DNA Barcoding of Mullets (Family Mugilidae) from Pakistan Reveals Surprisingly High Number of Unknown Candidate Species
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Barcode Reference Library and Taxonomical Nomenclature
2.2. Sample Collection and Identification
2.3. DNA Amplification and Sequencing
2.4. DNA Barcode-Based Species Identification
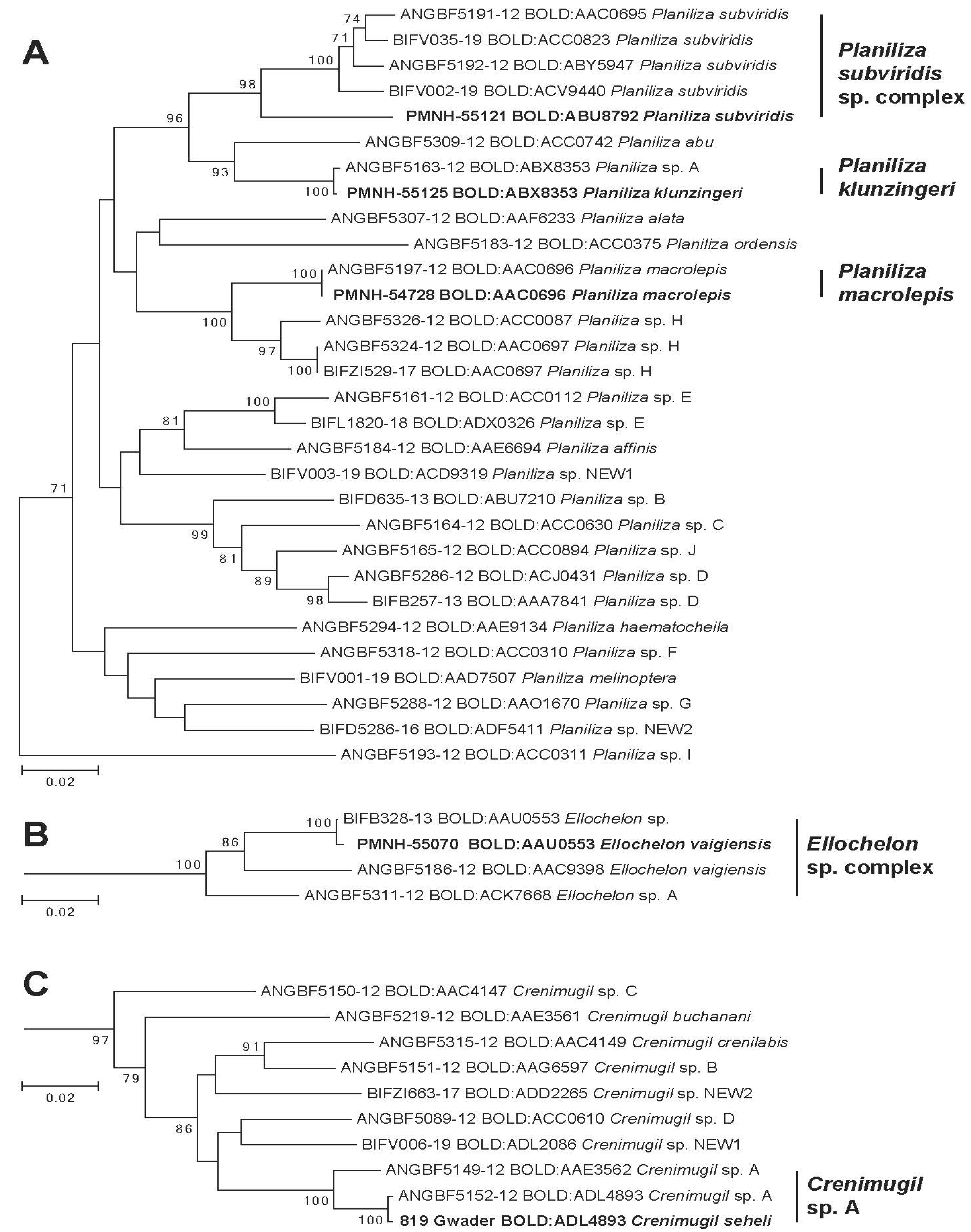
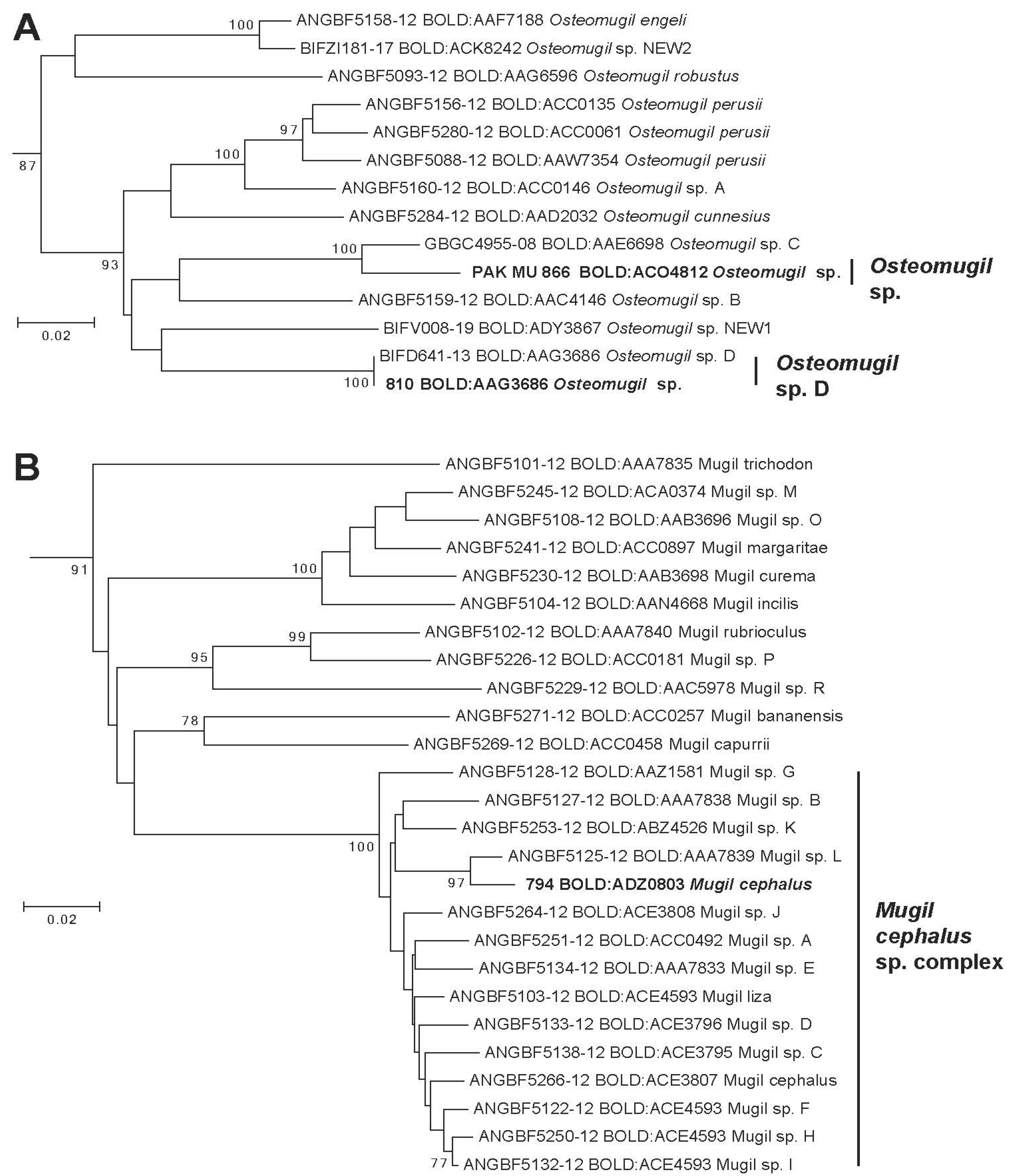
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hubert, N.; Meyer, C.P.; Bruggemann, H.J.; Guerin, F.; Komeno, R.J.; Espiau, B.; Causse, R.; Williams, J.T.; Planes, S. Cryptic diversity in Indo-Pacific coral-reef fishes revealed by DNA-barcoding provides new support to the centre-of-overlap hypothesis. PLoS ONE 2012, 7, e28987. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.D.; Borsa, P. Mitochondrial phylogeny of grey mullets (Acanthopterygii: Mugilidae) suggest high proportion of cryptic species. Comptes Rendus Biol. 2015, 338, 226–277. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.D.; Holmes, B.H.; Yearsley, G.K. DNA barcoding reveals a likely second species of Asian sea bass (barramundi) (Lates calcarifer). J. Fish Biol. 2008, 72, 458–463. [Google Scholar] [CrossRef]
- Ward, R.D.; Holmes, B.H.; White, W.T.; Last, P.R. DNA barcoding Australasian chondrichthyans: Results and potential uses in conservation. Mar. Freshw. Res. 2008, 59, 57–71. [Google Scholar] [CrossRef]
- Lara, A.; PONCE de LEÓN, J.L.; Rodriguez, R.; Casane, D.; Cote, G.; Bernatchez, L.; Garcíamachado, E.R.I.K. DNA barcoding of Cuban freshwater fishes: Evidence for cryptic species and taxonomic conflicts. Mol. Ecol. Resour. 2010, 10, 421–430. [Google Scholar] [CrossRef]
- Sriwattanarothai, N.; Steinke, D.; Ruenwongsa, P.; Hanner, R.; Panijpan, B. Molecular and morphological evidence supports the species status of the Mahachai fighter Betta sp. Mahachai and reveals new species of Betta from Thailand. J. Fish Biol. 2010, 77, 414–424. [Google Scholar] [CrossRef]
- Smith, K.L.; Harmon, L.J.; Shoo, L.P.; Melville, J. Evidence of constrained phenotypic evolution in a cryptic species complex of agamid lizards. Evolution 2011, 65, 976–992. [Google Scholar] [CrossRef]
- Puckridge, M.; Last, P.R.; White, W.T.; Andreakis, N. Phylogeography of the Indo West Pacific maskrays (Dasyatidae, Neotrygon): A complex example of chondrichthyan radiation in the Cenozoic. Ecol. Evol. 2013, 3, 217–232. [Google Scholar] [CrossRef]
- Borsa, P.; Arlyza, I.S.; Hoareau, T.B.; Shen, K.N. Diagnostic description and geographic distribution of four new cryptic species of the blue-spotted maskray species complex (Myliobatoidei: Dasyatidae; Neotrygon spp.) based on DNA sequences. J. Oceanol. Limnol. 2018, 36, 827–841. [Google Scholar] [CrossRef]
- Borsa, P.; Fauvelot, C.; Tiavouane, J.; Grulois, D.; Wabnitz, C.; Naguit, M.A. Andrefouet S Distribution of Noah’s giant clam, Tridacna noae. Mar. Biodivers. 2015, 45, 339–344. [Google Scholar] [CrossRef]
- Pante, E.; Puillandre, N.; Viricel, A.; Arnaud-Haond, S.; Aurelle, D.; Castelin, M.; Viard, F. Species are hypotheses: Avoid connectivity assessments based on pillars of sand. Mol. Ecol. 2015, 24, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Struck, T.H.; Cerca, J. Cryptic species and their evolutionary significance. Encycl. Life Sci. 2019, 1–9. [Google Scholar] [CrossRef]
- Fox, C.; Taylor, M.I.; Pereyra, R.; Rico, C. Mapping of the spawning grounds of Irish Sea gadoids using genetic identification of planktonic eggs. Mol. Ecol. 2005, 14, 879–884. [Google Scholar] [CrossRef]
- Hubert, N.; Hanner, R.; Holm, E.; Mandrak, N.E.; Taylor, E.; Burridge, M.; Zhang, J. Identifying Canadian freshwater fishes through DNA barcodes. PLoS ONE 2008, 3, e2490. [Google Scholar] [CrossRef] [PubMed]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.L.; Meier, R.; Winker, K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.R.; Underkoffler, K.E.; Sundberg, M.A. DNA barcoding provides support for a cryptic species complex within the globally distributed and fishery important opah (Lampris guttatus). Mol. Ecol. Resour. 2014, 14, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Froese, R.; Pauly, D. FishBase. 2021. Available online: www.fishbase.org (accessed on 5 March 2021).
- Whitfield, A.K.; Panfili, J.; Durand, J.D. A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Rev. Fish Biol. Fish. 2012, 22, 641–681. [Google Scholar] [CrossRef]
- Durand, J.D.; Hubert, N.; Shen, K.N.; Borsa, P. DNA barcoding grey mullets. Rev. Fish Biol. Fish. 2017, 27, 233–243. [Google Scholar] [CrossRef]
- Lee, S.C.; Chang, J.T.; Tsu, Y.Y. Genetic relationships of four Taiwan mullets (Pisces: Perciformes: Mugilidae). J. Fish Biol. 1995, 46, 159–162. [Google Scholar] [CrossRef]
- Papasotiropoulos, V.; Klossa-Kilia, E.; Kilias, G.; Alahiotis, S. Genetic divergence and phylogenetic relationships in grey mullets (Teleostei: Mugilidae) using allozyme data. Biochem. Genet. 2001, 39, 155–168. [Google Scholar] [CrossRef]
- Papasotiropoulos, V.; Klossa-Kilia, E.; Alahiotis, S.N.; Kilias, G. Molecular phylogeny of grey mullets (Teleostei: Mugilidae) in Greece: Evidence from sequence analysis of mtDNA segments. Biochem. Genet. 2007, 45, 623. [Google Scholar] [CrossRef]
- Turan, C.; Caliskan, M.; Kucuktas, H. Phylogenetic relationships of nine mullet species (Mugilidae) in the Mediterranean Sea. Hydrobiologia 2005, 532, 45–51. [Google Scholar] [CrossRef]
- Fraga, E.; Schneider, H.; Nirchio, M.; Santa-Brigida, E.; Rodrigues-Filho, L.F.; Sampaio, I. Molecular phylogenetic analyses of mullets (Mugilidae, Mugiliformes) based on two mitochondrial genes. J. Appl. Ichthyol. 2007, 23, 598–604. [Google Scholar] [CrossRef]
- Erguden, D.; Gurlek, M.; Yaglioglu, D.; Turan, C. Genetic Identification and Taxonomic Relationship of Mediterranean Mugilid Species Based on Mitochondrial 168 rDNA Sequence Data. J. Anim. Vet. Adv. 2010, 9, 336–341. [Google Scholar]
- Liu, J.Y.; Brown, C.L.; Yang, T.B. Phylogenetic relationships of mullets (Mugilidae) in China Seas based on partial sequences of two mitochondrial genes. Biochem. Syst. Ecol. 2010, 38, 647–655. [Google Scholar] [CrossRef]
- Shen, K.N.; Jamandre, B.W.; Hsu, C.C.; Tzeng, W.N.; Durand, J.D. Plio Pleistocene sea level and temperature fluctuations in the northwestern Pacific promoted speciation in the globally distributed flathead mullet Mugil cephalus. BMC Evol. Biol. 2011, 11, 1–17. [Google Scholar]
- Durand, J.D.; Shen, K.N.; Chen, W.J.; Jamandre, B.W.; Blel, H.; Diop, K.; Borsa, P. Systematics of the grey mullets (Teleostei: Mugiliformes: Mugilidae): Molecular phylogenetic evidence challenges two centuries of morphology-based taxonomy. Mol. Phylogenet. Evol. 2012, 64, 73–92. [Google Scholar] [CrossRef]
- Shen, K.; Durand, J.D. The biogeography of Mugilidae in India, South-East and East Asia. In Biology, Ecology and Culture of Grey Mullets (Mugilidae); Crosetti, D., Blaber, S., Eds.; CRC Press: Boca Racon, FL, USA, 2016; pp. 63–84. [Google Scholar] [CrossRef]
- Xia, R.; Durand, J.D.; Fu, C. Multilocus resolution of Mugilidae phylogeny (Teleostei: Mugiliformes) implications for the family’s taxonomy. Mol. Phylogenet. Evol. 2016, 96, 161–177. [Google Scholar] [CrossRef]
- Delrieu-Trottin, E.; Durand, J.D.; Limmon, G.; Sukmono, T.; Sugeha, H.Y.; Chen, W.J.; Fitriana, Y. Biodiversity inventory of the grey mullets (Actinopterygii:Mugilidae) of the Indo Australian Archipelago through the iterative use of DNA based species delimitation and specimen assignment methods. Evol. Appl. 2020, 13, 1451–1467. [Google Scholar] [CrossRef]
- Neves, J.M.; Almeida, J.P.; Sturaro, M.J.; FabrÉ, N.N.; Pereira, R.J.; Mott, T. Deep genetic divergence and paraphyly in cryptic species of Mugil fishes (Actinopterygii: Mugilidae). Syst. Biodivers. 2020, 18, 116–128. [Google Scholar] [CrossRef]
- Briggs, J.C. Fishes of worldwide (circumtropical) distribution. Copeia 1960, 3, 171–180. [Google Scholar] [CrossRef]
- Autem, M.; Bonhomme, F. Eléments de systématique biochimique chez les Mugilidés de Méditerranée. Biochem. Syst. Ecol. 1980, 8, 305–308. [Google Scholar] [CrossRef]
- Caldara, F.; Bargelloni, L.; Ostellari, L.; Penzo, E.; Colombo, L.; Patarnello, T. Molecular phylogeny of grey mullets based on mitochondrial DNA sequence analysis: Evidence of a differential rate of evolution at the intrafamily level. Mol. Phylogenet. Evol. 1996, 6, 416–424. [Google Scholar] [CrossRef]
- Rossi, A.R.; Capula, M.; Crosetti, D.; Campton, D.E.; Sola, L. Genetic divergence and phylogenetic inferences in five species of Mugilidae (Pisces: Perciformes). Mar. Biol. 1998, 131, 213–218. [Google Scholar] [CrossRef]
- Rossi, A.R.; Ungaro, A.; De Innocentiis, S.; Crosetti, D.; Sola, L. Phylogenetic analysis of Mediterranean Mugilids by allozymes and 16S mt-rRNA genes investigation: Are the Mediterranean species of Liza monophyletic? Biochem. Genet. 2004, 42, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Murgia, R.; Tola, G.; Archer, S.N.; Vallerga, S.; Hirano, J. Genetic identification of grey mullet species (Mugilidae) by analysis of mitochondrial DNA sequence: Application to identify the origin of processed ovary products (bottarga). Mar. Biotechnol. 2002, 4, 119–126. [Google Scholar] [CrossRef]
- Gornungm, E.; Colangelo, P.; Annesi, F. 5S ribosomal RNA genes in six species of Mediterranean grey mullets: Genomic organization and phylogenetic inference. Genome 2007, 50, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Imsiridou, A.; Minos, G.; Katsares, V.; Karaiskou, N.; Tsiora, A. Genetic identification and phylogenetic inferences in different Mugilidae species using 5S rDNA markers. Aquac. Res. 2007, 38, 1370–1379. [Google Scholar] [CrossRef]
- Semina, A.V.; Polyakova, N.E.; Makhotkin, M.A.; Brykov, V.A. Mitochondrial DNA divergence and phylogenetic relationships in mullets (Pisces: Mugilidae) of the Sea of Japan and the Sea of Azov revealed by PCR-RFLP-analysis. Russ. J. Mar. Biol. 2007, 33, 187–192. [Google Scholar] [CrossRef]
- Blel, H.; Chatti, N.; Besbes, R.; Farjallah, S.; Elouaer, A.; Guerbej, H.; Said, K. Phylogenetic relationships in grey mullets (Mugilidae) in a Tunisian lagoon. Aquac. Res. 2008, 39, 268–275. [Google Scholar] [CrossRef]
- Durand, J.D.; Whitfield, A.K. Biogeography and Distribution of Mugilidae in the Western, Central and Southern Regions of Africa. In Biology, Ecology and Culture of Grey Mullets (Mugilidae); Crosetti, D., Blaber, S., Eds.; CRC Press: Boca Racon, FL, USA, 2016; pp. 102–115. [Google Scholar] [CrossRef]
- Menezes, N.A.; Nirchio, M.; Oliveira, C.D.; Siccharamirez, R. Taxonomic review of the species of Mugil (Teleostei: Perciformes: Mugilidae) from the Atlantic South Caribbean and South America, with integration of morphological, cytogenetic and molecular data. Zootaxa 2015, 3918, 1–38. [Google Scholar] [CrossRef]
- Menezes, M.R.; Martins, M.; Naik, S. Interspecific genetic divergence in grey mullets from the Goa region. Aquaculture 1992, 105, 117–129. [Google Scholar] [CrossRef]
- Rahman, M.A.U.; Khan, S.A.; Lyla, P.S.; Kumar, C.P. DNA barcoding resolves taxonomic ambiguity in mugilidae of Parangipettai waters (Southeast Coast of India). Turk. J. Fish. Aquat. Sci. 2013, 13, 321–330. [Google Scholar]
- Qureshi, M.R. Marine Fishes of Karachi and the Coast of Sindh and Makran; Government of Pakistan Ministry of Food Agriculture; Government of Pakistan Press: Karachi, Pakistan, 1955; p. 80.
- Bianchi, G. Field Guide to the Commercial Marine and Brackish-Water Species of Pakistan; FAO species identification sheets for fishery purposes; prepared with the support of PAK/77/033 and FAO (FIRM) Regular Programme; FAO: Italy, Rome, 1985; p. 200. [Google Scholar]
- Fahmida, I. Mullets of Korangi Creek Karachi. Zool. Surv. Rec. Pak. 2002, 14, 11–18. [Google Scholar]
- Froese, R.; Pauly, D. Fish Base. World Wide Web Electronic Publication. 2010. Available online: www.fishbase.org (accessed on 10 September 2010).
- Psomadakis, P.N.; Osmany, H.B.; Moazzam, M. Field Identification Guide to the Living Marine Resources of Pakistan; FAO Species Identification Guide for Fishery Purposes; FAO: Italy, Rome, 2015; 386p. [Google Scholar]
- Durand, J.D.; Chen, W.J.; Shen, K.N.; Borsa, P. Genus-level taxonomic changes imposed by the mitochondrial phylogene.y of grey mullets (Teleostei: Mugilidae). Comptes Rendus Biol. 2012, 335, 687–697. [Google Scholar] [CrossRef]
- Thomson, J.M. The Mugilidae of the world. Mem. Qld. Mus. 1997, 41, 457–562. [Google Scholar]
- Ghasemzadeh, J. Phylogeny and Systematics of Indo-Pacific Mullets (Teleostei: Mugilidae) with Special Reference to the Mullets of Australia. Ph.D. Thesis, Macquarie University, Sydney, Australia, 1998. [Google Scholar]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.A.X.; Robertson, J. Marine eco regions of the world: A bio regionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D. DNA barcoding of Australia’s fish species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Senou, H.; Yoshino, T.; Okiyama, M. A review of the mullets with a keel on the back, Liza carinata complex (Pisces: Mugilidae). Publ. Seto Mar. Biol. Lab. 1987, 32, 303–321. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Panhwar, S.K.; Gao, T.; Han, Z.; Li, C.; Sun, D.; Song, N. New genetic evidence from three keel-backed liza species based on DNA Barcoding confirms morphology-based identification. Pak. J. Zool. 2017, 49, 1901–1907. [Google Scholar]
- Khedkar, G.D.; Jamdade, R.; Naik, S.; David, L.; Haymer, D. DNA barcodes for the fishes of the Narmada, one of India’s longest rivers. PLoS ONE 2014, 9, e101460. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.D. Implications of Molecular Phylogeny for the Taxonomy of Mugilidae. In Biology, Ecology and Culture of Grey Mullets (Mugilidae); Crosetti, D., Blaber, S., Eds.; CRC Press: Boca Racon, FL, USA, 2016; pp. 22–41. [Google Scholar] [CrossRef]
- Viet Tran, T.T.; Ke Phan, L.; Durand, J.D. Diversity and distribution of cryptic species within the Mugil cephalus species complex in Vietnam. Mitochondrial DNA Part A 2017, 28, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.N.; Chang, C.W.; Durand, J.D. Spawning segregation and philopatry are major prezygotic barriers in sympatric cryptic Mugil cephalus species. Comptes Rendus Biol. 2015, 338, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Knebelsberger, T.; Landi, M.; Neumann, H.; Kloppmann, M.; Sell, A.F.; Campbell, P.D.; Laakmann, S.; Raupach, M.J.; Carvalho, G.R.; Costa, F.O. A reliable DNA barcode reference library for the identification of the North European shelf fish fauna. Mol. Ecol. Resour. 2014, 14, 1060–1107. [Google Scholar] [CrossRef]
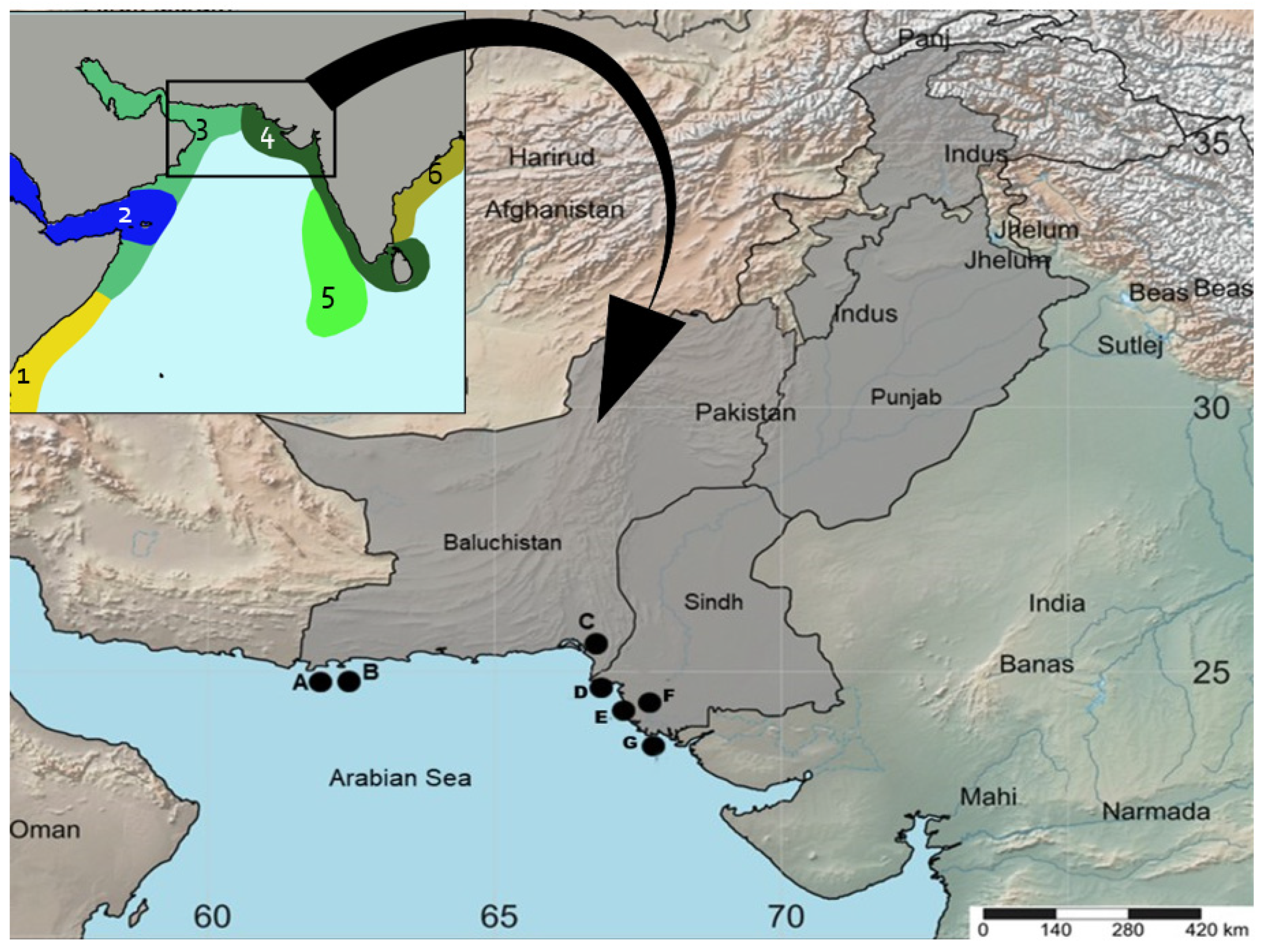
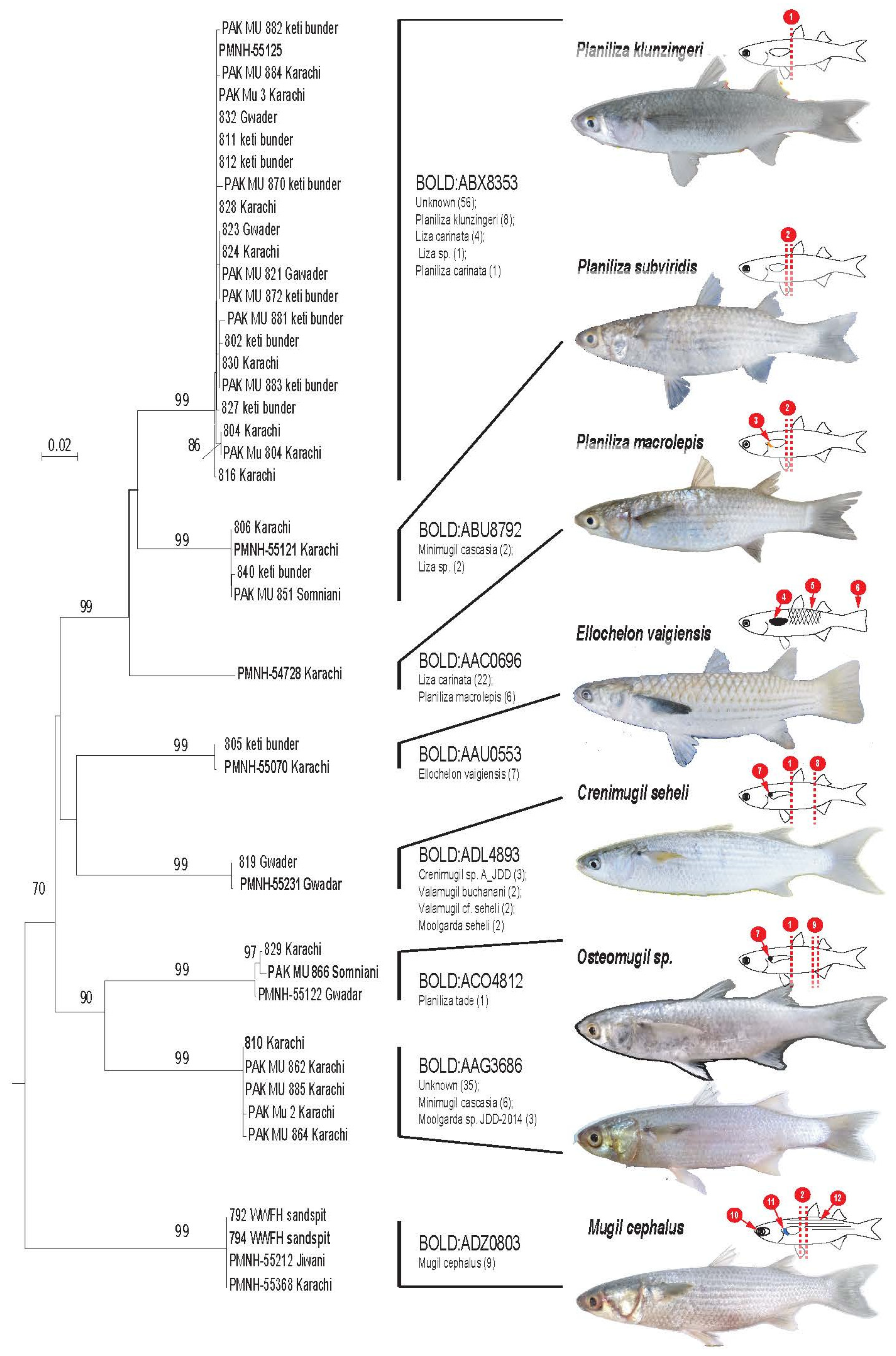
| Morpho Species | Code No. | Location | Co-Ordinates | Accession No. |
|---|---|---|---|---|
| Mugil cephalus | 792 | Kakapir, Karachi | 24°50′42″ N 66°54′01″ E | MT943713 |
| 794 | Kakapir, Karachi | 2450′42″ N 66°54′01″ E | MT943714 | |
| PMNH-55212 | Jiwani, Baluchistan | 25°10′59″ N 61°46′24″ E | MN511974 | |
| PMNH-55368 | Gwadar, Baluchistan | 24°06′53″ N 62°19′41″ E | MN511975 | |
| Planiliza macrolepis | PMNH-54728 | Ibrahim Haideri, Karachi | 24°47′39″ N 67°08′31″ E | MN512028 |
| Planiliza subviridis | PAK Mu 851 | Somniani, Baluchistan | 25°09′25” N 66°43′25” E | MT943724 |
| 806 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943723 | |
| 840 | Keti Bunder, Sindh | 24°07′49″ N 67°27′10″ E | MT943722 | |
| PMNH-55121 | Ibrahim Haideri, Karachi | 24°47′39″ N 67°08′31″ E | MN511966 | |
| Planiliza klunzingeri | PAK Mu 884 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943743 |
| PAK Mu 804 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943734 | |
| 824 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943727 | |
| 816 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943737 | |
| 830 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943735 | |
| 828 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943736 | |
| PaK Mu 3 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943740 | |
| PAK Mu 872 | Keti Bunder, Sindh | 24°07′49″ N 67°27′10″ E | MT943730 | |
| PAK Mu 870 | Keti Bunder, Sindh | 24°07′49″ N 67°27′10″ E | MT943731 | |
| PAK Mu 881 | Keti Bunder, Sindh | 24°07′49″ N 67°27′10″ E | MT943729 | |
| PAK Mu 882 | Keti Bunder, Sindh | 24°07′49″ N 67°27′10″ E | MT943728 | |
| PAK Mu 883 | Keti Bunder, Sindh | 24°07′49″ N 67°27′10″ E | MT943744 | |
| 802 | Keti Bunder, Sindh | 24°07′49″ N 67°27′10″ E | MT943742 | |
| 827 | Keti Bunder, Sindh | 24°07′49″ N 67°27′10″ E | MT943726 | |
| 811 | Keti Bunder, Sindh | 24°07′49″ N 67°27′10″ E | MT943739 | |
| 812 | Keti Bunder, Sindh | 24°07′49″ N 67°27′10″ E | MT943738 | |
| 832 | Gwadar, Baluchistan | 25°06′53″ N 62°19′41″ E | MT943725 | |
| PAK Mu 821 | Gwadar, Baluchistan | 25°06′53″ N 62°19′41″ E | MT943733 | |
| 823 | Gwadar, Baluchistan | 25°06′53″ N 62°19′41″ E | MT943732 | |
| PMNH 55125 | Gwadar, Baluchistan | 25°06′53″ N 62°19′41″ E | MN12027 | |
| Crenimugil seheli | 819 | Gwadar, Baluchistan | 25°06′53″ N 62°19′41″ E | MT943705 |
| PMNH-55231 | Gwadar, Baluchistan | 25°06′53″ N 62°19′41″ E | MN512029 | |
| Osteomugil sp. | 829 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943716 |
| PAK Mu 866 | Somniani, Baluchistan | 25°09′25” N 66°43′25” E | MT943715 | |
| PMNH-55122 | Gwadar, Baluchistan | 25°06′53″ N 62°19′41″ E | MN512031 | |
| 810 | Karachi Fish harbor | 24°50′57″ N 67°27′10″ E | MT943717 | |
| PAK Mu 864 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943718 | |
| PAK Mu 862 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943719 | |
| PAK Mu 2 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943721 | |
| PAK MU 885 | Karachi Fish harbor | 24°50′57″ N 66°58′35″ E | MT943720 | |
| Ellochelon vaigiensis | 805 | Keti Bunder, Sindh | 24°07′49″ N 67°27′10″ E | MT943712 |
| PMNH-55070 | French beach, Karachi | 24°50′32″ N 66°48′53″ E | MN511887 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, A.; Siddiqui, P.J.A.; Amir, S.A.; Durand, J.-D. DNA Barcoding of Mullets (Family Mugilidae) from Pakistan Reveals Surprisingly High Number of Unknown Candidate Species. Diversity 2021, 13, 232. https://doi.org/10.3390/d13060232
Hasan A, Siddiqui PJA, Amir SA, Durand J-D. DNA Barcoding of Mullets (Family Mugilidae) from Pakistan Reveals Surprisingly High Number of Unknown Candidate Species. Diversity. 2021; 13(6):232. https://doi.org/10.3390/d13060232
Chicago/Turabian StyleHasan, Ariba, Pirzada Jamal Ahmed Siddiqui, Shabir Ali Amir, and Jean-Dominique Durand. 2021. "DNA Barcoding of Mullets (Family Mugilidae) from Pakistan Reveals Surprisingly High Number of Unknown Candidate Species" Diversity 13, no. 6: 232. https://doi.org/10.3390/d13060232
APA StyleHasan, A., Siddiqui, P. J. A., Amir, S. A., & Durand, J.-D. (2021). DNA Barcoding of Mullets (Family Mugilidae) from Pakistan Reveals Surprisingly High Number of Unknown Candidate Species. Diversity, 13(6), 232. https://doi.org/10.3390/d13060232







