Genome-Wide Identification and Characterization of Cysteine-Rich Receptor-Like Protein Kinase Genes in Tomato and Their Expression Profile in Response to Heat Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. RNA Extraction and Expression Analysis
2.3. Expression Analysis of SlCRK Genes and GO Enrichment of Co-Expressed Genes of SlCRK Genes
2.4. Identification of SlCRK Family Members in the Tomato Genome
2.5. Phylogenetic Analysis
2.6. Structural Characterization and Promoter Analysis
3. Results
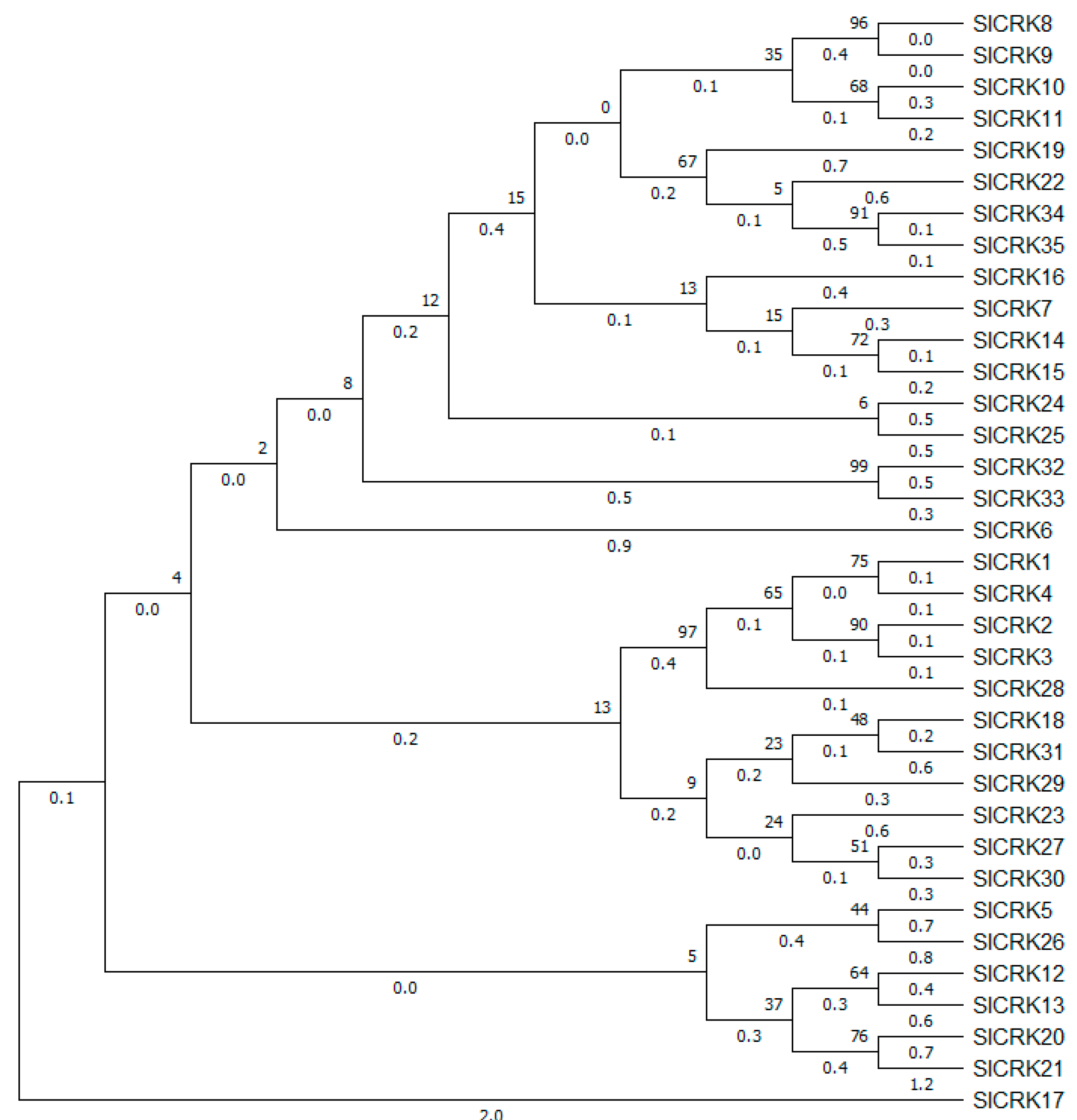
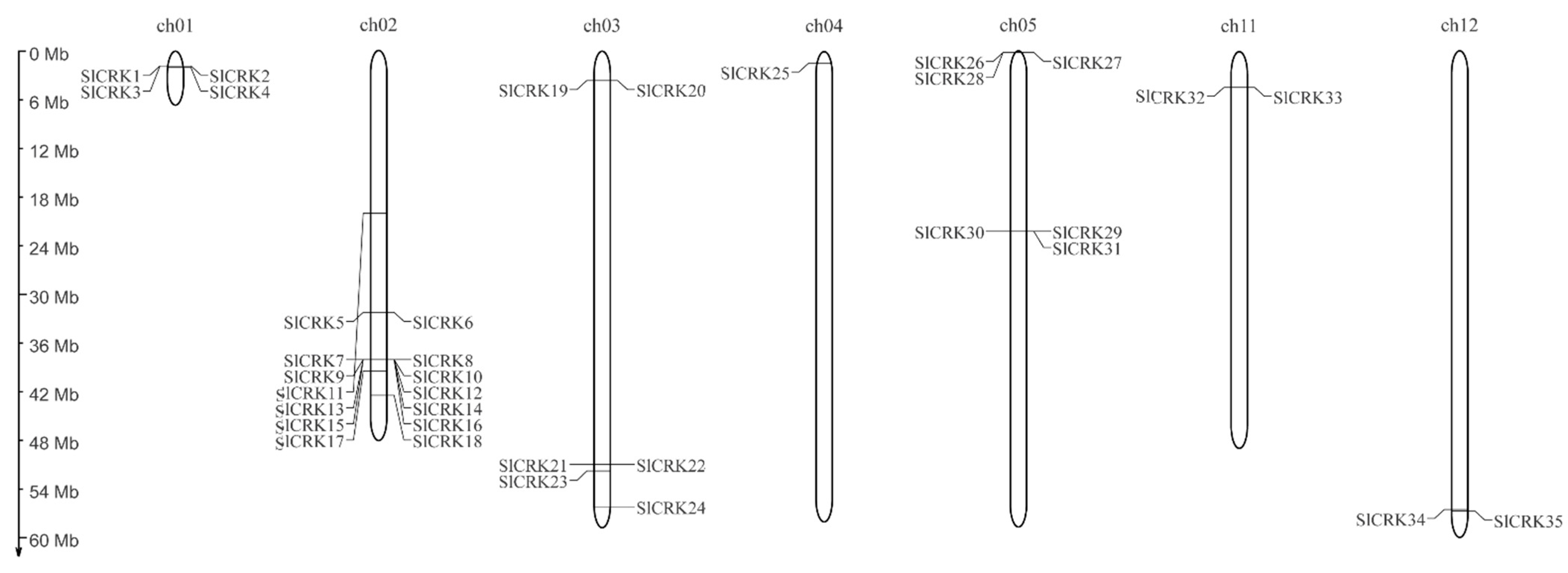
3.1. Identification, Phylogenetic Analysis and Chromosomal Localization of SlCRK Genes in Tomato
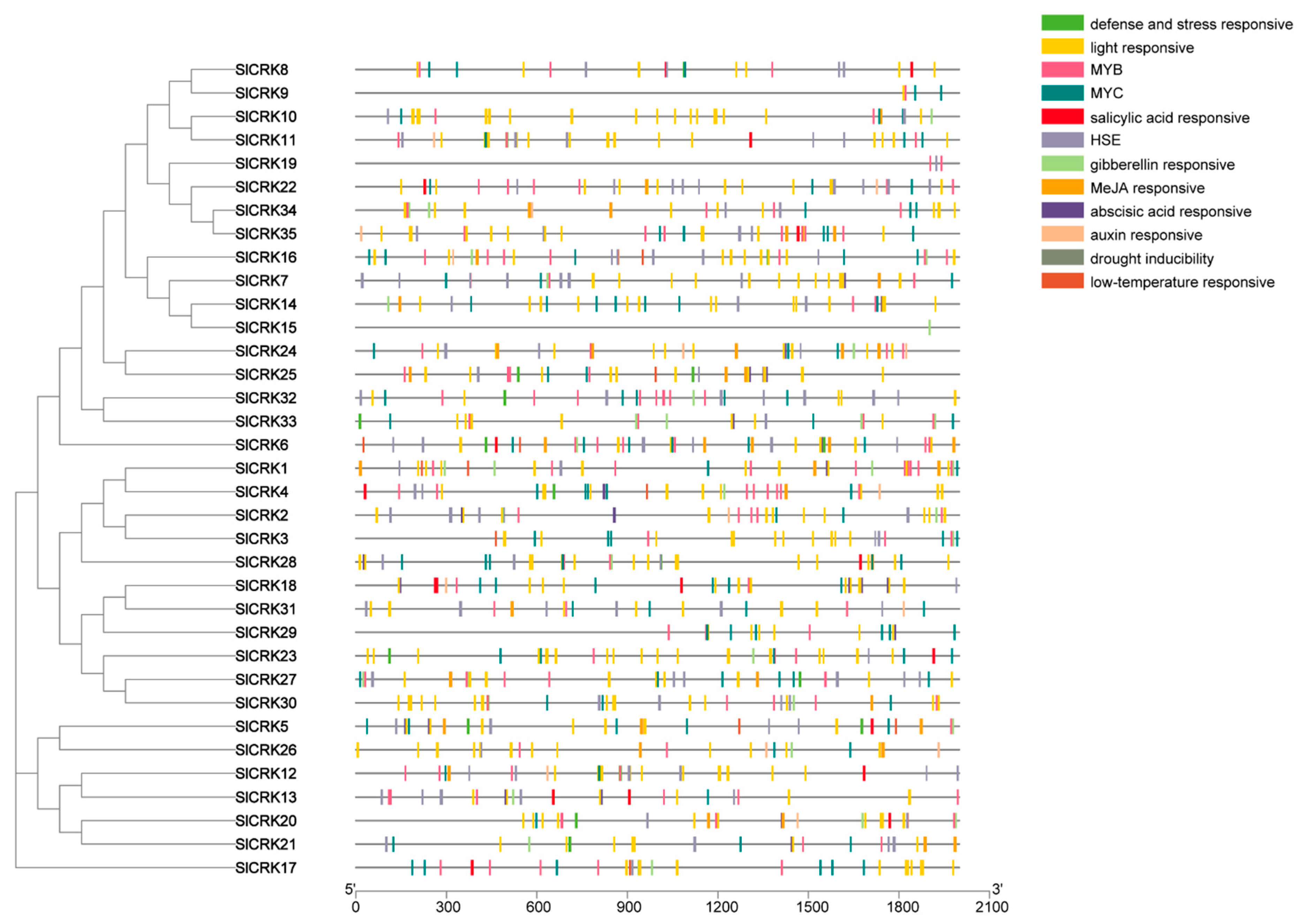
3.2. Analysis of Cis-Regulatory Elements in SlCRK Genes
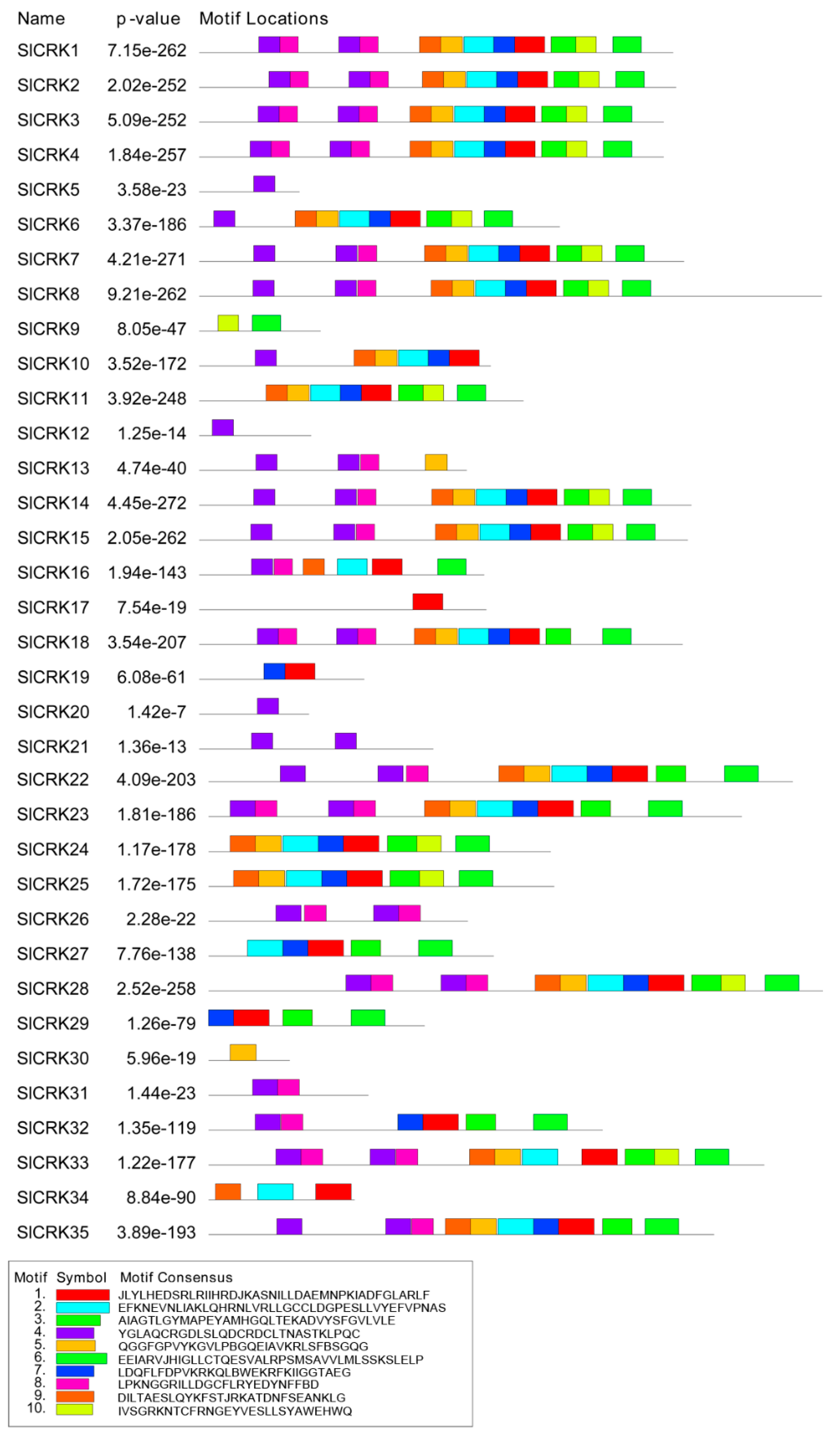
3.3. Structure Analysis of SlCRKs
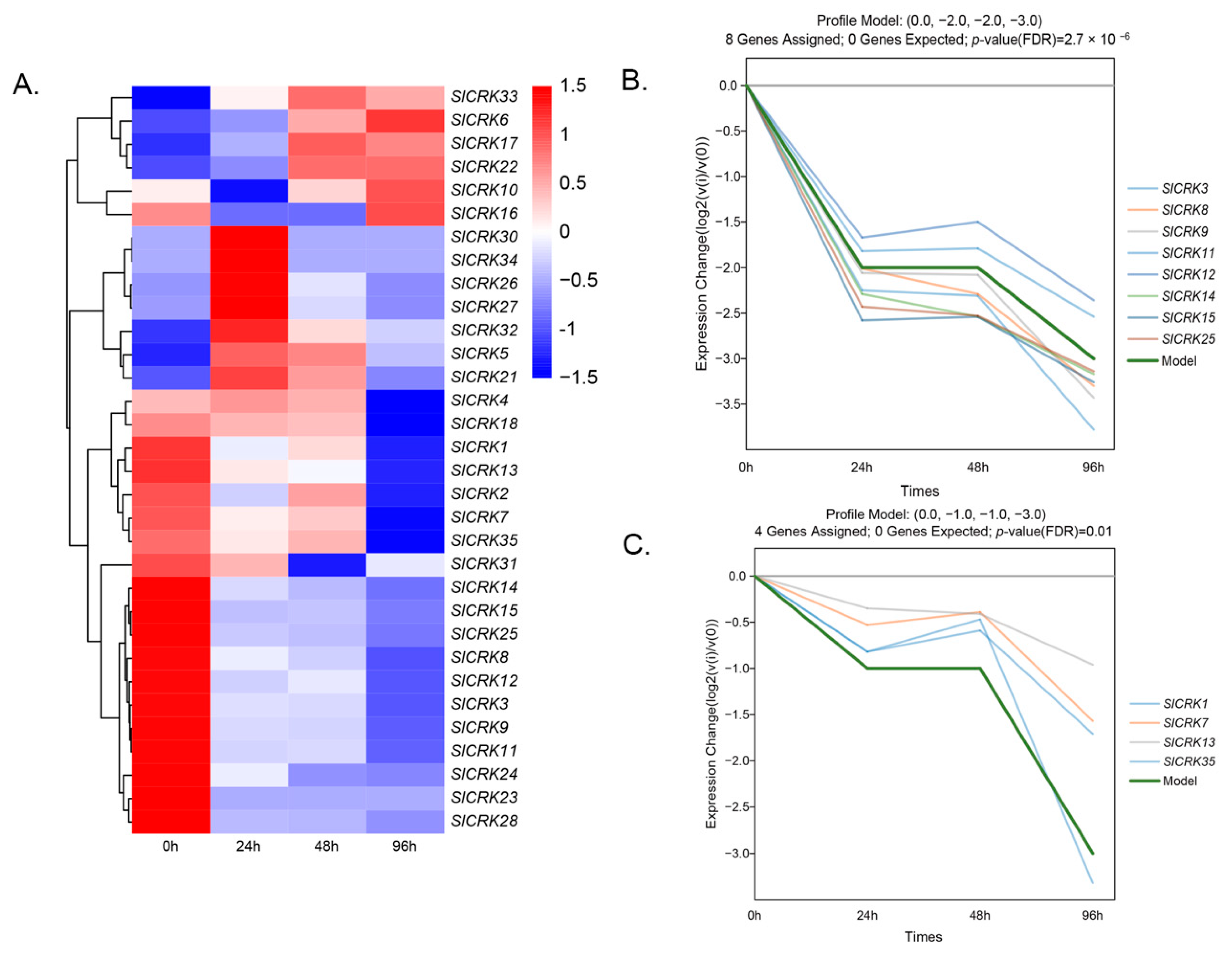
3.4. Expression Patterns of SlCRKs in Response to Heat Stress
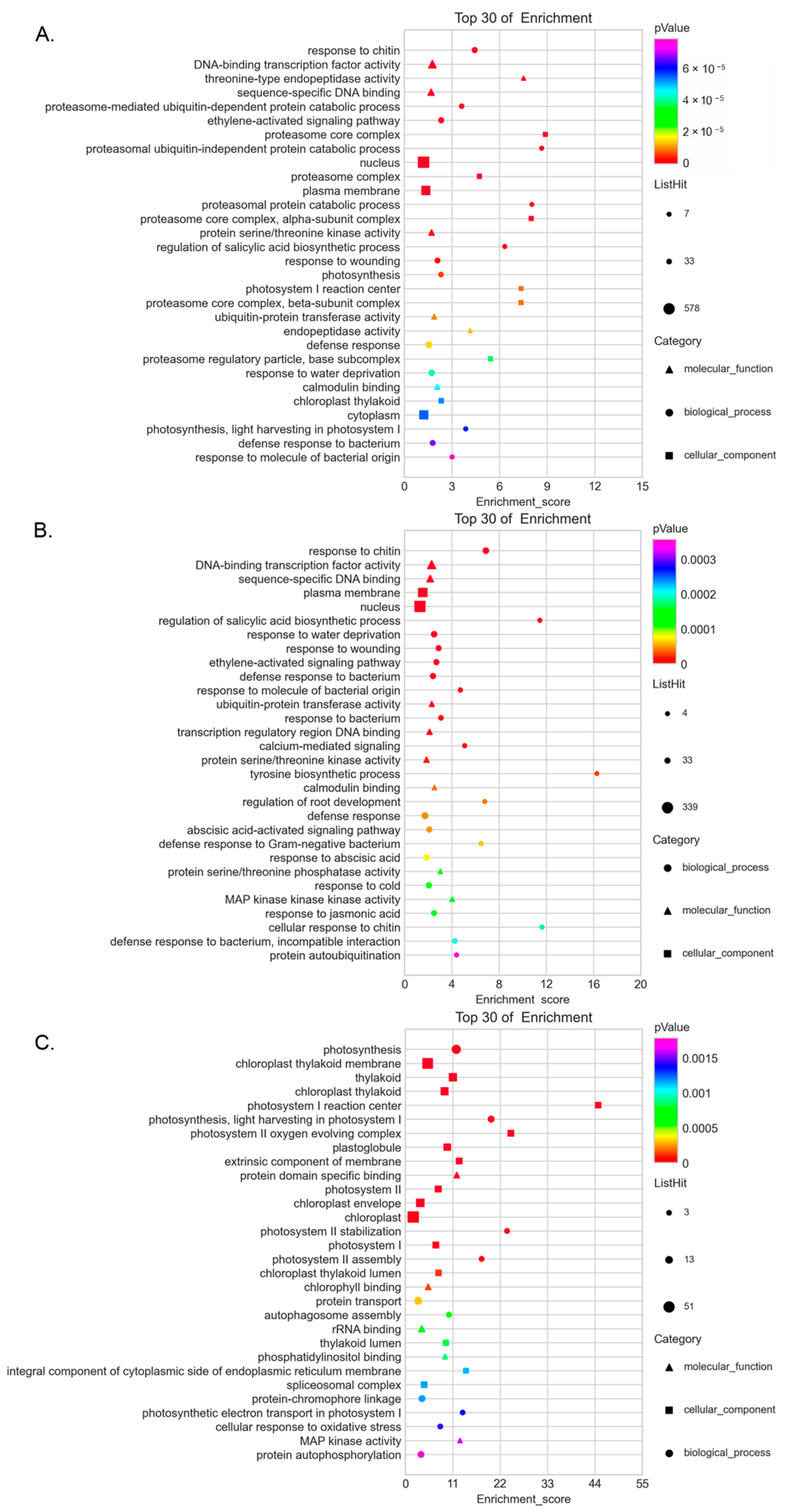
3.5. Enrichment of Co-Expressed Genes Associated with SlCRKs in Tomato under High Temperature Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, E.R.; Walker, J.C. Receptor-like protein kinases: The keys to response. Curr. Opin. Plant Biol. 2003, 6, 339–342. [Google Scholar] [CrossRef]
- Czernic, P.; Visser, B.; Sun, W.; Savouré, A.; Deslandes, L.; Marco, Y.; Van Montagu, M.; Verbruggen, N. Characterization of an Arabidopsis thaliana receptor-like protein kinase gene activated by oxidative stress and pathogen attack. Plant J. 1999, 18, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.H.; Bleecker, A.B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA 2001, 98, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z. A superfamily of proteins with novel cysteine-rich repeats. Plant Physiol. 2001, 126, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Wrzaczek, M.; Brosché, M.; Salojärvi, J.; Kangasjärvi, S.; Idänheimo, N.; Mersmann, S.; Robatzek, S.; Karpiński, S.; Karpińska, B.; Kangasjärvi, J. Transcriptional regulation of the CRK/DUF26 group of Receptor-like protein kinases by ozone and plant hormones in Arabidopsis. BMC Plant Biol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Du, L.; Chen, Z. Sensitization of defense responses and activation of programmed cell death by a pathogen-induced receptor-like protein kinase in Arabidopsis. Plant Mol. Biol. 2003, 53, 61–74. [Google Scholar] [CrossRef]
- Acharya, B.R.; Raina, S.; Maqbool, S.B.; Jagadeeswaran, G.; Mosher, S.L.; Appel, H.M.; Schultz, J.C.; Klessig, D.F.; Raina, R. Overexpression of CRK13, an Arabidopsis cysteine-rich receptor-like kinase, results in enhanced resistance to Pseudomonas syringae. Plant J. 2007, 50, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Seto, H.; Fujioka, S.; Yoshida, S.; Chory, J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 2001, 410, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, L.; Bauer, Z.; Boller, T. Both the Extracellular Leucine-Rich Repeat Domain and the Kinase Activity of FLS2 Are Required for Flagellin Binding and Signaling in Arabidopsis. Plant Cell 2001, 13, 1155–1163. [Google Scholar] [CrossRef]
- Bourdais, G.; Burdiak, P.ł.; Gauthier, A.; Nitsch, L.; Salojärvi, J.; Rayapuram, C.; Idänheimo, N.; Hunter, K.; Kimura, S.; Merilo, E.; et al. Large-Scale Phenomics Identifies Primary and Fine-Tuning Roles for CRKs in Responses Related to Oxidative Stress. PLoS Genet. 2015, 11. [Google Scholar] [CrossRef]
- Chen, K.; Fan, B.; Du, L.; Chen, Z. Activation of hypersensitive cell death by pathogen-induced receptor-like protein kinases from Arabidopsis. Plant Mol. Biol. 2004, 56, 271–283. [Google Scholar] [CrossRef]
- Yeh, Y.H.; Chang, Y.H.; Huang, P.Y.; Huang, J.B.; Zimmerli, L. Enhanced Arabidopsis pattern-triggered immunity by overexpression of cysteine-rich receptor-like kinases. Front. Plant Sci. 2015, 6, 322. [Google Scholar] [CrossRef]
- Idänheimo, N.; Gauthier, A.; Salojärvi, J.; Siligato, R.; Brosché, M.; Kollist, H.; Mähönen, A.P.; Kangasjärvi, J.; Wrzaczek, M. The Arabidopsis thaliana cysteine-rich receptor-like kinases CRK6 and CRK7 protect against apoplastic oxidative stress. Biochem. Biophys. Res. Commun. 2014, 445, 457–462. [Google Scholar] [CrossRef]
- Tanaka, H.; Osakabe, Y.; Katsura, S.; Mizuno, S.; Maruyama, K.; Kusakabe, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Abiotic stress-inducible receptor-like kinases negatively control ABA signaling in Arabidopsis. Plant J. 2012, 70, 599–613. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, G.; Shi, R.; Han, X.; Qi, L.; Wang, R.; Xiong, L.; Li, G. Arabidopsis cysteine-rich receptor-like kinase 45 functions in the responses to abscisic acid and abiotic stresses. Plant Physiol. Biochem. 2013, 67, 189–198. [Google Scholar] [CrossRef]
- Gu, J.; Sun, J.; Liu, N.; Sun, X.; Liu, C.; Wu, L.; Liu, G.; Zeng, F.; Hou, C.; Han, S.; et al. A novel cysteine-rich receptor-like kinase gene, TaCRK2, contributes to leaf rust resistance in wheat. Mol. Plant Pathol. 2020, 21, 732–746. [Google Scholar] [CrossRef]
- Xu, X.; Yu, T.; Xu, R.; Shi, Y.; Lin, X.; Xu, Q.; Qi, X.; Weng, Y.; Chen, X. Fine mapping of a dominantly inherited powdery mildew resistance major-effect QTL, Pm1. 1, in cucumber identifies a 41.1 kb region containing two tandemly arrayed cysteine-rich receptor-like protein kinase genes. Theor. Appl. Genet. 2016, 129, 507–516. [Google Scholar] [CrossRef]
- Felix Amuji, C.; Beaumont, L.J.; Atwell, B.J. The Effect of Co-occurring Heat and Water Stress on Reproductive Traits and Yield of Tomato (Solanum lycopersicum). Hortic. J. 2020, 89. [Google Scholar] [CrossRef]
- Shaheen, M.R.; Ayyub, C.M.; Amjad, M.; Waraich, E.A. Morpho-physiological evaluation of tomato genotypes under high temperature stress conditions. J. Sci. Food Agric. 2016, 96, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.L.; Russo, V.M.; Roberts, W.; Wann, E.V.; Raper, R.L. Cultural and Environmental Factors Governing Tomato Production: Local Production under Elevated Temperatures. HortScience Horts 2012, 47, 1022. [Google Scholar] [CrossRef]
- Frank, G.; Pressman, E.; Ophir, R.; Althan, L.; Shaked, R.; Freedman, M.; Shen, S.; Firon, N. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J. Exp. Bot. 2009, 60, 3891–3908. [Google Scholar] [CrossRef]
- Ding, H.; He, J.; Wu, Y.; Wu, X.; Ge, C.; Wang, Y.; Zhong, S.; Peiter, E.; Liang, J.; Xu, W. The Tomato Mitogen-Activated Protein Kinase SlMPK1 Is as a Negative Regulator of the High-Temperature Stress Response. Plant Physiol. 2018, 177, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhong, X.; Zhao, F.; Wang, Y.; Yan, B.; Li, Q.; Chen, G.; Mao, B.; Wang, J.; Li, Y.; et al. Overexpression of receptor-like kinase ERECTA improves thermotolerance in rice and tomato. Nat. Biotechnol. 2015, 33, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Z.; Li, D.; Li, C.; Wei, D.; Li, S.; Liu, Y.; Chen, T.H.H.; Yang, X. Comparative effects of glycinebetaine on the thermotolerance in codA- and BADH-transgenic tomato plants under high temperature stress. Plant Cell Rep. 2020, 39, 1525–1538. [Google Scholar] [CrossRef]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van De Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Goff, K.E.; Ramonell, K.M. The role and regulation of receptor-like kinases in plant defense. Gene Regul. Syst. Biol. 2007, 1, 167–175. [Google Scholar] [CrossRef]
- Ederli, L.; Madeo, L.; Calderini, O.; Gehring, C.; Moretti, C.; Buonaurio, R.; Paolocci, F.; Pasqualini, S. The Arabidopsis thaliana cysteine-rich receptor-like kinase CRK20 modulates host responses to Pseudomonas syringae pv. tomato DC3000 infection. J. Plant Physiol. 2011, 168, 1784–1794. [Google Scholar] [CrossRef]
- Burdiak, P.; Rusaczonek, A.; Witoń, D.; Głów, D.; Karpiński, S. Cysteine-rich receptor-like kinase CRK5 as a regulator of growth, development, and ultraviolet radiation responses in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 3325–3337. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Liang, S.; Wu, Z.; Bi, C.; Yu, Y.T.; Wang, X.F.; Zhang, D.P. Overexpression of an Arabidopsis cysteine-rich receptor-like protein kinase, CRK5, enhances abscisic acid sensitivity and confers drought tolerance. J. Exp. Bot. 2016, 67, 5009–5027. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Kim, Y.C.; Kwon, S.J.; Ryu, C.M.; Park, O.K. The arabidopsis cysteine-rich receptor-like kinase crk36 regulates immunity through interaction with the cytoplasmic kinase BIK1. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Zuo, C.; Liu, H.; Lv, Q.; Chen, Z.; Tian, Y.; Mao, J.; Chu, M.; Ma, Z.; An, Z.; Chen, B. Genome-Wide Analysis of the Apple (Malus domestica) Cysteine-Rich Receptor-Like Kinase (CRK) Family: Annotation, Genomic Organization, and Expression Profiles in Response to Fungal Infection. Plant Mol. Biol. Rep. 2020, 38, 14–24. [Google Scholar] [CrossRef]
- Delgado-Cerrone, L.; Alvarez, A.; Mena, E.; Ponce de León, I.; Montesano, M. Genome-wide analysis of the soybean CRK-family and transcriptional regulation by biotic stress signals triggering plant immunity. PLoS ONE 2018, 13, e0207438. [Google Scholar] [CrossRef]
- Chern, M.; Xu, Q.; Bart, R.S.; Bai, W.; Ruan, D.; Sze-To, W.H.; Canlas, P.E.; Jain, R.; Chen, X.; Ronald, P.C. A Genetic Screen Identifies a Requirement for Cysteine-Rich–Receptor-Like Kinases in Rice NH1 (OsNPR1)-Mediated Immunity. PLoS Genet. 2016, 12. [Google Scholar] [CrossRef]
- Du, D.; Liu, M.; Xing, Y.; Chen, X.; Zhang, Y.; Zhu, M.; Lu, X.; Zhang, Q.; Ling, Y.; Sang, X.; et al. Semi-dominant mutation in the cysteine-rich receptor-like kinase gene, ALS1, conducts constitutive defence response in rice. Plant Biol. 2019, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Freeling, M. Bias in Plant Gene Content Following Different Sorts of Duplication: Tandem, Whole-Genome, Segmental, or by Transposition. Annu. Rev. Plant Biol. 2009, 60, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.-H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef]
- Zhao, P.; Javed, S.; Shi, X.; Wu, B.; Zhang, D.; Xu, S.; Wang, X. Varying Architecture of Heat Shock Elements Contributes to Distinct Magnitudes of Target Gene Expression and Diverged Biological Pathways in Heat Stress Response of Bread Wheat. Front. Genet. 2020, 11. [Google Scholar] [CrossRef]
- Gray, W.M.; Östin, A.; Sandberg, G.; Romano, C.P.; Estelle, M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc. Natl. Acad. Sci. USA 1998, 95, 7197–7202. [Google Scholar] [CrossRef]
- de Wit, M.; Lorrain, S.; Fankhauser, C. Auxin-mediated plant architectural changes in response to shade and high temperature. Physiol. Plant 2014, 151, 13–24. [Google Scholar] [CrossRef]
- Stavang, J.A.; Gallego-Bartolomé, J.; Gómez, M.D.; Yoshida, S.; Asami, T.; Olsen, J.E.; García-Martínez, J.L.; Alabadí, D.; Blázquez, M.A. Hormonal regulation of temperature-induced growth in Arabidopsis. Plant J. 2009, 60, 589–601. [Google Scholar] [CrossRef]
- Clarke, S.M.; Mur, L.A.J.; Wood, J.E.; Scott, I.M. Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J. 2004, 38, 432–447. [Google Scholar] [CrossRef]
- Clarke, S.M.; Cristescu, S.M.; Miersch, O.; Harren, F.J.M.; Wasternack, C.; Mur, L.A.J. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol. 2009, 182, 175–187. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Zhang, Y.Q.; Zhang, G.Q.; Sun, Z.H.; Zhou, J.; Zhou, Y.H.; Xia, X.J.; Yu, J.Q.; Shi, K. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol. 2015, 17, 81–89. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Li, J.; Han, G.; Sun, C.; Sui, N. Research advances of MYB transcription factors in plant stress resistance and breeding. Plant Signal. Behav. 2019, 14, 1613131. [Google Scholar] [CrossRef]
- Feng, H.-L.; Ma, N.-N.; Meng, X.; Zhang, S.; Wang, J.-R.; Chai, S.; Meng, Q.-W. A novel tomato MYC-type ICE1-like transcription factor, SlICE1a, confers cold, osmotic and salt tolerance in transgenic tobacco. Plant Physiol. Biochem. 2013, 73, 309–320. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Gene ID | Gene Length, bp | Transcript Length, bp | CDS Length, bp | Protein Length, aa | pI | MW, kDa | Subcellular Location |
|---|---|---|---|---|---|---|---|---|
| SlCRK1 | Solyc01g007960 | 2678 | 2002 | 1956 | 651 | 7.49 | 72.17 | plas: 8, E.R.: 3, nucl: 1, extr: 1 |
| SlCRK2 | Solyc01g007970 | 3469 | 1968 | 1968 | 655 | 8.42 | 72.73 | plas: 9.5, cyto_plas: 5.5, E.R.: 4 |
| SlCRK3 | Solyc01g007980 | 2798 | 2108 | 1917 | 638 | 8.59 | 71.30 | plas: 9, E.R.: 2, nucl: 1, extr: 1 |
| SlCRK4 | Solyc01g007990 | 3007 | 2235 | 1917 | 638 | 8.05 | 70.72 | plas: 9.5, golg_plas: 6, vacu: 3 |
| SlCRK5 | Solyc02g067770 | 1478 | 705 | 414 | 137 | 4.88 | 15.40 | extr: 5, vacu: 5, E.R.: 3 |
| SlCRK6 | Solyc02g067780 | 2538 | 1652 | 1488 | 495 | 6.07 | 55.96 | chlo: 7, nucl: 3, cyto: 2, plas: 1 |
| SlCRK7 | Solyc02g079990 | 3067 | 2242 | 2001 | 666 | 6.52 | 74.19 | plas: 10.5, golg_plas: 6.5, vacu: 2 |
| SlCRK8 | Solyc02g080010 | 4369 | 2654 | 2571 | 856 | 7.02 | 96.25 | plas: 8.5, golg_plas: 5.5, vacu: 3, golg: 1.5 |
| SlCRK9 | Solyc02g080020 | 624 | 504 | 504 | 167 | 8.56 | 18.41 | chlo: 4, E.R.: 4, nucl: 2, mito: 2, cyto: 1 |
| SlCRK10 | Solyc02g080030 | 2272 | 1203 | 1203 | 400 | 5.29 | 45.05 | vacu: 9, cyto: 1, plas: 1, extr: 1, E.R.: 1 |
| SlCRK11 | Solyc02g080040 | 2630 | 1629 | 1338 | 445 | 8.16 | 49.77 | chlo: 3, nucl: 3, cyto: 3, plas: 2.5, golg_plas: 2.5, golg: 1.5 |
| SlCRK12 | Solyc02g080050 | 462 | 462 | 462 | 153 | 4.86 | 17.35 | chlo: 8, nucl: 2, extr: 2, cyto: 1 |
| SlCRK13 | Solyc02g080060 | 1156 | 1104 | 1104 | 367 | 8.81 | 41.31 | vacu: 5, extr: 4, golg: 2, nucl: 1, cyto: 1 |
| SlCRK14 | Solyc02g080070 | 4178 | 2364 | 2031 | 676 | 6.16 | 75.45 | plas: 8.5, golg_plas: 6.5, golg: 3.5 |
| SlCRK15 | Solyc02g080080 | 3451 | 2139 | 2016 | 671 | 6.02 | 74.63 | plas: 10.5, golg_plas: 6.5, golg: 1.5 |
| SlCRK16 | Solyc02g080090 | 2985 | 1176 | 1176 | 391 | 6.27 | 43.28 | chlo: 6, extr: 5, cyto: 2 |
| SlCRK17 | Solyc02g082310 | 2197 | 1185 | 1185 | 394 | 4.95 | 42.37 | nucl: 7, cyto: 5, chlo: 1 |
| SlCRK18 | Solyc02g086590 | 3939 | 2496 | 1995 | 664 | 8.56 | 72.79 | plas: 3, mito: 2, vacu: 2, E.R.: 2, golg: 2, chlo: 1, cyto: 1 |
| SlCRK19 | Solyc03g031540 | 2260 | 681 | 681 | 227 | 6.45 | 25.56 | cyto: 8, nucl: 3, mito: 2 |
| SlCRK20 | Solyc03g031550 | 453 | 453 | 453 | 150 | 6.11 | 17.16 | chlo: 4, extr: 4, vacu: 2.5, E.R._vacu: 2.5, mito: 2 |
| SlCRK21 | Solyc03g111530 | 2698 | 981 | 966 | 321 | 7.42 | 36.75 | plas: 7.5, golg_plas: 6, golg: 3.5, E.R.: 2 |
| SlCRK22 | Solyc03g111540 | 2863 | 2028 | 2028 | 675 | 6.17 | 75.90 | plas: 8.5, golg_plas: 6, vacu: 3, golg: 2.5 |
| SlCRK23 | Solyc03g112730 | 2717 | 2230 | 1851 | 616 | 6.00 | 67.94 | E.R.: 3, chlo: 2, nucl: 2, plas: 2, extr: 2, cyto: 1, mito: 1 |
| SlCRK24 | Solyc03g119340 | 3129 | 1676 | 1188 | 395 | 6.35 | 43.69 | nucl: 8, cyto: 3, chlo: 1, mito: 1 |
| SlCRK25 | Solyc04g007880 | 4040 | 1468 | 1200 | 399 | 9.57 | 44.41 | nucl: 7, cyto: 3, chlo: 1, mito: 1, pero: 1 |
| SlCRK26 | Solyc05g005050 | 1962 | 967 | 900 | 299 | 8.80 | 32.85 | vacu: 4, chlo: 3, plas: 3, extr: 3 |
| SlCRK27 | Solyc05g005060 | 1534 | 1209 | 990 | 329 | 7.13 | 37.11 | mito: 11.5, cyto_mito: 6.5, chlo: 1 |
| SlCRK28 | Solyc05g005070 | 3802 | 2434 | 2160 | 719 | 6.81 | 80.58 | plas: 9, nucl: 2, vacu: 2 |
| SlCRK29 | Solyc05g018910 | 1543 | 750 | 750 | 249 | 6.31 | 27.44 | cyto: 5, golg: 3, nucl: 2, E.R.: 2, chlo: 1 |
| SlCRK30 | Solyc05g018920 | 348 | 282 | 282 | 93 | 8.86 | 10.59 | chlo: 9, cyto: 2, vacu: 2 |
| SlCRK31 | Solyc05g018930 | 613 | 555 | 555 | 184 | 5.29 | 20.24 | chlo: 11, nucl: 1, mito: 1 |
| SlCRK32 | Solyc11g011870 | 2769 | 1368 | 1368 | 455 | 7.97 | 50.43 | cyto: 5.5, cyto_nucl: 3.5, plas: 3, mito: 2, chlo: 1, E.R.: 1 |
| SlCRK33 | Solyc11g011880 | 3209 | 1929 | 1929 | 642 | 8.36 | 71.20 | plas: 5.5, E.R.: 4, golg_plas: 4, vacu: 2, golg: 1.5 |
| SlCRK34 | Solyc12g087910 | 723 | 507 | 507 | 168 | 8.85 | 19.27 | cyto: 8, extr: 3, nucl: 2 |
| SlCRK35 | Solyc12g087920 | 3352 | 1755 | 1755 | 584 | 6.29 | 66.27 | chlo: 4, extr: 4, cyto: 2, vacu: 2, plas: 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Feng, Z.; Zhu, W.; Liu, J.; Zhang, Y. Genome-Wide Identification and Characterization of Cysteine-Rich Receptor-Like Protein Kinase Genes in Tomato and Their Expression Profile in Response to Heat Stress. Diversity 2021, 13, 258. https://doi.org/10.3390/d13060258
Liu Y, Feng Z, Zhu W, Liu J, Zhang Y. Genome-Wide Identification and Characterization of Cysteine-Rich Receptor-Like Protein Kinase Genes in Tomato and Their Expression Profile in Response to Heat Stress. Diversity. 2021; 13(6):258. https://doi.org/10.3390/d13060258
Chicago/Turabian StyleLiu, Yahui, Zhengxiang Feng, Weimin Zhu, Junzhong Liu, and Yingying Zhang. 2021. "Genome-Wide Identification and Characterization of Cysteine-Rich Receptor-Like Protein Kinase Genes in Tomato and Their Expression Profile in Response to Heat Stress" Diversity 13, no. 6: 258. https://doi.org/10.3390/d13060258
APA StyleLiu, Y., Feng, Z., Zhu, W., Liu, J., & Zhang, Y. (2021). Genome-Wide Identification and Characterization of Cysteine-Rich Receptor-Like Protein Kinase Genes in Tomato and Their Expression Profile in Response to Heat Stress. Diversity, 13(6), 258. https://doi.org/10.3390/d13060258








