | Lewis and Bowman, 1981 | G5 | X | X | |||
| Caecidotea stygia T | Packard, 1871 | G5 | X | X | X | X | X |
| Class Maxillopoda | |||||||
| Order Cyclopoida | |||||||
| Family Cyclopidae | |||||||
| Megacyclops donnaldsoni | (Chappuis, 1929) | G3 | X | X | X | X | |
| Order Harpacticoida | |||||||
| Family Canthocamptidae | |||||||
| Attheyella pilosa T | Chappuis, 1929 | GNR | X | X | X | ||
| Bryocamptus morrisoni | (Chappuis, 1928) | G3 | X | X | X | X | |
| Order Siphonostomatoida | |||||||
| Family Lernaeopodidae | |||||||
| Cauloxenus stygius | Cope, 1872 | G1 | X | X | |||
| Class Ostracoda | |||||||
| Order Podocopida | |||||||
| Family Entocytheridae | |||||||
| Sagittocythere barri | (Hart and Hobbs, 1961) | G5 | X | X | X | X | |
| Sagittocythere stygia T,E | Hart and Hart, 1966 | G1 | X | X | X | X | |
| Phylum Mollusca | |||||||
| Class Gastropoda | |||||||
| Order Neotaenioglossa | |||||||
| Family Hydrobiidae | |||||||
| Antroselates spiralis T | Hubricht, 1963 | G3 | X | X | X | X | |
| Phylum Chordata | |||||||
| Class Actinopterygii | |||||||
| Order Percopsiformes | |||||||
| Family Amblyopsidae | |||||||
| Amblyopsis spelaea T | DeKay, 1842 | G2; NT; S | X | X | X | X | X |
| Typhlichthys subterraneus | Girard, 1859 | G4; NT; S | X | X | X | X | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemiller, M.L.; Helf, K.; Toomey, R.S. Mammoth Cave: A Hotspot of Subterranean Biodiversity in the United States. Diversity 2021, 13, 373. https://doi.org/10.3390/d13080373
Niemiller ML, Helf K, Toomey RS. Mammoth Cave: A Hotspot of Subterranean Biodiversity in the United States. Diversity. 2021; 13(8):373. https://doi.org/10.3390/d13080373
Chicago/Turabian StyleNiemiller, Matthew L., Kurt Helf, and Rickard S. Toomey. 2021. "Mammoth Cave: A Hotspot of Subterranean Biodiversity in the United States" Diversity 13, no. 8: 373. https://doi.org/10.3390/d13080373
APA StyleNiemiller, M. L., Helf, K., & Toomey, R. S. (2021). Mammoth Cave: A Hotspot of Subterranean Biodiversity in the United States. Diversity, 13(8), 373. https://doi.org/10.3390/d13080373






