Moisture and Salinity Drive the Vegetation Composition of Wadi Hargan, Riyadh, Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
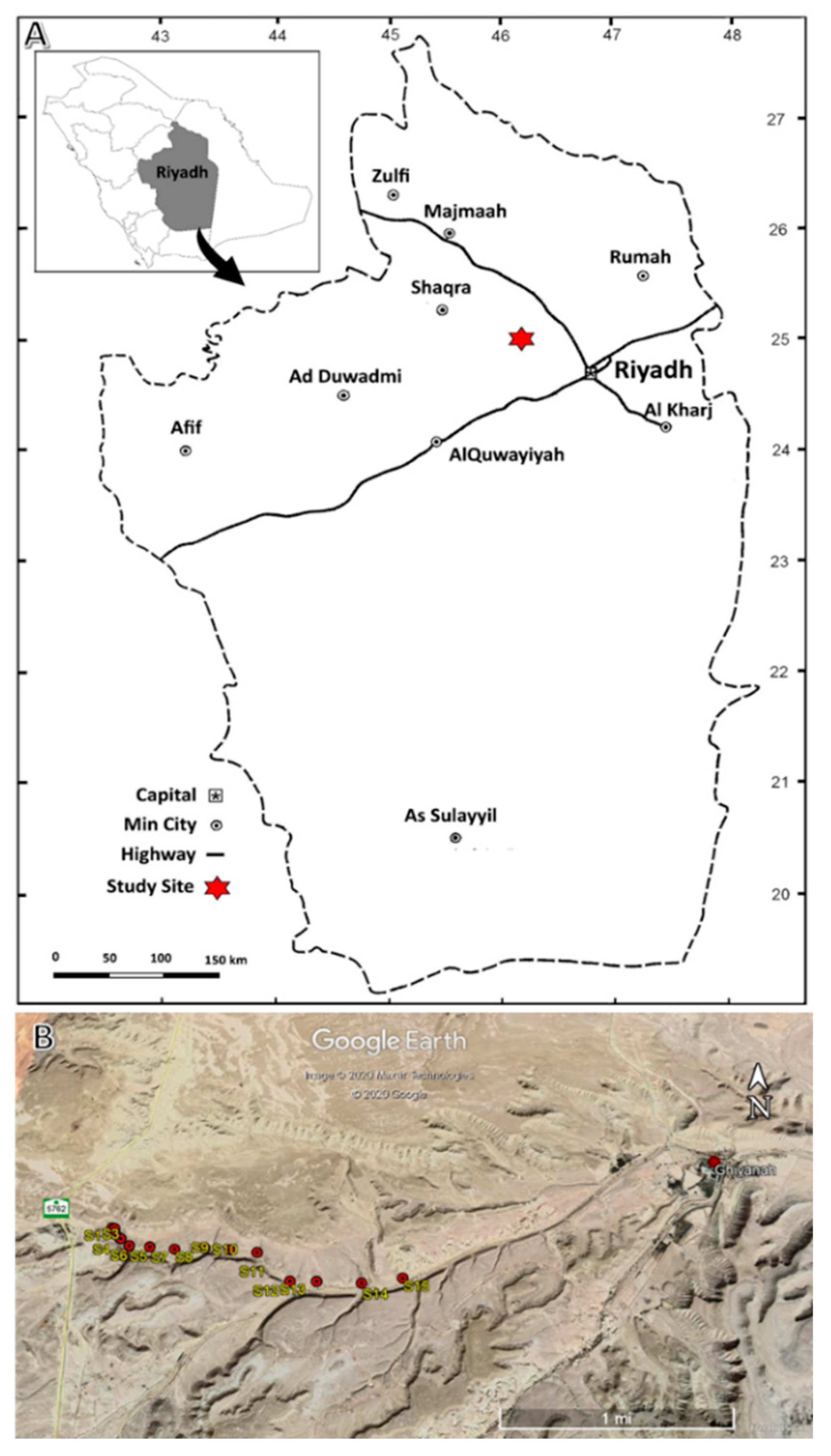
2.1. The Study Area
2.2. Vegetation Analysis
2.3. Soil Analysis
2.4. Data Analysis
3. Results
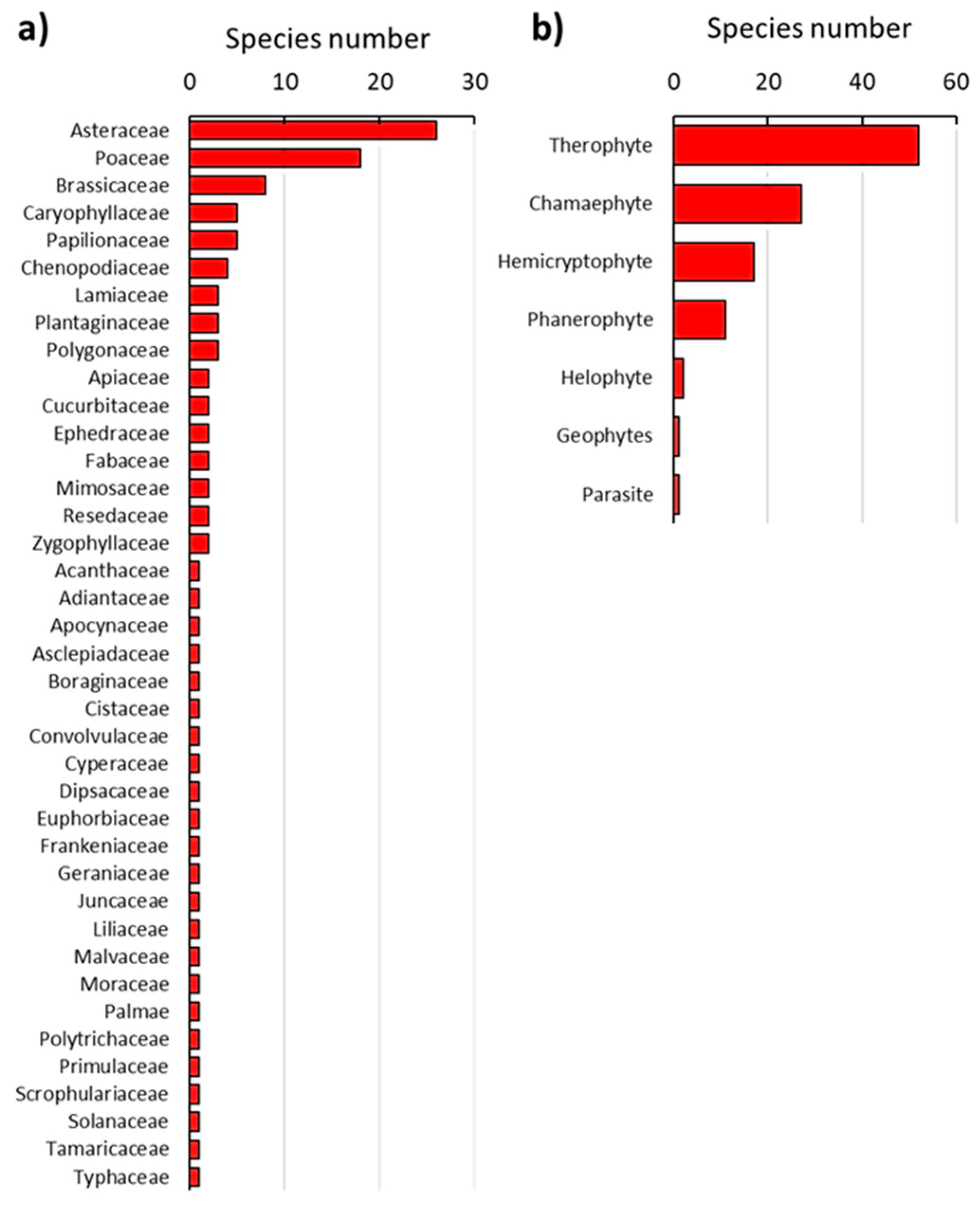
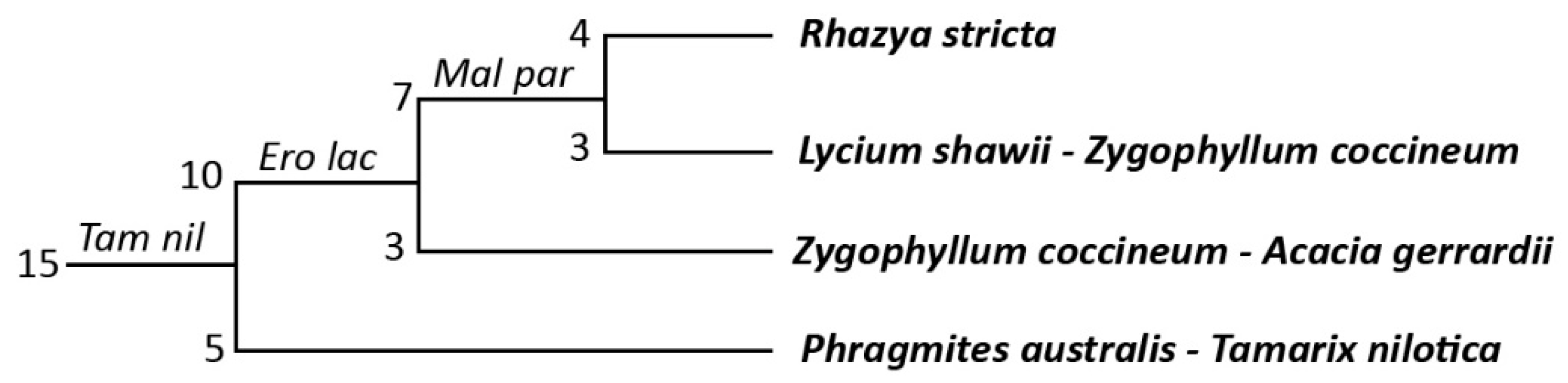
3.1. Vegetation Composition
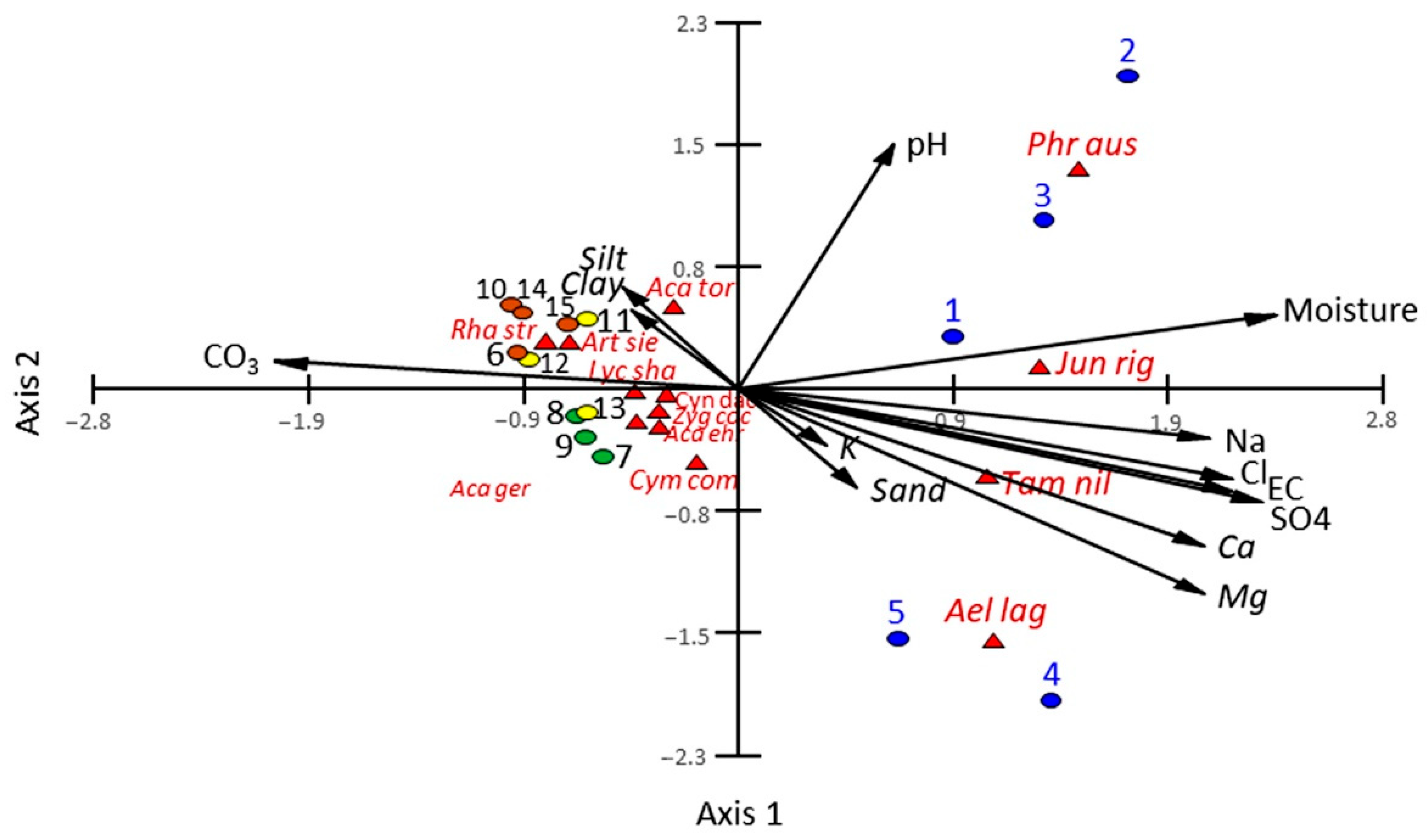
3.2. Vegetation-Soil Relationship
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Obaid, S.; Samraoui, B.; Thomas, J.; El-Serehy, H.A.; Alfarhan, A.H.; Schneider, W.; O’connell, M. An overview of wetlands of Saudi Arabia: Values, threats, and perspectives. Ambio 2017, 46, 98–108. [Google Scholar] [CrossRef]
- Hunter, M.L., Jr.; Acuña, V.; Bauer, D.M.; Bell, K.P.; Calhoun, A.J.; Felipe-Lucia, M.R.; Fitzsimons, J.A.; González, E.; Kinnison, M.; Lindenmayer, D. Conserving small natural features with large ecological roles: A synthetic overview. Biol. Conserv. 2017, 211, 88–95. [Google Scholar] [CrossRef]
- Springer, K.B.; Manker, C.R.; Pigati, J.S. Dynamic response of desert wetlands to abrupt climate change. Proc. Natl. Acad. Sci. USA 2015, 112, 14522–14526. [Google Scholar] [CrossRef] [PubMed]
- Patten, D.T.; Rouse, L.; Stromberg, J.C. Isolated spring wetlands in the Great Basin and Mojave Deserts, USA: Potential response of vegetation to groundwater withdrawal. Environ. Manag. 2008, 41, 398–413. [Google Scholar] [CrossRef]
- Villa, J.A.; Bernal, B. Carbon sequestration in wetlands, from science to practice: An overview of the biogeochemical process, measurement methods, and policy framework. Ecol. Eng. 2018, 114, 115–128. [Google Scholar] [CrossRef]
- Sutton-Grier, A.E.; Sandifer, P.A. Conservation of wetlands and other coastal ecosystems: A commentary on their value to protect biodiversity, reduce disaster impacts, and promote human health and well-being. Wetlands 2019, 39, 1295–1302. [Google Scholar] [CrossRef]
- Orimoloye, I.R.; Kalumba, A.M.; Mazinyo, S.P.; Nel, W. Geospatial analysis of wetland dynamics: Wetland depletion and biodiversity conservation of Isimangaliso Wetland, South Africa. J. King Saud Univ. Sci. 2020, 32, 90–96. [Google Scholar] [CrossRef]
- McKinstry, M.C.; Hubert, W.A.; Anderson, S.H. Wetland and Riparian Areas of the Intermountain West: Ecology and Management; University of Texas Press: Austin, TX, USA, 2004; Volume 4. [Google Scholar]
- Williams, W.D. Biodiversity in temporary wetlands of dryland regions. Int. Ver. Für Theor. Und Angew. Limnol. Verh. 2000, 27, 141–144. [Google Scholar] [CrossRef]
- Noby, K.; Kingma, S.; Heitkönig, I.M.A.; Bulte, E.H.; Naguib, M. Smelly wetlands in the Sahara: Role of sewage ponds in bird migration across Egypt. In Proceedings of Wias Annual Conference; Wageningen University: Lunteren, The Netherlands, 2020; p. 70. [Google Scholar]
- Tinley, K. Survey of Saudi Arabian Wetlands; IUCN/NCWCD Report; General Intelligence Presidency: Riyadh, Saudi Arabia, 1994. [Google Scholar]
- Schenk, H.J.; Holzapfel, C.; Hamilton, J.G.; Mahall, B.E. Spatial ecology of a small desert shrub on adjacent geological substrates. J. Ecol. 2003, 91, 383–395. [Google Scholar] [CrossRef]
- Zhang, D.-M.; Zhao, W.-Z.; Zhang, G.-F. Soil moisture and salt ionic composition effects on species distribution and diversity in semiarid inland saline habitats, northwestern China. Ecol. Res. 2018, 33, 505–515. [Google Scholar] [CrossRef]
- Peters, D.P.; Yao, J.; Sala, O.E.; Anderson, J.P. Directional climate change and potential reversal of desertification in arid and semiarid ecosystems. Glob. Chang. Biol. 2012, 18, 151–163. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.; Li, J.; Zhao, G.; Shangguan, Z. Effect of soil moisture and atmospheric humidity on both plant productivity and diversity of native grasslands across the Loess Plateau, China. Ecol. Eng. 2016, 94, 525–531. [Google Scholar] [CrossRef]
- Li, W.-Q.; Xiao-Jing, L.; Khan, M.A.; Gul, B. Relationship between soil characteristics and halophytic vegetation in coastal region of North China. Pak. J. Bot. 2008, 40, 1081–1090. [Google Scholar]
- Xi, H.; Feng, Q.; Zhang, L.; Si, J.; Chang, Z.; Yu, T.; Guo, R. Effects of water and salinity on plant species composition and community succession in Ejina Desert Oasis, northwest China. Environ. Earth Sci. 2016, 75, 138. [Google Scholar] [CrossRef]
- Mętrak, M.; Chachulski, Ł.; Navruzshoev, D.; Pawlikowski, P.; Rojan, E.; Sulwiński, M.; Suska-Malawska, M. Nature's patchwork: How water sources and soil salinity determine the distribution and structure of halophytic plant communities in arid environments of the Eastern Pamir. PLoS ONE 2017, 12, e0174496. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, G.; Abdelly, C.; Michalet, R. Interactive effects of climate and topography on soil salinity and vegetation zonation in North-African continental saline depressions. J. Veg. Sci. 2019, 30, 312–321. [Google Scholar] [CrossRef]
- Pringle, C.M. Threats to US public lands from cumulative hydrologic alterations outside of their boundaries. Ecol. Appl. 2000, 10, 971–989. [Google Scholar] [CrossRef]
- Pringle, C.M. Hydrologic connectivity and the management of biological reserves: A global perspective. Ecol. Appl. 2001, 11, 981–998. [Google Scholar] [CrossRef]
- Al-Amro, A.; El-Sheikh, M.; El-Sheikh, A. Vegetation analysis of some wetland habitats in central region of Saudi Arabia. Appl. Ecol. Environ. Res. 2018, 16, 3255–3269. [Google Scholar] [CrossRef]
- Galal, T.M.; Al-Yasi, H.M.; Fadl, M.A. Vegetation zonation along the desert-wetland ecosystem of Taif Highland, Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 3374–3383. [Google Scholar] [CrossRef] [PubMed]
- Vincent, P. Saudi Arabia: An Environmental Overview; Taylor & Francis: Leiden, The Netherlands, 2008. [Google Scholar]
- Bonham, C.D. Measurements for Terrestrial Vegetation; John Wiley & Sons: New York, NY, USA, 2013. [Google Scholar]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; Wiley and Sons: New York, NY, USA, 1974. [Google Scholar]
- Collenette, S. Wildflowers of Saudi Arabia; National Commission for Wildlife Conservation and Development (NCWCD): Riyadh, Saudi Arabia, 1999. [Google Scholar]
- Chaudhary, S.A. Flora of the Kingdom of Saudi Arabia; Ministry of Agriculture and Water: Riyadh, Saudi Arabia, 1999. [Google Scholar]
- Raunkiaer, C. Plant Life Forms; Clarendon Press: Oxford, UK, 1937. [Google Scholar]
- Zohary, M. Geobotanical Foundations of the Middle East; Gustav Fischer Verlag: Stuttgart, Germany, 1973. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Rowell, D. Soil Science: Methods and Applications; Longman Group: Essex, UK, 1994. [Google Scholar]
- Pierce, W.C.; Haenisch, E.L.; Sawyer, D.T. Quantitative Analysis; Wiley Toppen: Tokyo, Japan, 1958. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Interscience Publishers Inc.: New York, NY, USA, 1947. [Google Scholar]
- Allen, S.E.; Grimshaw, H.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK, 1974. [Google Scholar]
- Hill, M.O. Decorana—A FORTRAN Program for Detrended Correspondence Analysis and Reciprocal Averaging; Ecology and Systematics; Cornell University: Ithaca, NY, USA, 1979; pp. 14850–14852. [Google Scholar]
- Hill, M.O. Twinspan—A FORTRAN Program for Arranging Multivariate Data in an Ordered Two-Way Table by the Classification of the Individuals and Attributes. Ecology and Systematics; Cornell University: Ithaca, NY, USA, 1979; pp. 14850–14890. [Google Scholar]
- Gauch, H.G., Jr.; Whittaker, R.H. Hierarchical classification of community data. J. Ecol. 1981, 69, 537–558. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Smilauer, P. CANOCO Reference Manual and Canodraw for Windows User's Guide: Software for Canonical Community Ordination (Version 4.5); Biometris: Wageningen, The Netherlands, 2002; Available online: www.canoco.com (accessed on 1 April 2015).
- Zahran, M.A.; Willis, A.J. The Vegetation of Egypt, 2nd ed.; Springer Science & Business Media: Berlin, Germany, 2009. [Google Scholar]
- Abd El-Gawad, A.M. Ecology and allelopathic control of Brassica tournefortii in reclaimed areas of the Nile Delta, Egypt. Turk. J. Bot. 2014, 38, 347–357. [Google Scholar] [CrossRef]
- El-Shabasy, A.; Kasem, W. Systematic composition, species diversity and plant chorology at Wadi Tashar, Jazan, Saudi Arabia. J. Med. Plants Stud. 2018, 6, 83–88. [Google Scholar]
- Osman, A.K.; Al-Ghamdi, F.; Bawadekji, A. Floristic diversity and vegetation analysis of Wadi Arar: A typical desert Wadi of the Northern Border region of Saudi Arabia. Saudi J. Biol. Sci. 2014, 21, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Abdel Khalik, K.; Al-Gohary, I.; Al-Sodany, Y. Floristic composition and vegetation: Environmental relationships of wadi fatimah, mecca, Saudi Arabia. Arid Land Res. Manag. 2017, 31, 316–334. [Google Scholar] [CrossRef]
- Kasem, T.; Marei, A. Floristic Compositions and its affinities to phytogeographical regions in Wadi Khulab of Jazan, Saudi Arabia. Int. J. Plant Soil Sci. 2017, 16, 1–11. [Google Scholar] [CrossRef]
- El Ghazali, G.E.; Al-Soqeer, A.R.A.; El Tayeb, G.E. Floristic and ecological studies on the plant cover of Wadi Al Rummah, Qassim Region, Saudi Arabia. Int. Res. J. Plant Sci. 2013, 4, 310–318. [Google Scholar]
- Khalik, K.A.; El-Sheikh, M.; El-Aidarous, A. Floristic diversity and vegetation analysis of wadi Al-Noman, Mecca, Saudi Arabia. Turk. J. Bot. 2013, 37, 894–907. [Google Scholar] [CrossRef]
- Clayton, W. Poales. In Flowering Plants of the World; Heywood, V.H., Ed.; Oxford University Press: Oxford, UK, 1978; pp. 285–290. [Google Scholar]
- Jeffrey, C. Compositae. In Flowering Plants of the World; Heywood, V.H., Ed.; Oxford University Press: Oxford, UK, 1978; pp. 263–268. [Google Scholar]
- Al-Hashim, M.H.; Taha, M.M.N.; El-Asmar, H.M. Physiographic characteristics along the Ibex Protectorate: Remote sensing application, Hotet Bani Tamim, Central Saudi Arabia. J. Environ. Earth Sci. 2019, 9, 28–42. [Google Scholar]
- Alshammari, A.; Sharawy, S. Wild plants diversity of the Hema Faid Region (Ha'il Province, Saudi Arabia). Asian J. Plant Sci. 2010, 9, 447–454. [Google Scholar] [CrossRef][Green Version]
- Sharawy, S.M.; Alshammari, A.M. Checklist of poisonous plants and animals in Aja Mountain, Ha’il Region, Saudi Arabia. Aust. J. Basic Appl. Sci. 2009, 3, 2217–2225. [Google Scholar]
- Habibi, K. Group dynamics of the Nubian ibex (Capra ibex nubiana) in the Tuwayiq Canyons, Saudi Arabia. J. Zool. 1997, 241, 791–801. [Google Scholar] [CrossRef]
- Li, S.; Su, P.; Zhang, H.; Zhou, Z.; Xie, T.; Shi, R.; Gou, W. Distribution patterns of desert plant diversity and relationship to soil properties in the Heihe River Basin, China. Ecosphere 2018, 9, e02355. [Google Scholar] [CrossRef]
- Gong, Y.; Lv, G.; Guo, Z.; Chen, Y.; Cao, J. Influence of aridity and salinity on plant nutrients scales up from species to community level in a desert ecosystem. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Zhou, W.-L.; Yang, X.-Q.; Hao, P.; Liu, Q.-W.; Cao, D.-C.; Baribault, T.; Li, J.-W. Plant diversity and its maintenance in Populus euphratica riparian forests in the Ejina Oasis, China. Forest. Stud. China 2010, 12, 55–61. [Google Scholar] [CrossRef]
- Wang, Z. Research on desert water management and desert control. Eur. J. Remote Sens. 2020, 53, 1–13. [Google Scholar] [CrossRef]
- Rohit Katuri, J.; Trifonov, P.; Arye, G. Spatial distribution of salinity and sodicity in arid climate following long term brackish water drip irrigated olive orchard. Water 2019, 11, 2556. [Google Scholar] [CrossRef]
- Arora, S.; Dagar, J.C. Salinity tolerance indicators. In Research Developments in Saline Agriculture; Dagar, J.C., Yadav, R.K., Sharma, P.C., Eds.; Springer: Singapore, 2019; pp. 155–201. [Google Scholar]
- Aref, I.M.; El-Juhany, L.; Hegazy, S.S. Comparison of the growth and biomass production of six acacia species in Riyadh, Saudi Arabia after 4 years of irrigated cultivation. J. Arid Environ. 2003, 54, 783–792. [Google Scholar] [CrossRef]
- El-Sheikh, M.A.; Thomas, J.; Alatar, A.A.; Hegazy, A.K.; Abbady, G.A.; Alfarhan, A.H.; Okla, M.I. Vegetation of Thumamah Nature Park: A managed arid land site in Saudi Arabia. Rend. Lincei. 2013, 24, 349–367. [Google Scholar] [CrossRef]
- Shaltout, K.; Mady, M. Analysis of raudhas vegetation in central Saudi Arabia. J. Arid Environ. 1996, 34, 441–454. [Google Scholar] [CrossRef]
- Sardans, J.; Bartrons, M.; Margalef, O.; Gargallo-Garriga, A.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Sigurdsson, B.D.; Chen, H.Y.; Peñuelas, J. Plant invasion is associated with higher plant–soil nutrient concentrations in nutrient-poor environments. Glob. Chang. Biol. 2017, 23, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Incerti, G.; Cartenì, F.; Cesarano, G.; Sarker, T.C.; El-Gawad, A.; Ahmed, M.; D'Ascoli, R.; Bonanomi, G.; Giannino, F. Faster N release, but not C loss, from leaf litter of invasives compared to native species in Mediterranean ecosystems. Front. Plant Sci. 2018, 9, 534. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Plant Community | ||||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Stand no. | 5 | 3 | 3 | 4 | |
| Species no. | 40 | 21 | 21 | 20 | |
| Simpson index | 0.95 | 0.91 | 0.94 | 0.92 | |
| Shannon-evenness | 0.81 | 0.76 | 0.86 | 0.76 | |
| No. | Plant species | Average of importance value | |||
| 1 | Acacia ehrenbergiana Hayne | 7.20 ± 1.38 * | 21.25 ± 5.88 | 8.16 ± 3.82 | 17.27 ± 3.05 |
| 2 | Acacia gerrardii Benth. | 4.99 ± 1.90 | 30.67 ± 10.25 | 16.49 ± 3.13 | 5.09 ± 2.24 |
| 3 | Acacia tortilis (Forssk.) Hayne | 5.88 ± 2.55 | --- | 12.88 ± 6.02 | 18.65 ± 3.45 |
| 4 | Adiantum capillus-veneris L. | 4.42 ± 2.72 | --- | --- | --- |
| 5 | Aeluropus lagopoides (L.) Thwaites | 22.60 ± 7.78 | --- | --- | --- |
| 6 | Alhagi graecorum Boiss. | 1.34 ± 0.82 | 1.29 ± 0.67 | --- | 1.55 ± 0.68 |
| 7 | Artemisia monosperma Delile | --- | 5.90 ± 1.54 | --- | |
| 8 | Artemisia sieberi Besser | 1.17 ± 0.72 | --- | 6.98 ± 1.73 | 22.50 ± 5.54 |
| 9 | Artemisia pycnocephala (Less.) DC. | 0.69 ± 0.43 | --- | --- | --- |
| 10 | Astragalus spinosus (Forssk.) Muschl. | --- | --- | 6.07 ± 1.69 | 10.05 ± 2.77 |
| 11 | Atriplex leucoclada Boiss. | 1.90 ± 0.81 | --- | --- | --- |
| 12 | Blepharis ciliaris (L.) B.L.Burtt | 0.69 ± 0.43 | --- | --- | 3.26 ± 0.84 |
| 13 | Cenchrus ciliaris L. | 3.97 ± 1.02 | --- | 5.00 ± 2.34 | --- |
| 14 | Cucumis prophetarum L. | 0.56 ± 0.35 | --- | --- | --- |
| 15 | Citrullus colocynthis (L.) Schrad. | --- | --- | 1.90 ± 0.89 | --- |
| 16 | Cynodon dactylon (L.) Pers. | 3.14 ± 1.34 | 12.98 ± 3.368 | 11.00 ± 2.59 | --- |
| 17 | Cymbopogon commutatus (Steud.) Stapf | 2.27 ± 1.40 | 9.80 ± 2.93 | --- | 3.97 ± 1.74 |
| 18 | Ephedra foliata Boiss. ex C.A.Mey. | 0.56 ± 0.35 | --- | --- | --- |
| 19 | Ephedra ciliata Fisch. & C.A.Mey. | --- | 3.44 ± 0.96 | 7.22 ± 3.37 | --- |
| 20 | Fagonia bruguieri DC. | 1.92 ± 0.49 | 1.70 ± 0.89 | --- | 2.19 ± 0.97 |
| 21 | Farsetia aegyptia Turra | 1.26 ± 0.48 | --- | 1.90 ± 0.89 | 1.55 ± 0.68 |
| 22 | Ficus salicifolia Vahl | 2.05 ± 1.26 | --- | --- | --- |
| 23 | Gymnocarpos decandrus Forssk. | 1.84 ± 0.47 | --- | --- | 1.55 ± 0.68 |
| 24 | Haloxylon salicornicum (Moq.) Bunge ex Boiss. | 2.67 ± 1.64 | --- | --- | --- |
| 25 | Helianthemum lippii (L.) Dum. Cours. | 1.28 ± 0.48 | 2.91 ± 0.76 | 7.27 ± 0.30 | 1.81 ± |
| 26 | Hyparrhenia hirta (L.) Stapf | 3.16 ± 1.37 | 3.61 ± 0.97 | 5.61 ± 1.31 | --- |
| 27 | Juncus rigidus Desf. | 22.31 ± 3.60 | --- | --- | --- |
| 28 | Lycium shawii Roem. & Schult. | 5.77 ± 0.97 | 3.91 ± 2.03 | 31.22 ± 8.19 | 22.46 ± 9.88 |
| 29 | Moricandia sinaica (Boiss.) Boiss. | --- | 11.37 ± 3.76 | 1.84 ± 0.86 | 1.45 ± 0.64 |
| 30 | Ochradenus baccatus Delile | 4.82 ± 0.81 | 1.35 ± 0.70 | 11.62 ± 1.49 | 6.00 ± 1.52 |
| 31 | Panicum turgidum Forssk. | 1.32 ± 0.81 | 3.12 ± 1.63 | --- | --- |
| 32 | Pennisetum divisum (Forssk. ex J.F.Gmel.) Henrard | --- | --- | --- | 9.38 ± 4.12 |
| 33 | Pergularia tomentosa L. | 0.56 ± 0.35 | --- | 1.90 ± 0.89 | --- |
| 34 | Phoenix dactylifera L. | 3.25 ± 1.23 | --- | --- | --- |
| 35 | Phragmites australis (Cav.) Trin. ex Steud. | 29.97 ± 9.78 | --- | --- | --- |
| 36 | Polygonum aviculare L. | 0.56 ± 0.35 | --- | --- | --- |
| 37 | Prosopis farcta (Banks & Sol.) J.F.Macbr. | 1.32 ± 0.81 | --- | --- | --- |
| 38 | Pulicaria undulata (L.) Kostel. | 0.91 ± 0.56 | 3.26 ± 0.85 | 2.83 ± 1.32 | 1.36 ± 0.60 |
| 39 | Reseda muricata C.Presl | --- | --- | 4.23 ± 1.01 | --- |
| 40 | Rhanterium epapposum Oliv. | 2.69 ± 1.10 | 2.09 ± 1.09 | --- | --- |
| 41 | Rhazya stricta Decne. | --- | 14.81 ± 3.89 | 21.84 ± 7.19 | 47.34 ± 5.06 |
| 42 | Scirpoides holoschoenus (L.) Soják | 1.18 ± 0.72 | --- | --- | --- |
| 43 | Scrophularia deserti Delile | --- | 2.09 ± 1.09 | --- | --- |
| 44 | Stipagrostis plumosa Munro ex T.Anderson | --- | 3.30 ± 1.72 | --- | --- |
| 45 | Tamarix nilotica (Ehrenb.) Bunge | 27.71 ± 4.93 | --- | --- | --- |
| 46 | Teucrium polium L. | 0.59 ± 0.36 | --- | --- | --- |
| 47 | Typha domingensis Pers. | 5.49 ± 3.37 | --- | --- | --- |
| 48 | Zilla spinosa (L.) Prantl | 0.56 ± 0.35 | 1.70 ± 0.89 | 4.62 ± 1.15 | 4.22 ± 1.09 |
| 49 | Zygophyllum coccineum L. | 15.40 ± 3.46 | 59.41 ± 13.27 | 29.41 ± 0.71 | 18.36 ± 5.63 |
| Parameters | Plant Community | F Value | p Value | |||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| Clay (%) | 7.01 ± 1.46 *AB | 5.34 ± 0.44 B | 11.39 ± 1.93 A | 6.54 ± 1.14 AB | 3.788 | 0.0213 * |
| Silt (%) | 6.86 ± 1.25 A | 5.66 ± 0.66 A | 9.51 ± 2.17 A | 7.31 ± 1.19 A | 1.273 | 0.3028 |
| Sand (%) | 86.13 ± 2.60 AB | 89.00 ± 0.57 A | 79.10 ± 3.95 B | 86.15 ± 1.86 AB | 2.721 | 0.0633 |
| pH | 8.30 ± 0.10 A | 8.16 ± 0.15 A | 8.45 ± 0.08 A | 8.22 ± 0.12 A | 1.093 | 0.3683 |
| EC (dS m−1) | 2.50 ± 0.15 A | 0.85 ± 0.23 B | 0.42 ± 0.02 BC | 0.30 ± 0.05 C | 52.000 | <0.0001 *** |
| CO3 (%) | 0.69 ± 0.08 B | 1.35 ± 0.18 A | 1.20 ± 0.08 A | 1.43 ± 0.15 A | 6.366 | 0.0020 ** |
| Cl (meq/L) | 4.54 ± 0.74 A | 2.15 ± 0.38 B | 1.38 ± 0.44 B | 0.60 ± 0.07 B | 13.034 | <0.0001 *** |
| SO4 (meq/L) | 23.91 ± 2.65 A | 2.49 ± 0.51 B | 0.69 ± 0.09 B | 1.06 ± 0.21 B | 69.373 | <0.0001 *** |
| Ca (meq/L) | 16.90 ± 2.43 A | 2.79 ± 0.53 B | 1.44 ± 0.20 B | 2.61 ± 0.34 B | 33.846 | <0.0001 *** |
| Mg (meq/L) | 8.15 ± 1.39 A | 2.28 ± 0.73 B | 0.69 ± 0.09 B | 1.51 ± 0.43 B | 17.343 | <0.0001 *** |
| Na (meq/L) | 4.59 ± 0.99 A | 1.13 ± 0.54 B | 0.42 ± 0.16 B | 0.31 ± 0.07 B | 12.500 | <0.0001 *** |
| K (meq/L) | 0.38 ± 0.08 A | 0.40 ± 0.13 A | 0.20 ± 0.03 A | 0.35 ± 0.03 A | 1.288 | 0.2978 |
| Moisture (%) | 18.76 ± 2.70 A | 0.97 ± 0.12 B | 0.65 ± 0.09 B | 0.94 ± 0.14 B | 43.734 | <0.0001 *** |
| Variable | Axis 1 | Axis 2 | Axis 3 |
|---|---|---|---|
| Eigenvalues | 0.631 | 0.369 | 0.288 |
| Percentage | 28.513 | 16.684 | 13.016 |
| Cum. Percentage | 28.513 | 45.197 | 58.213 |
| Species-environmental correlations | 0.999 | 0.987 | 0.994 |
| Clay | −0.190 | 0.200 | 0.111 |
| Silt | −0.203 | 0.257 | −0.153 |
| Sand | 0.207 | −0.241 | 0.028 |
| pH | 0.271 | 0.610 | 0.083 |
| EC | 0.867 | −0.252 | 0.301 |
| CO3 | −0.813 | 0.070 | 0.185 |
| Cl | 0.865 | −0.220 | 0.121 |
| SO4 | 0.917 | −0.277 | −0.002 |
| Ca | 0.814 | −0.384 | −0.099 |
| Mg | 0.815 | −0.505 | 0.019 |
| Na | 0.826 | −0.120 | 0.053 |
| K | 0.154 | −0.137 | 0.354 |
| Moisture | 0.940 | 0.183 | −0.036 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-ElGawad, A.M.; Assaeed, A.M.; Al-Rowaily, S.L.; Dar, B.M.; Malik, J.A. Moisture and Salinity Drive the Vegetation Composition of Wadi Hargan, Riyadh, Saudi Arabia. Diversity 2021, 13, 587. https://doi.org/10.3390/d13110587
Abd-ElGawad AM, Assaeed AM, Al-Rowaily SL, Dar BM, Malik JA. Moisture and Salinity Drive the Vegetation Composition of Wadi Hargan, Riyadh, Saudi Arabia. Diversity. 2021; 13(11):587. https://doi.org/10.3390/d13110587
Chicago/Turabian StyleAbd-ElGawad, Ahmed M., Abdulaziz M. Assaeed, Saud L. Al-Rowaily, Basharat M. Dar, and Jahangir A. Malik. 2021. "Moisture and Salinity Drive the Vegetation Composition of Wadi Hargan, Riyadh, Saudi Arabia" Diversity 13, no. 11: 587. https://doi.org/10.3390/d13110587
APA StyleAbd-ElGawad, A. M., Assaeed, A. M., Al-Rowaily, S. L., Dar, B. M., & Malik, J. A. (2021). Moisture and Salinity Drive the Vegetation Composition of Wadi Hargan, Riyadh, Saudi Arabia. Diversity, 13(11), 587. https://doi.org/10.3390/d13110587










