Diversity, Status and Phenology of the Dragonflies and Damselflies of Cyprus (Insecta: Odonata)
Abstract
:1. Introduction
2. Study Area
3. Methods
4. Results
4.1. Status
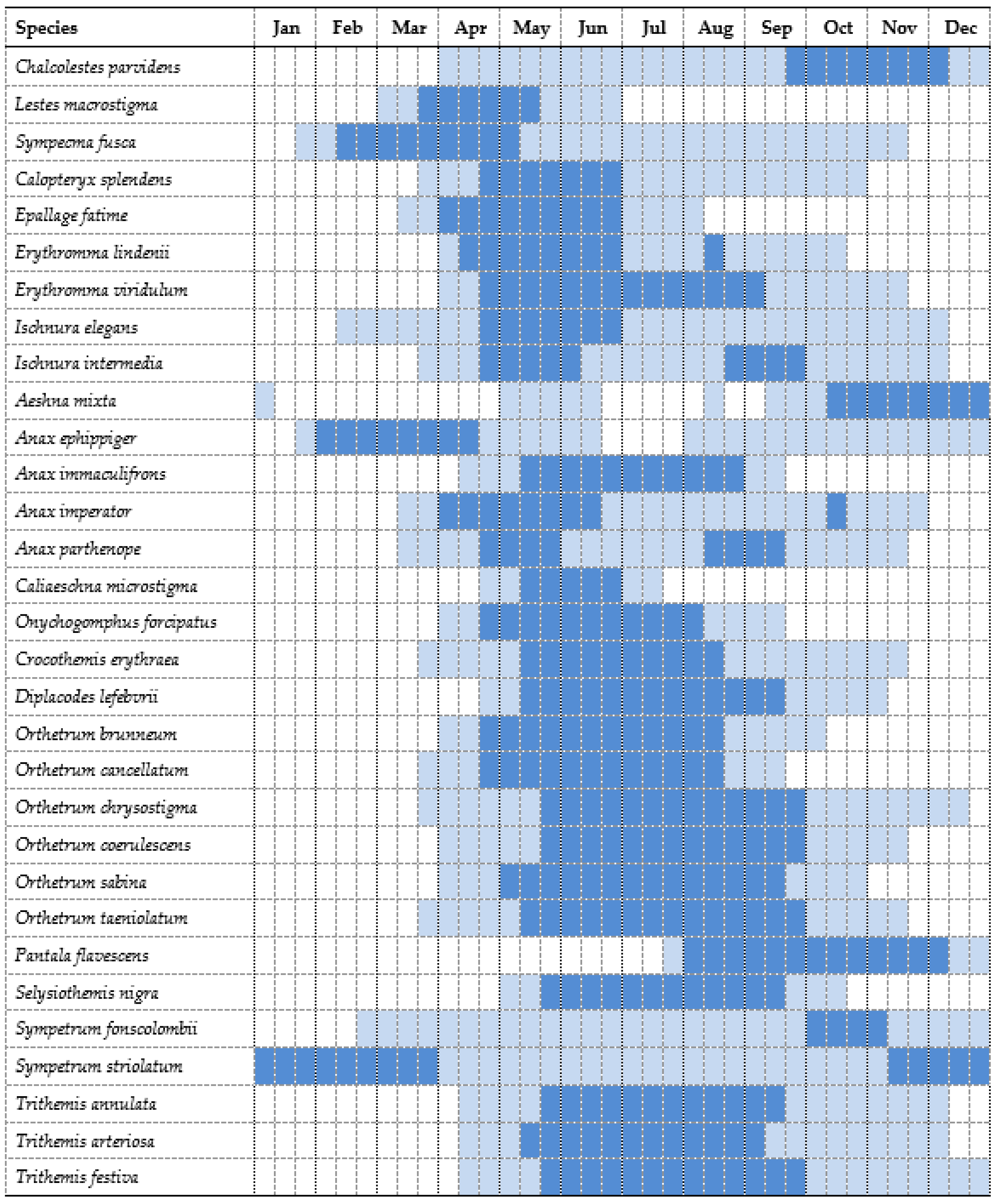
4.2. Phenology
5. Discussion
5.1. Status
5.2. Yearly Variation in Abundance
5.3. Phenology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Total Records | Total Ind. | Earliest Sighting | Latest Sighting |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chalcolestes parvidens | 86/2189 | 71/354 | 122/1626 | 76/685 | 120/1780 | 111/2799 | 144/2716 | 730 | 12,149 | 20-iii-2014 | 02-ii-2015 * |
| Lestes barbarus | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3/3 | 3 | 3 | 14-viii-2019 | 31-viii-2019 |
| Lestes macrostigma | 6/964 | 8/263 | 4/121 | 5/145 | 6/20 | 13/577 | 15/903 | 57 | 2993 | 04-iv-2019 | 16-vi-2017 |
| Sympecma fusca | 55/1937 | 86/3089 | 59/349 | 165/4121 | 35/175 | 101/2882 | 77/1414 | 578 | 13,967 | year-round | year-round |
| Calopteryx splendens | 178/4809 | 118/2140 | 173/3948 | 135/1653 | 121/2173 | 202/2548 | 227/2817 | 1154 | 20,088 | 07-iii-2014 | 21-xi-2019 |
| Epallage fatime | 110/2084 | 83/1387 | 87/2128 | 84/434 | 54/710 | 94/738 | 144/717 | 656 | 8198 | 07-iii-2013 | 20-viii-2013 |
| Erythromma lindenii | 13/53 | 19/361 | 14/216 | 17/197 | 19/197 | 23/133 | 13/45 | 118 | 1202 | 05-iv-2019 | 11-xi-2016 |
| Erythromma viridulum | 5/9 | 2/12 | 6/23 | 17/2205 | 9/907 | 19/284 | 16/276 | 74 | 3716 | 03-iv-2018 | 12-xi-2015 |
| Ischnura elegans | 299/10,197 | 379/5232 | 425/9197 | 436/5815 | 348/7653 | 467/6841 | 439/11,234 | 2793 | 56,169 | year-round | year-round |
| Ischnura intermedia | 49/364 | 35/242 | 78/1069 | 93/798 | 77/780 | 84/926 | 64/404 | 480 | 4583 | 05-iii-2017 | 05-xii-2019 |
| Ischnura pumilio | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0 | 0 | ||
| Aeshna affinis | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0 | 0 | ||
| Aeshna isoceles | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 15/58 | 15 | 58 | 13-iv-2019 | 08-vii-2019 |
| Aeshna mixta | 46/212 | 63/207 | 85/315 | 35/66 | 82/163 | 79/194 | 75/240 | 465 | 1397 | 06-iii-2013 | 16-i-2016 |
| Anax ephippiger | 51/2508 | 20/294 | 10/37 | 15/104 | 34/142 | 26/268 | 54/1928 | 210 | 5281 | 26-i-2017 | 21-xii-2019 |
| Anax immaculifrons | 12/20 | 27/54 | 34/71 | 55/96 | 26/36 | 45/74 | 29/33 | 228 | 384 | 13-iv-2016 | 27-ix-2015 |
| Anax imperator | 37/78 | 10/21 | 7/12 | 12/17 | 16/18 | 16/21 | 11/13 | 109 | 180 | 06-iii-2013 | 28-xi-2014 |
| Anax parthenope | 133/482 | 154/771 | 131/454 | 139/375 | 141/551 | 182/444 | 149/581 | 1029 | 3658 | 04-ii-2014 | 21-xii-2018 |
| Caliaeschna microstigma | 9/14 | 9/10 | 2/2 | 8/20 | 8/11 | 14/77 | 11/10 | 61 | 144 | 24-iv-2014 | 13-vii-2013 |
| Onychogomphus forcipatus | 93/667 | 34/60 | 76/330 | 19/35 | 31/71 | 56/114 | 113/155 | 422 | 1432 | 20-iii-2013 | 16-x-2013 |
| Brachythemis impartita | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0 | 0 | ||
| Crocothemis erythraea | 283/2393 | 296/2747 | 302/2389 | 301/1301 | 261/1326 | 391/2122 | 420/2230 | 2254 | 14,508 | 20-ii-2014 | 15-xiii-2014 |
| Diplacodes lefebvrii | 29/822 | 34/197 | 25/437 | 17/94 | 14/169 | 23/140 | 20/153 | 162 | 2012 | 21-iv-2016 | 17-xi-2018 |
| Orthetrum brunneum | 112/1122 | 105/404 | 107/540 | 95/265 | 118/465 | 148/524 | 120/302 | 805 | 3622 | 20-iii-2014 | 28-x-2017 |
| Orthetrum cancellatum | 21/234 | 18/133 | 19/814 | 24/663 | 28/380 | 32/366 | 31/197 | 173 | 2787 | 29-iii-2013 | 14-ix-2013 |
| Orthetrum chrysostigma | 193/2071 | 177/1599 | 248/3470 | 220/2062 | 196/1022 | 291/1341 | 355/2265 | 1680 | 13,830 | 02-iii-2018 | 15-i-2018 * |
| Orthetrum coerulescens | 92/1186 | 130/1854 | 180/1706 | 182/1417 | 226/2171 | 279/2176 | 266/2434 | 1355 | 12,944 | 21-iii-2018 | 24-xi-2013 |
| Orthetrum sabina | 89/398 | 85/650 | 54/708 | 72/1299 | 78/749 | 74/955 | 89/793 | 541 | 5552 | 15-iii-2018 | 24-xi-2013 |
| Orthetrum taeniolatum | 38/506 | 31/94 | 47/163 | 32/71 | 13/21 | 31/76 | 48/333 | 240 | 1264 | 23-iii-2014 | 27-xi-2013 |
| Pantala flavescens | 1/1 | 13/24 | 2/5 | 9/13 | 13/17 | 45/74 | 146/233 | 229 | 367 | 09-vi-2018 | 11-i-2015 * |
| Selysiothemis nigra | 23/1069 | 34/1006 | 31/2299 | 42/1269 | 35/828 | 32/300 | 34/547 | 231 | 7318 | 20-iv-2018 | 19-x-2018 |
| Sympetrum fonscolombii | 173/10,088 | 206/7825 | 254/11,290 | 185/4329 | 249/7306 | 238/7283 | 299/17,861 | 1604 | 65,982 | year-round | year-round |
| Sympetrum meridionale | 1/11 | 2/2 | 0/0 | 0/0 | 0/0 | 0/0 | 1/0 | 4 | 13 | - | - |
| Sympetrum striolatum | 162/4536 | 172/1536 | 129/590 | 196/1756 | 136/1204 | 258/1766 | 226/4009 | 1279 | 15,397 | year-round | year-round |
| Trithemis annulata | 294/12,382 | 275/6533 | 279/6213 | 286/4089 | 252/3839 | 321/5223 | 343/7248 | 2050 | 45,527 | year-round | year-round |
| Trithemis arteriosa | 72/716 | 60/137 | 68/224 | 124/331 | 103/343 | 176/639 | 268/1397 | 871 | 3787 | 23-iii-2018 | 06-i-2018 * |
| Trithemis festiva | 172/3130 | 215/2196 | 137/2432 | 121/1259 | 218/754 | 239/961 | 1239/1764 | 1239 | 12,496 | 26-iii-2018 | 09-i-2016 * |
| Number of records | 2937 | 2893 | 3273 | 3233 | 2970 | 4089 | 4504 | 23,899 | - | ||
| Total adult count | 67,252 | 41,434 | 53,178 | 36,984 | 35,981 | 42,866 | 65,313 | - | 343,008 |
References
- Theobald, E.; Ettinger, A.; Burgess, H.; De Bey, L.; Schmidt, N.; Froehlich, H.; Wagner, C.; HilleRisLambers, J.; Tewksbury, J.; Harsch, M.A.; et al. Global change and local solutions. Tapping the unrealized potential of citizen science for biodiversity research. Biol. Conserv. 2015, 181, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Termaat, T.; van Strien, A.J.; van Grunsven, R.H.A.; De Knijf, G.; Bjelke, U.; Burbach, K.; Conze, K.-J.; Goffart, P.; Hepper, D.; Kalkman, V.J.; et al. Distribution trends of European dragonflies under climate change. Divers. Distrib. 2019, 25, 936–950. [Google Scholar] [CrossRef] [Green Version]
- Bried, J.T.; Ries, L.; Smith, B.; Patten, M.; Abbott, J.; Ball-Damerow, J.; Cannings, R.; Cordero-Rivera, A.; Cordoba-Aguilar, A.; De Marco, P., Jr.; et al. Towards global volunteer monitoring of Odonate abundance. BioScience 2020, 70, 914–923. [Google Scholar] [CrossRef]
- Kalkman, V.J.; van Pelt, G.J. The distribution and flight period of the dragonflies of Turkey. Brachytron 2006, 10, 83–153. [Google Scholar]
- Boudot, J.-P.; Kalkman, V.J. (Eds.) Atlas of the European Dragonflies and Damselflies; KNNV Publishing: Zeist, The Netherlands, 2015; p. 381. [Google Scholar]
- Boudot, J.-P.; Borisov, S.N.; De Knijf, G.; van Grunsven, R.; Schröter, A.; Kalkman, V.J. Atlas of the dragonflies and damselflies of West and Central Asia. Brachytron 2021, 3–248. [Google Scholar]
- Lopau, W.; Adena, J. Die Libellenfauna von Cypern Naturkundliche Reiseberichte. Heft 19; Private Publikation: Gnarrenburg, Germany, 2002; p. 73. [Google Scholar]
- Flint, P. Observations of dragonflies (Odonata) from northern Cyprus. Libellula 2019, 38, 1–28. [Google Scholar]
- Ministry of Agriculture, Rural Development and the Environment. Previously. Available online: https://www.cyprus.gov.cy/moa (accessed on 3 January 2020).
- Water Development Department. Dams of Cyprus. Ministry of Agriculture, Natural Resources and Environment. 2009. Available online: https://www.cyprus.gov.cy/moa/wdd (accessed on 2 July 2020).
- Kier, G.; Kreft, H.; Ming Lee, T.; Jetz, W.; Ibisch, P.L.; Nowick, C.; Mutke, J.; Barthlott, W. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. USA 2009, 106, 9322–9327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudot, J.-P.; Kalkman, V.J.; Azpilicueta Amorín, M.; Bogdanovic, T.; Cordero Rivera, A.; Degabriele, G.; Dommanget, J.-L.; Ferreira, S.; Garrigos, B.; Jovic, M.; et al. Atlas of the Odonata of the Mediterranean and North Africa. Libellula 2009, 1 (Suppl. 9), 1–256. [Google Scholar]
- De Knijf, G.; Sparrow, D.J.; Dimitriou, A.C.; Kent, R.; Kent, H.; Siedle, K.; Lewis, J.; Crossley, L. Distribution, ecology and status of a threatened species Ischnura intermedia (Insecta: Odonata), new for Europe. Int. J. Odonatol. 2016, 19, 257–274. [Google Scholar] [CrossRef]
- Vilenica, M.; Kulijer, D.; Gligorovic, B.; Gligorovic, A.; De Knijf, G. Distribution, habitat requirements and vulnerability of Caliaeschna microstigma (Odonata: Aeshnidae) at the north-western edge of the species’ range. Odonatologica 2021, in press. [Google Scholar]
- Sparrow, D.J.; De Knijf, G.; Smith, M.S.; Sparrow, R.; Michaelides, M.; Konis, D.; Siedle, K. The circumtropical Pantala flavescens is a regular visitor to Cyprus and reproducing on the island (Odonata: Libellulidae). Odonatologica 2020, 49, 289–311. [Google Scholar]
- Kalkman, V.J. Orthetrum sabina (Schneider, 1845). In Atlas of European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KNNV Publishing: Zeist, The Netherlands, 2015; pp. 283–285. [Google Scholar]
- Sparrow, D.J.; Makris, C.; Sparrow, R.; Michaelides, M.; Konis, D.; De Knijf, G. First records of Aeshna isoceles and the rediscovery of Lestes barbarus on Cyprus (Odonata: Lestidae, Aeshnidae). Not. Odonatol. 2020, 9, 185–195. [Google Scholar]
- Navas, R.P.L. Insect Orientalia. Serie 9. Mem. Accad. Pontif. Nuovi Lincei 1932, 2, 913–919. [Google Scholar]
- Boudot, J.-P.; Prentice, S. Calopteryx virgo (Linnaeus, 1758). In Atlas of European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KNNV Publishing: Zeist, The Netherlands, 2015; pp. 73–74. [Google Scholar]
- DG Environment. Reporting under Article 17 of the Habitats Directive: Explanatory Notes and Guidelines for the Period 2013–2018; DG Environment: Brussels, Belgium, 2017; p. 188. [Google Scholar]
- Care, N.; Chester, E.T.; Robson, B.J. Flow regime change alters shredder identity but not leaf litter decomposition in headwater streams affected by severe, permanent drying. Freshw. Biol. 2021, 66, 1813–1830. [Google Scholar] [CrossRef]
- Boudot, J.-P.; Raab, R. Lestes macrostigma (Eversmann, 1836). In Atlas of European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KNNV Publishing: Zeist, The Netherlands, 2015; pp. 58–60. [Google Scholar]
- Kalkman, V.J.; Marinov, M.; Kutsarov, Y. Epallage fatime (Charpentier, 1840). In Atlas of European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KNNV Publishing: Zeist, The Netherlands, 2015; pp. 78–79. [Google Scholar]
- Kalkman, V.J.; Jovic, M. Caliaeschna microstigma (Schneider, 1845). In Atlas of European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KNNV Publishing: Zeist, The Netherlands, 2015; pp. 184–185. [Google Scholar]
- Jödicke, R. Die Binsenjungfern und Winterlibellen Europas/Lestidae; Westarp Wissenschaften/Die Neue Brehm-Bücherei (Bd. 631): Magdeburg, Germany, 1997; p. 277. [Google Scholar]
- De Knijf, G.; Demolder, H. Early spring observations of Odonata from Cyprus. Libellula 2013, 32, 59–74. [Google Scholar]
- Kalkman, V.J.; Sacha, D.; David, S. Sympetrum striolatum (Charpentier, 1840). In Atlas of European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KNNV Publishing: Zeist, The Netherlands, 2015; pp. 309–311. [Google Scholar]
- Samraoui, B.; Bouzid, R.; Boulahbal, R.; Corbet, P.S. Postponed reproductive maturation in upland refuges maintains life-cycle continuity during the hot, dry season in Algerian dragonflies (Anisoptera). Int. J. Odonatol. 1998, 1, 118–135. [Google Scholar] [CrossRef]
- Samraoui, B.; Corbet, P.S. The Odonata of Numidia, Northeastern Algeria. Part II. Seasonal ecology. Int. J. Odonatol. 2000, 3, 27–39. [Google Scholar] [CrossRef]
- Dijkstra, K.-D.B.; Schröter, A.; Lewington, R. Field Guide to the Dragonflies of Britain and Europe, 2nd ed.; Bloomsbury Nature Guides: London, UK, 2020; p. 336. [Google Scholar]
- Boudot, J.-P.; Dyatlova, E. Chalcolestes parvidens (Artobolevskij, 1929). In Atlas of European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KNNV Publishing: Zeist, The Netherlands, 2015; pp. 52–53. [Google Scholar]
- Tarboton, W.; Tarboton, M. A Guide to the Dragonflies & Damselflies of South Africa; Struik Nature: Cape Town, South Africa, 2015; p. 224. [Google Scholar]
- Boudot, J.-P. Trithemis festiva (Rambur, 1842). In Atlas of European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KNNV Publishing: Zeist, The Netherlands, 2015; pp. 317–318. [Google Scholar]

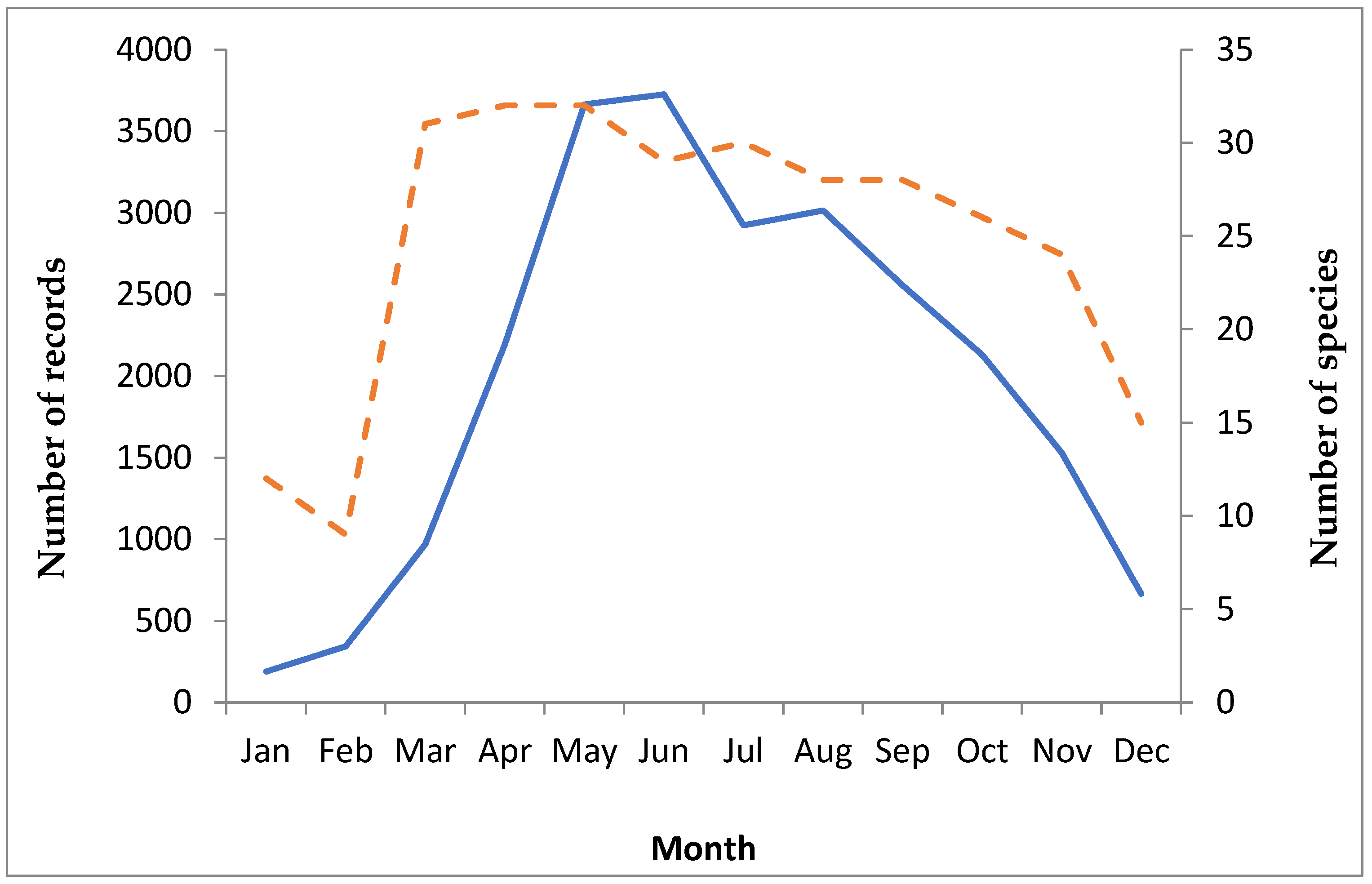

| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2013–2019 | |
|---|---|---|---|---|---|---|---|---|
| Number of visits | 865 | 1088 | 1042 | 1049 | 1062 | 1315 | 1456 | 7877 |
| Number of sites | 171 | 126 | 139 | 162 | 214 | 237 | 310 | 703 |
| Number of records | 2937 | 2893 | 3273 | 3233 | 2970 | 4089 | 4504 | 23,899 |
| Count of adults | 67,252 | 41,434 | 53,178 | 36,984 | 35,981 | 42,866 | 65,313 | 343,008 |
| Species | Total | % | Status | Criteria |
|---|---|---|---|---|
| Sympetrum fonscolombii | 282 | 40.1 | very common | |
| Crocothemis erythraea | 247 | 35.1 | very common | |
| Trithemis annulata | 231 | 32.9 | very common | |
| Ischnura elegans | 216 | 30.7 | very common | |
| Sympetrum striolatum | 202 | 28.7 | very common | |
| Trithemis arteriosa | 193 | 27.5 | very common | |
| Orthetrum chrysostigma | 178 | 25.3 | very common | |
| Anax parthenope | 152 | 21.6 | very common | ≥150 loc |
| Orthetrum brunneum | 143 | 20.3 | Common | |
| Orthetrum coerulescens | 138 | 19.6 | Common | |
| Calopteryx splendens | 134 | 19.1 | Common | |
| Sympecma fusca | 124 | 17.6 | Common | |
| Epallage fatime | 117 | 16.6 | Common | |
| Anax ephippiger | 117 | 16.6 | Common | |
| Pantala flavescens | 109 | 15.5 | Common | ≥100 loc |
| Aeshna mixta | 95 | 13.5 | Rather scarce | |
| Onychogomphus forcipatus | 93 | 13.2 | Rather scarce | |
| Trithemis festiva | 90 | 12.8 | Rather scarce | |
| Chalcolestes parvidens | 88 | 12.5 | Rather scarce | |
| Orthetrum taeniolatum | 76 | 10.8 | Rather scarce | |
| Orthetrum sabina | 73 | 10.4 | Rather scarce | |
| Anax imperator | 58 | 8.3 | Rather scarce | ≥50 loc |
| Anax immaculifrons | 47 | 6.7 | Scarce | |
| Selysiothemis nigra | 41 | 5.8 | Scarce | |
| Diplacodes lefebvrii | 39 | 5.5 | Scarce | |
| Orthetrum cancellatum | 36 | 5.1 | Scarce | |
| Erythromma lindenii | 32 | 4.6 | Scarce | |
| Caliaeschna microstigma | 28 | 4.0 | Scarce | |
| Ischnura intermedia | 24 | 3.4 | Scarce | |
| Erythromma viridulum | 19 | 2.7 | Scarce | |
| Lestes macrostigma | 10 | 1.4 | Scarce | ≥10 loc |
| Sympetrum meridionale | 4 | 0.6 | extremely rare | |
| Aeshna isoceles | 2 | 0.3 | extremely rare | |
| Lestes barbarus | 1 | 0.1 | extremely rare | <10 loc |
| Ischnura pumilio | 0 | 0.0 | no longer present | |
| Aeshna affinis | 0 | 0.0 | no longer present | |
| Brachythemis impartita | 0 | 0.0 | no longer present |
| 30-Year Period | 1901/02–1930/31 | 1931/32–1960/61 | 1961/62–1990/91 | 1991/92–2020/21 |
|---|---|---|---|---|
| Average annual rainfall mm | 559 | 524 | 503 | 476 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparrow, D.J.; De Knijf, G.; Sparrow, R.L. Diversity, Status and Phenology of the Dragonflies and Damselflies of Cyprus (Insecta: Odonata). Diversity 2021, 13, 532. https://doi.org/10.3390/d13110532
Sparrow DJ, De Knijf G, Sparrow RL. Diversity, Status and Phenology of the Dragonflies and Damselflies of Cyprus (Insecta: Odonata). Diversity. 2021; 13(11):532. https://doi.org/10.3390/d13110532
Chicago/Turabian StyleSparrow, David J., Geert De Knijf, and Rosalyn L. Sparrow. 2021. "Diversity, Status and Phenology of the Dragonflies and Damselflies of Cyprus (Insecta: Odonata)" Diversity 13, no. 11: 532. https://doi.org/10.3390/d13110532
APA StyleSparrow, D. J., De Knijf, G., & Sparrow, R. L. (2021). Diversity, Status and Phenology of the Dragonflies and Damselflies of Cyprus (Insecta: Odonata). Diversity, 13(11), 532. https://doi.org/10.3390/d13110532






