Effects of Dams on Vertebrate Diversity: A Global Analysis
Abstract
:1. Introduction
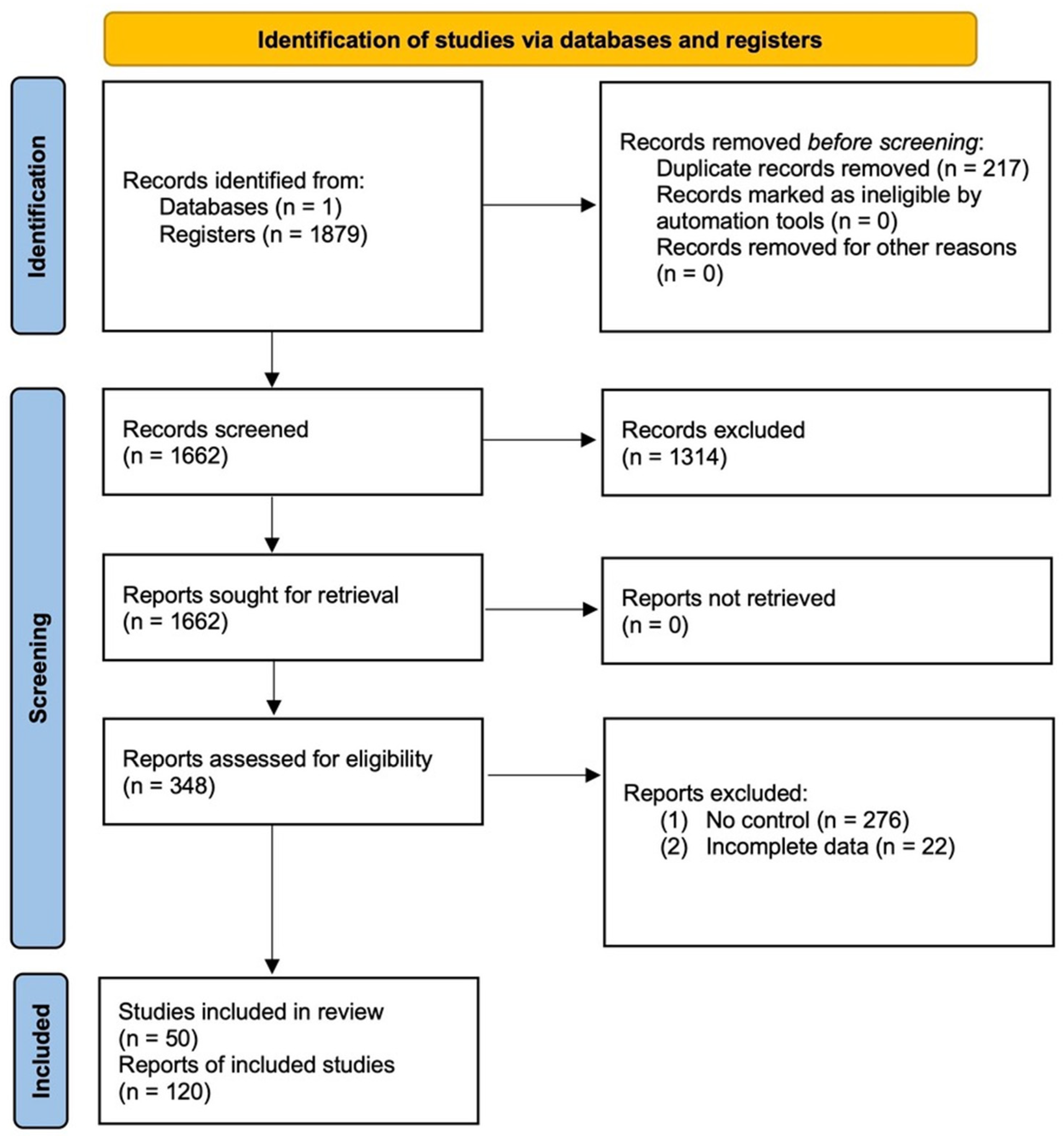
2. Materials and Methods
2.1. Literature Search and Inclusion Criteria
2.2. Calculation of Effect Size and Moderators
2.3. Model and Publication Bias
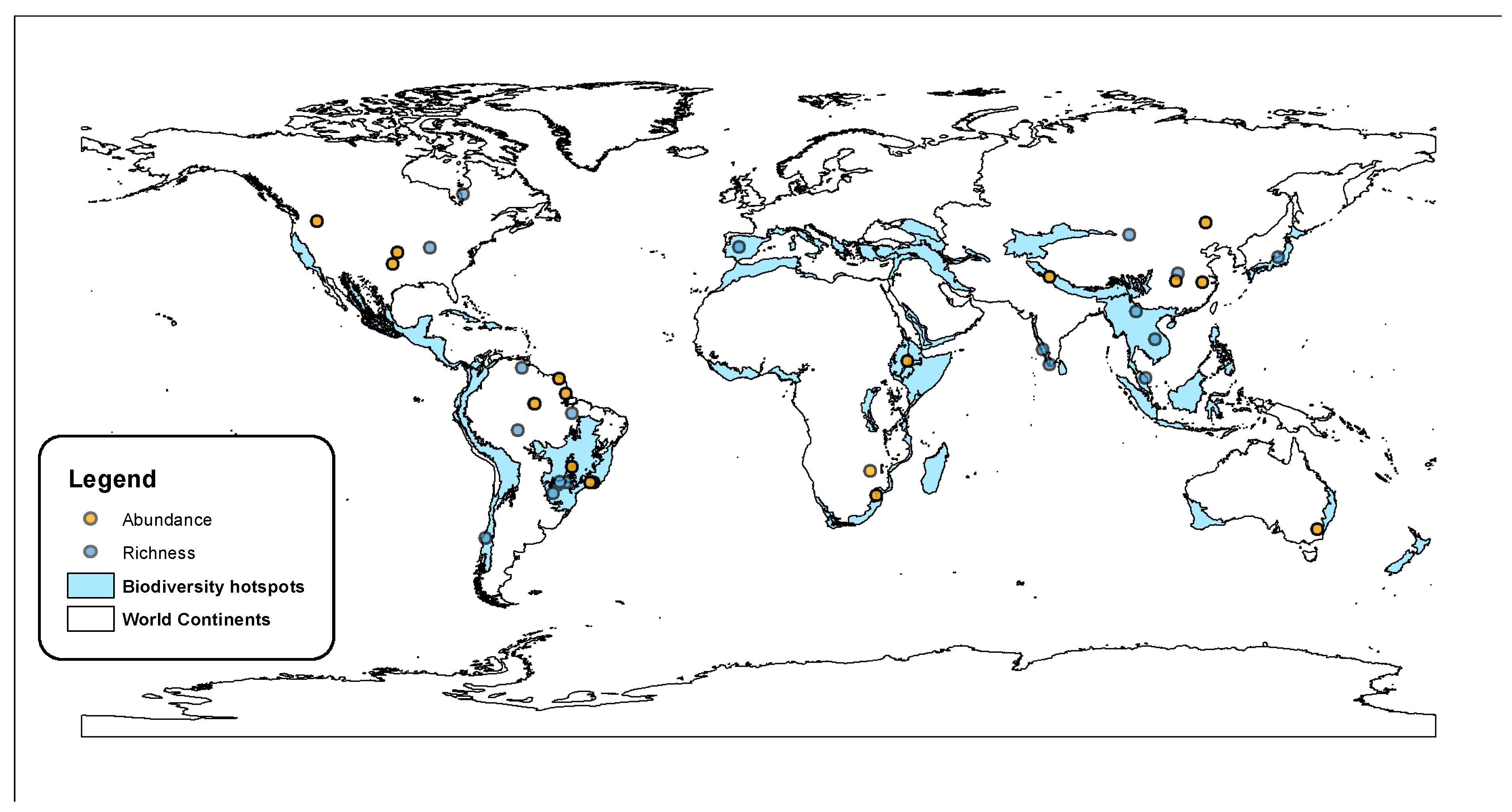
3. Results
3.1. Vertebrate Richness
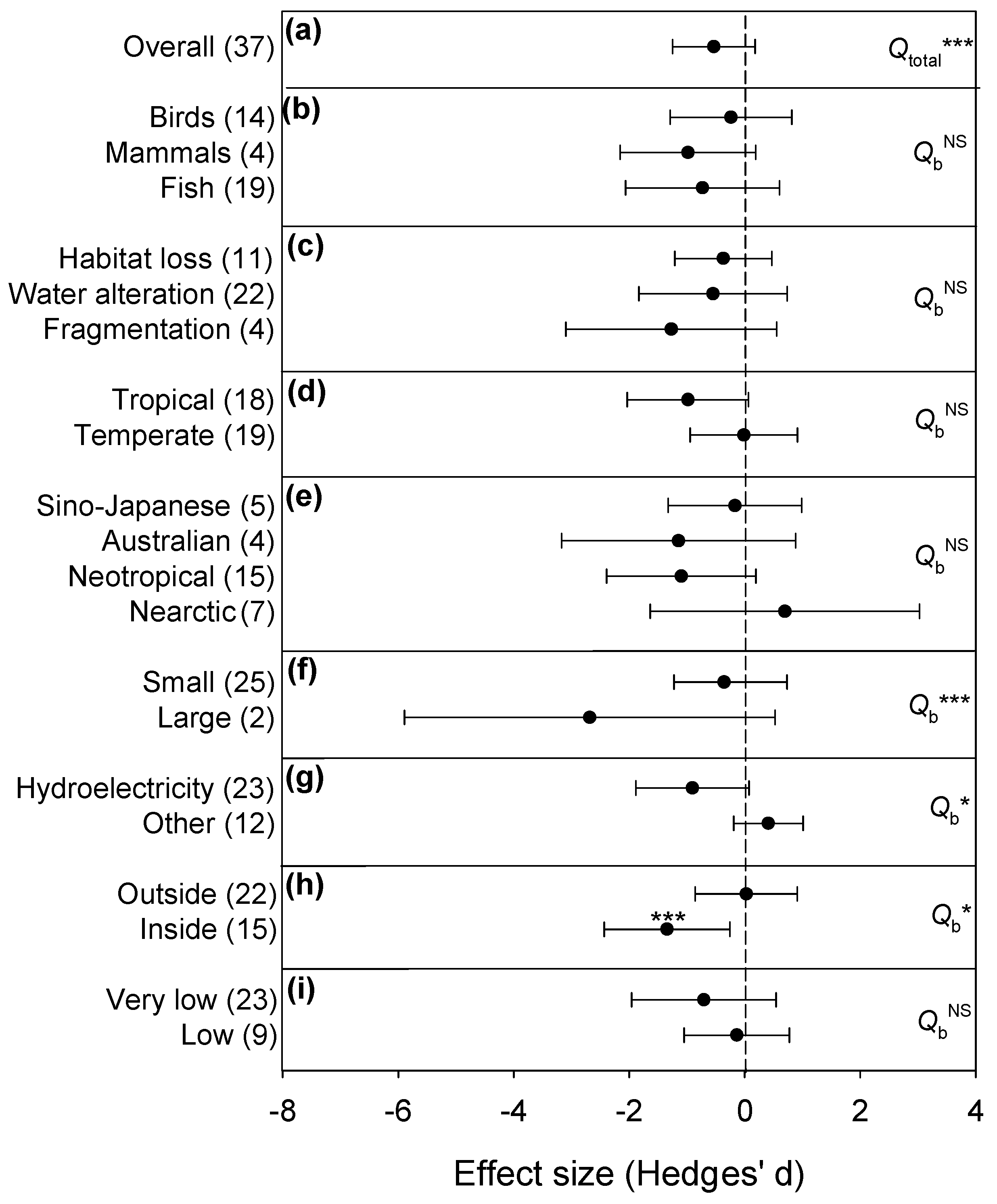
3.2. Vertebrate Abundance
3.3. Differential Effects on Aquatic and Terrestrial Vertebrates
3.4. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
References
- Kaunda, C.S.; Kimambo, C.Z.; Nielsen, T.K. Hydropower in the Context of Sustainable Energy Supply: A Review of Technologies and Challenges. ISRN Renew. Energy 2012, 2012, 730631. [Google Scholar] [CrossRef]
- Khagram, S. Dams and Development: Transnational Struggles for Water and Power; Cornell University Press: Ithaca, NY, USA, 2004. [Google Scholar]
- ICOLD. World Register of Dams. Available online: https://www.icold-cigb.org/GB/world_register/general_synthesis.asp (accessed on 8 August 2021).
- Downing, J.A.; Prairie, Y.T.; Cole, J.J.; Duarte, C.M.; Tranvik, L.J.; Striegl, R.G.; McDowell, W.H.; Kortelainen, P.; Caraco, N.F.; Melack, J.M.; et al. The global abundance and size distribution of lakes, ponds, and impoundments. Limnol. Oceanogr. 2006, 51, 2388–2397. [Google Scholar] [CrossRef] [Green Version]
- Lehner, B.; Liermann, C.R.; Revenga, C.; Vörösmarty, C.; Fekete, B.; Crouzet, P.; Döll, P.; Endejan, M.; Frenken, K.; Magome, J.; et al. High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front. Ecol. Environ. 2011, 9, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, M.; van Soesbergen, A.; Sáenz, L. GOODD, a global dataset of more than 38,000 georeferenced dams. Sci. Data 2020, 7, 31. [Google Scholar] [CrossRef]
- FAO. Núcleo de Base de Datos Principal de AQUASTAT. Available online: http://www.fao.org/aquastat/statistics (accessed on 8 August 2021).
- Zarfl, C.; Lumsdon, A.E.; Berlekamp, J.; Tydecks, L.; Tockner, K. A global boom in hydropower dam construction. Aquat. Sci. 2014, 77, 161–170. [Google Scholar] [CrossRef]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and Flow Regulation of the World’s Large River Systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAllister, D.E.; Craig, J.F.; Davidson, N.; Delany, S.; Seddon, M.; Biodiversity Impacts of Large Dams. A Contributing Paper to the World Commission on Dams. Available online: http://www.damsreport.org/docs/kbase/contrib/env245.pdf (accessed on 5 May 2021).
- Poff, N.L.; Hart, D.D. How dams vary and why it matters for the emerging science of dam removal: An ecological classifi-cation of dams is needed to characterize how the tremendous variation in the size, operational mode, age, and number of dams in a river basin influences the potential for restoring regulated rivers via dam removal. BioScience 2002, 52, 659–668. [Google Scholar] [CrossRef] [Green Version]
- New, T.; Xie, Z. Impacts of large dams on riparian vegetation: Applying global experience to the case of China’s Three Gorges Dam. Biodivers. Conserv. 2008, 17, 3149–3163. [Google Scholar] [CrossRef]
- Pandit, M.K.; Grumbine, R.E. Potential Effects of Ongoing and Proposed Hydropower Development on Terrestrial Biological Diversity in the Indian Himalaya. Conserv. Biol. 2012, 26, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, J.; Han, X.; Xie, Z.; Gao, X. Three-Gorges dam—experiment in habitat fragmentation? Science 2003, 300, 1239–1240. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.M.; McCully, P.; Pringle, C.M. Global-Scale Environmental Effects of Hydrological Alterations: Introduction. BioScience 2000, 50, 746–751. [Google Scholar] [CrossRef] [Green Version]
- Bednarek, A.T. Undamming Rivers: A Review of the Ecological Impacts of Dam Removal. Environ. Manag. 2001, 27, 803–814. [Google Scholar] [CrossRef]
- Morita, K.; Yamamoto, S. Effects of Habitat Fragmentation by Damming on the Persistence of Stream-Dwelling Charr Populations. Conserv. Biol. 2002, 16, 1318–1323. [Google Scholar] [CrossRef]
- Liermann, C.R.; Nilsson, C.; Robertson, J.; Ng, R.Y. Implications of Dam Obstruction for Global Freshwater Fish Diversity. BioScience 2012, 62, 539–548. [Google Scholar] [CrossRef]
- Cody, M.L. Habitat Selection in Birds: The Roles of Vegetation Structure, Competitors, and Productivity. BioScience 1981, 31, 107–113. [Google Scholar] [CrossRef]
- Granjon, L.; Cosson, J.-F.; Judas, J.; Ringuet, S. Influence of tropical rainforest fragmentation on mammal communities in French Guiana: Short-term effects. Acta Oecologica 1996, 17, 673–684. [Google Scholar]
- Naiman, R.J.; Dudgeon, D. Global alteration of freshwaters: Influences on human and environmental well-being. Ecol. Res. 2010, 26, 865–873. [Google Scholar] [CrossRef]
- Guzy, J.C.; Eskew, E.A.; Halstead, B.J.; Price, S.J. Influence of damming on anuran species richness in riparian areas: A test of the serial discontinuity concept. Ecol. Evol. 2018, 8, 2268–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, A.R.; Infante, D.M.; Daniel, W.M.; Wehrly, K.E.; Wang, L.; Brenden, T.O. Assessment of dam effects on streams and fish assemblages of the conterminous USA. Sci. Total Environ. 2017, 586, 879–889. [Google Scholar] [CrossRef]
- Nilsson, C.; Dynesius, M. Ecological effects of river regulation on mammals and birds: A review. Regul. Rivers: Res. Manag. 1994, 9, 45–53. [Google Scholar] [CrossRef]
- Cuttelod, A.; García, N.; Malak, D.A.; Temple, H.J.; Katariya, V. The Mediterranean: A biodiversity hotspot under threat. In Wildlife in a Changing World—An Analysis of the 2008 IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2009; Volume 89, p. 9. [Google Scholar]
- Kano, Y.; Dudgeon, D.; Nam, S.; Samejima, H.; Watanabe, K.; Grudpan, C.; Grudpan, J.; Magtoon, W.; Musikasinthorn, P.; Nguyen, P.T.; et al. Impacts of Dams and Global Warming on Fish Biodiversity in the Indo-Burma Hotspot. PLoS ONE 2016, 11, e0160151. [Google Scholar] [CrossRef] [Green Version]
- Swihart, R.K.; Gehring, T.M.; Kolozsvary, M.B.; Nupp, T.E. Responses of ‘resistant’ vertebrates to habitat loss and fragmentation: The importance of niche breadth and range boundaries. Divers. Distrib. 2002, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Bohada-Murillo, M.; Castaño-Villa, G.J.; Fontúrbel, F.E. The effects of forestry and agroforestry plantations on bird diversity: A global synthesis. Land Degrad. Dev. 2019, 31, 646–654. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Gurevitch, J.; Curtis, P.S.; Jones, M.H. Meta-analysis in ecology. Adv. Ecol. Res. 2001, 32, 199–247. [Google Scholar] [CrossRef]
- Holt, B.G.; Lessard, J.-P.; Borregaard, M.K.; Fritz, S.A.; Araújo, M.B.; Dimitrov, D.; Fabre, P.-H.; Graham, C.H.; Graves, G.R.; Jønsson, K.A.; et al. An Update of Wallace’s Zoogeographic Regions of the World. Science 2012, 339, 74–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global Biodiversity: Indicators of Recent Declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Global SDG Indicators Database. United Nations Statistics Division. Available online: https://unstats.un.org/sdgs/indicators/database/ (accessed on 12 November 2020).
- FAO. AQUASTAT. Available online: http://www.fao.org/aquastat/en/databases/dams (accessed on 2 August 2021).
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. When Does it Make Sense to Perform a Meta-Analysis. In Introduction to Meta-Analysis, 1st ed.; John Wiley & Sons, Ltd.: Chchester, UK, 2009; pp. 357–364. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Jennions, M.D.; Møller, A.P. Publication bias in ecology and evolution: An empirical assessment using the ‘trim and fill’ method. Biol. Rev. 2002, 77, 211–222. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Évid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Cheng, F.; Li, W.; Castello, L.; Murphy, B.R.; Xie, S. Potential effects of dam cascade on fish: Lessons from the Yangtze River. Rev. Fish Biol. Fish. 2015, 25, 569–585. [Google Scholar] [CrossRef]
- Dynesius, M.; Nilsson, C. Fragmentation and Flow Regulation of River Systems in the Northern Third of the World. Science 1994, 266, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Reitan, O.; Thingstad, P.G. Responses of birds to damming-a review of the influence of lakes, dams and reservoirs on bird ecology. Ornis Nor. 1999, 22, 3–37. [Google Scholar]
- Palmeirim, A.F.; Benchimol, M.; Vieira, M.V.; Peres, C.A. Small mammal responses to Amazonian forest islands are modulated by their forest dependence. Oecologia 2018, 187, 191–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Bueno, A.S.; Peres, C.A. The role of baseline suitability in assessing the impacts of land-use change on biodiversity. Biol. Conserv. 2020, 243, 108396. [Google Scholar] [CrossRef]
- Bueno, A.S.; Dantas, S.M.; Henriques, L.M.P.; Peres, C.A. Ecological traits modulate bird species responses to forest fragmentation in an Amazonian anthropogenic archipelago. Divers. Distrib. 2018, 24, 387–402. [Google Scholar] [CrossRef] [Green Version]
- Dalecky, A.; Chauvet, S.; Ringuet, S.; Claessens, O.; Judas, J.; Larue, M.; Cosson, J.-F. Large mammals on small islands: Short term effects of forest fragmentation on the large mammal fauna in French Guiana. Rev. D’écologie 2002, 57, 145. [Google Scholar]
- Naniwadekar, R.; Vasudevan, K. Impact of Dams on Riparian Frog Communities in the Southern Western Ghats, India. Diversity 2014, 6, 567–578. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Xu, J.; Zeng, G.; Sang, L.; Liu, Q.; Yin, Z.; Dai, J.; Yin, D.; Liang, J.; et al. Effects of dam construction on biodiversity: A review. J. Clean. Prod. 2019, 221, 480–489. [Google Scholar] [CrossRef]
- Benchimol, M.; Peres, C.A. Widespread Forest Vertebrate Extinctions Induced by a Mega Hydroelectric Dam in Lowland Amazonia. PLoS ONE 2015, 10, e0129818. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, R.; Agostinho, A.A.; Ferreira, E.A.; Pavanelli, C.S.; Suzuki, H.I.; Lima, D.P.; Gomes, L.C. Effects of the hydrological regime on the ichthyofauna of riverine environments of the Upper Paraná River floodplain. Braz. J. Biol. 2009, 69, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Lees, A.C.; Peres, C.A.; Fearnside, P.M.; Schneider, M.; Zuanon, J.A.S. Hydropower and the future of Amazonian biodiversity. Biodivers. Conserv. 2016, 25, 451–466. [Google Scholar] [CrossRef]
- Laurance, W.F. Forest destruction in tropical Asia. Curr. Sci. 2007, 93, 1544–1550. [Google Scholar]
- Possingham, H.; Andelman, S.J.; Burgman, M.A.; Medellín, R.A.; Master, L.L.; Keith, D.A. Limits to the use of threatened species lists. Trends Ecol. Evol. 2002, 17, 503–507. [Google Scholar] [CrossRef] [Green Version]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2018, 94, 849–873. [Google Scholar] [CrossRef] [Green Version]
- Jones, I.L.; Bull, J.W. Major dams and the challenge of achieving “No Net Loss” of biodiversity in the tropics. Sustain. Dev. 2019, 28, 435–443. [Google Scholar] [CrossRef] [Green Version]

| Group | Class | Order | Families |
|---|---|---|---|
| Fish | Actinopterygii | 22 | 57 |
| Herpetofauna | Amphibia | 1 | 7 |
| Reptilia | 1 | 4 | |
| Birds | Aves | 21 | 57 |
| Mammals | Mammalia | 8 | 18 |
| Moderator | Level | Mean Effect | 95% Conf Int | Q Value |
|---|---|---|---|---|
| (a) Species richness | ||||
| Overall effect (45) | 0.219 | −0.179–0.617 | 103.77 *** | |
| Disturbance | Stream alteration (40) | 0.250 | −0.185–0.685 | 2.96 NS |
| Fragmentation (5) | −0.182 | −0.741–0.376 | ||
| Zone | Tropical (20) | 0.646 | −0.171–1.464 | 2.01 NS |
| Temperate (25) | 0.054 | −0.252–0.361 | ||
| Zoogeo | Neotropical (21) | 0.698 | −0.081–1.477 | 2.82 NS |
| Nearctic (9) | 0.015 | −0.635–0.666 | ||
| Sino-Japanese (10) | −0.012 | −0.561–0.538 | ||
| Size | Small (16) | 0.798 | −0.206–0.319 | 2.08 NS |
| Large (13) | 0.057 | −0.267–1.862 | ||
| Purpose | Hydroelectricity (27) | 0.434 | −0.134–1.003 | 1.68 NS |
| Other (12) | −0.128 | −0.863–0.608 | ||
| Hotspot | Outside (17) | 0.298 | −0.342–0.938 | 0.12 NS |
| Inside (26) | 0.156 | −0.396–0.708 | ||
| Red index | Low (15) | −0.106 | −0.489–0.277 | 2.19 NS |
| Very low (28) | 0.411 | −0.205–1.026 | ||
| (b) Abundance | ||||
| Overall effect (18) | 0.665 | −2.097–0.766 | 100.11 *** | |
| Disturbance | Stream alteration (18) | 0.665 | −2.097–0.766 | N/A |
| Zone | Tropical (10) | 0.983 | −3.059–1.093 | 1.19 NS |
| Temperate (8) | 0.073 | −0.641–0.787 | ||
| Zoogeo | Neotropical (10) | −0.983 | −3.059–1.093 | 1.88 NS |
| Nearctic (4) | −3.933 | −19.179–11.313 | ||
| Sino-Japanese (4) | 0.037 | −0.454–0.528 | ||
| Size | Small (12) | −0.748 | −2.418–0.921 | N/A |
| Purpose | Hydroelectricity (10) | −0.983 | −3.059–1.093 | 1.19 NS |
| Other (8) | 0.073 | −0.641–0.787 | ||
| Hotspot | Outside (12) | −0.032 | −1.112–1.048 | 1.76 NS |
| Inside (6) | −1.887 | −5.256–1.483 | ||
| Red index | Low (4) | 0.037 | −0.454–0.529 | 0.99 NS |
| Very low (12) | −1.203 | −3.930–1.524 | ||
| Moderator | Level | Mean Effect | 95% Conf Int | Q Value |
|---|---|---|---|---|
| (a) Species richness | ||||
| Overall effect (36) | −0.886 | −1.403–−0.369 | 192.97 *** | |
| Taxa | Birds (23) | −0.608 | −1.187–−0.029 | |
| Mammals (8) | −2.057 | −3.791–−0.323 | ||
| Herpeto (5) | −0.623 | −1.911–0.665 | ||
| Disturbance | Stream alteration (6) | 0.203 | −1.134–1.539 | 21.06 *** |
| Fragmentation (16) | −1.977 | −2.694–−1.259 | ||
| Habitat loss (14) | −0.236 | −0.761–0.289 | ||
| Zone | Tropical (24) | −1.304 | −1.956–−0.652 | 7.46 ** |
| Temperate (12) | −0.054 | −0.784–0.676 | ||
| Zoogeo | Neotropical (17) | −1.799 | −2.591–−1.008 | 22.10 *** |
| Nearctic (6) | 0.541 | −0.310–1.392 | ||
| Oriental (6) | −0.439 | −1.111–0.232 | ||
| Australian (6) | −0.340 | −1.721–1.040 | ||
| Size | Small (23) | −0.565 | −1.165–0.034 | 4.36 * |
| Large (10) | −1.799 | −2.966–−0.634 | ||
| Purpose | Hydroelectricity (32) | −1.001 | −1.555–−0.446 | N/A |
| Hotspot | Outside (22) | −1.237 | −2.016–−0.458 | 3.34 NS |
| Inside (14) | −0.407 | −0.962–0.148 | ||
| Red index | Low (7) | −0.556 | −1.250–0.138 | 0.01 NS |
| Very low (22) | −0.573 | −1.323–0.177 | ||
| (b) Abundance | ||||
| Overall effect (18) | −0.383 | −1.206–0.440 | 125.57 *** | |
| Taxa | Birds (14) | −0.239 | −1.294–0.817 | 1.47 NS |
| Mammals (4) | −0.982 | −2.157–0.193 | ||
| Disturbance | Fragmentation (4) | −1.271 | −3.097–0.555 | 2.24 NS |
| Habitat loss (11) | −0.370 | −1.210–0.469 | ||
| Zone | Tropical (7) | −0.981 | −1.855–−0.107 | 1.76 NS |
| Temperate (11) | −0.067 | −1.379–1.245 | ||
| Zoogeo | Neotropical (4) | 1.271 | −3.097–0.555 | 0.02 NS |
| Australian (4) | −1.15 | −3.173–0.882 | ||
| Size | Small (13) | 0.007 | −0.979–0.993 | N/A |
| Purpose | Hydroelectricity (12) | −0.756 | −1.961–0.449 | 7.85 ** |
| Other (4) | 0.906 | 0.181–1.632 | ||
| Hotspot | Outside (10) | 0.066 | −1.321–1.454 | 2.71 NS |
| Inside (8) | −0.994 | −1.453–−0.534 | ||
| Red index | Low (5) | −0.376 | −2.494–1.742 | 0.01 NS |
| Very low (10) | −0.303 | −1.681–1.076 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohada-Murillo, M.; Castaño-Villa, G.J.; Fontúrbel, F.E. Effects of Dams on Vertebrate Diversity: A Global Analysis. Diversity 2021, 13, 528. https://doi.org/10.3390/d13110528
Bohada-Murillo M, Castaño-Villa GJ, Fontúrbel FE. Effects of Dams on Vertebrate Diversity: A Global Analysis. Diversity. 2021; 13(11):528. https://doi.org/10.3390/d13110528
Chicago/Turabian StyleBohada-Murillo, Mauricio, Gabriel J. Castaño-Villa, and Francisco E. Fontúrbel. 2021. "Effects of Dams on Vertebrate Diversity: A Global Analysis" Diversity 13, no. 11: 528. https://doi.org/10.3390/d13110528
APA StyleBohada-Murillo, M., Castaño-Villa, G. J., & Fontúrbel, F. E. (2021). Effects of Dams on Vertebrate Diversity: A Global Analysis. Diversity, 13(11), 528. https://doi.org/10.3390/d13110528








