Floral Trait and Mycorrhizal Similarity between an Endangered Orchid and Its Natural Hybrid
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Morphological Analysis
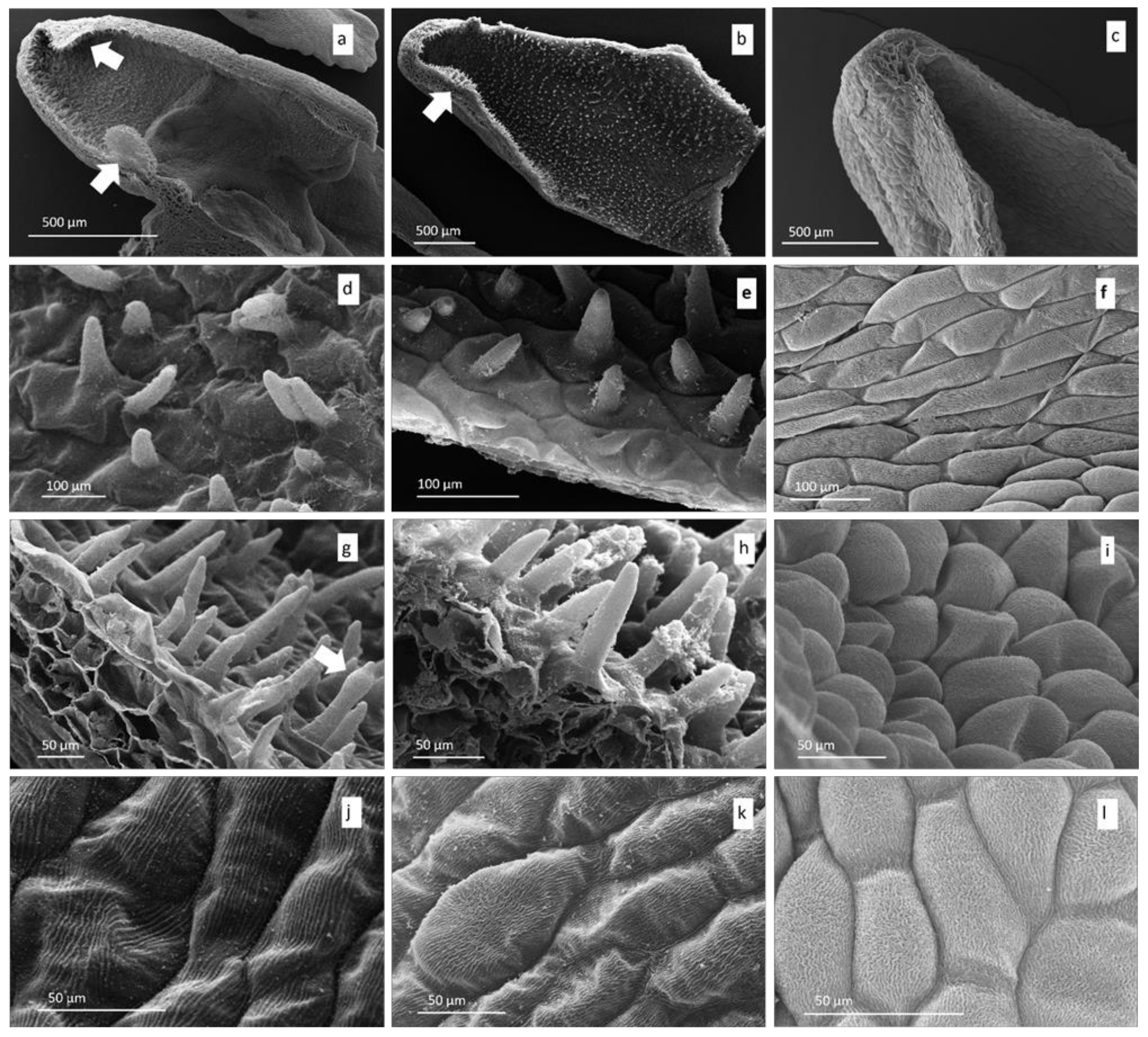
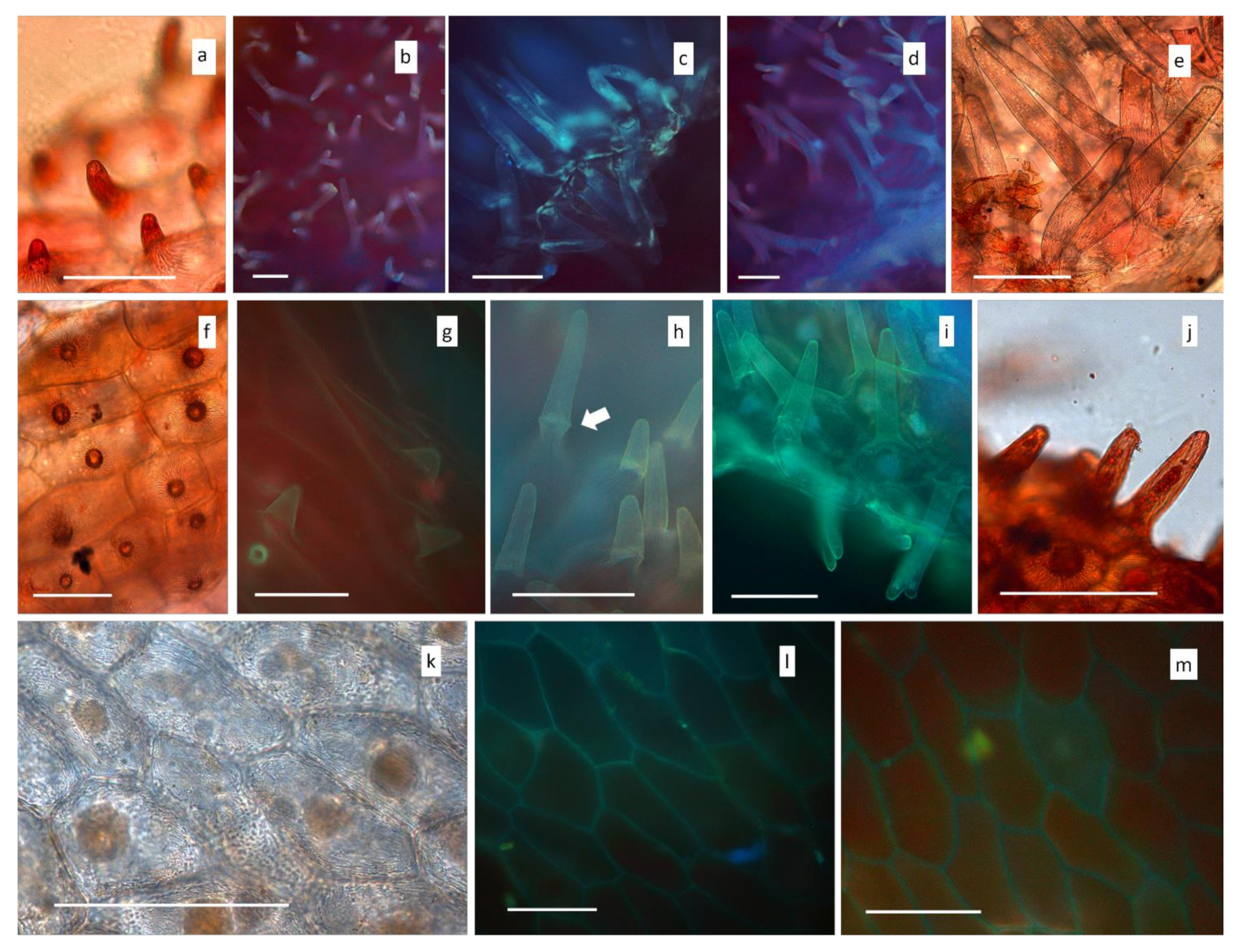
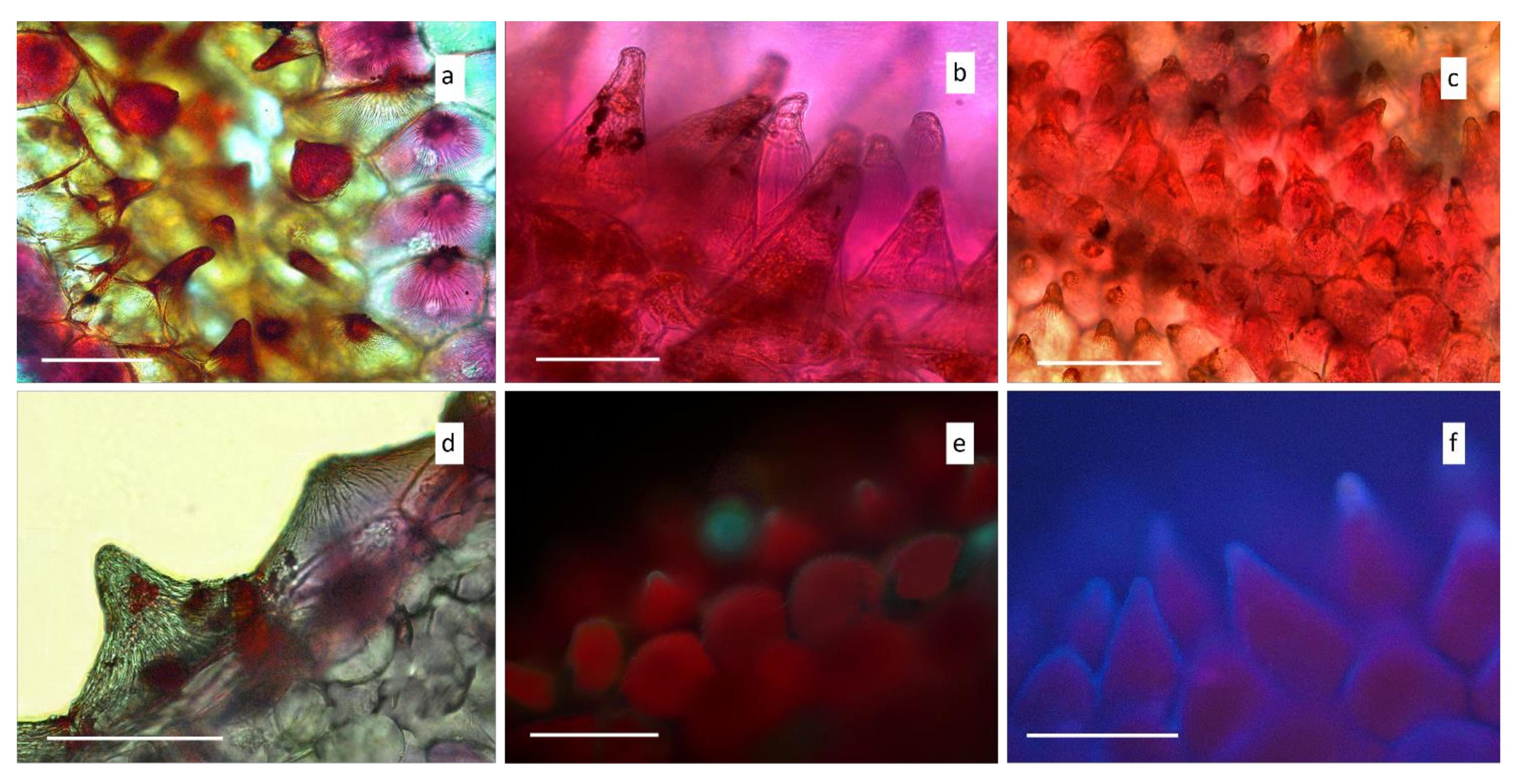
Light and Scanning Electron Microscopy
2.3. Analysis of Essential Oils
2.3.1. Isolation of Volatile Fraction
2.3.2. Fractionation and Alkylthiolation of Alkenes
2.3.3. GC-FID Analysis
2.3.4. GC-MS Analysis
2.3.5. Identification of the Components of the Volatile Fractions
2.4. Fungal Metabarcoding from Roots
2.4.1. DNA Extraction and Amplification
2.4.2. Data Analysis and Bioinformatics
3. Results
3.1. Microscopy
3.2. Chemical Composition of Essential Oils
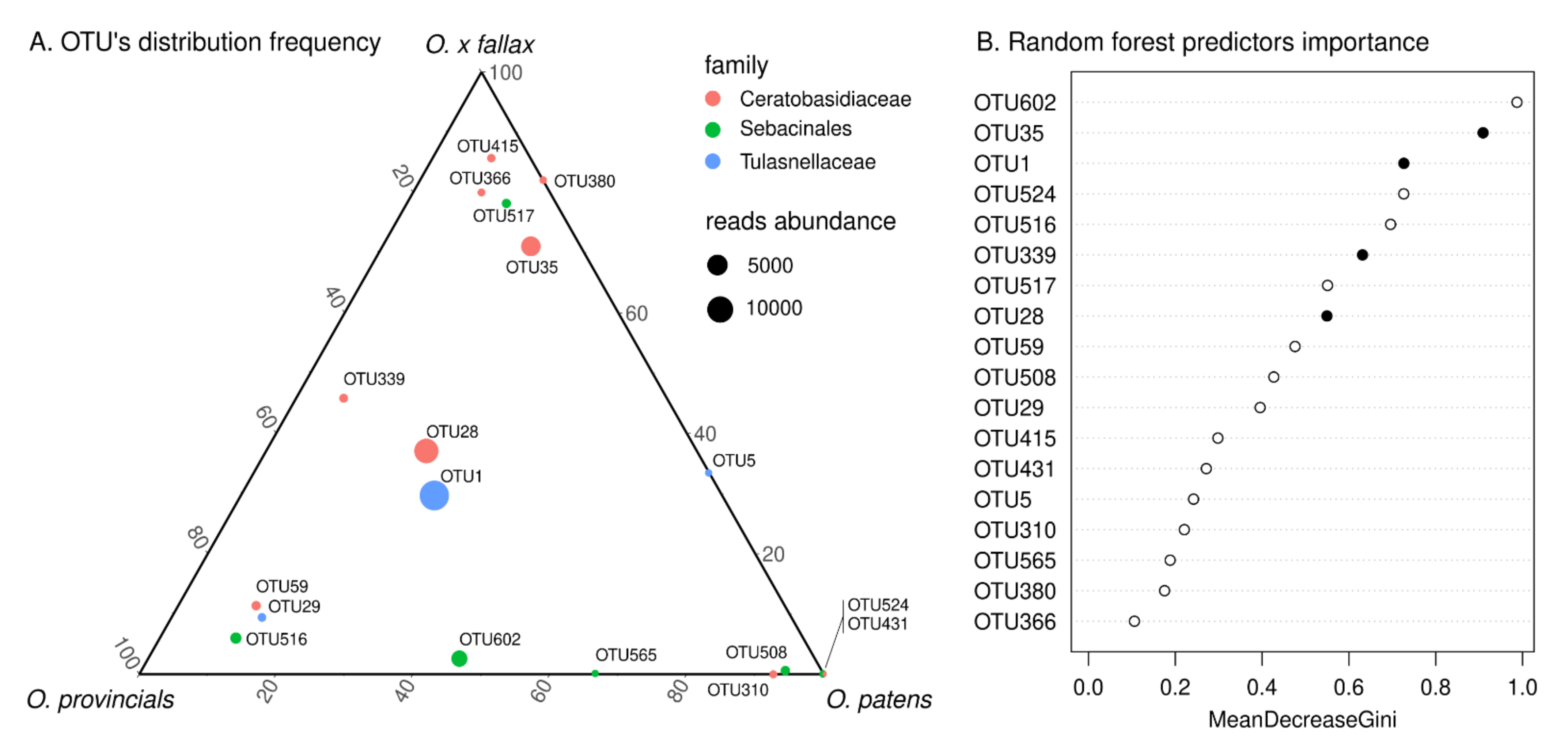
3.3. Fungal Metabarcoding from Roots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, E. Introgressive Hybridization; Wiley: New York, NY, USA, 1949. [Google Scholar]
- Stebbins, G.L. The role of hybridization in evolution. Proc. Am. Philos. Soc. 1959, 103, 231–251. [Google Scholar]
- Soltis, D.E.; Soltis, P.S. Polyploidy: Recurrent formation and genome evolution. Trends Ecol. Evol. 1999, 14, 348–352. [Google Scholar] [CrossRef]
- Cozzolino, S.; Nardella, A.; Impagliazzo, S.; Widmer, A.; Lexer, C. Hybridization and conservation of Mediterranean orchids: Should we protect the orchid hybrids or the orchid hybrid zones? Biol. Conserv. 2006, 129, 14–23. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, Z.; Liu, B. The effects of hybridization and genome doubling in plant evolution via allopolyploidy. Mol. Biol. Rep. 2020, 47, 5549–5558. [Google Scholar] [CrossRef]
- Kim, J.K.; Bae, S.E.; Lee, S.J.; Yoon, M.G. New insight into hybridization and unidirectional introgression between Ammodytes japonicus and Ammodytes Heian (Trachiniformes, Ammodytidae). PLoS ONE 2017, 12, e0178001. [Google Scholar] [CrossRef] [PubMed]
- Todesco, M.; Pascual, M.A.; Owens, G.L.; Ostevik, K.L.; Moyers, B.T.; Hübner, S.; Heredia, S.M.; Hahn, M.A.; Caseys, C.; Bock, D.G.; et al. Hybridization and extinction. Evol. Appl. 2016, 9, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Ellstrand, N.C.; Schierenbeck, K.A. Hybridization as a stimulus for the evolution of invasiveness in plants. Proc. Natl. Acad. Sci. USA 2000, 97, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Ferdy, J.B.; Austerlitz, F. Extinction and introgression in a community of partially cross-fertile plant species. Am. Nat. 2002, 160, 74–86. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Leary, R.F.; Spruell, P.; Wenburg, J.K. The problems with hybrids: Setting conservation guidelines. Trends Ecol. Evol. 2001, 16, 613–622. [Google Scholar] [CrossRef]
- Arnold, M.L. Natural Hybridization and Evolution; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Bersweden, L.; Viruel, J.; Schatz, B.; Harland, J.; Gargiulo, R.; Cowan, R.S.; Calevo, J.; Juan, A.; Clarkson, J.J.; Leitch, A.R.; et al. Microsatellites and petal morphology reveal new patterns of admixture in Orchis hybrid zones. Am. J. Bot. 2021, 108, 1388–1404. [Google Scholar] [CrossRef] [PubMed]
- Coyne, J.A.; Orr, H.A. Speciation; Sinauer: Sunderland, MA, USA, 2004. [Google Scholar]
- Mota, M.R.; Pinheiro, F.; Leal, B.S.S.; Wendt, T.; Palma-Silva, C. The role of hybridization and introgression in maintaining species integrity and cohesion in naturally isolated inselberg bromeliad populations. Plant Biol. 2019, 21, 122–132. [Google Scholar] [CrossRef]
- Abbott, R.J.; Albach, D.; Ansell, S.; Arntzen, J.W.; Baird, S.J.E.; Bierne, N.; Boughman, J.; Brelsford, A.; Buerkle, C.A.; Buggs, R.; et al. Hybridization and speciation. J. Evol. Biol. 2013, 26, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Burgess, K.S.; Zheng, W.; Tao, Z.B.; Li, D.Z.; Gao, L.M. Incomplete reproductive isolation between Rhododendron taxa enables hybrid formation and persistence. J. Integr. Plant Biol. 2019, 61, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Willing, B.; Willing, E. Bibliographie über die Orchideen Europas und der Mittelmeerländer. Englera 1985, 5, 1–280. [Google Scholar] [CrossRef]
- Scopece, G.; Musacchio, A.; Widmer, A.; Cozzolino, S. Patterns of reproductive isolation in Mediterranean deceptive orchids. Evolution 2007, 61, 2623–2642. [Google Scholar] [CrossRef]
- Scopece, G.; Widmer, A.; Cozzolino, S. Evolution of postzygotic reproductive isolation in a guild of deceptive orchids. Am. Nat. 2008, 171, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, G.; Bellusci, F.; Musacchio, A. Genetic integrity of sympatric hybridising plant species: The case of Orchis italica and O. anthropophora. Plant Biol. 2009, 11, 434–441. [Google Scholar] [CrossRef]
- Cozzolino, S.; Widmer, A. Orchid diversity: An evolutionary consequence of deception? Trends Ecol. Evol. 2005, 20, 487–494. [Google Scholar] [CrossRef]
- Kretzschmar, H.; Eccarius, W.; Dietrich, H. The Orchid Genera Anacamptis, Orchis, Neotinea; Echinomedia Verlag: Bürgel, Germany, 2007. [Google Scholar]
- Jacquemyn, H.; Brys, R.; Honnay, O.; Roldán-Ruiz, I.; Lievens, B.; Wiegand, T. Nonrandom spatial structuring of orchids in a hybrid zone of three Orchis species. New Phytol. 2012, 193, 454–464. [Google Scholar] [CrossRef]
- Joffard, N.; Massol, F.; Grenié, M.; Montgelard, C.; Schatz, B. Effect of pollination strategy, phylogeny and distribution on pollination niches of Euro-Mediterranean orchids. J. Ecol. 2019, 107, 478–490. [Google Scholar] [CrossRef]
- Schatz, B.; Genoud, D.; Claessens, J.; Kleynen, J. Orchid–pollinator network in Euro-Mediterranean region: What we know, what we think we know, and what remains to be done. Acta Oecol. 2020, 107, 103605. [Google Scholar] [CrossRef]
- Moccia, M.D.; Widmer, A.; Cozzolino, S. The strength of reproductive isolation in two hybridizing food-deceptive orchid species. Mol. Ecol. 2007, 16, 2855–2866. [Google Scholar] [CrossRef]
- Cozzolino, S.; Scopece, G. Specificity in pollination and consequences for postmating reproductive isolation in deceptive Mediterranean orchids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 3037–3046. [Google Scholar] [CrossRef]
- Stebbins, G.L. Chromosomal Evolution in Higher Plants; Edward Arnol: London, UK, 1971. [Google Scholar]
- De hert, K.; Jacquemyn, H.; Van Glabeke, S.; Roldán-Ruiz, I.; Vandepitte, K.; Leus, L.; Honnay, O. Reproductive isolation and hybridization in sympatric populations of three Dactylorhiza species (Orchidaceae) with different ploidy levels. Ann. Bot. 2012, 109, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.N. Terrestrial Orchids from Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 2nd ed.; Academic Press: Cambridge, UK, 2008. [Google Scholar]
- Hollick, P.S.; Taylor, R.J.; McComb, J.A.; Dixon, K.W. If orchid mycorrhizal fungi are so specific, how do natural hybrids cope? Selbyana 2005, 26, 159–170. [Google Scholar]
- Jacquemyn, H.; Merckx, V.; Brys, R.; Tyteca, D.; Cammue, B.P.A.; Honnay, O.; Lievens, B. Analysis of network architecture reveals phylogenetic constraints on mycorrhizal specificity in the genus Orchis (Orchidaceae). New Phytol. 2011, 192, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Shefferson, R.P.; Bunch, W.; Cowden, C.C.; Lee, Y.I.; Kartzinel, T.R.; Yukawa, T.; Downing, J.; Jiang, H. Does evolutionary history determine specificity in broad ecological interactions? J. Ecol. 2019, 107, 1582–1593. [Google Scholar] [CrossRef]
- Calevo, J.; Voyron, S.; Ercole, E.; Girlanda, M. Is the Distribution of Two Rare Orchis Sister Species Limited by Their Main Mycobiont? Diversity 2020, 12, 262. [Google Scholar] [CrossRef]
- Těšitelová, T.; Jersáková, J.; Roy, M.; Kubátová, B.; Těšitel, J.; Urfus, T.; Trávníček, P.; Suda, J. Ploidy-specific symbiotic interactions: Divergence of mycorrhizal fungi between cytotypes of the Gymnadenia conopsea group (Orchidaceae). New Phytol. 2013, 199, 1022–1033. [Google Scholar] [CrossRef]
- Calevo, J.; Gargiulo, R.; Bersweden, L.; Viruel, J.; González-Montelongo, C.; Rhebbas, K.; Boutabia, L.; Fay, M.F. Molecular evidence of species- and subspecies-level distinctions in the rare Orchis patens s.l. and implications for conservation. Biodivers. Conserv. 2021, 30, 1293–1314. [Google Scholar] [CrossRef]
- Pellegrino, G.; Cozzolino, S.; D’Emerico, S.; Grunanger, P. The taxonomic position of the controversial taxon Orchis clandestina (Orchidaceae): Karyomorphological and molecular analyses. Bot. Helv. 2000, 110, 101–107. [Google Scholar]
- Talamond, P.; Verdeil, J.L.; Conéjéro, G. Secondary metabolite localization by autofluorescence in living plant cells. Molecules 2015, 20, 5024–5037. [Google Scholar] [CrossRef] [PubMed]
- Stern, W.L.; Curry, K.J.; Whitten, W.M. Staining Fragrance Glands in Orchid Flowers. Bull. Torrey Bot. Club 1986, 113, 288–297. [Google Scholar] [CrossRef]
- Chieco, C.; Rotondi, A.; Morrone, L.; Rapparini, F.; Baraldi, R. An ethanol-based fixation method for anatomical and micro-morphological characterization of leaves of various tree species. Biotech. Histochem. 2013, 88, 109–119. [Google Scholar] [CrossRef]
- Bateman, R.M.; Molnár, V.A.; Sramkó, G. In situ morphometric survey elucidates the evolutionary systematics of the Eurasian Himantoglossum clade (Orchidaceae: Orchidinae). PeerJ 2017, 5, e2893. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, M.K.; Şenel, G.; Şeker, Ş.S. Comparison of labellum and spur papillae in Dactylorhiza (Orchidaceae) from Anatolia. Braz. J. Bot. 2020, 43, 367–377. [Google Scholar] [CrossRef]
- Robustelli della Cuna, F.S.; Calevo, J.; Bari, E.; Giovannini, A.; Boselli, C.; Tava, A. Characterization and Antioxidant Activity of Essential Oil of Four Sympatric Orchid Species. Molecules 2019, 24, 3878. [Google Scholar] [CrossRef]
- Robustelli della Cuna, F.S.; Giovannini, A.; Braglia, L.; Sottani, C.; Preda, S. Chemical Composition of the Essential Oil from Leaves and Flowers of Passiflora sexocellata and Passiflora trifasciata (Passifloraceae). Nat. Prod. Commun. 2021, 16, 1–7. [Google Scholar] [CrossRef]
- Carlson, D.A.; Roan, C.S.; Yost, R.A.; Hector, J. Dimethyl disulphide derivatives of long chain alkenes, alkadienes and alkatrienes for gas chromatography/mass spectrometry. Anal. Chem. 1989, 61, 1564–1571. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Joulain, D.; Konig, W.A. (Eds.) The atlas of spectral data of sesquiterpene hydrocarbons. In NIST/EPA/NIH Mass Spectral Database; Version 2.1 Perkin-Elmer Instrument LLC, Copyright ©. 2000; E. B. Verlag: Hamburg, Germany, 1998. [Google Scholar]
- Taylor, D.L.; McCormick, M.K. Internal transcribed spacer primers and sequences for improved characterization of basidiomycetous orchid mycorrhizas. New Phytol. 2008, 177, 1020–1033. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Voyron, S.; Ercole, E.; Ghignone, S.; Perotto, S.; Girlanda, M. Fine-scale spatial distribution of orchid mycorrhizal fungi in the soil of host-rich grasslands. New Phytol. 2017, 213, 1428–1439. [Google Scholar] [CrossRef]
- Zang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Hoiland, K.; Kjoller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi—Recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Koljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 3030, 1312–1313. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 3 September 2021).
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 3 September 2021).
- Hamilton, N.E.; Ferry, M. ggtern: Ternary Diagrams Using ggplot2. J. Stat. Softw. 2018, 87, 1–17. [Google Scholar] [CrossRef]
- Ho, T.K. Random decision forests. In Proceedings of the 3rd International Conference on Document Analysis and Recognition, Montreal, QC, Canada, 14–16 August 1995; Volume 1, pp. 278–282. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Bell, A.; Roberts, D.; Hawkins, J.; Rudall, P.; Box, M.; Bateman, R. Comparative micromorphology of nectariferous and nectarless labellar spurs in selected clades of subtribe Orchidinae (Orchidaceae). Bot. J. Linn. Soc. 2009, 160, 369–387. [Google Scholar] [CrossRef]
- Naczk, A.M.; Kowalkowska, A.K.; Wiśniewska, N.P.; Haliński, L.P.; Kapusta, M.; Czerwicka, M. Floral anatomy, ultrastructure and chemical analysis in Dactylorhiza incarnata/maculate complex (Orchidaceae). Bot. J. Linn. Soc. 2018, 187, 512–536. [Google Scholar] [CrossRef]
- Rieseberg, L.H.; Raymond, O.; Rosenthal, D.M.; Lai, Z.; Livingstone, K.; Nakazato, T.; Durphy, J.L.; Schwarzbach, A.E.; Donovan, L.A.; Lexer, C. Major ecological transitions in wild sunflowers facilitated by hybridization. Science 2003, 301, 1211–1216. [Google Scholar] [CrossRef]
- Kowalkowska, A.K.; Pawłowicz, M.; Guzanek, P.; Krawczyńska, A.T. Floral nectary and osmophore of Epipactis helleborine (L.) Crantz (Orchidaceae). Protoplasma 2018, 255, 1811–1825. [Google Scholar] [CrossRef] [PubMed]
- Stpiczyńska, M.; Davies, K.; Kamińska, M. Comparative anatomy of the nectar spur in selected species of Aeridinae (Orchidaceae). Ann. Bot. 2010, 107, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.S.; Pais, M.S. Ultrastructural aspects of the nectary spur of Limodorum abortivum (L.) Sw. (Orchidaceae). Ann. Bot. 1992, 70, 325–331. [Google Scholar] [CrossRef]
- Stpiczyńska, M.; Matusiewicz, J. Anatomy and ultrastructure of spur nectary of Gymnadenia conopsea L. (Orchidaceae). Acta Soc. Bot. Pol. 2001, 70, 267–272. [Google Scholar] [CrossRef][Green Version]
- Pridgeon, A.M.; Stern, W.L. Osmophores of Scaphosepalum (Orchidaceae). Bot. Gaz. 1985, 146, 115–123. [Google Scholar] [CrossRef]
- Stern, W.L.; Curry, K.J.; Pridgeon, A.M. Osmophores of Stanhopea (Orchidaceae). Am. J. Bot. 1987, 74, 1323–1331. [Google Scholar] [CrossRef]
- Pais, M.S.S.; Figueiredo, A.C.S. Floral nectaries from Limodorum abortivum (L.) Sw. and Epipactis atropurpurea Rafin (Orchidaceae): Ultrastructural changes in plastids during the secretory process. Apidologie 1994, 25, 615–626. [Google Scholar] [CrossRef]
- Stpiczyńska, M. The structure of nectary of Platanthera bifolia L. (Orchidaceae). Acta Soc. Bot. Pol. 1997, 62, 5–9. [Google Scholar] [CrossRef]
- Kowalkowska, A.K.; Margońska, H.B.; Kozieradzka-Kiszkurno, M.; Bohdanowicz, J. Studies on the ultrastructure of a three-spurred fumeauxiana form of Anacamptis pyramidalis. Plant Syst. Evol. 2012, 298, 1025–1035. [Google Scholar] [CrossRef]
- Wiśniewska, N.; Kowalkowska, A.K.; Kozieradzka-Kiszkurno, M.; Krawczyńska, A.T.; Bohdanowicz, J. Floral features of two species of Bulbophyllum section Lepidorhiza Schltr.: B. levanae Ames and B. nymphopolitanum Kraenzl. (Bulbophyllinae Schltr. Orchidaceae). Protoplasma 2018, 255, 485–499. [Google Scholar] [CrossRef]
- Davies, K.L.; Stpiczynska, M.; Turner, M.P. A rudimentary labellar speculum in Cymbidium lowianum (Rchb.f.) Rchb.f. and Cymbidium devonianum Paxton (Orchidaceae). Ann. Bot. 2006, 97, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Stpiczyńska, M. Osmophores of the fragrant orchid Gymnadenia conopsea L. (Orchidaceae). Acta Soc. Bot. Pol. 2001, 70, 91–96. [Google Scholar] [CrossRef]
- Teixeira Sde, P.; Borba, E.L.; Semir, J. Lip anatomy and its implications for the pollination mechanisms of Bulbophyllum species (Orchidaceae). Ann. Bot. 2004, 93, 499–505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waelti, M.O.; Muhlemann, K.; Widmer, A.; Schiestl, F.P. Floral odour and reproductive isolation in two species of Silene. J. Evol. Biol. 2008, 21, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Alija, A.J.; Bresgen, N.; Sommerburg, O.; Langhans, C.D.; Siems, W.; Eckl, P.M. Cyto- and genotoxic potential of beta-carotene and cleavage products under oxidative stress. Biofactors 2005, 24, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Dafni, A. Floral mimicry-mutualism and unidirectional exploitation of insects by plants. In The Plant Surface and Insects; Southwood, T.R.E., Juniper, B.E., Eds.; Edward Arnold: London, UK, 1986; pp. 81–90. [Google Scholar]
- G.I.R.O.S.-Gruppo Italiano per la Ricerca Sulle Orchidee Spontanee • Leggi Argomento-Generi: Anacamptis, Neotinea, Orchis (giros.it). Available online: http://www.giros.it/forum/viewtopic.php?f=131&t=1498&sid=f261715f17a25c493beedc63a8b740fb (accessed on 1 October 2021).
- Cozzolino, S.; Schiestl, F.P.; Müller, A.; De Castro, O.; Nardella, A.M.; Widmer, A. Evidence for pollinator sharing in Mediterranean nectar-mimic orchids: Absence of premating barriers? Proc. Biol. Sci. 2005, 272, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.D.; Nadeau, J.A.; Zhang, X.S.; Bui, A.Q.; Halevy, A.H. Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell 1993, 5, 419–432. [Google Scholar] [PubMed]


| Orchis patens | Orchis provincialis | Orchis × fallax | Identification e | ||||
|---|---|---|---|---|---|---|---|
| # | Compound a | RI b | RI c | % d | % | % | |
| 1 | Benzaldehyde | 953 | 957 | 0.67 ± 0.4 | 1.92 ± 0.2 | 1.28 ± 0.1 | RI, NIST |
| 2 | Undecane | 1100 | 1100 | 0.67 ± 0.5 | 0.47 ± 0.3 | 2.20 ± 0.1 | RI, NIST |
| 3 | Nonanal | 1101 | 1104 | 0.81 ± 0.9 | 2.46 ± 0.1 | 2.55 ± 0.3 | RI, NIST |
| 4 | Cinnamaldehyde | 1270 | 1271 | 1.45 ± 0.1 | - | - | RI, NIST |
| 5 | Nonanoic acid | 1276 | 1274 | - | 2.51 ± 0.2 | 1.00 ± 0.1 | RI, NIST |
| 6 | Tetradecane | 1400 | 1400 | 0.42 ± 0.2 | 0.74 ± 0.3 | - | RI, NIST |
| 7 | trans-Cinnamic acid | 1428 | 1428 | - | 2.36 ± 0.5 | - | RI, NIST |
| 8 | Unidentified | - | 1496 | - | 1.89 ± 0.3 | 4.65 ± 0.4 | - |
| 9 | Pentadecane | 1500 | 1500 | 20.15 ± 0.3 | - | - | RI, NIST |
| 10 | Dodecanoic acid | 1567 | 1563 | 1.34 ± 0.2 | 1.10 ± 0.1 | 1.35 ± 0.1 | RI, NIST |
| 11 | Hexadecane | 1600 | 1600 | 1.49 ± 0.7 | 1.57 ± 0.2 | 2.21 ± 0.3 | RI, NIST |
| 12 | Oxo-β-ionone | 1665 | 1665 | 2.99 ± 0.4 | 20.66 ± 0.3 | 8.38 ± 0.1 | RI, NIST |
| 13 | Heptadecane | 1700 | 1700 | 3.83 ± 0.1 | 4.02 ± 0.2 | 7.34 ± 0.2 | RI, NIST |
| 14 | Octadecane | 1800 | 1800 | 6.38 ± 0.1 | 5.63 ± 0.3 | 8.97 ± 0.1 | RI, NIST |
| 15 | Nonadecane | 1900 | 1900 | 6.06 ± 0.2 | 4.69 ± 01 | 8.49 ± 0.1 | RI, NIST |
| 16 | Hexadecanoic acid | 1968 | 1963 | 11.37 ± 0.1 | 10.37 ± 0.1 | 14.48 ± 0.3 | RI, NIST |
| 17 | Eicosane | 2000 | 2000 | 4.43 ± 0.4 | 4.62 ± 0.3 | 5.60 ± 0.1 | RI, NIST |
| 18 | Heneicosane | 2100 | 2100 | 4.60 ± 0.2 | 2.94 ± 0.2 | 3.25 ± 0.7 | RI, NIST |
| 19 | Docosane | 2200 | 2200 | 4.79 ± 0.5 | 4.36 ± 0.1 | 4.11 ± 0.2 | RI, NIST |
| 20 | 9-tricosene | 2279 | 2279 | 1.17 ± 0.2 | 0.56 ± 0.4 | 0.82 ± 0.2 | MS, RI |
| 21 | 7-tricosene | 2300 | 2290 | - | 0.98 ± 0.1 | - | MS, RI |
| 22 | Tricosane | 2300 | 2300 | 2.63 ± 0.1 | 2.63 ± 0.3 | 3.00 ± 0.3 | RI, NIST |
| 23 | Tetracosane | 2400 | 2400 | 1.25 ± 0.4 | 2.08 ± 0.2 | 1.49 ± 0.8 | RI, NIST |
| 24 | 9-pentacosene | 2474 | 2474 | 3.24 ± 0.1 | 0.81 ± 0.5 | - | MS, RI |
| 25 | 7-pentacosene | 2483 | 2489 | 0.74 ± 0.5 | - | - | MS, RI |
| 26 | Pentacosane | 2500 | 2500 | 7.16 ± 0.1 | 8.61 ± 0.2 | 7.69 ± 0.1 | MS, RI |
| 27 | Hexacosane | 2600 | 2600 | 0.48 ± 0.4 | 1.33 ± 0.1 | 1.17 ± 0.1 | RI, NIST |
| 28 | 12-heptacosene | 2671 | 2670 | 0.50 ± 0.6 | - | - | MS, RI |
| 29 | 9-heptacosene | 2676 | 2675 | 6.77 ± 0.3 | 4.19 ± 0.1 | 2.89 ± 0.1 | MS, RI |
| 30 | 7-heptacosene | 2683 | 2683 | 1.32 ± 0.1 | 0.79 ± 0.4 | - | MS, RI |
| 31 | Heptacosane | 2700 | 2700 | 3.28 ± 0.1 | 5.70 ± 0.1 | 7.06 ± 0.3 | RI, NIST |
| Acids | 12.71 | 16.34 | 16.84 | ||||
| Aldehydes | 2.92 | 4.38 | 3.83 | ||||
| Saturated hydrocharbons | 67.63 | 49.40 | 62.59 | ||||
| Unsaturated hydrocharbons | 13.74 | 7.33 | 3.72 | ||||
| Ketones | 2.99 | 20.66 | 8.38 | ||||
| Unidentified | - | 1.89 | 4.65 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calevo, J.; Bazzicalupo, M.; Adamo, M.; Robustelli della Cuna, F.S.; Voyron, S.; Girlanda, M.; Duffy, K.J.; Giovannini, A.; Cornara, L. Floral Trait and Mycorrhizal Similarity between an Endangered Orchid and Its Natural Hybrid. Diversity 2021, 13, 550. https://doi.org/10.3390/d13110550
Calevo J, Bazzicalupo M, Adamo M, Robustelli della Cuna FS, Voyron S, Girlanda M, Duffy KJ, Giovannini A, Cornara L. Floral Trait and Mycorrhizal Similarity between an Endangered Orchid and Its Natural Hybrid. Diversity. 2021; 13(11):550. https://doi.org/10.3390/d13110550
Chicago/Turabian StyleCalevo, Jacopo, Miriam Bazzicalupo, Martino Adamo, Francesco Saverio Robustelli della Cuna, Samuele Voyron, Mariangela Girlanda, Karl J. Duffy, Annalisa Giovannini, and Laura Cornara. 2021. "Floral Trait and Mycorrhizal Similarity between an Endangered Orchid and Its Natural Hybrid" Diversity 13, no. 11: 550. https://doi.org/10.3390/d13110550
APA StyleCalevo, J., Bazzicalupo, M., Adamo, M., Robustelli della Cuna, F. S., Voyron, S., Girlanda, M., Duffy, K. J., Giovannini, A., & Cornara, L. (2021). Floral Trait and Mycorrhizal Similarity between an Endangered Orchid and Its Natural Hybrid. Diversity, 13(11), 550. https://doi.org/10.3390/d13110550









