Turbid Coral Reefs: Past, Present and Future—A Review
Abstract
1. Introduction
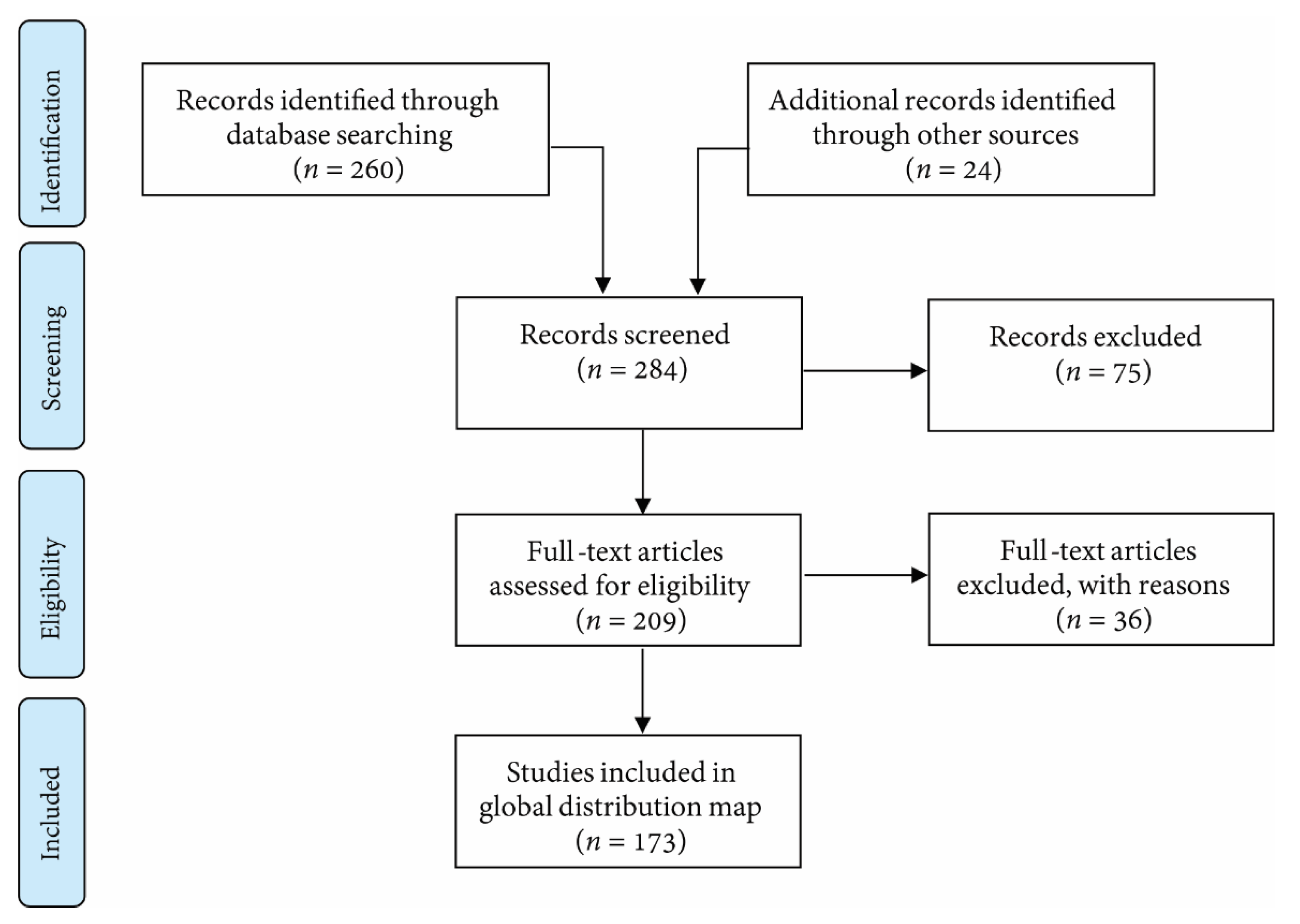
2. Methods–Searching for Turbid Reefs (in the Literature)
3. Defining a Turbid Reef
4. The Past—Holocene Paleoecological Reconstructions of Turbid Coral Reefs
5. The Present (1900 to Present Day)
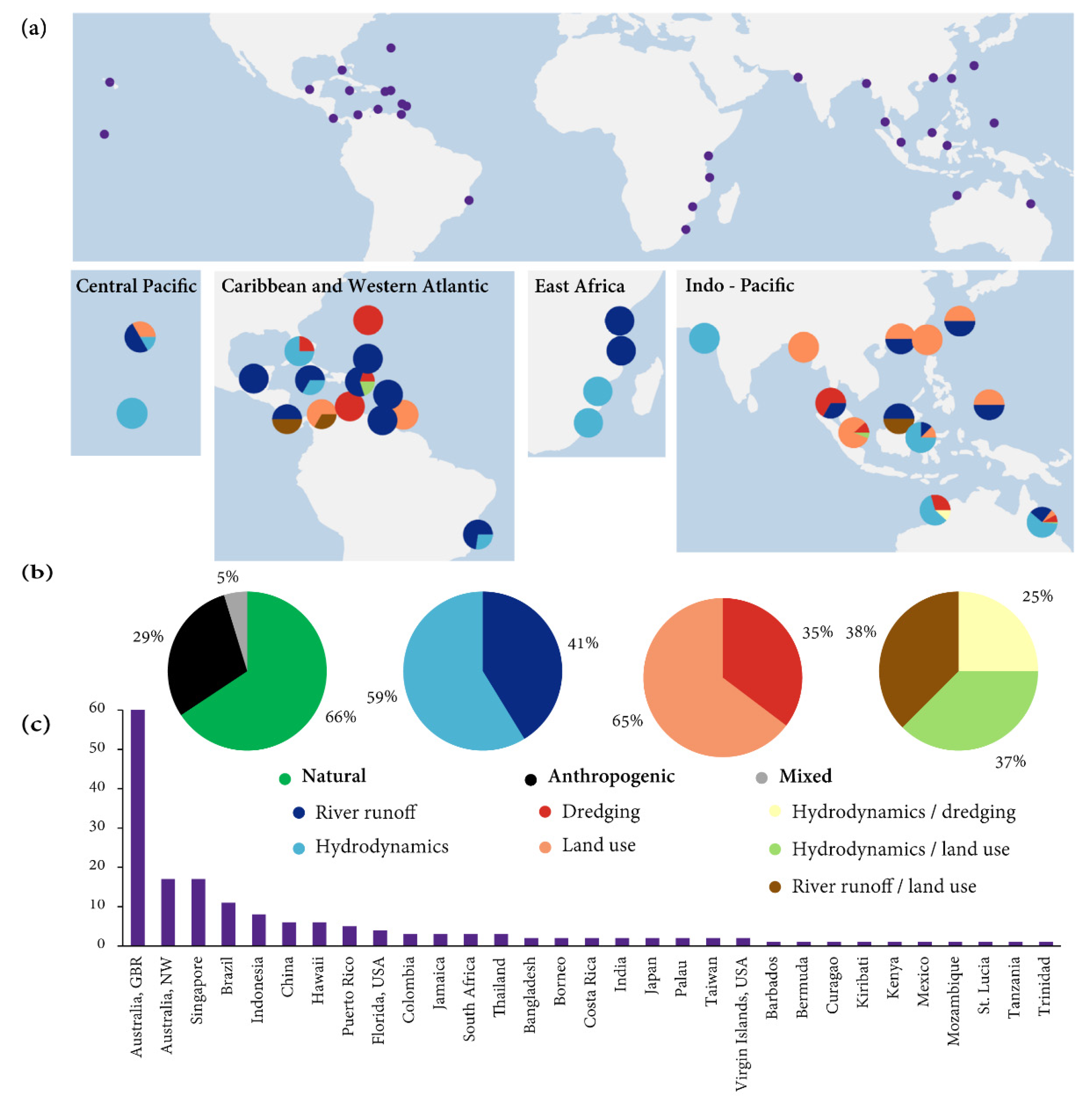
5.1. Global Distribution, Sources of Turbidity and Environmental Setting
5.2. Paluma Shoals Reef Complex, Great Barrier Reef, Australia—Natural (Persistent) Turbid Reef
5.3. The Southern Islands Group, Singapore—Anthropogenic (Transitional) Turbid Reef
5.4. PSRC vs. Singapore
6. The Future—Facing Local and Global Stressors
6.1. High Sediment Loads
6.2. Eutrophication
6.3. Warming Oceans
6.4. Increased Storm Severity and Ocean Acidification
6.5. Sea-Level Rise
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Van Duin, E.H.S.; Blom, G.; Los, F.J.; Maffione, R.; Zimmerman, R.; Cerco, C.F.; Dortch, M.; Best, E.P.H. Modeling underwater light climate in relation to sedimentation, resuspension, water quality and autotrophic growth. Hydrobiologia 2001, 444, 25–42. [Google Scholar] [CrossRef]
- Flores, F.; Hoogenboom, M.O.; Smith, L.D.; Cooper, T.F.; Abrego, D.; Negri, A.P. Chronic exposure of corals to fine sediments: Lethal and sub-lethal impacts. PLoS ONE 2012, 7, e37795. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.S. Responses of coral reefs and reef organisms to sedimentation. Mar. Ecol. Prog. Ser. 1990, 62, 185–202. [Google Scholar] [CrossRef]
- Anthony, K.R.N. Coral suspension feeding on fine particulate matter. J. Exp. Mar. Biol. Ecol. 1999, 232, 85–106. [Google Scholar] [CrossRef]
- Macdonald, R.K.; Ridd, P.V.; Whinney, J.C.; Larcombe, P.; Neil, D.T. Towards environmental management of water turbidity within open coastal waters of the Great Barrier Reef. Mar. Pollut. Bull. 2013, 74, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Sully, S.; van Woesik, R. Turbid reefs moderate coral bleaching under climate-related temperature stress. Glob. Chang. Biol. 2020, 26, 1367–1373. [Google Scholar] [CrossRef]
- Morgan, K.M.; Perry, C.T.; Johnson, J.A.; Smithers, S.G. Nearshore turbid-zone corals exhibit high bleaching tolerance on the Great Barrier Reef following the 2016 ocean warming event. Front. Mar. Sci. 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Larcombe, P.; Costen, A.; Woolfe, K.J. The hydrodynamic and sedimentary setting of nearshore coral reefs, Central Great Barrier Reef shelf, Australia: Paluma Shoals, a case study. Sedimentology 2001, 48, 811–835. [Google Scholar] [CrossRef]
- Sanders, D.; Baron-Szabo, R.C. Scleractinian assemblages under sediment input: Their characteristics and relation to the nutrient input concept. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 216, 139–181. [Google Scholar] [CrossRef]
- Palmer, S.E.; Perry, C.T.; Smithers, S.G.; Gulliver, P. Internal structure and accretionary history of a nearshore, turbid-zone coral reef: Paluma Shoals, central Great Barrier Reef, Australia. Mar. Geol. 2010, 276, 14–29. [Google Scholar] [CrossRef]
- Weber, M.; De Beer, D.; Lott, C.; Polerecky, L.; Kohls, K.; Abed, R.M.M.; Ferdelman, T.G.; Fabricius, K.E. Mechanisms of damage to corals exposed to sedimentation. Proc. Natl. Acad. Sci. USA 2012, 109, E1558–E1567. [Google Scholar] [CrossRef]
- Gilmour, J. Experimental investigation into the effects of suspended sediment on fertilisation, larval survival and settlement in a scleractinian coral. Mar. Biol. 1999, 135, 451–462. [Google Scholar] [CrossRef]
- Fabricius, K.E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.M.; Perry, C.T.; Smithers, S.G.; Johnson, J.A.; Daniell, J.J. Evidence of extensive reef development and high coral cover in nearshore environments: Implications for understanding coral adaptation in turbid settings. Sci. Rep. 2016, 6, 29616. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, V.; Rodríguez, S.C.; López-Victoria, M.; Zapata, F.A.; Zea, S.; Galindo-Martínez, C.T.; Iglesias-Prieto, R.; Pollock, J.; Medina, M. Unraveling the structure and composition of Varadero Reef, an improbable and imperiled coral reef in the Colombian Caribbean. PeerJ 2017, 2017, e4119. [Google Scholar] [CrossRef]
- Goodkin, N.; Switzer, A.; McCorry, D.; DeVantier, L.; True, J.; Hughen, K.; Angeline, N.; Yang, T. Coral communities of Hong Kong: Long-lived corals in a marginal reef environment. Mar. Ecol. Prog. Ser. 2011, 426, 185–196. [Google Scholar] [CrossRef]
- Wilson, B.; Blake, S.; Ryan, D.; Hacker, J. Reconnaissance of species-rich coral reefs in a muddy, macro-tidal, enclosed embayment, Talbot Bay, Kimberley, Western Australia. J. R. Soc. West. Aust. 2011, 94, 251–265. [Google Scholar]
- Browne, N.; Braoun, C.; McIlwain, J.; Nagarajan, R.; Zinke, J. Borneo coral reefs subject to high sediment loads show evidence of resilience to various environmental stressors. PeerJ 2019, 7, e7382. [Google Scholar] [CrossRef] [PubMed]
- Browne, N.K.; Smithers, S.G.; Perry, C.T. Carbonate and terrigenous sediment budgets for two inshore turbid reefs on the central Great Barrier Reef. Mar. Geol. 2013, 346, 101–123. [Google Scholar] [CrossRef]
- Anthony, K.; Larcombe, P. Coral Reefs in Turbid Waters: Sediment-Induced Stresses in Corals and Likely Mechanisms of Adaptation. In Proceedings of the Ninth International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; pp. 239–244. [Google Scholar]
- Perry, C.T.; Smithers, S.G.; Palmer, S.E.; Larcombe, P.; Johnson, K.G. 1200 year paleoecological record of coral community development from the terrigenous inner shelf of the Great barrier reef. Geology 2008, 36, 691–694. [Google Scholar] [CrossRef]
- Wagner, D.E.; Kramer, P.; Woesik, R. Van Species composition, habitat, and water quality influence coral bleaching in southern Florida. Mar. Ecol. Prog. Ser. 2010, 408, 65–78. [Google Scholar] [CrossRef]
- Perry, C.T.; Smithers, S.G. Evidence for the episodic “turn on” and “turn off” of turbid-zone coral reefs during the late Holocene sea-level highstand. Geology 2010, 38, 119–122. [Google Scholar] [CrossRef]
- Santodomingo, N.; Novak, V.; Pretkovic, V.; Marshall, N.; Di Martino, E.; Capelli, E.L.G.; Rosler, A.; Reich, S.; Braga, J.C.; Renema, W.; et al. A divers patch reef from turbid habitats in the middle miocene (East Kalimantan, Indonesia). Palaios 2015, 30, 128–149. [Google Scholar] [CrossRef]
- Solihuddin, T.; Collins, L.B.; Blakeway, D.; O’ Leary, M.J. Holocene coral reef growth and sea level in a macrotidal, high turbidity setting: Cockatoo Island, Kimberley Bioregion, northwest Australia. Mar. Geol. 2015, 359, 50–60. [Google Scholar] [CrossRef]
- Johnson, J.A.; Perry, C.T.; Smithers, S.G.; Morgan, K.M.; Santodomingo, N.; Johnson, K.G. Palaeoecological records of coral community development on a turbid, nearshore reef complex: Baselines for assessing ecological change. Coral Reefs 2017, 36, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Potts, D.; Jacobs, J. Evolution of Reef-Building Scleractinian Corals in Turbid Environments: A Paleo-Ecological Hypothesis. In Proceedings of the International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; pp. 249–254. [Google Scholar]
- Perry, C.T.; Larcombe, P. Marginal and non-reef-building coral environments. Coral Reefs 2003, 22, 427–432. [Google Scholar] [CrossRef]
- Richards, Z.; Bryce, M.; Bryce, C. The composition and structure of shallow benthic reef communities in the Kimberley, north-west Australia. Rec. West. Aust. Mus. Suppl. 2018, 85, 75. [Google Scholar] [CrossRef]
- Segal, B.; Castro, C.B. Coral community structure and sedimentation at different distances from the coast of the Abrolhos Bank, Brazil. Braz. J. Oceanogr. 2011, 59, 119–129. [Google Scholar] [CrossRef]
- Tan, K.S.; Acerbi, E.; Lauro, F.M.; Siang Tan, K.; Acerbi, E.; Lauro, F.M.; Tan, K.S.; Acerbi, E.; Lauro, F.M. Marine habitats and biodiversity of Singapore’s coastal waters: A review. Reg. Stud. Mar. Sci. 2016, 8, 340–352. [Google Scholar] [CrossRef]
- Foster, T.; Smith, A.; Jury, M.; Driscoll, A. Overview of PIANC report 108—Dredging and port construction around coral reefs. In Proceedings of the Coasts and Ports 2011: Diverse and Developing: Proceedings of the 20th Australasian Coastal and Ocean Engineering Conference and the 13th Australasian Port and Harbour Conference, Perth, Australia, 28–30 September 2011; pp. 573–578. [Google Scholar]
- Smith, A.; Foster, T.; Corcoran, E.; Monkivitch, J. Dredging and material relocation in sensitive coral environments. In Proceedings of the Eighteenth World Dredging Congress (WODCON XVIII), Lake Buena Vista, FL, USA, 27 May–1 June 2007; pp. 945–955. [Google Scholar]
- Dsikowitzky, L.; Ferse, S.; Schwarzbauer, J.; Vogt, T.S.; Irianto, H.E. Impacts of megacities on tropical coastal ecosystems—The case of Jakarta, Indonesia. Mar. Pollut. Bull. 2016, 110, 621–623. [Google Scholar] [CrossRef]
- Orpin, A.R.; Haig, D.W.; Woolfe, K.J. Sedimentary and foraminiferal facies in Exmouth Gulf, in arid tropical northwestern Australia. Aust. J. Earth Sci. 1999, 46, 607–621. [Google Scholar] [CrossRef]
- Corlett, R.T. The ecological transformation of Singapore, 1819-1990. J. Biogeogr. 1992, 19, 411–420. [Google Scholar] [CrossRef]
- Van Woesik, R.; Done, T.J. Coral communities and reef growth in the southern Great Barrier Reef. Coral Reefs 1997, 16, 103–115. [Google Scholar] [CrossRef]
- Perry, C.T.; Alvarez-Filip, L.; Graham, N.A.J.; Mumby, P.J.; Wilson, S.K.; Kench, P.S.; Manzello, D.P.; Morgan, K.M.; Slangen, A.B.A.; Thomson, D.P.; et al. Loss of coral reef growth capacity to track future increases in sea level. Nature 2018, 558, 396–400. [Google Scholar] [CrossRef]
- Januchowski-Hartley, F.A.; Bauman, A.G.; Morgan, K.M.; Seah, J.C.L.; Huang, D.; Todd, P.A. Accreting coral reefs in a highly urbanized environment. Coral Reefs 2020, 39, 717–731. [Google Scholar] [CrossRef]
- Morgan, K.M.; Moynihan, M.A.; Sanwlani, N.; Switzer, A.D. Light Limitation and Depth-Variable Sedimentation Drives Vertical Reef Compression on Turbid Coral Reefs. Front. Mar. Sci. 2020, 7, 571256. [Google Scholar] [CrossRef]
- Chou, L.M.; Huang, D.; Tan, K.S.; Toh, T.C.; Goh, B.P.L.; Tun, K. Singapore. In World Seas: An Environmental Evaluation, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 539–558. [Google Scholar] [CrossRef]
- Pickering, C.; Byrne, J. The benefits of publishing systematic quantitative literature reviews for PhD candidates and other early-career researchers. High. Educ. Res. Dev. 2014, 33, 534–548. [Google Scholar] [CrossRef]
- Pickering, C.; Grignon, J.; Steven, R.; Guitart, D.; Pickering, C.; Grignon, J.; Steven, R.; Guitart, D.; Byrne, J. Studies in Higher Education Publishing not perishing: How research students transition from novice to knowledgeable using systematic quantitative literature reviews. Stud. High. Educ. 2015, 40, 1756–1769. [Google Scholar] [CrossRef]
- Jones, R.; Ricardo, G.F.; Negri, A.P. Effects of sediments on the reproductive cycle of corals. Mar. Pollut. Bull. 2015, 100, 13–33. [Google Scholar] [CrossRef]
- Jones, R.; Bessell-Browne, P.; Fisher, R.; Klonowski, W.; Slivkoff, M. Assessing the impacts of sediments from dredging on corals. Mar. Pollut. Bull. 2016, 102, 9–29. [Google Scholar] [CrossRef]
- Jones, R.; Giofre, N.; Luter, H.M.; Neoh, T.L.; Fisher, R.; Duckworth, A. Responses of corals to chronic turbidity. Sci. Rep. 2020, 10, 4762. [Google Scholar] [CrossRef] [PubMed]
- Burt, J.A.; Camp, E.F.; Enochs, I.C.; Johansen, J.L.; Morgan, K.M.; Riegl, B.; Hoey, A.S. Insights from extreme coral reefs in a changing world. Coral Reefs 2020, 39, 495–507. [Google Scholar] [CrossRef]
- Risk, M.J.; Edinger, E. Impacts of Sediment on Coral Reefs. Encycl. Earth Sci. Ser. 2011, 575–586. [Google Scholar] [CrossRef]
- Risk, M.J. Assessing the effects of sediments and nutrients on coral reefs. Curr. Opin. Environ. Sustain. 2014, 7, 108–117. [Google Scholar] [CrossRef]
- Erftemeijer, P.L.A.; Riegl, B.; Hoeksema, B.W.; Todd, P.A. Environmental impacts of dredging and other sediment disturbances on corals: A review. Mar. Pollut. Bull. 2012, 64, 1737–1765. [Google Scholar] [CrossRef] [PubMed]
- Camp, E.F.; Schoepf, V.; Mumby, P.J.; Hardtke, L.A.; Rodolfo-Metalpa, R.; Smith, D.J.; Suggett, D.J. The future of coral reefs subject to rapid climate change: Lessons from natural extreme environments. Front. Mar. Sci. 2018, 5, 4. [Google Scholar] [CrossRef]
- Bartley, R.; Bainbridge, Z.T.; Lewis, S.E.; Kroon, F.J.; Wilkinson, S.N.; Brodie, J.E.; Silburn, D.M. Relating sediment impacts on coral reefs to watershed sources, processes and management: A review. Sci. Total Environ. 2014, 468–469, 1138–1153. [Google Scholar] [CrossRef]
- Browne, N.K.; Smithers, S.G.; Perry, C.T. Coral reefs of the turbid inner-shelf of the Great Barrier Reef, Australia: An environmental and geomorphic perspective on their occurrence, composition and growth. Earth Sci. Rev. 2012, 115, 1–20. [Google Scholar] [CrossRef]
- Todd, P.A.; Heery, E.C.; Loke, L.H.L.; Thurstan, R.H.; Kotze, D.J.; Swan, C. Towards an urban marine ecology: Characterizing the drivers, patterns and processes of marine ecosystems in coastal cities. Oikos 2019, 128, 1215–1242. [Google Scholar] [CrossRef]
- Heery, E.C.; Hoeksema, B.W.; Browne, N.K.; Reimer, J.D.; Ang, P.O.; Huang, D.; Friess, D.A.; Chou, L.M.; Loke, L.H.L.; Saksena-Taylor, P.; et al. Urban coral reefs: Degradation and resilience of hard coral assemblages in coastal cities of East and Southeast Asia. Mar. Pollut. Bull. 2018, 135, 654–681. [Google Scholar] [CrossRef]
- Storlazzi, C.D.; Elias, E.; Field, M.E.; Presto, M.K. Numerical modeling of the impact of sea-level rise on fringing coral reef hydrodynamics and sediment transport. Coral Reefs 2011, 30, 83–96. [Google Scholar] [CrossRef]
- Van Rijn, L.C.; Ribberink, J.S.; Van Der Werf, J.; Walstra, D.J.R. Coastal sediment dynamics: Recent advances and future research needs. J. Hydraul. Res. 2013, 51, 475–493. [Google Scholar] [CrossRef]
- e Castro, C.B.; Segal, B.; Negrão, F.; Calderon, E.N. Four-year monthly sediment deposition on turbid southwestern atlantic coral reefs, with a comparison of benthic assemblages. Braz. J. Oceanogr. 2012, 60, 49–63. [Google Scholar] [CrossRef]
- Evans, R.D.; Wilson, S.K.; Fisher, R.; Ryan, N.M.; Babcock, R.; Blakeway, D.; Bond, T.; Dorji, P.; Dufois, F.; Fearns, P.; et al. Early recovery dynamics of turbid coral reefs after recurring bleaching events. J. Environ. Manag. 2020, 268, 110666. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.D.; Leitão, R.L.L.; Ribeiro, F.V.; Moraes, F.C.; Neves, L.M.; Bastos, A.C.; Pereira-Filho, G.H.; Kampel, M.; Salomon, P.S.; Sá, J.A.; et al. Sustained mass coral bleaching (2016–2017) in Brazilian turbid-zone reefs: Taxonomic, cross-shelf and habitat-related trends. Coral Reefs 2019, 38, 801–813. [Google Scholar] [CrossRef]
- Hennige, S.J.; Smith, D.J.; Walsh, S.J.; McGinley, M.P.; Warner, M.E.; Suggett, D.J. Acclimation and adaptation of scleractinian coral communities along environmental gradients within an Indonesian reef system. J. Exp. Mar. Bio. Ecol. 2010, 391, 143–152. [Google Scholar] [CrossRef]
- Dikou, A.; van Woesik, R. Survival under chronic stress from sediment load: Spatial patterns of hard coral communities in the southern islands of Singapore. Mar. Pollut. Bull. 2006, 52, 1340–1354. [Google Scholar] [CrossRef]
- Dikou, A. Skeletal linear extension rates of the foliose scleractinian coral Merulina ampliata (Ellis & Solander, 1786) in a turbid environment. Mar. Ecol. 2009, 30, 405–415. [Google Scholar] [CrossRef]
- Hossain, M.M.; Islam, M.H. Status of the Biodiversity of St. Martin’S Island, Bay of Bengal, Bangladesh. Pak. J. Mar. Sci. 2006, 15, 201–210. [Google Scholar]
- Storlazzi, C.D.; Field, M.E.; Bothner, M.H. The use (and misuse) of sediment traps in coral reef environments: Theory, observations, and suggested protocols. Coral Reefs 2011, 30, 23–38. [Google Scholar] [CrossRef]
- Schleyer, M.H.; Celliers, L. Coral dominance at the reef-sediment interface in marginal coral communities at Sodwana Bay, South Africa. Mar. Freshw. Res. 2003, 54, 967–972. [Google Scholar] [CrossRef]
- Loiola, M.; Cruz, I.C.S.; Lisboa, D.S.; Mariano-Neto, E.; Leão, Z.M.A.N.; Oliveira, M.D.M.; Kikuchi, R.K.P. Structure of marginal coral reef assemblages under different turbidity regime. Mar. Environ. Res. 2019, 147, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.D. Late Holocene island reef development on the inner zone of the northern Great Barrier Reef: Insights from Low Isles Reef. Aust. J. Earth Sci. 2008, 55, 669–683. [Google Scholar] [CrossRef]
- Leonard, N.D.; Lepore, M.L.; Zhao, J.; Rodriguez-Ramirez, A.; Butler, I.R.; Clark, T.R.; Roff, G.; McCook, L.; Nguyen, A.D.; Feng, Y.; et al. Re-evaluating mid-Holocene reef “turn-off” on the inshore Southern Great Barrier Reef. Quat. Sci. Rev. 2020, 244, 106518. [Google Scholar] [CrossRef]
- Alvarado, J.J.; Fernández, C.; Cortés, J. Water quality conditions on coral reefs at the marino ballena national park, pacific costa RICA. Bull. Mar. Sci. 2009, 84, 137–152. [Google Scholar]
- Gilmour, J.P.; Cooper, T.F.; Fabricius, K.E.; Smith, L.D. Early Warning Indicators of Change in the Condition of Corals and Coral Communities in Response to Key Anthropogenic Stressors in the Pilbara, Western Australia; Australian Institute of Marine Science: Townsville, Australia, 2006; p. 108.
- Roff, G.; Clark, T.R.; Reymond, C.E.; Zhao, J.; Feng, Y.; McCook, L.J.; Done, T.J.; Pandolfi, J.M. Palaeoecological evidence of a historical collapse of corals at Pelorus Island, inshore Great Barrier Reef, following European settlement. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122100. [Google Scholar] [CrossRef]
- Ryan, E.J.; Smithers, S.G.; Lewis, S.E.; Clark, T.R.; Zhao, J.X. Chronostratigraphy of Bramston Reef reveals a long-term record of fringing reef growth under muddy conditions in the central Great Barrier Reef. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 441, 734–747. [Google Scholar] [CrossRef]
- Ryan, E.; Smithers, S.; Lewis, S.; Clark, T.; Zhao, J. The Variable Influences of Sea Level, Sedimentation and Exposure on Holocene Reef Development over a Cross-Shelf Transect, Central Great Barrier Reef. Diversity 2018, 10, 110. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Zhao, J.; Feng, Y. Uranium-thorium dating of coral mortality and community shift in a highly disturbed inshore reef (Weizhou Island, northern South China Sea). Sci. Total Environ. 2021, 752, 141866. [Google Scholar] [CrossRef]
- Yamano, H.; Inoue, T.; Adachi, H.; Tsukaya, K.; Adachi, R.; Baba, S. Holocene sea-level change and evolution of a mixed coral reef and mangrove system at Iriomote Island, southwest Japan. Estuar. Coast. Shelf Sci. 2019, 220, 166–175. [Google Scholar] [CrossRef]
- Dechnik, B.; Bastos, A.C.; Vieira, L.S.; Webster, J.M.; Fallon, S.; Yokoyama, Y.; Nothdurft, L.; Sanborn, K.; Batista, J.; Moura, R.; et al. Holocene reef growth in the tropical southwestern Atlantic: Evidence for sea level and climate instability. Quat. Sci. Rev. 2019, 218, 365–377. [Google Scholar] [CrossRef]
- Cortes, J.; Macintyre, I.G.; Glynn, P.W. Holocene growth history of an eastern Pacific fringing reef, Punta Islotes, Costa Rica. Coral Reefs 1994, 13, 65–73. [Google Scholar] [CrossRef]
- Tudhope, A.W.; Scoffin, T.P. Growth and structure of fringing reefs in a muddy environment, south Thailand. J. Sediment. Res. A Sediment. Petrol. Process. 1994, 64, 752–764. [Google Scholar] [CrossRef]
- Johnson, J.A.; Perry, C.T.; Smithers, S.G.; Morgan, K.M.; Woodroffe, S.A. Reef shallowing is a critical control on benthic foraminiferal assemblage composition on nearshore turbid coral reefs. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 533. [Google Scholar] [CrossRef]
- Perry, C.T.; Smithers, S.G. Taphonomic signatures of turbid-zone reef development: Examples from Paluma Shoals and Lugger Shoal, inshore central Great Barrier Reef, Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 242, 1–20. [Google Scholar] [CrossRef]
- Roche, R.C.; Perry, C.T.; Smithers, S.G.; Leng, M.J.; Grove, C.A.; Sloane, H.J.; Unsworth, C.E. Mid-Holocene sea surface conditions and riverine influence on the inshore Great Barrier Reef. Holocene 2014, 24, 885–897. [Google Scholar] [CrossRef]
- Perry, C.T.; Smithers, S.G.; Gulliver, P.; Browne, N.K. Evidence of very rapid reef accretion and reef growth under high turbidity and terrigenous sedimentation. Geology 2012, 40, 719–722. [Google Scholar] [CrossRef]
- Solihuddin, T.; O’Leary, M.J.; Blakeway, D.; Parnum, I.; Kordi, M.; Collins, L.B. Holocene reef evolution in a macrotidal setting: Buccaneer Archipelago, Kimberley Bioregion, Northwest Australia. Coral Reefs 2016, 35, 783–794. [Google Scholar] [CrossRef]
- Ryan, E.J.; Lewis, S.E.; Smithers, S.G.; Clark, T.R.; Zhao, J.-X. Multi-scale records of reef development and condition provide context for contemporary changes on inshore reefs. Glob. Planet. Chang. 2016, 146, 162–178. [Google Scholar] [CrossRef]
- Lewis, S.E.; Wüst, R.A.J.; Webster, J.M.; Shields, G.A.; Renema, W.; Lough, J.M.; Jacobsen, G. Development of an inshore fringing coral reef using textural, compositional and stratigraphic data from Magnetic Island, Great Barrier Reef, Australia. Mar. Geol. 2012, 299–302, 18–32. [Google Scholar] [CrossRef]
- Huang, D.; Tun, K.P.P.; Chou, L.M.; Todd, P.A. An inventory of zooxanthellate scleractinian corals in Singapore, including 33 new records. RAFFLES Bull. Zool. 2009, 22, 69–80. [Google Scholar]
- Poquita-Du, R.C.; Quek, Z.B.R.; Jain, S.S.; Schmidt-Roach, S.; Tun, K.; Heery, E.C.; Chou, L.M.; Todd, P.A.; Huang, D. Last species standing: Loss of Pocilloporidae corals associated with coastal urbanization in a tropical city state. Mar. Biodivers. 2019, 49, 1727–1741. [Google Scholar] [CrossRef]
- Browne, N.K.; Smithers, S.G.; Perry, C.T. Spatial and temporal variations in turbidity on two inshore turbid reefs on the Great Barrier Reef, Australia. Coral Reefs 2013, 32, 195–210. [Google Scholar] [CrossRef]
- Jones, R.; Fisher, R.; Bessell-Browne, P. Sediment deposition and coral smothering. PLoS ONE 2019, 14, e0216248. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, G.F.; Jones, R.J.; Clode, P.L.; Humanes, A.; Negri, A.P. Suspended sediments limit coral sperm availability. Sci. Rep. 2015, 5, 18084. [Google Scholar] [CrossRef]
- Ridgway, T.; Inostroza, K.; Synnot, L.; Trapon, M.; Twomey, L.; Westera, M. Temporal patterns of coral cover in the offshore Pilbara, Western Australia. Mar. Biol. 2016, 163, 1–9. [Google Scholar] [CrossRef]
- Pollock, F.J.; Lamb, J.B.; Field, S.N.; Heron, S.F.; Schaffelke, B.; Shedrawi, G.; Bourne, D.G.; Willis, B.L. Sediment and turbidity associated with offshore dredging increase coral disease prevalence on nearby reefs. PLoS ONE 2014, 9, e0165541. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, J.P. Acute sedimentation causes size-specific mortality and asexual budding in the mushroom coral, Fungia fungites. Mar. Freshw. Res. 2002, 53, 805–812. [Google Scholar] [CrossRef]
- Stoddart, J.A.; Blakeway, D.R.; Grey, K.A.; Stoddart, S.E. Rapid High-Precision Monitoring of Coral Communities to Support Reactive Management of Dredging in Mermaid Sound, Dampier, Western Australia. Corals of the Dampier Harbour: Their Survival and Reproduction During the Dredging Programs of 2004. 2005. Available online: http://www.mscience.net.au/wp-content/uploads/2015/06/cotdh_web_03_coral_monitoring.pdf (accessed on 3 June 2021).
- Kordi, M.N.; O’Leary, M. Geomorphic classification of coral reefs in the north western Australian shelf. Reg. Stud. Mar. Sci. 2016, 7, 100–110. [Google Scholar] [CrossRef]
- Wilson, B. Kimberley marine biota. History and environment. Rec. West. Aust. Museum 2014, 84, 1. [Google Scholar] [CrossRef]
- Fisher, R.; Bessell-Browne, P.; Jones, R. Synergistic and antagonistic impacts of suspended sediments and thermal stress on corals. Nat. Commun. 2019, 10, 2346. [Google Scholar] [CrossRef] [PubMed]
- Richards, Z.T.; Garcia, R.A.; Wallace, C.C.; Rosser, N.L.; Muir, P.R. A diverse assemblage of reef corals thriving in a dynamic intertidal reef setting (Bonaparte archipelago, Kimberley, Australia). PLoS ONE 2015, 10, e0117791. [Google Scholar] [CrossRef]
- van Katwijk, M.M.; Meier, N.F.; van Loon, R.; van Hove, E.M.; Giesen, W.B.J.T.; van der Velde, G.; den Hartog, C. Sabaki River sediment load and coral stress: Correlation between sediments and condition of the Malindi-Watamu reefs in Kenya (Indian Ocean). Mar. Biol. 1993, 117, 675–683. [Google Scholar] [CrossRef]
- Reuter, M.; Bosellini, F.R.; Budd, A.F.; Ćorić, S.; Piller, W.E.; Harzhauser, M. High coral reef connectivity across the Indian Ocean is revealed 6–7 Ma ago by a turbid-water scleractinian assemblage from Tanzania (Eastern Africa). Coral Reefs 2019, 38, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.T. Coral reefs in a high-latitude, siliciclastic barrier island setting: Reef framework and sediment production at Inhaca Island, southern Mozambique. Coral Reefs 2003, 22, 485–497. [Google Scholar] [CrossRef]
- Riegl, B.; Heine, C.; Branch, G. Function of funnel-shaped coral growth in a high-sedimentation environment. Mar. Ecol. Prog. Ser. 1996, 145, 87–93. [Google Scholar] [CrossRef]
- Riegl, B.; Branch, G.M. Effects of sediment on the energy budgets of four scleractinian (Bourne 1900) and five alcyonacean (Lamouroux 1816) corals. J. Exp. Mar. Biol. Ecol. 1995, 186, 259–275. [Google Scholar] [CrossRef]
- Dallmeyer, D.G.; Porter, J.W.; Smith, G.J. Effects of particulate peat on the behavior and physiology of the Jamaican reef-building coral Montastrea annularis. Mar. Biol. 1982, 68, 229–233. [Google Scholar] [CrossRef]
- Mallela, J.; Perry, C.T. Calcium carbonate budgets for two coral reefs affected by different terrestrial runoff regimes, Rio Bueno, Jamaica. Coral Reefs 2007, 26, 129–145. [Google Scholar] [CrossRef]
- Carricart-Ganivet, J.P.; Merino, M. Growth responses of the reef-building coral Montastraea annularis along a gradient of continental influence in the southern Gulf of Mexico. Bull. Mar. Sci. 2001, 68, 133–146. [Google Scholar]
- Siegle, E.; Costa, M.B. Nearshore Wave Power Increase on Reef-Shaped Coasts Due to Sea-Level Rise. Earth’s Futur. 2017, 5, 1054–1065. [Google Scholar] [CrossRef]
- Moura, R.L.; Amado-Filho, G.M.; Moraes, F.C.; Brasileiro, P.S.; Salomon, P.S.; Mahiques, M.M.; Bastos, A.C.; Almeida, M.G.; Silva, J.M.; Araujo, B.F.; et al. An extensive reef system at the Amazon River mouth. Sci. Adv. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- López-Victoria, M.; Rodríguez-Moreno, M.; Zapata, F.A. A paradoxical reef from Varadero, Cartagena Bay, Colombia. Coral Reefs 2015, 34, 231. [Google Scholar] [CrossRef]
- Roitman, S.; López-Londoño, T.; Joseph Pollock, F.; Ritchie, K.B.; Galindo-Martínez, C.T.; Gómez-Campo, K.; González-Guerrero, L.A.; Pizarro, V.; López-Victoria, M.; Iglesias-Prieto, R.; et al. Surviving marginalized reefs: Assessing the implications of the microbiome on coral physiology and survivorship. Coral Reefs. 2020, 39, 795–807. [Google Scholar] [CrossRef]
- Junjie, R.K.; Browne, N.K.; Erftemeijer, P.L.A.; Todd, P.A. Impacts of sediments on coral energetics: Partitioning the effects of turbidity and settling particles. PLoS ONE 2014, 9, e107195. [Google Scholar] [CrossRef]
- Guest, J.R.; Low, J.; Tun, K.; Wilson, B.; Ng, C.; Raingeard, D.; Ulstrup, K.E.; Tanzil, J.T.I.; Todd, P.A.; Toh, T.C.; et al. Coral community response to bleaching on a highly disturbed reef. Sci. Rep. 2016, 6, 20717. [Google Scholar] [CrossRef]
- Larsen, T.C.; Browne, N.K.; Erichsen, A.C.; Tun, K.; Todd, P.A. Modelling for management: Coral photo-physiology and growth potential under varying turbidity regimes. Ecol. Modell. 2017, 362, 1–12. [Google Scholar] [CrossRef]
- Lui, G.C.Y.; Setiawan, W.; Todd, P.A.; Erftemeijer, P.L.A. Among-genotype variation for sediment rejection in the reef-building coral Diploastrea heliopora (Lamarck, 1816). Raffles Bull. Zool. 2012, 60, 529–535. [Google Scholar]
- Goh, N.K.C.; Chou, L.M. Growth of Five Species of Gorgonians (Sub-Class Octocorallia) in the Sedimented Waters of Singapore. Mar. Ecol. 1995, 16, 337–346. [Google Scholar] [CrossRef]
- Smithers, S.; Larcombe, P. Late Holocene initiation and growth of a nearshore turbid-zone coral reef: Paluma Shoals, central Great Barrier Reef, Australia. Coral Reefs 2003, 22, 499–505. [Google Scholar] [CrossRef]
- Perry, C.T. Structure and development of detrital reef deposits in turbid nearshore environments, Inhaca Island, Mozambique. Mar. Geol. 2005, 214, 143–161. [Google Scholar] [CrossRef]
- Hoitink, A.J.F. Tidally-induced clouds of suspended sediment connected to shallow-water coral reefs. Mar. Geol. 2004, 208, 13–31. [Google Scholar] [CrossRef]
- Restrepo, J.D.; Zapata, P.; Díaz, J.M.; Garzón-Ferreira, J.; García, C.B. Fluvial fluxes into the Caribbean Sea and their impact on coastal ecosystems: The Magdalena River, Colombia. Glob. Planet. Chang. 2006, 50, 33–49. [Google Scholar] [CrossRef]
- Perry, C.T.; Smithers, S.G.; Gulliver, P. Rapid vertical accretion on a “young” shore-detached turbid zone reef: Offshore Paluma Shoals, central Great Barrier Reef, Australia. Coral Reefs 2013, 32, 1143–1148. [Google Scholar] [CrossRef]
- Morgan, K.M.; Perry, C.T.; Arthur, R.; Williams, H.T.P.; Smithers, S.G. Projections of coral cover and habitat change on turbid reefs under future sea-level rise. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200541. [Google Scholar] [CrossRef]
- Morgan, K.M.; Perry, C.T.; Smithers, S.G.; Johnson, J.A.; Gulliver, P. Transitions in coral reef accretion rates linked to intrinsic ecological shifts on turbid-zone nearshore reefs. Geology 2016, 44, 995–998. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Fabricius, K.E. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Bio. Ecol. 2000, 252, 221–253. [Google Scholar] [CrossRef]
- Heron, S.F.; Maynard, J.A.; Van Hooidonk, R.; Eakin, C.M. Warming Trends and Bleaching Stress of the World’s Coral Reefs 1985–2012. Sci. Rep. 2016, 6, 38402. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G.; et al. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Loya, Y.; Sakai, K.; Yamazato, K.; Nakano, Y.; Sambali, H.; Van Woesik, R. Coral bleaching: The winners and the losers. Ecol. Lett. 2001, 4, 122–131. [Google Scholar] [CrossRef]
- Chou, L.M. Marine habitats in one of the world’s busiest harbours. In The Environment in Asia Pacific Harbours; Springer: Cham, The Netherlands, 2006; pp. 377–391. [Google Scholar]
- Yap, W.Y.; Lam, J.S.L. 80 million-twenty-foot-equivalent-unit container port? Sustainability issues in port and coastal development. Ocean Coast. Manag. 2013, 71, 13–25. [Google Scholar] [CrossRef]
- Lai, S.; Loke, L.H.L.; Hilton, M.J.; Bouma, T.J.; Todd, P.A. The effects of urbanisation on coastal habitats and the potential for ecological engineering: A Singapore case study. Ocean Coast. Manag. 2015, 103, 78–85. [Google Scholar] [CrossRef]
- Min Sin, T.; Peng Ang, H.; Buurman, J.; Chin Lee, A.; Lin Leong, Y.; Keat Ooi, S.; Steinberg, P.; Lay-Ming Teo, S. The urban marine environment of Singapore. Reg. Stud. Mar. Sci. 2016, 8, 331–339. [Google Scholar] [CrossRef]
- DOS. SingStat Website—Singapore Population. Available online: https://www.singstat.gov.sg/modules/infographics/population (accessed on 20 July 2020).
- Tun, K. Optimisation of Reef Survey Methods and Application of Reef Metrics and Biocriteria for the Monitoring of Sediment-Impacted Reefs. Ph.D. Thesis, National University of Singapore, Singapore, 2012. [Google Scholar]
- Crawfurd, J. Journal of an Embassy from the Governor-General of India to the Courts of Siam and Cochin China: Exhibiting a View of the Actual State of Those Kingdoms, 2nd ed.; Colburn, H., Ed.; National Art Library (Great Britain), Forster Collection: London, UK, 1830. [Google Scholar]
- Hilton, M.J.; Manning, S.S. Conversion of Coastal Habitats* in Singapore: Indications of Unsustainable Development. Environ. Conserv. 1995, 22, 307–322. [Google Scholar] [CrossRef]
- Guest, J.R.; Tun, K.; Low, J.; Vergés, A.; Marzinelli, E.M.; Campbell, A.H.; Bauman, A.G.; Feary, D.A.; Chou, L.M.; Steinberg, P.D. 27 years of benthic and coral community dynamics on turbid, highly urbanised reefs off Singapore. Sci. Rep. 2016, 6, 36260. [Google Scholar] [CrossRef] [PubMed]
- Hilton, M.J.; Chou, L.M. Sediment facies of a low-energy, meso-tidal, fringing reef, Singapore. Singap. J. Trop. Geogr. 1999, 20, 111–130. [Google Scholar] [CrossRef]
- Browne, N.K.; Tay, J.K.L.; Low, J.; Larson, O.; Todd, P.A. Fluctuations in coral health of four common inshore reef corals in response to seasonal and anthropogenic changes in water quality. Mar. Environ. Res. 2015, 105, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.S.Y.; Chan, Y.K.S.; Ng, C.S.L.; Tun, K.P.P.; Darling, E.S.; Huang, D. Comparing patterns of taxonomic, functional and phylogenetic diversity in reef coral communities. Coral Reefs 2018, 37, 737–750. [Google Scholar] [CrossRef]
- Ng, C.S.L.; Toh, T.C.; Chou, L.M. Coral restoration in Singapore’s sediment-challenged sea. Reg. Stud. Mar. Sci. 2016, 8, 422–429. [Google Scholar] [CrossRef]
- De’ath, G.; Fabricius, K.E.; Sweatman, H.; Puotinen, M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. USA 2012, 109, 17995–17999. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.R.; Baird, A.H.; Maynard, J.A.; Muttaqin, E.; Edwards, A.J.; Campbell, S.J.; Yewdall, K.; Affendi, Y.A.; Chou, L.M. Contrasting Patterns of Coral Bleaching Susceptibility in 2010 Suggest an Adaptive Response to Thermal Stress. PLoS ONE 2012, 7, e33353. [Google Scholar] [CrossRef] [PubMed]
- Tun, K.; Chou, L.M.; Low, J.; Yeemin, T.; Phongsuwan, N.; Setiasih, N.; Wilson, J.; Affendi, Y.A.; Kee Alfian, A.A.; Lane, D.; et al. A regional overview on the 2010 coral bleaching event in Southeast Asia. Status Coral Reefs East Asian Seas Reg. 2010, 2010, 9–27. [Google Scholar]
- Australian Bureau of Statistics, Australian Government. 2016 Census Priv. Policy. 2020. Available online: https://quickstats.censusdata.abs.gov.au/census_services/getproduct/census/2016/quickstat/3016?opendocument (accessed on 21 September 2020).
- Cheal, A.J.; MacNeil, M.A.; Emslie, M.J.; Sweatman, H. The threat to coral reefs from more intense cyclones under climate change. Glob. Chang. Biol. 2017, 23, 1511–1524. [Google Scholar] [CrossRef]
- Smithers, S.G.; Hopley, D.; Parnell, K.E. Fringing and Nearshore Coral Reefs of the Great Barrier Reef: Episodic Holocene. Source J. Coast. Res. 2006, 22, 175–187. [Google Scholar] [CrossRef]
- Anthony, K.R.N. Enhanced particle-feeding capacity of corals on turbid reefs (Great Barrier Reef, Australia). Coral Reefs 2000, 19, 59–67. [Google Scholar] [CrossRef]
- Browne, N.K.; Tay, J.; Todd, P.A. Recreating pulsed turbidity events to determine coral-sediment thresholds for active management. J. Exp. Mar. Bio. Ecol. 2015, 466, 98–109. [Google Scholar] [CrossRef]
- Chow, G.S.E.; Chan, Y.K.S.; Jain, S.; Huang, D. Light limitation selects for depth generalists in urbanised reef coral communities. Mar. Environ. Res. 2019, 147, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Van Maren, D.S.; Liew, S.C.; Hasan, G.M.J.J.; Sebastiaan Van Maren, D.; Liew, S.C.; Hasan, G.M.J.J. The role of terrestrial sediment on turbidity near Singapores coral reefs. Cont. Shelf Res. 2014, 76, 75–88. [Google Scholar] [CrossRef]
- Browne, N.K. Spatial and temporal variations in coral growth on an inshore turbid reef subjected to multiple disturbances. Mar. Environ. Res. 2012, 77, 71–83. [Google Scholar] [CrossRef]
- Birrell, C.L.; McCook, L.J.; Willis, B.L. Effects of algal turfs and sediment on coral settlement. Mar. Pollut. Bull. 2005, 51, 408–414. [Google Scholar] [CrossRef]
- Ricardo, G.F.; Jones, R.J.; Negri, A.P.; Stocker, R. That sinking feeling: Suspended sediments can prevent the ascent of coral egg bundles. Sci. Rep. 2016, 6, 21567. [Google Scholar] [CrossRef] [PubMed]
- Perez, K.; Rodgers, K.S.; Jokiel, P.L.; Lager, C.V.; Lager, D.J. Effects of terrigenous sediment on settlement and survival of the reef coral Pocillopora damicornis. PeerJ 2014, 2, e387. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Connolly, S.R.; Hoegh-Guldberg, O. Bleaching, energetics, and coral mortality risk: Effects of temperature, light, and sediment regime. Limnol. Oceanogr. 2007, 52, 716–726. [Google Scholar] [CrossRef]
- Welle, P.D.; Small, M.J.; Doney, S.C.; Azevedo, I.L. Estimating the effect of multiple environmental stressors on coral bleaching and mortality. PLoS ONE 2017, 12, e0175018. [Google Scholar] [CrossRef] [PubMed]
- Ogston, A.S.; Field, M.E. Predictions of Turbidity Due to Enhanced Sediment Resuspension Resulting from Sea-Level Rise on a Fringing Coral Reef: Evidence from Molokai, Hawaii. J. Coast. Res. 2010, 26, 1027–1037. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Poloczanska, E.S.; Skirving, W.; Dove, S. Coral reef ecosystems under climate change and ocean acidification. Front. Mar. Sci. 2017, 4, 158. [Google Scholar] [CrossRef]
- Oppenheimer, M.; Glavovic, B.C.; Hinkel, J.; van de Wal, R.; Magnan, A.K.; Abd-Elgawad, A.; Cai, R.; Cifuentes-Jara, M.; DeConto, R.M.; Ghosh, T.; et al. Sea Level Rise and Implications for Low Lying Islands, Coasts and Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Guinotte, J.M.; Buddemeier, A.R.W.; Kleypas, A.J.A. Future coral reef habitat marginality: Temporal and spatial effects of climate change in the Pacific basin. Coral Reefs 2003, 22, 551–558. [Google Scholar] [CrossRef]
- Tomascik, T.; Sander, F. Effects of eutrophication on reef-building corals—II. Structure of scleractinian coral communities on fringing reefs, Barbados, West Indies. Mar. Biol. 1987, 94, 53–75. [Google Scholar] [CrossRef]
- Loya, Y. The coral reefs of Eilat—Past, present and future: Three decades of coral community structure studies. Coral Health Dis. 2004, 1–34. [Google Scholar] [CrossRef]
- Wittenberg, M.; Hunte, W. Effects of eutrophication and sedimentation on juvenile corals. Mar. Biol. 1992, 138, 131–138. [Google Scholar] [CrossRef]
- Duprey, N.N.; Yasuhara, M.; Baker, D.M. Reefs of tomorrow: Eutrophication reduces coral biodiversity in an urbanized seascape. Glob. Chang. Biol. 2016, 22, 3550–3565. [Google Scholar] [CrossRef] [PubMed]
- Hennige, S.; Smith, D.; Perkins, R.; Consalvey, M.; Paterson, D.; Suggett, D. Photoacclimation, growth and distribution of massive coral species in clear and turbid waters. Mar. Ecol. Prog. Ser. 2008, 369, 77–88. [Google Scholar] [CrossRef]
- Marshall, S.M.; Orr, A.P. Sedimantation on Low Isles Reef and its relation to coral growth. Sci. Rep. 1931, 1, 93–133. [Google Scholar]
- Stafford-Smith, M.G. Sediment-rejection efficiency of 22 species of Australian scleractinian corals. Mar. Biol. 1993, 115, 229–243. [Google Scholar] [CrossRef]
- Browne, N.K.; Precht, E.; Last, K.S.; Todd, P.A. Photo-physiological costs associated with acute sediment stress events in three near-shore turbid water corals. Mar. Ecol. Prog. Ser. 2014, 502, 129–143. [Google Scholar] [CrossRef]
- Sofonia, J.J.; Anthony, K.R.N. High-sediment tolerance in the reef coral Turbinaria mesenterina from the inner Great Barrier Reef lagoon (Australia). Estuar. Coast. Shelf Sci. 2008, 78, 748–752. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Hoogenboom, M.O.; Connolly, S.R. Adaptive variation in coral geometry and the optimization of internal colony light climates. Funct. Ecol. 2005, 19, 17–26. [Google Scholar] [CrossRef]
- Tisthammer, K.H.; Timmins-Schiffman, E.; Seneca, F.O.; Nunn, B.L.; Richmond, R.H. Physiological and molecular responses suggest local adaptation of the lobe coral Porites lobata to the nearshore environment. bioRxiv 2019, 786673. [Google Scholar] [CrossRef]
- Tisthammer, K.H.; Timmins-Schiffman, E.; Seneca, F.O.; Nunn, B.L.; Richmond, R.H. Physiological and molecular responses of lobe coral indicate nearshore adaptations to anthropogenic stressors. Sci. Rep. 2021, 11, 3423. [Google Scholar] [CrossRef]
- Oelsner, G.P.; Stets, E.G. Recent trends in nutrient and sediment loading to coastal areas of the conterminous U.S.: Insights and global context. Sci. Total Environ. 2019, 654, 1225–1240. [Google Scholar] [CrossRef]
- Fong, C.R.; Gaynus, C.J.; Carpenter, R.C. Extreme rainfall events pulse substantial nutrients and sediments from terrestrial to nearshore coastal communities: A case study from French Polynesia. Sci. Rep. 2020, 10, 2955. [Google Scholar] [CrossRef] [PubMed]
- Stanley, G.D. The evolution of modern corals and their early history. Earth Sci. Rev. 2003, 60, 195–225. [Google Scholar] [CrossRef]
- Stat, M.; Carter, D.; Hoegh-Guldberg, O. The evolutionary history of Symbiodinium and scleractinian hosts-Symbiosis, diversity, and the effect of climate change. Perspect. Plant Ecol. Evol. Syst. 2006, 8, 23–43. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Gattuso, J.P.; Dallot, S.; Jaubert, J. Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 2000, 19, 103–113. [Google Scholar] [CrossRef]
- D’Angelo, C.; Wiedenmann, J. Impacts of nutrient enrichment on coral reefs: New perspectives and implications for coastal management and reef survival. Curr. Opin. Environ. Sustain. 2014, 7, 82–93. [Google Scholar] [CrossRef]
- Koop, K.; Booth, D.; Broadbent, A.; Brodie, J.; Bucher, D.; Capone, D.; Coll, J.; Dennison, W.; Erdmann, M.; Harrison, P.; et al. ENCORE: The effect of nutrient enrichment on coral reefs. Synthesis of results and conclusions. Mar. Pollut. Bull. 2001, 42, 91–120. [Google Scholar] [CrossRef]
- DeCarlo, T.M.; Gajdzik, L.; Ellis, J.; Coker, D.J.; Roberts, M.B.; Hammerman, N.M.; Pandolfi, J.M.; Monroe, A.A.; Berumen, M.L. Nutrient-supplying ocean currents modulate coral bleaching susceptibility. Sci. Adv. 2020, 6, eabc5493. [Google Scholar] [CrossRef]
- Wiedenmann, J.; D’Angelo, C.; Smith, E.G.; Hunt, A.N.; Legiret, F.E.; Postle, A.D.; Achterberg, E.P. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Chang. 2013, 3, 160–164. [Google Scholar] [CrossRef]
- Burkepile, D.E.; Shantz, A.A.; Adam, T.C.; Munsterman, K.S.; Speare, K.E.; Ladd, M.C.; Rice, M.M.; Ezzat, L.; McIlroy, S.; Wong, J.C.Y.; et al. Nitrogen Identity Drives Differential Impacts of Nutrients on Coral Bleaching and Mortality. Ecosystems 2020, 23, 798–811. [Google Scholar] [CrossRef]
- Sawall, Y.; Teichberg, M.C.; Seemann, J.; Litaay, M.; Jompa, J.; Richter, C. Nutritional status and metabolism of the coral Stylophora subseriata along a eutrophication gradient in Spermonde Archipelago (Indonesia). Coral Reefs 2011, 30, 841–853. [Google Scholar] [CrossRef]
- Costa, O.S.; Leão, Z.M.A.N.; Nimmo, M.; Attrill, M.J. Nutrification impacts on coral reefs from northern Bahia, Brazil. Island Ocean Deep. Biol. 2000, 440, 307–315. [Google Scholar] [CrossRef]
- Vega Thurber, R.L.; Burkepile, D.E.; Fuchs, C.; Shantz, A.A.; Mcminds, R.; Zaneveld, J.R. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob. Chang. Biol. 2014, 20, 544–554. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Cooper, T.F.; Humphrey, C.; Uthicke, S.; De’ath, G.; Davidson, J.; LeGrand, H.; Thompson, A.; Schaffelke, B. A bioindicator system for water quality on inshore coral reefs of the Great Barrier Reef. Mar. Pollut. Bull. 2012, 65, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Babcock, R.; Smith, L. Effects of sedimentation on coral settlement and survivorship. Mar. Biol. 2000, I, 1–4. [Google Scholar]
- Houlbrèque, F.; Ferrier-Pagès, C. Heterotrophy in tropical scleractinian corals. Biol. Rev. 2009, 84, 1–17. [Google Scholar] [CrossRef]
- Moynihan, M.A.; Martin, P.; Morgan, K.; Baker, D.M.; Goodkin, N. In situ measurements of coral-associated nitrogen fixation from turbid reefs. In Proceedings of the Fall Meeting 2018, Washington, DC, USA, 10–14 December 2018. [Google Scholar]
- Smith, E.G.; Gurskaya, A.; Hume, B.C.C.; Voolstra, C.R.; Todd, P.A.; Bauman, A.G.; Burt, J.A. Low Symbiodiniaceae diversity in a turbid marginal reef environment. Coral Reefs 2020, 39, 545–553. [Google Scholar] [CrossRef]
- Lough, J.M.; Barnes, D.J. Comparisons of skeletal density variations in Porites from the central Great Barrier Reef. J. Exp. Mar. Bio. Ecol. 1992, 155, 1–25. [Google Scholar] [CrossRef]
- Ng, C.S.L.; Lim, J.X.; Sam, S.Q.; Kikuzawa, Y.P.; Toh, T.C.; Wee, T.W.; Sim, W.T.; Ng, N.K.; Huang, D.; Chou, L.M. Variability in skeletal bulk densities of common hard corals in Southeast Asia. Coral Reefs 2019, 38, 1133–1143. [Google Scholar] [CrossRef]
- Risk, M.J.; Sammarco, P.W. Cross-shelf trends in skeletal density of the massive coral Porites lobata from the Great Barrier Reef. Mar. Ecol. Prog. Ser. 1991, 69, 195–200. [Google Scholar] [CrossRef]
- Fabricius, K.E.; De’ath, G.; Puotinen, M.L.; Done, T.; Cooper, T.F.; Burgess, S.C. Disturbance gradients on inshore and offshore coral reefs caused by a severe tropical cyclone. Limnol. Oceanogr. 2008, 53, 690–704. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Mollica, N.R.; Guo, W.; Cohen, A.L.; Huang, K.F.; Foster, G.L.; Donald, H.K.; Solow, A.R. Ocean acidification affects coral growth by reducing skeletal density. Proc. Natl. Acad. Sci. USA 2018, 115, 1754–1759. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Effects of suspensoids (turbidity) on penetration of solar radiation in aquatic ecosystems. Perspect. South. Hemisph. Limnol. 1985, 195–208. [Google Scholar] [CrossRef]
- Nyström, M.; Folke, C. Spatial resilience of coral reefs. Ecosystems 2001, 4, 406–417. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef] [PubMed]
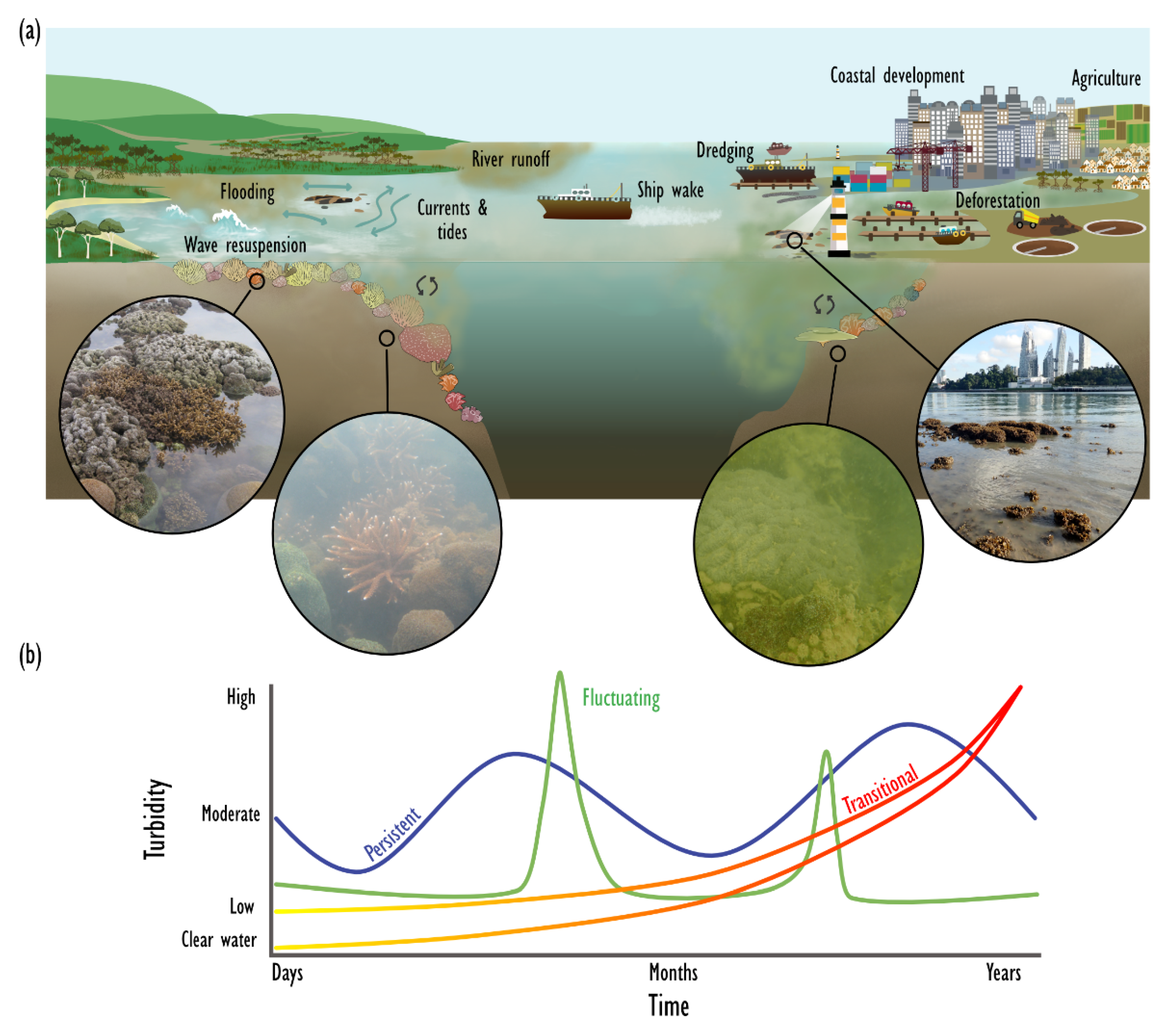
| Paluma Shoals Reef Complex | Singapore | ||
|---|---|---|---|
| Nearest urban development | Townsville ~30 km, 195,084 people (in 2020) [145] | Singapore < 6 km, 5.69 million (in 2020) [133] | |
| Reef initiation period | 1700–1000 YBP [26,117,121] | No data available | |
| Stressors | Global | Cyclones, heat waves, crown-of-thorns starfish [14,142,146,147] | Heat waves |
| Local | N/A | Dredging, coastal development, ship traffic [138,143] | |
| Sea surface temperature (°C) | 25–28 [148] | 27–31 [134,149,150] | |
| Turbidity regime | Natural-persistent (wind-waves, tidal currents, river plums) [8,89,123] | Anthropogenic-transitional (dredging, coastal development) [41,131,132] | |
| Turbidity (NTU) | 15–50 [8,10,19,40] | 4.8–6.6 [149,151] | |
| Sedimentation rate (average) 1 | 60.5 g m2 d−1 [53] | 176 g m2 d−1 [149] | |
| Coral genera 2 | Montipora (50%), Acropora (15%), Turbinaria (12%), Porites (1.5%), Lobophyllia, Stylophora, Seriatopora, Pavona, Goniastrea, Favia, Favites Platygyra, Goniopora, Galaxea, Psammocora, Cyphastrea, Hydnophora, Symphyllia, Echinopora, Pachyseris, Alveopora, Fungia, Euphyllia [7] | Pectinia (11–19%), Pachyseris (7–14%), Merulina (6–12%), Montipora (7%), Porites (6%), Echinopora (4%), Platygyra (4%), Acropora, Pocillopora, Pavona, Goniastrea, Favia, Favites, Lobophyllia, Goniopora, Galaxea, Montastraea, Diploastrea, Cyphastrea, Hydnophora, Symphyllia, Echinophyllia, Oxypora, Leptoseris, Leptastrea, Fungia [87,137,150] | |
| Coral cover (average) | 38% [14,19] | 31% [113,140] | |
| Reef geomorphology | Fringing (inner-shelf, coastal reefs) and offshore patch reefs [10] | Fringing or patch reefs near the southern islands [31] | |
| Coral growth depth range | <6 m [123] | <6 m [62] | |
| Reef area | ~16 km2 [26] | ~9.5 km2 [131] | |
| Carbonate budget (CaCO3) | ~6.9 kg m2 year−1 [19] | ~3.7 kg m2 year−1 [39] | |
| Reef accretion potential (average, based on carbonate budget values) | 2.97 mm year−1 [19] | 1.55 mm year−1 [74] | |
| Threat | Resilience Attributes | Outstanding Questions |
|---|---|---|
| Increasing sediment loads | Sediment-tolerant corals (e.g., morphological adaptation, enhanced photo-acclimatization to low light, heterotrophic feeding) | What are the molecular components that improve a coral’s ability to grow, adapt and acclimate to turbid conditions? |
| Higher energy hydrodynamic setting | Is there a threshold energy level that is more likely to support turbid reef growth and development? | |
| Eutrophication | Remote settings (e.g., >50 km from urban areas) | How do nutrient inputs influence coral growth and skeletogensis, and what are the consequences for longer-term reef development? How will bioerosion intensity change with increased eutrophication? |
| Effective conservation, management and regulation plan | What is the coral community threshold to nutrient input? What are the best ways to control nutrient flow into coastal catchments? | |
| Warming oceans | Persistent turbid reefs where corals have adapted to low light and where suspended sediments may reduce stress from UV radiation | What is the relationship between suspended sediment concentrations and reduced stress from UV (during bleaching events)? |
| A higher proportion of heterotrophic corals that can utilize this energy resource during bleaching events | By how much does heterotrophy extend the survival rate of bleached corals and improve recovery rates? | |
| Heat-tolerant symbionts | How do survival and recovery rates differ among different coral/symbiont clade associations? | |
| Storm severity | Higher skeletal density | To what extent does lower coral skeletal density influence mechanical damage during a storm event? |
| Massive and encrusting corals reef communities-dominated reef | How does the ratio of branching to encrusting to massive influence rates of coral dislodgement (with cyclone energy)? What has more influence on rates of coral dislodgement during storm events: coral community structure or substrate strength? | |
| Ocean acidification | Unknown | How do turbidity and/or sedimentation affect coral physiology under different OA scenarios? |
| Sea-level rise | Higher net carbonate production | What is the vertical growth potential (i.e., carbonate budgets) of present day turbid coral communities? |
| The reef structure is at/or close to sea level | What are the SLR projections for tropical coastal settings where most of the turbid reefs are located? | |
| Will corals be able to colonize algal/sediment substrates as accommodation space above reefs increase? | ||
| How will SLR change turbidity conditions and sedimentation on reefs? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zweifler, A.; O’Leary, M.; Morgan, K.; Browne, N.K. Turbid Coral Reefs: Past, Present and Future—A Review. Diversity 2021, 13, 251. https://doi.org/10.3390/d13060251
Zweifler A, O’Leary M, Morgan K, Browne NK. Turbid Coral Reefs: Past, Present and Future—A Review. Diversity. 2021; 13(6):251. https://doi.org/10.3390/d13060251
Chicago/Turabian StyleZweifler (Zvifler), Adi, Michael O’Leary, Kyle Morgan, and Nicola K. Browne. 2021. "Turbid Coral Reefs: Past, Present and Future—A Review" Diversity 13, no. 6: 251. https://doi.org/10.3390/d13060251
APA StyleZweifler, A., O’Leary, M., Morgan, K., & Browne, N. K. (2021). Turbid Coral Reefs: Past, Present and Future—A Review. Diversity, 13(6), 251. https://doi.org/10.3390/d13060251






