Environmental and Management Control over the Submontane Grassland Plant Communities in Central Slovakia
Abstract
1. Introduction
- (i)
- How is plant species richness related to evaluated environmental variables?
- (ii)
- Does management affect species richness of the studied grasslands?
- (iii)
- Does species composition respond to different grassland management?
2. Materials and Methods
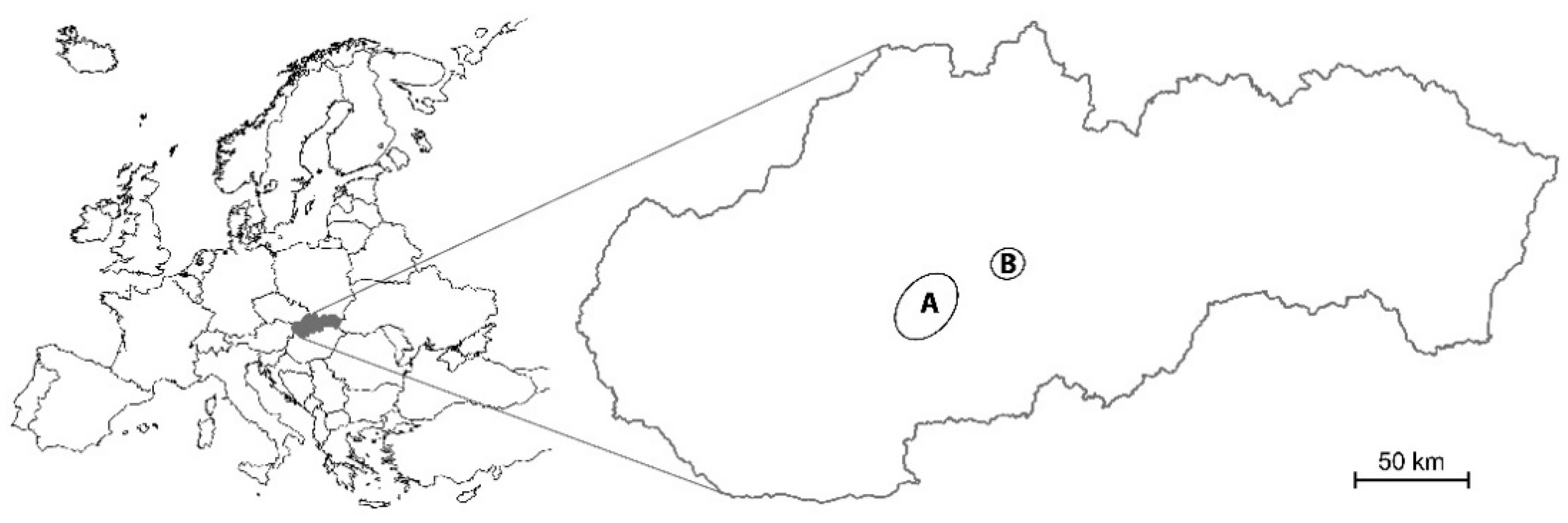
2.1. Study Area
2.2. Field Data Collection
2.3. Soil Analyses
2.4. Data Analyses
3. Results
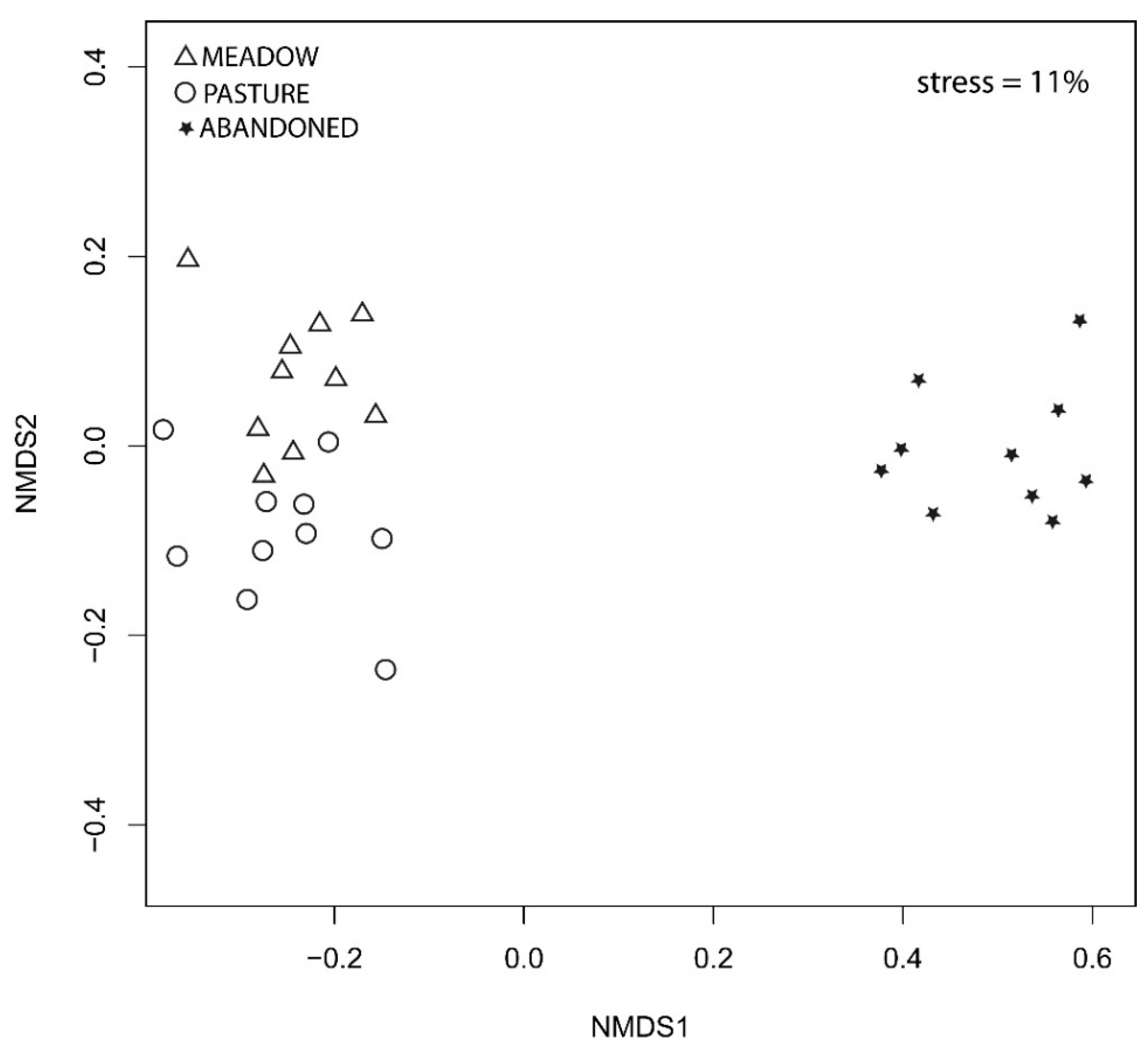
3.1. Floristic Diversity of Grasslands
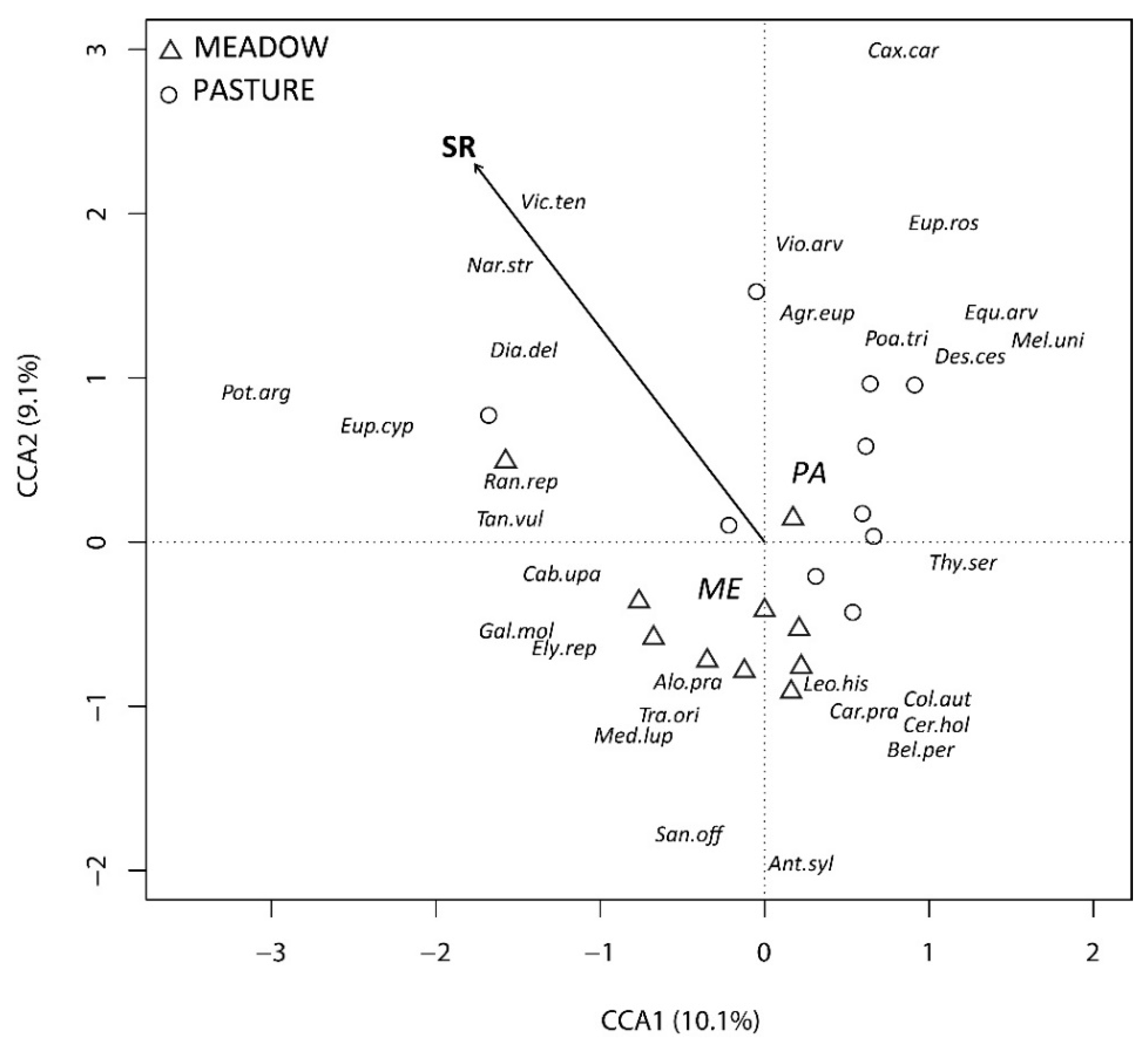
3.2. Determinants of Diversity and Community Composition
4. Discussion
4.1. Floristic Diversity of Grasslands
4.2. Determinants of Diversity and Community Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| MEADOW | PASTURE | ABANDONED | |
|---|---|---|---|
| Light | 7.04 | 7.03 | 6.77 |
| Temperature | 5.41 | 5.42 | 5.20 |
| Continentality | 3.71 | 3.65 | 3.67 |
| Moisture | 4.95 | 4.92 | 7.06 |
| Soil reaction | 6.12 | 5.88 | 6.29 |
| Nutrients | 4.76 | 4.54 | 5.31 |
References
- Fischer, M.; Wipf, S. Effect of low-intensity grazing on the species-rich vegetation of traditionally mown subalpine meadows. Biol. Conserv. 2002, 104, 1–11. [Google Scholar] [CrossRef]
- Myklestad, A.; Satersdal, M. The importance of traditional meadow usement techniques for conservation of vascular plant species richness in Norway. Biol. Conserv. 2004, 118, 133–139. [Google Scholar] [CrossRef]
- Merunková, K.; Chytrý, M. Environmental control of species richness and composition in upland grasslands of the southern Czech Republic. Plant Ecol. 2012, 213, 591–602. [Google Scholar] [CrossRef]
- Öckinger, E.; Smith, H.G. Semi-natural grasslands as population sources for pollinating insects in agricultural landscapes. J. Appl. Ecol. 2007, 44, 50–59. [Google Scholar] [CrossRef]
- Bazzoffi, P. Soil erosion tolerance and water runoff control: Minimum environmental standards. Reg. Environ. Chang. 2009, 9, 169–179. [Google Scholar] [CrossRef]
- Chytrý, M.; Dražil, T.; Hájek, M.; Kalníková, V.; Preislerová, Z.; Šibík, J.; Ujházy, K.; Axmanová, I.; Bernátová, D.; Blanár, D.; et al. The most species-rich plant communities in the Czech Republic and Slovakia (with new world records). Preslia 2015, 87, 217–278. [Google Scholar]
- Crofts, A.; Jefferson, R.G. The Lowland Grassland Management Handbook, 2nd ed.; English Nature/The Wildlife Trusts: Peterborough, UK, 1994. [Google Scholar]
- Allan, E.; Manning, P.; Alt, F.; Binkenstein, J.; Blaser, S.; Blüthgen, N.; Blüthgen, N.; Böhm, S.; Grassein, F.; Hölzel, N.; et al. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 2015, 18, 834–843. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Mitteleuropas mit den Alpen in Ökologischer, Dynamischer und Historischer Sicht; Ulmer: Stuttgart, Germany, 1996. [Google Scholar]
- Janišová, M.; Michalcová, D.; Bacaro, G.; Ghisla, A. Landscape effects on diversity of semi-natural grasslands. Agric. Ecosyst. Environ. 2014, 182, 47–58. [Google Scholar] [CrossRef]
- Dorresteijn, I.; Loos, J.; Hanspach, J.; Fischer, J. Socioecological drivers facilitating biodiversity conservation in traditional farming landscapes. Ecosyst. Health Sustain. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Pykälä, J.; Luoto, M.; Heikkinen, R.K.; Kontula, T. Plant species richness and persistence of rare plants in abandoned semi-natural grasslands in northern Europe. Basic Appl. Ecol. 2005, 6, 25–33. [Google Scholar] [CrossRef]
- Sutcliffe, L.; Batáry, P.; Kormann, U.; Báldi, A.; Dicks, L.; Herzon, I.; Kleijn, D.; Tryjanowski, P.; Apostolova, I.; Arlettaz, R.; et al. Harnessing the biodiversity value of Central and Eastern European farmland. Divers. Distrib. 2015, 21, 722–730. [Google Scholar] [CrossRef]
- Collins, S.L.; Knapp, A.K.; Briggs, J.M.; Blair, J.M.; Steinauer, E.M. Modulation of diversity by grazing and mowing in native tallgrass prairie. Science 1998, 280, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Nekrošienė, R.; Skuodienė, R. Changes in floristic composition of meadow phytocenoses, as landscape stability indicators, in protected areas in Western Lithuania. Pol. J. Environ. Stud. 2012, 21, 703–711. [Google Scholar]
- Steinshamn, H.; Grøva, L.; Adler, S.A.; Brunberg, E.; Lande, U.S. Effects of grazing abandoned grassland on herbage production and utilization, and sheep preference and performance. Front. in Environ. Sci. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Klaus, V.H.; Kleinebecker, T.; Prati, D.; Gossner, M.M.; Alt, F.; Boch, S.; Gockel, S.; Hempe, A.; Langef, M.; Müller, J.; et al. Does organic grassland farming benefit plant and arthropod diversity at the expense of yield and soil fertility? Agric. Ecosyst. Environ. 2013, 177, 1–9. [Google Scholar] [CrossRef]
- Kruse, M.; Stein-Bachinger, K.; Gottwald, F.; Schmidt, E.; Heinken, T. Influence of grassland management on the biodiversity of plants and butterflies on organic suckler cow farms. Tuexenia 2016, 36, 97–119. [Google Scholar]
- Marini, L.; Fontana, P.; Klimek, S.; Battisti, A.; Gaston, K.J. Impact of farm size and topography on plant and insect diversity of managed grasslands in the Alps. Biol. Conserv. 2009, 142, 394–403. [Google Scholar] [CrossRef]
- Poschlod, P.; Bakker, J.P.; Kahmen, S. Changing land use and its impact on biodiversity. Basic Appl. Ecol. 2005, 6, 93–98. [Google Scholar] [CrossRef]
- Niedrist, G.; Tasser, E.; Lüth, C.; Dalla Via, J.; Tappeiner, U. Plant diversity declines with recent land use changes in European Alps. Plant Ecol. 2009, 202, 195–210. [Google Scholar] [CrossRef]
- Tälle, M.; Fogelfors, H.; Westerberg, I.; Milberg, P. The conservation benefit of mowing vs grazing for management of species-rich grasslands: A multi-site, multi-year field experiment. Nord. J. Bot. 2015, 33, 761–768. [Google Scholar] [CrossRef]
- Gilhaus, K.; Boch, S.; Fischer, M.; Hölzel, N.; Kleinebecker, T.; Prati, D.; Rupprecht, D.; Schmitt, B.; Klaus, V.H. Grassland management in Germany: Effects on plant diversity and vegetation composition. Tuexenia 2017, 37, 379–397. [Google Scholar]
- Chabuz, W.; Kulik, M.; Sawicka-Zugaj, W.; Zołkiewski, P.; Warda, M.; Pluta, M.; Lipiec, A.; Bochniak, A.; Zdulski, J. Impact of the type of use of permanent grasslands areas in mountainous regions on the floristic diversity of habitats and animal welfare. Glob. Ecol. Conserv. 2019, 19, e00629. [Google Scholar] [CrossRef]
- Chytrý, M.; Danihelka, J.; Ermakov, N.; Hájek, M.; Hájková, P.; Kočí, M.; Kubešová, S.; Lustyk, P.; Otýpková, Z.; Popov, D.; et al. Plant species richness in continental southern Siberia: Effects of pH and climate in the context of the species pool hypothesis. Glob. Ecol. Biogeogr. 2007, 16, 668–678. [Google Scholar] [CrossRef]
- Crawley, M.J.; Johnston, A.E.; Silvertown, J.; Dodd, M.; de Mazancourt, C.; Heard, M.S.; Henman, D.F.; Edwards, G.R. Determinants of species richness in the Park Grass Experiment. Am. Nat. 2005, 165, 179–192. [Google Scholar] [CrossRef]
- Critchley, C.N.R.; Chambers, B.J.; Fowbert, J.A.; Bhogal, A.; Rose, S.C.; Sanderson, R.A. Plant species richness, functional type and soil properties of grasslands and allied vegetation in English environmentally sensitive areas. Grass Forage Sci. 2002, 57, 82–92. [Google Scholar] [CrossRef]
- Hejcman, M.; Klaudisová, M.; Schellberg, J.; Honsová, D. The Rengen Grassland Experiment: Plant species composition after 64 years of fertilizer application. Agric. Ecosyst. Environ. 2007, 122, 259–266. [Google Scholar] [CrossRef]
- Roem, W.J.; Berendse, F. Soil acidity and nutrient supply ratio as possible factors determining changes in plant species diversity in grassland and heathland communities. Biol. Conserv. 2000, 92, 151–161. [Google Scholar] [CrossRef]
- Bruun, H.H. Patterns of species richness in dry grassland patches in an agricultural landscape. Ecography 2000, 23, 641–650. [Google Scholar] [CrossRef]
- Akatov, V.; Chefranov, S.; Akatova, T. The Relationship between Local Species Richness and Species Pool: A Case Study from the High Mountains of the Greater Caucasus. Plant. Ecol. 2005, 181, 9–22. [Google Scholar] [CrossRef]
- Halada, Ľ.; David, S.; Hreško, J.; Klimantová, A.; Bača, A.; Rusňák, T.; Buraľ, M.; Vadel, Ľ. Changes in grassland management and plant diversity in a marginal region of the Carpathian Mts. in 1999–2015. Sci. Total Environ. 2017, 609, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Janišová, M.; Hájková, P.; Hegedüšová, K.; Hrivnák, R.; Kliment, J.; Michálková, D.; Ružičková, H.; Řezníčková, M.; Tichý, L.; Škodová, I.; et al. Travinnobylinná Vegetácia Slovenska—Elektronický Expertný Systém na Identifikáciu Syntaxónov; Botanický ústav SAV: Bratislava, Slovakia, 2007. [Google Scholar]
- Janišová, M. Vegetation-environment relationships in dry calcareous grassland. Ekologia 2005, 24, 25–44. [Google Scholar]
- Imrichová, Z. Impact of management practices on diversity of grasslands in agricultural region of middle Slovakia. Ekologia 2006, 25 (Suppl. S1), 76–84. [Google Scholar]
- Pavlů, V.; Hejcman, M.; Pavlů, L.; Gaisler, J. Restoration of grazing management and its effect on vegetation in an upland grassland. Appl. Veg. Sci. 2007, 10, 375–382. [Google Scholar] [CrossRef]
- Janišová, M.; Uhliarová, E.; Hlásny, T.; Turisová, I. Vegetation-environment relationships in grassland communities of central Slovakia. Tuexenia 2010, 30, 423–443. [Google Scholar]
- Kopeć, M.; Zarzycki, J.; Gondek, K. Species diversity of submontane grasslands: Effects of topographic and soil factors. Pol. J. Ecol. 2010, 58, 285–295. [Google Scholar]
- Galvánek, D.; Lepš, J. The effect of management on productivity, litter accumulation and seedling recruitment in a Carpathian mountain grassland. Plant Ecol. 2012, 213, 523–533. [Google Scholar] [CrossRef]
- Pruchniewicz, D.; Żołnierz, L. The influence of environmental factors and management methods on the vegetation of mesic grasslands in a central European mountain range. Flora 2014, 209, 687–692. [Google Scholar] [CrossRef]
- Duffková, R.; Hakrová, P.; Brom, J.; Fučík, P. Effects of management practices in highland pastures on agronomic and environmental objectives. Appl. Ecol. Environ. Res. 2017, 1, 1677–1695. [Google Scholar] [CrossRef]
- Gaisler, J.; Pavlů, L.; Nwaogu, C.; Pavlů, K.; Hejcman, M.; Pavlů, V.V. Long-term effects of mulching, traditional cutting and no management on plant species composition of improved upland grassland in the Czech Republic. Grass Forage Sci. 2019, 74, 463–475. [Google Scholar] [CrossRef]
- Faško, P.; Šťastný, P.; Lapin, M.; Šramková, N. Mean monthly precipitation totals. In Landscape Atlas of the Slovak Republic, 1st ed.; Slovak Environmental Agency, Ministry of Environment of the Slovak Republic: Banská Bystrica, Slovakia, 2002; pp. 100–101. [Google Scholar]
- Lapin, M.; Faško, P.; Melo, M.; Šťastný, P.; Tomlain, J. 2002 Cimatic regions. In Landscape Atlas of the Slovak Republic, 1st ed.; Slovak Environmental Agency, Ministry of Environment of the Slovak Republic: Banská Bystrica, Slovakia, 2002; pp. 94–95. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde, 3rd ed.; Springer: Vienna, Austria, 1964. [Google Scholar]
- Westhoff, V.; van der Maarel, E. The Braun-Blanquet approach. In Ordination and Classification of Communities; Whittaker, R.H., Ed.; Dr. W. Junk: Dordrecht, The Netherlands, 1973; pp. 617–726. [Google Scholar]
- Marhold, K.; Hindák, F. (Eds.) Checlist of Non-Vascular and Vascular Plants of Slovakia; Veda: Bratislava, Slovakia, 1998. [Google Scholar]
- Novikmec, M.; Svitok, M.; Kočický, D.; Šporka, F.; Bitušík, P. Surface Water Temperature and Ice Cover of Tatra Mountains Lakes Depend on Altitude, Topographic Shading, and Bathymetry. Arct. Antarc. Alp. Res. 2013, 45, 77–87. [Google Scholar] [CrossRef]
- Hrivnáková, K.; Makovníková, J.; Barančíková, G.; Bezák, P.; Bezáková, Z.; Dodok, R.; Grečo, V.; Chlpík, J.; Kobza, J.; Lištjak, M.; et al. Jednotné Pracovné Postupy Rozborov pôd; Výskumný ústav pôdoznalectva a ochrany pôdy: Bratislava, Slovakia, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 September 2020).
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 1992, 18, 1–258. [Google Scholar]
- Eliáš, P.; Dítě, D.; Kliment, J.; Hrivnák, R. Red list of ferns and flowering plants of Slovakia, 5th edition. Biologia 2015, 70, 218–228. [Google Scholar] [CrossRef]
- Uhliarová, E.; Janišová, M.; Ujházy, K. Arrhenatherion. In Travinnobylinná Vegetácia Slovenska—Elektronický Expertný Systém na Identifikáciu Syntaxónov; Janišová, M., Ed.; Botanický Ústav SAV: Bratislava, Slovakia, 2007; pp. 142–145. [Google Scholar]
- Stanová, V.; Valachovič, M. (Eds.) Katalóg Biotopov Slovenska; DAPHNE—Inštitút Aplikovanej Ekológie: Bratislava, Slovakia, 2002. [Google Scholar]
- Hájková, P. Calthion palustris Tüxen 1937. In Travinnobylinná Vegetácia Slovenska—Elektronický Expertný Systém na Identifikáciu Syntaxónov; Janišová, M., Ed.; Botanický Ústav SAV: Bratislava, Slovakia, 2007; pp. 134–162. [Google Scholar]
- Klimek, S.; Richter Kemmermann, A.; Hofmann, M.; Isselstein, J. Plant species richness and composition in managed grasslands: The relative importance of field management and environmental factors. Biol. Conserv. 2007, 134, 559–570. [Google Scholar] [CrossRef]
- Tälle, M.; Deák, B.; Poschlod, P.; Valkó, O.; Westerberg, L.; Milberg, P. Grazing vs. mowing: A meta-analysis of biodiversity benefits for grassland management. Agric. Ecosyst. Environ. 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Cousins, S.A.O.; Lindborg, R.; Mattsson, S. Land use history and site location are more important for grassland species richness than local soil properties. Nord. J. Bot. 2009, 27, 483–489. [Google Scholar] [CrossRef]
- Cousins, S.A.O.; Eriksson, O. Plant species occurrences in a rural hemiboreal landscape: Effects of remnant habitats, site history, topography and soil. Ecography 2001, 24, 461–469. [Google Scholar] [CrossRef]
- Wellstein, C.; Otte, A.; Waldhardt, R. Impact of site and management on the diversity of central European mesic grassland. Agric. Ecosyst. Environ. 2007, 122, 203–210. [Google Scholar] [CrossRef]
- Pärtel, M.; Bruun, H.H.; Sammul, M. Biodiversity in temperate European grasslands: Origin and conservation. In Integrating Efficient Grassland Farming and Biodiversity, Grassland Science in Europe; Lillak, R., Viiralt, R., Linke, A., Geherman, V., Eds.; Estonian Grassland Society: Tartu, Estonia, 2005; Volume 10, pp. 1–14. [Google Scholar]
- Chytrý, M.; Tichý, L.; Roleček, J. Local and regional patterns of species richness in Central European vegetation types along the pH/calcium gradient. Folia Geobot. 2003, 38, 429–442. [Google Scholar] [CrossRef]
- Janssens, F.; Peeters, A.; Tallowin, J.R.B.; Bakker, R.M.; Fillat, F.; Oomes, M.J.M. Relation between soil chemical factors and grasslands diversity. Plant Soil 1998, 202, 69–78. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; West, J.B.; Hobbie, S.E.; Reich, P.B. Plant diversity, CO2, and N influence inorganic and organic n leaching in grasslands. Ecology 2007, 88, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Gowing, D.; Wallace, H. Available soil phosphorus in semi-natural grasslands: Assessment methods and community tolerances. Biol. Conserv. 2009, 142, 1074–1083. [Google Scholar] [CrossRef]
- Michalcová, D.; Gilbert, J.C.; Lawson, C.S.; Gowing, D.J.G. The combined effect of waterlogging, extractable P and soil pH on a-diversity: A case study on mesotrophic grasslands in the UK. Plant Ecol. 2011, 212, 879–888. [Google Scholar] [CrossRef]
- Marini, L.; Scotton, M.; Klimek, S.; Isselstein, J.; Pecile, A. Effects of local factors on plant species richness and composition of Alpine meadows. Agric. Ecosyst. Environ. 2007, 119, 281–288. [Google Scholar] [CrossRef]
- Soons, M.B.; Hefting, M.M.; Dorland, E.; Lamers, L.P.M.; Versteeg, C.; Bobbink, R. Nitrogen effects on plant species richness in herbaceous communities are more widespread and stronger than those of phosphorus. Biol. Conserv. 2017, 212, 390–397. [Google Scholar] [CrossRef]
- Ren, Z.; Li, Q.; Chu, C.; Zhao, L.; Zhang, J.; Ai, D.; Yang, Y.; Wang, G. Effects of resource additions on species richness and ANPP in an alpine meadow community. J. Plant. Ecol. 2010, 3, 25–31. [Google Scholar] [CrossRef]
- Roth, T.; Kohli, L.; Rihm, B.; Achermann, B. Nitrogen deposition is negatively related to species richness and species composition of vascular plants and bryophytes in Swiss mountain grassland. Agric. Ecosyst. Environ. 2013, 178, 121–126. [Google Scholar] [CrossRef]
- Li, X.; Nie, Y.; Song, X.; Zhang, R.; Wang, G. Patterns of species diversity and functional diversity along the southto north-facing slope gradient in a sub-alpine meadow. Community Ecol. 2011, 2, 179–187. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, G.; Bao, W.; Chen, L.; Pei, S.; Fan, N. Effects of topographic factors on the plant species richness and distribution pattern of alpine meadow in source region of Lancang River, Southwest China. Chin. J. Ecol. 2019, 31, 2767–2774. [Google Scholar]
- Pittarello, M.; Lonati, M.; Ravetto Enri, S.; Lombardi, G. Environmental factors and management intensity affect in different ways plant diversity and pastoral value of alpine pastures. Ecol. Indic. 2020, 115, 106429. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Ecology of Central Europe, 4th ed.; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Raatikainen, K.J.; Oldén, A.; Käyhkö, N.; Mönkkönen, M.; Halme, P. Contemporary spatial and environmental factors determine vascular plant species richness on highly fragmented meadows in Central Finland. Landsc. Ecol. 2018, 33, 2169–2187. [Google Scholar] [CrossRef]
- Kahmen, S.; Poschlod, P.; Schreiber, K.F. Conservation management of calcareous grasslands. Changes in plant species composition and response of functional traits during 25 years. Biol. Conserv. 2002, 104, 319–328. [Google Scholar] [CrossRef]
- Köhler, M.; Hiller, G.; Tischew, S. Year-round horse grazing supports typical vascular plant species, orchids and rare bird communities in a dry calcareous grassland. Agric. Ecosyst. Environ. 2016, 234, 48–57. [Google Scholar] [CrossRef]
- Bennie, J.; Hill, M.O.; Baxter, R.; Huntley, B. Influence of slope and aspect on long-term vegetation change in British chalk grasslands. J. Ecol. 2006, 94, 355–368. [Google Scholar] [CrossRef]
| Variable | Abbreviation | MEADOW | PASTURE | ABANDONED |
|---|---|---|---|---|
| Average (min., max.) | ||||
| Altitude | ALT | 626 (490, 765) | 626.7 (481, 767) | 616 (502, 743) |
| pH | pH | 5.6 (4.9, 6.6) | 5.5 (5.0, 6.3) | 6.1 (5.3, 7.1) |
| Electric conductivity (μS·cm−1) | EC | 207 (106, 379) | 263 (172, 575) | 569 (220, 1110) |
| Phosphorus (mg·kg−1) | P | 9.77 (2.9, 24.1) | 11.2 (1.8, 32.5) | 4.7 (1.5, 11.5) |
| Nitrogen (% w) | N | 0.37 (0.24, 0.53) | 0.45 (0.38, 0.56) | 0.62 (0.23, 1.17) |
| Carbon (% w) | C | 4.07 (2.60, 6.11) | 5.13 (3.86, 6.24) | 7.96 (4.00, 15.00) |
| Slope inclination | INC | 8.5 (0.5, 14.5) | 12.1 (5.0, 21.0) | 2.6 (0.5, 14.5) |
| Solar radiation input (103 Wh·y−1) | SR | 1033 (917, 1132) | 1020 (884, 1135) | 1051 (1003, 1173) |
| N (number of sites) | 10 | 10 | 10 | |
| Species richness | 36 (28, 45) | 38 (30, 48) | 29 (18, 42) | |
| Shannon diversity (H) | 2.80 (2.40, 3.22) | 3.00 (2.51, 3.62) | 2.41 (1.44, 2.91) | |
| Overall | MEADOW | PASTURE | ABANDONED | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Richness | H | Richness | H | Richness | H | Richness | H |
| pH | −0.37 | −0.35 | 0.16 | 0.09 | −0.08 | 0.24 | −0.74 | −0.51 |
| P | −0.03 | −0.02 | −0.68 | −0.81 | 0.15 | 0.05 | 0.02 | −0.23 |
| N | −0.08 | −0.18 | −0.10 | −0.29 | −0.53 | −0.52 | 0.91 | 0.73 |
| ALT | 0.16 | 0.12 | −0.05 | −0.06 | −0.02 | −0.18 | 0.76 | 0.63 |
| INC | 0.15 | 0.20 | −0.20 | −0.19 | −0.67 | −0.43 | −0.24 | −0.07 |
| SR | −0.14 | −0.17 | −0.41 | −0.15 | −0.02 | −0.15 | 0.11 | −0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diviaková, A.; Stašiov, S.; Pondelík, R.; Pätoprstý, V.; Novikmec, M. Environmental and Management Control over the Submontane Grassland Plant Communities in Central Slovakia. Diversity 2021, 13, 30. https://doi.org/10.3390/d13010030
Diviaková A, Stašiov S, Pondelík R, Pätoprstý V, Novikmec M. Environmental and Management Control over the Submontane Grassland Plant Communities in Central Slovakia. Diversity. 2021; 13(1):30. https://doi.org/10.3390/d13010030
Chicago/Turabian StyleDiviaková, Andrea, Slavomír Stašiov, Radovan Pondelík, Vladimír Pätoprstý, and Milan Novikmec. 2021. "Environmental and Management Control over the Submontane Grassland Plant Communities in Central Slovakia" Diversity 13, no. 1: 30. https://doi.org/10.3390/d13010030
APA StyleDiviaková, A., Stašiov, S., Pondelík, R., Pätoprstý, V., & Novikmec, M. (2021). Environmental and Management Control over the Submontane Grassland Plant Communities in Central Slovakia. Diversity, 13(1), 30. https://doi.org/10.3390/d13010030





