Abstract
The East Asian marginal seas are among the most productive fisheries grounds. However, in recent decades they experienced massive proliferations of jellyfish that pose vast challenges for the management of harvested fish stocks. In the Korean Peninsula, the common bloom-formers Scyphozoan species Aurelia coerulea and Nemopilema nomurai are of major concern due to their detrimental effects on coastal socio-ecological systems. Here, we used pluriannual field observations spanning over 14 years to test the extent of climate influence on the interannual variability and bloom dynamics of A. coerulea and N. nomurai. To depict climate-jellyfish interactions we assessed partitioning effects, direct/indirect links, and the relative importance of hydroclimate forces on the variability of these species. We show that jellyfish interannual patterns and bloom dynamics are shaped by forces playing out at disparate scales. While abundance changes and earlier blooms of A. coerulea were driven by local environmental conditions, N. nomurai interannual patterns and bloom dynamics were linked with regional climate processes. Our results provide a synoptic picture of cascading effects from large scale climate to jellyfish dynamics in the Korean Peninsula that may affect fisheries sustainability due to the prominent detrimental impact these species have in the region.
1. Introduction
In the marine realm, the physical environment, energy pathways, and biogeochemical fluxes are shaped by the climate influence on water column structure and nutrient dynamics, which heavily impact plankton communities [1]. By virtue of their trophic position, modifications in the structure or biomass of plankton permeate the entire food web, affecting trophic cycling and fish recruitment [2,3]. Hence, the pivotal role played by plankton in food web stability makes these organisms fundamental indicators of the ecosystem state. In the last decades, however, global anthropogenic changes have altered marine ecosystem structure and functioning [4], raising risks to ecosystem services and human welfare.
The East Asian marginal seas are exposed to the influence of hemispheric-wide climate phenomena, i.e., Pacific Decadal Oscillation (PDO), El Niño Southern Oscillation (ENSO), and East Asia winter monsoon (EAWM). These climate phenomena mold regional weather patterns at interannual and decadal scales, thereby affecting temporal changes of marine taxa and food web dynamics. The East Asian marginal seas are characterized by high nutrient load promoted by hydrographic features that boosts large primary and secondary production, thus favoring environmental conditions for fishery [5,6]. Indeed, this region is one of the world’s most productive fisheries grounds [5]. Projected scenarios of global change, however, forewarn the potential impact climate changes (i.e., warming) may have on marine ecosystem assets and services of this region [7,8,9]. Partly triggered by such changes, conspicuous jellyfish proliferations have been observed in the area, posing challenges for the management of harvested fish stocks. These pervasive events have wide detrimental impacts for socio-ecological coastal ecosystems through their influence on trophic dynamics and carbon fluxes, and on human industries, i.e., fisheries. In the Korean Peninsula, massive jellyfish proliferations have generated wide socio-ecologic awareness, which has fostered scientific efforts aiming at their control [10,11].
The common bloom-formers jellyfish Aurelia coerulea and Nemopilema nomurai are of major concern, as they heavily impair fisheries, clog the intake water screens of nuclear electric power plants, and can produce envenomation events [10,11,12,13]. The first Scyphozoan belong to the moon jellyfish genus Aurelia, which has long been considered cosmopolitan and able of local adaptation due to its phenotypic plasticity [14]. However, recent molecular studies have shown that this genus is a species-complex embracing numerous locally adapted species [15], from which Aurelia coerulea von Lendenfeld, 1884 inhabits waters Korean waters [16,17]. The occurrence of this species has also been reported from the Northwest Pacific oceans, California coast, and some sites of the Mediterranean Sea [16,18,19]. The second Scyphozoan, Nemopilema nomurai Kishinouye 1922, is one of the largest jellyfish species in the world, able to reach a bell diameter of ca. 2 m and body weight of ca. 200 kg WW [5] and references therein. The population centre of N. nomurai is located in the northwest Yellow and East China seas, from which medusae stages are transported by the Korea/Tsushima Strait (i.e., a branch of the Kuroshio Current) dispersing individuals toward northern and eastern Korean waters and Japan Sea [20,21,22,23,24]. Although proliferations of this species have long been recorded, their intensity and recurrence have increased in the last two decades severely affecting local fisheries [5,25,26]. Here, we investigate the influence of climate phenomena governing East Asian marginal seas on these two Scyphozoan species in South Korean waters. To tackle these questions, we used pluriannual field observations spanning over the period 2006–2019 to assess underlying forces driving bloom dynamics of A. coerulea and N. nomurai, and to quantify partitioning effects, direct/indirect links, and the relative importance of hydroclimate forces on the interannual patterns of these species.
2. Materials and Methods
2.1. Hydroclimate Conditions in East Asian Marginal Seas
Hydroclimate patterns in the Korean Peninsula are mainly dominated by two atmospheric pressure systems, the Siberian high and the Aleutian low, e.g., high of Siberian in winter and high of Aleutian in summer [27], while interannual and decadal scales weather patterns are further influenced by the PDO, ENSO, and EAWM [28]. The surface oceanic structure of the Korean Peninsula is governed by cold and warm currents. In the northeastern region, the southward flowing North Korean Cold Current (NKCC), a branch of the Liman Current, while in the western and southeastern regions a branch of the Tsushima Current, the Yellow Sea Warm Current (YSWC) and the East Korea Warm Current (EKWC) flow northward. The latter warm current system is in turn influenced by the Kuroshio–Oyashio Extension (KOE), having the Tsushima current branch, which have shown a rapid warming phase during the late twentieth century, i.e., 2-fold higher than the global mean sea surface temperature (SST) change [29].
2.2. Biological Data
Jellyfish data were collected under the national Korean jellyfish monitoring network (KoJeM) launched in 2006 and headed by the National Institute of Fisheries Science (NIFS), Busan, Republic of Korea, to track jellyfish biomass changes in coastal waters of South Korea. Data are compiled by NIFS and supported by ca. 300 voluntarily trained fisherman and officers spread over 11 coastal areas covering the entire South Korean coastline: Gyeonggi, Incheon, Chungnam, Jeonbuk, Jeonnam, Gyeongnam, Busan, Ulsan, Gyeongbuk, Gangwon, Jeju. Data were collected at a weekly frequency in a binary format, presence/absence of jellyfish, from May to December. Quantitative data denote the spatial coverage of jellyfish in the peninsula. This proxy of jellyfish bloom is used as warning signal for beach users and fishing activities. We analyzed records of the two main Scyphozoan jellyfish, Aurelia coerulea and Nemopilema nomurai, covering the period 2006–2019. Chlorophyll data correspond to seasonal samples collected in February, May, August, and November by the National Institute of Fisheries Science (NIFS, 2006–2010) and the Korean Marine Environment Management Corporation (KOEM, 2011–2019). In Figure 1, we show average conditions of sea surface temperature and chlorophyll during the peak of these jellyfish in the region.
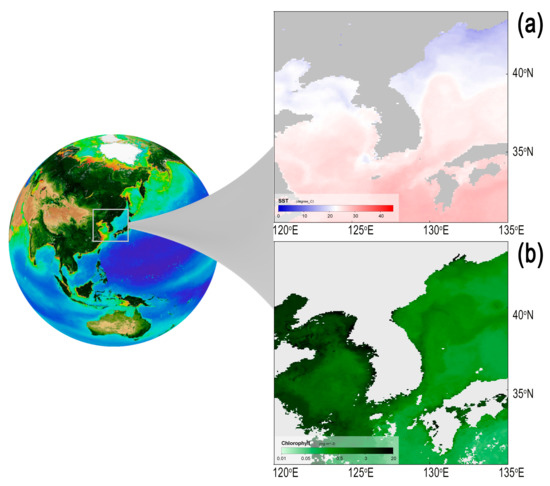
Figure 1.
Regional hydrological conditions in the Korean Peninsula. (a) Sea surface temperature (SST) and (b) surface chlorophyll concentration in Korean waters depicted from satellite data in 2009 September, which correspond to the month of largest bloom intensity in our field survey. Color scale in each panel displays SST in degree Celsius and chlorophyll in mg·m−3. Biosphere globe was downloaded from SeaWiFS Project https://oceancolor.gsfc.nasa.gov/SeaWiFS/ (accessed on 27 January 2021). SST and Chlorophyll data were downloaded from https://oceandata.sci.gsfc.nasa.gov (accessed on 27 January 2021).
2.3. Physical Data
To analyze the long-term interannual variability of jellyfish, we used the climate signals shaping seasonal and interannual weather patterns in the northwest Pacific region, that is, PDO, ENSO, and EAWM. The PDO index is the leading empirical orthogonal function (EOF) of SST variability in the North Pacific Ocean, poleward of 20° N [30]. The ENSO index is represented by the Niño-3.4 SST defined as a 5-month running mean over the region (5° N–5° S, 170–120° W) [31]. The EAWM index is described as the pressure gradient between the Aleutian low and Siberian high, which affects the variability of the winter monsoon in mid-latitude East Asia [32].
We also used relevant local hydrological drivers, e.g., sea surface temperature (SST) and precipitation, as proxy of salinity changes for the period 2006–2019. These data were obtained from the NCEP/NCAR reanalysis dataset provided by the US National Centers for Environmental Prediction/National Center for Atmospheric Research. The selected area extents from 34.00 to 38.00° N and from 125.50 to 129.50° E. Details of the dataset used are given in Table S1.
2.4. Statistical Analyses
First, prior to statistical analyses data harmonization was performed following standard statistical procedures, i.e., time series data were averaged at a monthly frequency and then standardized to zero mean and unit variance. Time series showing strong seasonality, e.g., SST, were seasonal detrended before standardization. Then, linear regressions were performed in time series to remove temporal trends and residual values were retained for analysis.
Second, to assess periods with predominantly positive or negative anomalies of climate and hydrographic conditions, we used the cumulative sum method (cusum). To do this, we cumulated the standardized values of time series over time. Each data point, yt, corresponding to time t (t from 1 to n) was added to the preceding data point according to the equation: . Subsequently, major shifts in a time-series were detected using the cumulative sum of standardized ordinary least square residuals (OLS-based CUSUM test), which disclose significant modifications in time series [33,34].
Third, we assessed interannual patterns of the jellyfish bloom dynamics, as characterized by their magnitude, spatial extent, and phenology. The magnitude of blooms was described by a semi-quantitative index ranging from 0 to 4. The scale was selected based on the spatial coverage of the bloom at time, t, in the South Korean coastal waters, where 0 indicates absence of jellyfish, 1 denotes low bloom magnitude with the presence of the species covering less than 25% of coasts, 2 refers to medium magnitude, with the presence of the species covering 25% to 50% of coasts, 3 indicates high bloom magnitude, with a spatial coverage between 50% to 75% of coasts, and 4 defines extreme bloom, with the presence of the species extending in more than 75% of coasts. Interannual variations in the phenology of jellyfish were assessed by the timing of bloom descriptors: start, peak, and end of bloom, which correspond to 15%, 50%, and 85% of cumulated jellyfish annual data, respectively. These bloom metrics allow evaluating bloom dynamics and have proven useful to detect shifts in the timing and patterns of population growth [35]. The KoJeM started in 2006, but during the first two years sampling consistency was intermittent. First and second years were omitted from our analysis, thus only the period 2008 to 2019 was considered for the assessment of phenological variability to avoid artifacts in bloom metrics analysis. Bloom descriptors (start, peak, end of bloom) were first regressed against a proxy of regional temperature. This variable is a composite vector of in-phase ENSO and PDO anomalies. In addition, we used Pearson moment correlation analysis to test the effect of local environmental variables, i.e., sea surface temperature and precipitation, on bloom dynamics. To avoid misinterpretation of results from correlations we accounted for autocorrelation in time series by adjusting the number of degrees of freedom (df) in the statistical test.
Finally, we applied structural equation modelling (SEM) to quantify partitioning effects of hydroclimate forces driving interannual variations of jellyfish bloom intensity. This technique allows depicting direct and indirect effects, and their relative importance. The strength and sign of links and the quantification of the model structure were determined by simple and partial multivariate regression and 1000 replicates of Monte Carlo permutation tests, while Chi-square values were used to assess robustness and fit of the overall path model [36]. The strength of the relationship between causal and response variables was described by the individual path coefficients, i.e., partial regression coefficients.
Data were analyzed using R software [37], including the packages pracma [38], pheatmap [39], ggplot2 [40], and strucchange [33] for data formatting and multivariate statistics, and AMOS (version 26.0) for SEM. Moreover, we used Grapher 14TM from Golden Software and SeaDas 8.0.0 (the SeaWiFS Data Analysis System, NASA Goddard Space Flight Center, Ocean Ecology Laboratory, Ocean Biology Processing Group, 2018) for computation and visualization.
3. Results and Discussion
3.1. Climate and Hydrographic Patterns in the Korean Peninsula
Climate phenomena governing weather patterns in the Korean Peninsula showed conspicuous monthly and interannual variability over the period 2006–2019 and a main change around 2011–2012 after which trends of time series were reversed (Figure 2). PDO was characterized by two main periods, before 2012 a dominant downward pattern was superposed to marked monthly variability, including two peaks of positive values in 2007 and 2009–2010 (Figure 2a). This period was followed by a dominant upward trend starting in 2013 that reached highest values in 2014–2016 and slightly decreased afterwards, although positive values remained dominant. ENSO variability showed a similar pattern, with two main periods superposed to markedly interannual variations. Before 2012, ENSO dynamics showed two positive peaks, in 2007 and 2009, followed by an upward trend after 2013, which reached maximum values in 2015, and was followed by a slight decrease with two short peaks in 2016 and 2019 (Figure 2b). Lastly, the EAWM showed a strong seasonal variability with the highest values concurrent with the shift of main trends in PDO and ENSO, in 2011 and 2012 (Figure 2c). The observed local hydroclimate conditions in the Korean Peninsula also displayed two main periods. The first was characterized by a downward temperature trend, marked interannual precipitation and higher-than-average chlorophyll concentration. Such trends were reversed ca. 2012, with temperature displaying an upward trend along with a decline of chlorophyll concentration, whereas precipitation showed the highest annual values in 2012 and during the last years of the study period (Figure 2d–f). These results support previous investigations that have shown a large influence of PDO and ENSO upon East Asian marginal seas, which evolves at interannual and decadal scales with ENSO modulating interannual changes and PDO shaping decadal variations [41,42]. It is worth noticing that the coherent temporal pattern of PDO and ENSO enhanced their effect and favored high temperatures after 2012, which were further concurrent with a decline in chlorophyll.
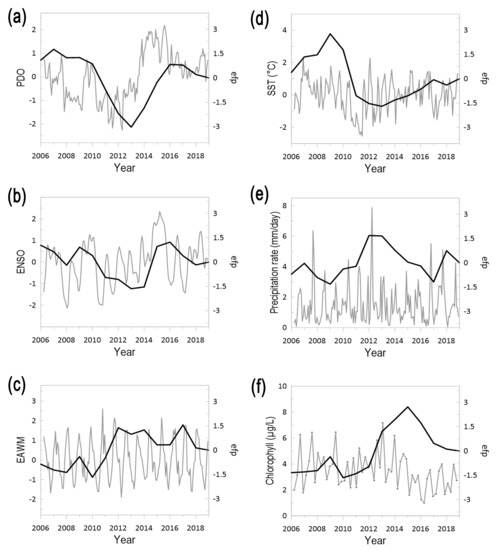
Figure 2.
Interannual changes of climate indices and hydrographic factors governing Korean waters over the period 2006–2019. (a) Pacific Decadal Oscillation (PDO), (b) El Niño Southern Oscillation (ENSO), (c) East Asia winter monsoon (EAWM), (d) sea surface temperature (SST), (e) precipitation rate, and (f) chlorophyll. For each variable it is shown the interannual variability (grey line) and structural changes denoted by the cumulative sum of ordinary least square residuals (OLS-based CUSUM process, black line).
The PDO is acknowledged as a the most prominent driver of the decadal leading pattern of annual mean SST in the North Pacific, and further display a modulation effect on the relationship between ENSO–EAWM, which increase (decrease) during the negative (positive) PDO phase [28,32,42]. In the East Asian marginal seas, the PDO influence is mainly driven by the Aleutian Low dynamics and westerlies, the intensity of which increase during the positive phase of PDO, thereby favoring lower temperatures in the northern and mid-latitude northwestern Pacific, while enhancing SST in the eastern Pacific coast [27,43]. This temporal pattern is characterized by multi-year periods of stable sign (positive or negative) that are separated by abrupt sign reversals [44], such low frequency oscillations have ample effect on the pelagic ecosystems in the North Pacific Ocean [45,46,47,48]. Although the PDO effect is conspicuous over long-term scales, our results pointed out that its impact is also noticeable at short term scales, i.e., interannual and seasonal, supporting previous observations in the southern East China Sea, where the positive phase of the PDO shapes seasonal and interannual dynamics of pelagic copepods, including key species such as Calanus sinicus [49,50].
3.2. Interannual Variability of Magnitude and Timing of Jellyfish Blooms in Korean Waters
The interannual variability of the two Scyphozoan species showed marked differences. Aurelia coerulea displayed a recurrent annual pattern with an average magnitude index of 1.37 ± 0.73 and characterized by two periods of maximal intensity in 2009–2010 and 2014–2016 (Figure 3a). During the blooming period, this species was always present throughout Korean waters, with an average spatial coverage of 21.66% ± 16.32%, although during the largest recorded blooms the species covered more than 50% of South Korean coasts (Figure 3b). After 2012 however, we noticed a general decrease in bloom intensity with a marked overall decline after 2016. The observed decline in bloom intensity might result from a combined effect of rising temperatures and precipitation. Indeed, empirical evidence has shown a negative impact of prolonged high temperatures on strobilation and polyp dynamics, as ephyra production is more sensitive to high temperatures than food limitation [51,52]. It is therefore plausible that the observed warmer winters in the recent decade have impaired polyp strobilation and ephyra release, two processes that are fundamental for the magnitude of jellyfish blooms. In addition, during the same period environmental conditions also displayed high precipitation, thus dropping salinity, which concurrent with high temperatures has a significant negative effect on the swimming behavior and settlement of A. coerulea planulae [53,54]. Moreover, we do not discard that the decline of the species may be partly linked to jellyfish preventive measures taken at the national level, such as a polyp removal program implemented from 2012 to 2015 in spots of eastern and southern coasts, e.g., Lake Shihwa, Lake Saemangeum, and Masan Bay [55,56].
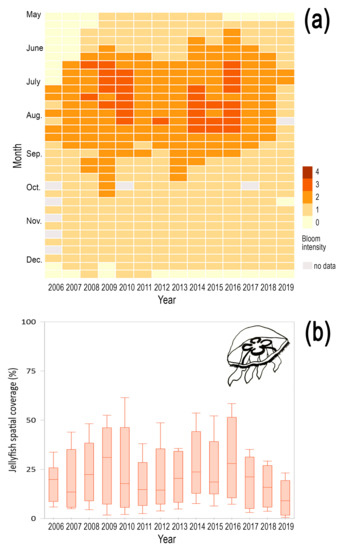
Figure 3.
Seasonal and interannual variability of the spatial coverage of Aurelia coerulea in Korean waters. (a) The magnitude of bloom is denoted by means of bloom intensity proxy from 0 to 4, based on the spatial coverage (%) during the bloom of the species, (b) box plots show maximum and minimum of annual jellyfish spatial coverage from 2006 to 2019.
Nemopilema nomurai showed intermittent annual variations with a decrease in bloom magnitude in the last ten years. The bloom intensity index showed an annual average intensity of 1.26 ± 0.86, with an average spatial extent of 19.20 ± 20.91%. Massive blooms were recorded in 2006–2009, whereas their magnitude declined in the years 2012–2013, 2015–2016 and 2019 (Figure 4a). Massive blooms of N. nomurai showed broad spatial extent, covering by times more than 60% of Korean coasts, i.e., 2006, 2007, and 2012, and exceptionally reaching up to 85% of the coasts, such as during the strongest bloom of 2009, which also lasted until late November (Figure 4b). Anomalous cold winters in East Asia are promoted by coherent patterns of PDO and La Niña [41], which also favor high primary production. These environmental settings are associated to increased N. nomurai strobila formation and ephyra production [57,58,59], thus suggesting a match hypothesis in the nursery area (Bohai Sea, Chanjiang River estuary) between the peak of primary producers and N. nomurai ephyra production [60]. This scenario allows therefore hypothesizing a larger number of N. nomurai transported from nursery areas to Korean coasts.
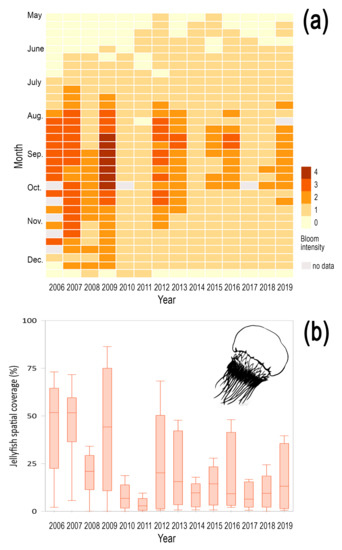
Figure 4.
Seasonal and interannual variability of the spatial coverage of Nemopilema nomurai in Korean waters. (a) The magnitude of bloom is indicated from 0 to 4 based on the spatial coverage (%) during the bloom of the species, (b) box plots show maximum and minimum of annual jellyfish spatial coverage from 2006 to 2019.
The blooms dynamics, as described by the phenology descriptors (start, peak, end of bloom, and duration) stressed marked differences in both, interannual patterns and driving factors affecting the two species. The two jellyfish appeared during late spring, although A. coerulea bloom started six weeks earlier than N. nomurai bloom (Figure 5a,c). We found that a large percentage of the interannual variations of the start of bloom in A. coerulea was shaped by local SST, as shown by the linear regression (R = 0.65) (Figure 5b; Table 1). Increased temperature may act directly on the metabolic rate of Aurelia sp. and has been referred as a leading factor on somatic and growth during early spring [14]. In clear contrast, the start of the bloom for N. nomurai in Korean waters was not related to local temperature conditions (Figure 5d), likely because in this region the blooms dynamics of the species depends on individuals advected by regional currents [24,26].
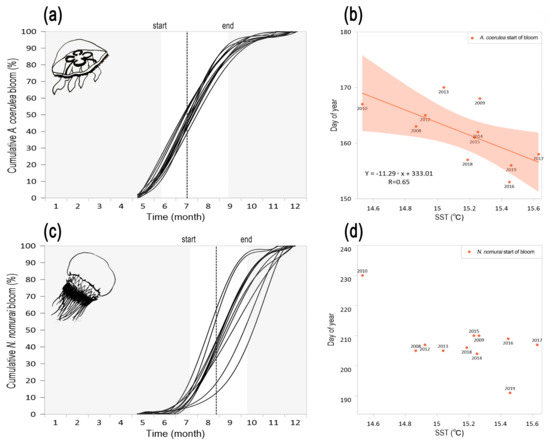
Figure 5.
Interannual variability of the seasonal population growth of scyphozoan Aurelia coerulea (top panel) and Nemopilema nomurai (bottom panel) in Korean waters over the period 2008–2019. (a,c) Curve lines show the cumulative percentage of bloom in different years from May to December. The vertical dotted line indicates the average peak of bloom in 2008–2019 (A. coerulea-3 July week, N. nomurai-4 August week). Gray filled areas show the non-bloom periods. (b,d) Linear regression with 95% confidence interval shows a significant relationship between the timing of start of bloom in A. coerulea and sea surface temperature (SST) (p < 0.05).

Table 1.
Phenological variability of the scyphozoan species Aurelia coerulea and Nemopilema nomurai in Korean waters. Bloom dynamics was summarized by bloom metrics descriptors averaged over the period 2008–2019. Coefficients of driving forces (p < 0.05) are shown in parentheses. Regional temperature (Reg. temp.) denotes a composite variable of in-phase ENSO and PDO anomalies. Prec. = precipitation. SST: sea surface temperature.
The peak of bloom in A. coerulea appeared earlier, seven weeks, than the peak of N. nomurai and showed a significant link with local precipitation (R = −0.64), which we used here as a proxy of salinity (Table 1). Indeed, summer heavy rain events may affect A. coerulea population growth, as shown in Lake Sihwa, western South Korea, due to an abrupt salinity decline (ca. 20 psu) [61]. Hong et al. (2013) hypothesized that such salinity changes foster a detrimental impact on young medusae recruitment with the consequent decline of medusa bloom [61]. In contrast, the peak of N. nomurai was linked to regional temperature variability governed by PDO and ENSO (Table 1). Lastly, the end of bloom in A. coerulea, occurred almost four weeks earlier than N. nomurai end of bloom. Consistent with the close connection of A. coerulea bloom dynamics with local hydrological conditions, our results showed that the end of bloom for A. coerulea was driven by SST (R = −0.64), while no relationship was found between the end of bloom of N. nomurai with local hydroclimate variables. Our results support former assessments of the relationship climate-jellyfish in this region that showed a larger influence of SST on A. coerulea than in other jellyfish species, including N. nomurai [62]. Regarding the length of bloom of these two jellyfish, computed as the difference between start and end of bloom thresholds, we found that although A. coerulea showed an earlier appearance (day of year 163) than N. nomurai (day of year 205) and reached faster the peak of bloom (A. coerulea 35 days, N. nomurai 42 days), the bloom duration in the two species was 77 days in A. coerulea and 87 days in N. nomurai. This suggests that the bloom dynamics of N. nomurai decreased faster after reaching the peak of bloom (N. nomurai 35 days after the peak, A. coerulea 52 days after the peak). Overall, the observed differences in phenological patterns stress the close link of A. coerulea with local temperature conditions, while the lack of connection between N. nomurai and local environmental conditions support a link with physical advection from the species nursery area. Indeed, modelling approaches tracking N. nomurai distribution using combined velocity fields showed a close link between the species species presence in the Korean Peninsula and the advection of water masses from the Yellow Sea [63]. The dynamics of these jellyfish species in Korean waters is therefore shaped by hydroclimate forces playing out at disparate scales (i.e., local for A. coerulea, regional for N. nomurai) in the west Pacific region.
3.3. Climate and Jellyfish Dynamics in the Korean Peninsula
The structural equation model provided a synoptic picture of mediator factors linking large scale climate forces and regional/local environmental factors ultimately influencing jellyfish interannual variability. Our analysis also revealed that these pathways varied for the two species (Figure 6). The model output for A. coerulea displayed a positive link with PDO. Such link was both direct and indirect, through the influence of PDO on SST. The path coefficients linking A. coerulea with PDO and SST were 0.48 and 0.18, respectively. The model further pointed out a lesser, but significant effect of chlorophyll (path coefficient: 0.20). While chlorophyll was influenced by the variability of PDO, ENSO, and SST (path coefficients: −0.42, 0.28, −0.22, respectively), although no significant relationships were detected with the EAWM. In contrast, the SEM model for N. nomurai displayed a different relationship between climate and this species, confirming a dominant role of factors playing out at large scale and a lesser influence from local variables. We found that abundance changes of the species are driven by PDO (path coefficient; −0.74), and ENSO (path coefficient: 0.49), as leading drivers of the interannual variability, whereas local hydrological factors were less important. Only SST showed a significant interaction (path coefficient: 0.27).
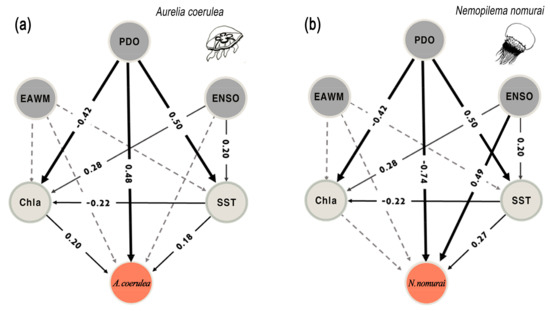
Figure 6.
Path diagrams showing the interactions in the climate-jellyfish network and their effects on the bloom pattern of Aurelia coerulea (a) and Nemopilema nomurai (b) in Korean waters from 2008 to 2019. Solid paths denote statistically significant (p < 0.05) whereas dashed lines indicate not. The standardized coefficients at each significant path allow the comparison of the strength of direct and indirect links. SEM models explained 45% of A. coerulea (p < 0.001) and 56% of N. nomurai (p < 0.001).
These results agree with former investigations on the climate-marine ecosystem coupling in the northwestern Pacific Ocean, where compelling evidence has shown that phenological events and biomass changes in plankton communities are molded by large scale climate patterns. For instance, earlier phytoplankton blooms noticed during the negative PDO phase along with a close link between the abundance of diatoms with spring temperature, from March to April [46], while in Korean waters, cascading effects linking climate, hydrographic changes and zooplankton biomass and structure have been associated with strong ENSO events in 1977, 1989, and 1998 [64]. Likewise, warm and cold hydrographic regimes driven by the PDO strongly influence diversity, biomass and phenology of phytoplankton communities in the Yellow Sea (i.e., Gyeonggi bay) [65].
Jellyfish proliferations are major threats for fishing resources in the Korean Peninsula. In particular, massive proliferations of these two Scyphozoans, A. coerulea and N. nomurai, have broad implications in fishing industries, as by times they have produced a drop of ca. 25% of the annual fisheries production in South Korea, i.e., during the years 2010–2012 where they caused a roughly loss of US$68.2–204.6 million [66]. Moreover, the massive N. nomurai proliferation heavily reduced the catch stock in 2009 with a yielding an average loss of 100 ton of fish catch per month in eastern and southern Korea [67]. Moreover, envenomation events caused by jellyfish in this region have shown a marked increase in the last decade, from 2007 to 2019 the number of cases was four-fold higher [68,69], including a first fatal case caused by N. nomurai in 2012 [70], thus becoming a risk factor for tourism [71]. Our results there provide a synoptic picture of the cascading effects from large scale climate to the jellyfish dynamics in the Korean Peninsula and have implications for fisheries sustainability due to the prominent detrimental impact these species have in the region. We conclude that an effective management to reduce economic losses fostered by N. nomurai can only be achieved through multilateral efforts, as the population dynamics of the species is coupled with regional hydrographic patterns, while local preventive measures can help dropping the impact of A. coerulea.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d13050214/s1, Table S1: Hydroclimate variable and their sources used in this study.
Author Contributions
Conceptualization, S.-H.L. and J.C.M.; Data curation, S.-H.L. and K.-Y.K.; Formal analysis, S.-H.L. and J.C.M.; Funding acquisition, J.-S.H.; Software, S.-H.L. and J.C.M.; Supervision, J.-S.H. and J.C.M.; Validation, J.C.M.; Visualization, S.-H.L. Writing—original draft, S.-H.L.; Writing—review & editing, J.-S.H., K.-Y.K. and J.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Taiwan Ocean University overseas fellowship Taiwan-France to S.-H.L., J1081381195, the Ministry of Science and Technology of Taiwan to J.-S.H., grants no. MOST 107-2621-M-019-001, MOST 108-2621-M-019-003, and MOST 109-2621-M-019-002 and the Center of Excellence for Ocean Engineering grant no. 109J13801-51.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Details of data used in this study are given in Supplementary Materials.
Acknowledgments
This study was conducted as a part of S.-H.L. Ph.D. dissertation and was supported by the National Taiwan Ocean University. We also thank four anonymous reviewers for their thoughtful and constructive comments that helped improving the manuscript. The authors wish to thank people involved in the Korean jellyfish monitoring network (KoJeM) whose commitment to sampling and technical support has made this long-term survey possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Molinero, J.C.; Reygondeau, G.; Bonnet, D. Climate variance influence on the non-stationary plankton dynamics. Mar. Environ. Res. 2013, 89, 91–96. [Google Scholar] [CrossRef]
- Taylor, A.H.; Allen, J.I.; Clark, P.A. Extraction of a weak climatic signal by an ecosystem. Nature 2002, 416, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Beaugrand, G.; Reid, P.C. Long-term changes in phytoplankton, zooplankton and salmon related to climate. Glob. Chang. Biol. 2003, 9, 801–817. [Google Scholar] [CrossRef]
- Bindoff, N.L.; Cheung, W.W.L.; Kairo, J.G.; Aristegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing Ocean, Marine Ecosystems, and Dependent Communities. IPCC Spec. Rep. Ocean Cryosph. Chang. Clim. 2019, 447–588. [Google Scholar]
- Uye, S.I. Blooms of the giant jellyfish Nemopilema nomurai: A threat to the fisheries sustainability of the East Asian Marginal Seas. Plankt. Benthos Res. 2008, 3, 125–131. [Google Scholar] [CrossRef]
- Tseng, H.C.; You, W.L.; Huang, W.; Chung, C.C.; Tsai, A.Y.; Chen, T.Y.; Lan, K.W.; Gong, G.C. Seasonal Variations of Marine Environment and Primary Production in the Taiwan Strait. Front. Mar. Sci. 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Jung, S.; Pang, I.C.; Lee, J.H.; Lee, K. Climate-change driven range shifts of anchovy biomass projected by bio-physical coupling individual based model in the marginal seas of East Asia. Ocean Sci. J. 2016, 51, 563–580. [Google Scholar] [CrossRef]
- Szuwalski, C. Comment on “Impacts of historical warming on marine fisheries production”. Science 2019, 365, 979–983. [Google Scholar] [CrossRef]
- Han, I.-S.; Lee, J.-S. Change the Annual Amplitude of Sea Surface Temperature due to Climate Change in a Recent Decade around the Korean Peninsula. J. Korean Soc. Mar. Environ. Saf. 2020, 26, 233–241. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Lee, J.-S.; Kim, D.-H. A Study on Direction of Industrial Utilization for Jellyfish in Korea. J. Fish. Mar. Sci. Educ. 2014, 26, 587–596. [Google Scholar] [CrossRef][Green Version]
- National Institute of Fisheries Science. Report on the Jellyfish Appearance in Korean Coastal Waters in 2019, E2019-ME-004; National Institute of Fisheries Science Printing Office: Busan, Korea, 2019. [Google Scholar]
- Lee, J.H.; Choi, H.W.; Chae, J.; Kim, D.S.; Lee, S.B. Performance analysis of intake screens in power plants on mass impingement of marine organisms. Ocean Polar Res. 2006, 28, 385–393. [Google Scholar] [CrossRef]
- Yoon, E.-A.; Cha, C.-P.; Hwang, D.-J.; Yoon, Y.-H.; Shin, H.-H.; Gwak, D.-S. Inter-annual occurrence variation of the large jellyfish Nemopilema nomurai due to the changing marine environment in the East China Sea. J. Korean Soc. Fish. Technol. 2012, 48, 242–255. [Google Scholar] [CrossRef]
- Lucas, C.H. Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia 2001, 451, 229–246. [Google Scholar] [CrossRef]
- Dawson, M.N. Macro-morphological variation among cryptic species of the moon jellyfish, Aurelia (Cnidaria: Scyphozoa). Mar. Biol. 2003, 143, 369–379. [Google Scholar] [CrossRef]
- Ki, J.S.; Hwang, D.S.; Shin, K.; Yoon, W.D.; Lim, D.; Kang, Y.S.; Lee, Y.; Lee, J.S. Recent moon jelly (Aurelia sp.1) blooms in Korean coastal waters suggest global expansion: Examples inferred from mitochondrial COI and nuclear ITS-5.8S rDNA sequences. ICES J. Mar. Sci. 2008, 65, 443–452. [Google Scholar] [CrossRef]
- Seo, Y.; Chae, J.; Ki, J.S. The complete mitochondrial genome of the jellyfish Aurelia coerulea (Cnidaria and Scyphozoa) with phylogenetic analysis. Mitochondrial DNA Part B Resour. 2020, 5, 1929–1930. [Google Scholar] [CrossRef]
- Dawson, M.N.; Jacobs, D.K. Molecular evidence for cryptic species of Aurelia aurita (Cnidaria, Scyphozoa). Biol. Bull. 2001, 200, 92–96. [Google Scholar] [CrossRef]
- Scorrano, S.; Aglieri, G.; Boero, F.; Dawson, M.N.; Piraino, S. Unmasking Aurelia species in the Mediterranean Sea: An integrative morphometric and molecular approach. Zool. J. Linn. Soc. 2017, 180, 243–267. [Google Scholar] [CrossRef]
- Kawahara, M.; Uye, S.I.; Ohtsu, K.; Iizumi, H. Unusual population explosion of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in East Asian waters. Mar. Ecol. Prog. Ser. 2006, 307, 161–173. [Google Scholar] [CrossRef]
- Yoon, W.D.; Yang, J.Y.; Shim, M.B.; Kang, H.K. Physical processes influencing the occurrence of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) around Jeju Island, Korea. J. Plankton Res. 2008, 30, 251–260. [Google Scholar] [CrossRef][Green Version]
- Moon, J.H.; Pang, I.C.; Yang, J.Y.; Yoon, W.D. Behavior of the giant jellyfish Nemopilema nomurai in the East China Sea and East/Japan Sea during the summer of 2005: A numerical model approach using a particle-tracking experiment. J. Mar. Syst. 2010, 80, 101–114. [Google Scholar] [CrossRef]
- Kitajima, S.; Hasegawa, T.; Nishiuchi, K.; Kiyomoto, Y.; Taneda, T.; Yamada, H. Temporal fluctuations in abundance and size of the giant jellyfish Nemopilema nomurai medusae in the northern East China Sea, 2006–2017. Mar. Biol. 2020, 167, 1–10. [Google Scholar] [CrossRef]
- Kitajima, S.; Iguchi, N.; Honda, N.; Watanabe, T.; Katoh, O. Distribution of Nemopilema nomurai in the southwestern Sea of Japan related to meandering of the Tsushima Warm Current. J. Oceanogr. 2015, 71, 287–296. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, D.; Keesing, J.K. Jellyfish blooms in China: Dominant species, causes and consequences. Mar. Pollut. Bull. 2010, 60, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.D.; Lee, H.E.; Han, C.; Chang, S.J.; Lee, K. Abundance and distribution of Nemopilema nomurai (Scyphozoa, Rhizostomeae) in Korean waters in 2005–2013. Ocean Sci. J. 2014, 49, 183–192. [Google Scholar] [CrossRef]
- Park, Y.H.; Yoon, J.H.; Youn, Y.H.; Vivier, F. Recent warming in the western north pacific in relation to rapid changes in the atmospheric circulation of the Siberian high and aleutian low systems. J. Clim. 2012, 25, 3476–3493. [Google Scholar] [CrossRef]
- Park, W.S. Interannual and interdecadal variations of sea surface temperature in the East Asian Marginal Seas. Prog. Oceanogr. 2000, 47, 191–204. [Google Scholar] [CrossRef]
- Toda, M.; Watanabe, M. Mechanisms of enhanced ocean surface warming in the Kuroshio region for 1951–2010. Clim. Dyn. 2020, 54, 4129–4145. [Google Scholar] [CrossRef]
- Mantua, N.J.; Hare, S.R.; Zhang, Y.; Wallace, J.M.; Francis, R.C. A Pacific Interdecadal Climate Oscillation with Impacts on Salmon Production. Bull. Am. Meteorol. Soc. 1997, 78, 1069–1079. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Stepaniak, D.P. Indices of El Niño evolution. J. Clim. 2001, 14, 1697–1701. [Google Scholar] [CrossRef]
- Jhun, J.G.; Lee, E.J. A new East Asian winter monsoon index and associated characteristics of the winter monsoon. J. Clim. 2004, 17, 711–726. [Google Scholar] [CrossRef]
- Zeileis, A.; Leisch, F.; Hornik, K.; Kleiber, C. Strucchange: An R package for testing for structural change in linear regression models. J. Stat. Softw. 2002, 7, 1–38. [Google Scholar] [CrossRef]
- Fernández de Puelles, M.L.; Molinero, J.C. Increasing zooplankton variance in the late 1990s unveils hydroclimate modifications in the Balearic Sea, Western Mediterranean. Mar. Environ. Res. 2013, 86, 76–80. [Google Scholar] [CrossRef]
- Greve, W.; Prinage, S.; Zidowitz, H.; Nast, J.; Reiners, F. On the phenology of North Sea ichthyoplankton. ICES J. Mar. Sci. 2005, 62, 1216–1223. [Google Scholar] [CrossRef]
- Alsterberg, C.; Eklöf, J.S.; Gamfeldt, L.; Havenhand, J.N.; Sundbäck, K. Consumers mediate the effects of experimental ocean acidification and warming on primary producers. Proc. Natl. Acad. Sci. USA 2013, 110, 8603–8608. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing, R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Borchers, H.W. Practical Numerical Math Functions. R Package Version 2.2.2. 2018. Available online: https://CRAN.R-project.org/package=pracma (accessed on 17 January 2021).
- Kolde, R. Pheatmap: Pretty Heatmaps. R Package Version 1.0.12. 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 21 January 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kim, J.W.; Yeh, S.W.; Chang, E.C. Combined effect of El Niño-Southern Oscillation and Pacific Decadal Oscillation on the East Asian winter monsoon. Clim. Dyn. 2014, 42, 957–971. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Wilson, R.; Panagiotopoulos, F.; Wu, B. On the long-term interannual variability of the east Asian winter monsoon. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef]
- Mantua, N.J. Pacific–Decadal Oscillation (PDO). Encycl. Glob. Environ. Chang. 2002, 1, 592–594. [Google Scholar]
- D’orgeville, M.; Peltier, W.R. On the Pacific Decadal Oscillation and the Atlantic Multidecadal Oscillation: Might they be related? Geophys. Res. Lett. 2007, 34, 1–5. [Google Scholar] [CrossRef]
- Mackas, D.L.; Greve, W.; Edwards, M.; Chiba, S.; Tadokoro, K.; Eloire, D.; Mazzocchi, M.G.; Batten, S.; Richardson, A.J.; Johnson, C.; et al. Changing zooplankton seasonality in a changing ocean: Comparing time series of zooplankton phenology. Prog. Oceanogr. 2012, 97–100, 31–62. [Google Scholar] [CrossRef]
- Chiba, S.; Batten, S.; Sasaoka, K.; Sasai, Y.; Sugisaki, H. Influence of the Pacific Decadal Oscillation on phytoplankton phenology and community structure in the western North Pacific. Geophys. Res. Lett. 2012, 39, 2–7. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, Y.; Huang, W.; Smol, J.P.; Tang, Q.; Sun, L. The Pacific decadal oscillation and changes in anchovy populations in the Northwest Pacific. J. Asian Earth Sci. 2015, 114, 504–511. [Google Scholar] [CrossRef]
- Shin, A.; Yoon, S.C.; Lee, S.I.; Park, H.W.; Kim, S. The relationship between fishing characteristics of Pacific bluefin tuna (Thunnus orientalis) and ocean conditions around Jeju Island. Fish. Aquat. Sci. 2018, 21, 1–12. [Google Scholar] [CrossRef]
- Molinero, J.C.; Tseng, L.C.; Lopez-Lopez, L.L.; Sommer, U.; Souissi, S.; Hwang, J.S. Climate-driven winter variations of Calanus sinicus abundance in the East China Sea. Fish. Oceanogr. 2016, 25, 555–564. [Google Scholar] [CrossRef]
- Molinero, J.C.; Tseng, L.C.; Abbate, C.L.; Ramirez-Romero, E.; Hwang, J.S. Interannual changes in zooplankton echo subtropical and high latitude climate effects in the southern East China Sea. PLoS ONE 2018, 13, e0197382. [Google Scholar] [CrossRef]
- Goldstein, J.; Augustin, C.B.; Bleich, S.; Holst, S. A matter of tolerance: Distribution potential of scyphozoan polyps in a changing environment. Mar. Ecol. 2017, 38, 1–10. [Google Scholar] [CrossRef]
- Loveridge, A.; Lucas, C.H.; Pitt, K.A. Shorter, warmer winters may inhibit production of ephyrae in a population of the moon jellyfish Aurelia aurita. Hydrobiologia 2021, 848, 739–749. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zheng, S.; Sun, S.; Zhang, F. Effect of temperature and food type on asexual reproduction in Aurelia sp.1 polyps. Hydrobiologia 2015, 754, 169–178. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, L.; Liu, Q.; Sun, T. Effects of salinity and temperature on the recruitment of Aurelia coerulea planulae. Mar. Biol. Res. 2018, 14, 454–461. [Google Scholar] [CrossRef]
- Kim, B. Distribution and Removal Effects of Polyps of the Moon Jellyfish, Aurelia coerulea (Cnidaria: Scyphozoa) in Korean coastal area. Ph.D. Thesis, Department of Fisheries Science Graduate School, Kunsan National University, Gunsan, Korea, 2018. [Google Scholar]
- Yoon, W.; Chae, J.; Koh, B.S.; Han, C. Polyp Removal of a Bloom Forming Jellyfish, Aurelia coerulea, in Korean Waters and Its Value Evaluation. Ocean Sci. J. 2018, 53, 499–507. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, S.; Jin, X.; Li, C. Associations of large jellyfish distributions with temperature and salinity in the Yellow Sea and East China Sea. Hydrobiologia 2012, 690, 81–96. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, F.; Sun, S.; Wang, S.; Li, C. Effects of duration at low temperature on asexual reproduction in polyps of the scyphozoan Nemopilema nomurai (Scyphozoa: Rhizostomeae). Hydrobiologia 2015, 754, 97–111. [Google Scholar] [CrossRef]
- Lee, H.E.; Han, C.H.; Kim, B.H.; Yoon, W.D. Effects of temperature and salinity on the asexual reproduction of Nemopilema nomurai (Scyphozoa: Rhizostomeae). Ocean Sci. J. 2017, 52, 573–579. [Google Scholar] [CrossRef]
- Xu, Y.; Ishizaka, J.; Yamaguchi, H.; Siswanto, E.; Wang, S. Relationships of interannual variability in SST and phytoplankton blooms with giantjellyfish (Nemopilema nomurai) outbreaks in the Yellow Sea and East China Sea. J. Oceanogr. 2013, 69, 511–526. [Google Scholar] [CrossRef]
- Hong, H.P.; Han, C.H.; Yoo, J.K. Population dynamics of jellyfish Aurelia aurita (s.l.) in Sihwa Lake. Ocean Polar Res. 2013, 35, 205–217. [Google Scholar] [CrossRef]
- Kim, B.-T.; Eom, K.-H.; Han, I.-S.; Park, H.-J. An Analysis of the Impact of Climatic Elements on the Jellyfish Blooms. J. Fish. Mar. Sci. Educ. 2015, 27, 1755–1763. [Google Scholar] [CrossRef][Green Version]
- Choi, J.G.; Jo, Y.H.; Moon, I.J.; Park, J.; Kim, D.W.; Lippmann, T.C. Physical forces determine the annual bloom intensity of the giant jellyfish Nemopilema nomurai off the coast of Korea. Reg. Stud. Mar. Sci. 2018, 24, 55–65. [Google Scholar] [CrossRef]
- Kang, Y.S.; Jung, S.; Zuenko, Y.; Choi, I.; Dolganova, N. Regional differences in the response of mesozooplankton to oceanographic regime shifts in the northeast Asian marginal seas. Prog. Oceanogr. 2012, 97–100, 120–134. [Google Scholar] [CrossRef]
- Jahan, R.; Choi, J.K. Climate Regime Shift and Phytoplankton Phenology in a Macrotidal Estuary: Long-Term Surveys in Gyeonggi Bay, Korea. Estuaries Coasts 2014, 37, 1169–1187. [Google Scholar] [CrossRef]
- Kim, D.H.; Seo, J.N.; Yoon, W.D.; Suh, Y.S. Estimating the economic damage caused by jellyfish to fisheries in Korea. Fish. Sci. 2012, 78, 1147–1152. [Google Scholar] [CrossRef]
- Kim, H.; Song, S.H.; Lee, S.; Kim, J.-B.; Yoo, J.-T.; Jang, D.-S. Dominant causes on the catch fluctuation of a set net fishery in the mid-south sea of Korea. J. Korean Soc. Fish. Technol. 2013, 49, 250–260. [Google Scholar] [CrossRef]
- Health Insurance Review and Assessment Service Korea, HIRAsabo Vol.141. Available online: http://www.hirasabo.or.kr (accessed on 11 December 2020).
- Ministry of Oceans and Fisheries. Jellyfish Envenomation to Bather in Korea Beaches; Marine Leisure Tourism Division: Sejong, Korea, 2019. [Google Scholar]
- Kim, J.H.; Han, S.B.; Durey, A. Fatal Pulmonary Edema in a Child after Jellyfish Stings in Korea. Wilderness Environ. Med. 2018, 29, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Abukhalifeh, A.N.; Chandran, K. Understanding the Perception of Safety and Security of Tourists at Jeju Island, South Korea. Rev. Integr. Bus. Econ. Res. 2020, 9, 107–114. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).