Abstract
Species coexistence is one of the most important concepts in ecology for understanding how biodiversity is shaped and changed. In this study, we investigated the mechanism by which two small cyprinid fishes (H. leucisculus and H. bleekeri) coexist by analyzing their niche segregation and morphological differences in the upper Yangtze River. Morphological analysis indicated that H. leucisculus has posteriorly located dorsal fins, whereas H. bleekeri has a more slender body, bigger eyes, longer anal fin base, and a higher head. Niche segregation analysis showed spatial and trophic niche segregation between these two species: on the spatial scale, H. leucisculus was more widely distributed than H. bleekeri, indicating that H. leucisculus is more of a generalist in the spatial dimension; on the trophic scale, H. bleekeri had a wider niche than H. leucisculus. Therefore, these two species adopt different adaptation mechanisms to coexist
1. Introduction
Species coexistence is one of the most important concepts in ecology for understanding how biodiversity is shaped and changed [1,2]. The mechanism that enable species—especially closely related, ecologically similar species—to coexist remains of interest in community ecology [3]. Although similar species commonly coexist in nature, classical niche theory suggests that complete niche overlap is evolutionally impossible [4,5]. Niche segregation, a process by which competing species evolve different patterns of resource-use under evolutionary pressures, is widely used to explain how similar species coexist [6,7,8]. Variation in resource-use can generally be separated into three aspects: space, time and food [9,10]. However, competing species tend to be segregated by at least one niche dimension [8,10,11].
Studies across diverse taxa, including birds [12], invertebrates [3,13], microbes [14,15], plants [16,17], fishes [1,8,18,19], amphibians and reptiles [20,21], have found that niche segregation is the main mechanism promoting coexistence. Rossier [22] confirmed the fish undergo spatial and temporal separation by identifying the spatial (distance from the shore) and seasonal (summer–winter) distributions of the ichthyofauna in the littoral zones of Lake Geneva. Knickle and Rose [5] studied the spatial and temporal movement patterns of sympatric juvenile Gadus morhua and Gadus ogac, using high-resolution radio-acoustic positioning in a coastal area of Newfoundland. Carniatto et al. [1] confirmed two sympatric, morphologically similar species of Moenkhausia with segregated food resources. Leray et al. [23] demonstrated that dietary partitioning promotes the coexistence of two species of damselfish that commonly co-occur in branching corals.
Ecomorphology is a branch of environmental science that studies the relationship between organisms’ morphologies and ecologies [24,25,26]. Fish morphology is related to environmental factors like habitat, diet and predation risk [27,28,29]. Morphological variation in aquatic systems can promote resource partitioning among competitors, and facilitate coexistence among closely related fishes [30,31]. Therefore, morphological measurements may be a reliable methodology for predicting factors of a fish’s niche, such as its habitat and diet preferences [25,27,32,33,34].
Hemiculter leucisculus and Hemiculter bleekeri are two ecologically similar species that are dominant in many bodies of water in China [35]. They are extremely similar in morphology and diet, and mainly stay in the upper water column [36]. Both are omnivores whose diets mainly consist of aquatic insects, algae, plant detritus, Oligochaeta, and the Cladocera and Copepod Crustacea [36]. H. leucisculus is a small resident fish that spawns between May and June. It has a high fecundity and adhesive eggs. H. bleekeri spawns between May and June, but has pelagic eggs. Congeneric and ecologically similar species may be considered as good models for studies on niche segregation among co-occurring species [7,37,38,39]. There have been some studies on species coexistence, but very few on the coexistence between two sympatric freshwater fish species that are widely distributed, ecologically similar, and phylogenetically closely related.
This study investigated niche segregation and morphological variation between the two small cyprinid fishes H. leucisculus and H. bleekeri in the Upper Yangtze River. The goals of the present study were to (i) identify the niche segregation between these fishes in the basin of the upper Yangtze River and use it to elucidate the mechanisms governing how these two closely related and widely distributed congeners coexist, and (ii) examine the morphological variation between H. bleekeri and H. leucisculus, to determine whether morphological features might be indicators of species niche [33].
2. Materials and Methods
2.1. Study Area and Fish Sampling
The upper Yangtze River is 4500 km long from its headwaters to Yichang in Hubei Province; it accounts for around 2/3 of the total length of the Yangtze River and has a drainage area of 1.0 × 106 km2 [40]. It exhibits significant habitat heterogeneity across its reaches as a result of its meteorological, hydrological, physiographical, and geological variations [41]. These reaches include large drops, deep gorges and rushing flows, and the main tributaries include the Yalong River, Min River, Tuo River, Jialing River and Wujiang River. This study covered the main stream of the Yangtze River from the Panzhihua to Yichang reaches, and their tributaries (Figure 1).
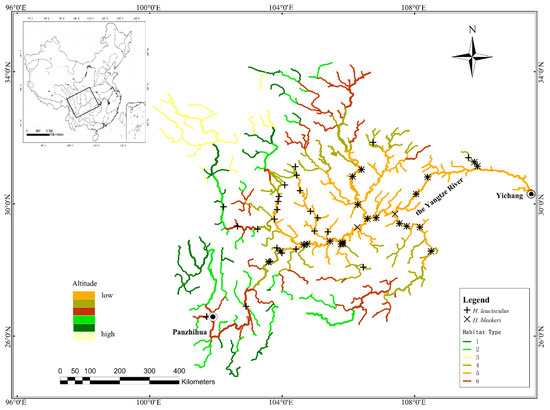
Figure 1.
Six habitat types in the upper Yangtze River from Yichang to Panzhihua, and distribution of H. leucisculus and H. bleekeri in this region. + indicates H. leucisculus and × indicates H. bleekeri; 1—sinuous headwater; 2—sinuous tributary; 3—steep headwater; 4—middle and upper sinuous river; 5—low altitude sinuous river; 6—upstream river habitat.
Surveys investigating the temporal niche of H. leucisculus and H. bleekeri were conducted twice (May–June and September–October) each year from 2012 to 2016 in three reaches in the upper Yangtze River: Yibin (28°41′23″ N, 104°32′01″ E), Hejiang (28°48′26″ N, 105°50′42″ E) and Mudong (29°34′42″ N, 106°50′26″ E) (Figure 2). Each survey was conducted for 15–20 days of each season, using at least two local fishing boats for each reach.
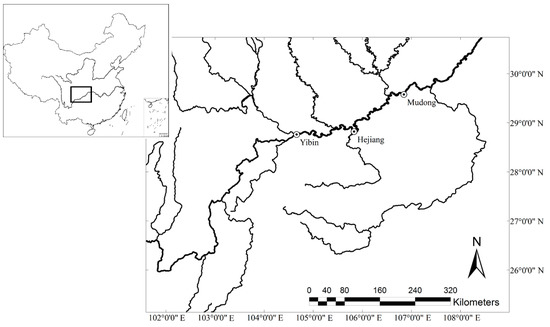
Figure 2.
Sampling sites along the upper Yangtze River, China.
Fishes were collected using local fishing boats with stationary gill nets (35 m long × 5 m high, with mesh size of 1.0 to 6.0 cm). Fishermen deployed the stationary gill nets at 06:00 a.m. and retrieved them at 06:00 a.m. the next day. The specimens were identified based on the description of Ding [42]. Each specimen was counted and weighed (to the nearest 0.1 g) and its body length was measured (to the nearest mm). First, a piece of dorsal muscle tissue was dissected from each specimen, oven-dried at 60 °C for more than 48 h, and powdered to prepare it for stable isotope analysis (SIA). Then, fishes were placed into 10% neutral buffered formaldehyde solution and transported to the laboratory for morphological measurement. The research followed the guidelines specified by the research permits from the Institute of Hydrobiology, Chinese Academy of Sciences (permit number Y216011001).
2.2. Morphological Measurement
Samples of H. leucisculus and H. bleekeri for morphological measurements were collected from Mudong town in the upper Yangtze River during the period May–June 2016 (Figure 3). A total of 58 specimens of these two species were measured (29 of each species), and 20 morphometric characteristics were measured for each specimen [43] (Figure 4). All measurements were taken by the same investigator to minimize artificial error, and morphometric characteristics were measured to the nearest 0.01 mm with a digital vernier caliper, except for body length, which was accurate to 1 mm.

Figure 3.
Photos of the studied species, Hemiculter leucisculus (a) and Hemiculter bleekeri (b).
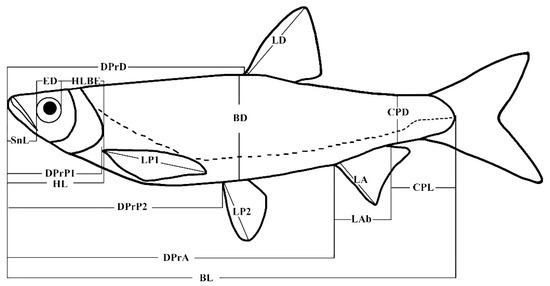
Figure 4.
Morphometric parameters investigated for H. leucisculus and H. bleekeri. BL, Body length; BD, Body depth; ED, Eye diameter; HL, Head length; HD, Head depth; DprD, Predorsal fin distance; DPrP1, Prepectoral fin distance—P1 is an abbreviation for pectoral fin; LP1, Pectoral fin length; DPrP2, Prepelvic fin distance—P2 is an abbreviation for pelvic fin; LP2, Pelvic fin length; DPrA, Pre-anal fin distance; LD, Dorsal fin length; LA, Anal fin length; LAb, Anal fin base length; CPL, Caudal peduncle length; CPD, Caudal peduncle depth [43].
A previous study identified the different morphological traits between the two species by analyzing the morphometric data of the specimens collected from the different locations (Table S1) [44]. Those results coincided with the results of the present study, demonstrating that the two species evidently had different morphological traits no matter where the specimens were collected. Moreover, many studies have performed morphometric analysis on fish using 22–32 specimens of a species [45,46,47,48]. Therefore, the number of specimens analyzed in the present study was enough for a robust morphometric analysis.
2.3. Spatial Niche
2.3.1. Data Sources
Our data consisted of a 1:250,000 drainage map and 90-m high-resolution Shuttle Radar Topography Mission (SRTM) Digital Elevation Models (DEM). The drainage map was provided by the National Geomatics Center of China and the SRTM DEM data were provided by the National Aeronautics and Space Administration (NASA) and the USGS National Imagery and Mapping Agency (NIMA) at 90-m resolution (http://srtm.csi.cgiar.org) [49]. These data provided information on the river, sub-catchment boundaries and altitude needed to calculate parameters. The information concerning the H. leucisculus and H. bleekeri distributions were obtained from surveys from our research group and other literature.
2.3.2. Technical Procedure for Classifying River Habitats
The habitat in this study was analyzed on the macro scale. The following habitat classification methods were used. First, appropriate classification indexes were chosen based on references. Second, the sub-catchment was generated on the ArcGIS platform based on the SRTM DEM data and the river drainage map. Then, the values of indexes within each sub-catchment were calculated with ArcGIS software, the indexes were analyzed by the cluster analysis method in R software, and the number of habitat types was determined according to the clustering results. Finally, each habitat type was named according to the dominant habitat factors.
2.3.3. Choice and Calculation of Habitat Classification Index
According to the scale characteristics of the habitats, the classification index should be able to reflect the medium- or large-scale characteristics of the river system, such as the physical form, water system structure, and scale. River slope, sinuosity, drainage density, stream order and altitude were selected as the classification indexes, based on a literature review [50] and the river characteristics.
Stream order [50] and altitude indexes were directly extracted from the 1:250,000 drainage map and SRTM DEM data in ArcGIS software.
The slope (m/km) of a reach was calculated using Equation (1) [51]:
The sinuosity of a reach was calculated using Equation (2) [52]:
Drainage density (km/km2) was calculated using Equation (3) [52]:
The parameters, such as straightline downvalley distance, channel distance, channel length and drainage area, used in Equations (1)–(3), were directly extracted from the 1:250,000 drainage map and SRTM DEM data in ArcGIS software.
2.4. Trophic Niche
A total of 14 samples for each species were randomly selected from Mudong town in the upper Yangtze River, from May to June 2016 (Figure 2), for stable isotopes (δ13C and δ15N) analysis. Firstly, we grouped all of the collected samples according to their standard body length, and randomly selected samples from each group. Thus, the average body length of the selected samples was approximately equal to the average body length of the population (Table S2). Our aim was to make the selected samples as representative of the population as possible. One dorsal muscle tissue (about 0.3 mg) was used for stable isotope analysis (SIA).
δ13C and δ15N were measured with a Delta Plus (Finnigan, Bremen, Germany) continuous-flow isotope ratio mass spectrometer coupled to a Carlo Erba NA2500 elemental analyzer (Carlo Erba Reagenti, Milan, Italy) at the Institute of Hydrobiology, Chinese Academy of Sciences. Stable isotope ratios were expressed in δ, a deviation from the international standards of parts per thousand (‰) that was calculated according to the following equation: δ X = [(Rsample/Rstandard) −1] × 1000, where δ is the measure of heavy to light; the standards are Vienna Pee Dee Belemnite for carbon and atmospheric N2 for nitrogen [53]; X is 13C or 15N; and R is the corresponding ratio 15N/14N or 13C/12C. Three working standards (USGS40, USGS41 and UREA-Thermo) were employed to calibrate the analyzer. The average standard errors of replicate measurements for δ13C and δ15N were both less than 0.3‰. After obtaining δ13C and δ15N, we corrected the values according to the fitting function obtained from the fitting of the measured and standard values of the three working standards.
2.5. Statistical Analysis
To eliminate any effect of size (length) on the dataset, the morphometric parameters were transformed according to Lahnsteiner and Jagsch [54]: Transformed morphometric parameter =. One-way analysis of variance (ANOVA) was firstly used to test whether those traits significantly differed between two species. Then a principal component analysis (PCA) was used to reduce the dimensionality of the variables, transform interdependent variables into significant and independent components [43,55], clarify the greater part of the variation, and extract new composite variables [56]. According to Kaiser–Meyer–Olkin (KMO) measures of sampling adequacy, and Bartlett’s test of sphericity (Yakubu and Okunsebor, 2011), the factor analysis of the transformed morphological data set was valid (χ2 = 1028.608; P < 0.01). To avoid super factorization, and to select variables that better represent morphology, only components with eigenvalue scores greater than 1.000 were considered, following the Kaiser–Guttman criteria [57]. The extracted principal components (PCs) were rotated using varimax rotation for simplifying factors, which could help interpret the factors or rotated PCs. Finally, an independent sample t-test was used to analyze traits comprising PC1, then the means of these traits were compared. The above procedures were all carried out using IBM SPSS Statistics (version 20.0, Armonk, New York, NY, USA).
In this study, catch per unit effort (CPUE) was expressed as g·boat−1·day−1 [58]. We investigated the CPUE of the two species based on the same sampling methods, using the same time for each location. Therefore, the CPUE results could be used to compare the population density of the two fish species in each location. The data for all five years were divided into spring and autumn seasons for each site, and then the CPUE data were compared between (a) different species from the same season and (b) different seasons in the same location.
One-way ANOVA was conducted to test whether there were significant differences in the δ13C and δ15N values between H. leucisculus and H. bleekeri. We also calculated the δ13C range (CR), the δ15N range (NR), the total area of the convex hull encompassing the data points (TA), and the corrected standard ellipse area (SEAc) for H. leucisculus and H. bleekeri [59,60,61], and then compared the trophic niches between these two species. All metrics were calculated using the R statistical computing package “siar” [62].
3. Results
3.1. Morphological Measurements
The results of the one-way ANOVA showed that, among the 21 morphometric parameters, the following 10 were significantly different between H. leucisculus and H. bleekeri: relative body depth (BD/BL), relative head depth (HD/HL), relative snout length (SnL/HL), ratio of snout length to eye diameter (SnL/ED), relative eye diameter (ED/HL), relative dorsal fin length (LD/HL), relative pelvic fin length (LP2/BL), relative anal fin base length (LAb/BL), relative pre-dorsal fin distance (DPrD/BL) and relative pre-anal fin distance (DPrA/BL).
The principal component analysis of 21 morphometric parameters extracted six factors with eigenvalues > 1, explaining 75.59% of the variance (Table 1). The first principal component (PC1) accounted for 26.00% of the variation, and the following PCs (PC2–6) accounted for 18.31%, 10.68%, 7.76%, 7.41% and 5.41%. For parsimony, only those factors with loadings above 0.60 were considered significant in this study.

Table 1.
Percentage of explained variance and weights of 21 morphometric characteristics (corrected based on standard body/head length) in six principal components (PCs), and the mean and standard deviation of morphometric characteristics in PC1. See Figure 4 for abbreviations. Sample number (n) = 58.
Comparison of the mean and standard deviations of morphological traits in PC1 (Table 1) showed that H. leucisculus had greater values than H. bleekeri for BD/BL and DPrD/BL, but had smaller values for the measurements HD/HL, ED/HL and LAb/BL. Therefore, H. leucisculus had posteriorly located dorsal fins, whereas H. bleekeri had a slender body, bigger eyes, a longer anal fin base and a higher head.
3.2. Spatial Niche
We identified 521 stream segments, according to the rules of river habitats classification [63], with lengths ranging from 0.216 to 337.904 km. Some sections of the river were short because there were many river junctions and the segmentation points were close to each other; these were mainly distributed in areas with good water systems. On the contrary, some rivers were poorly developed with fewer river intersections, yielding a longer river section.
According to the clustering results and the comprehensive characteristics of rivers, the river habitats in the upper Yangtze River were divided into six categories (Figure 1), containing 59, 60, 36, 104, 163 and 99 stream segments, respectively. The results of one-way ANOVA showed that river slope, sinuosity, drainage density, stream order and altitude were all significant in the six categories (P < 0.01). The six habitat types were named according to the differences in their index characteristics: sinuous headwater habitat, sinuous tributary habitat, steep headwater habitat, middle and upper sinuous river habitat, low altitude sinuous river habitat and upstream river habitat (Table 2).

Table 2.
Characteristics of six habitat types in the upper Yangtze River from Yichang to Panzhihua.
The habitat classification in the upper Yangtze River, from the Yichang to Panzhihua reaches, and the distribution of H. leucisculus and H. bleekeri, suggested that H. leucisculus was distributed in types 2, 4, 5 and 6, mainly in types 4 and 5, and H. bleekeri was distributed in types 4 and 5 (Figure 1).
3.3. Temporal Niche
Surveys over the entire sampling period, from 2012 to 2016, yielded 2460.2 g of H. leucisculus and 4870.6 g of H. bleekeri in total. The CPUE of H. leucisculus in the Mudong reach was 92.8 g·boat−1·day−1 in spring, and 137.7 g·boat−1·day−1 in autumn; the CPUE of H. bleekeri was 61.5 g·boat−1·day−1 in spring and 159.1 g·boat−1·day−1 in autumn. The CPUE of H. leucisculus in the Hejiang reach was 7.4 g·boat−1·day−1 in spring and 0.8 g·boat−1·day−1 in autumn; the CPUE of H. bleekeri was 58.9 g·boat−1·day−1 in spring and 34.8 g·boat−1·day−1 in autumn. The CPUE of H. bleekeri in autumn was 8.2 g·boat−1·day−1 in the Yibin reach. The stationary gill nets did not capture H. leucisculus or H. bleekeri in spring in the Yibin reach, or H. leucisculus in autumn in the Yibin reach.
The CPUE of H. bleekeri was higher than that of H. leucisculus in all reaches except the Mudong reach in spring. In the Mudong and Yibin reaches, the CPUEs of H. leucisculus and H. bleekeri in spring were lower than those in autumn (Figure 5).
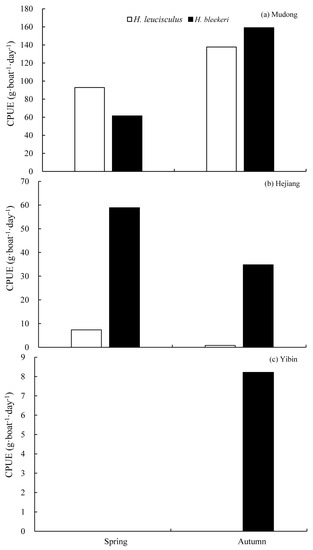
Figure 5.
CPUE of H. leucisculus and H. bleekeri in the Mudong, Hejiang, and Yibin reaches in spring (May–June) and autumn (September–October), 2012–2016 ((a): Mudong, (b): Hejiang, (c): Yibin). The blank square represents H. leucisculus and the solid square represents H. bleekeri.
3.4. Trophic Niche
According to the one-way ANOVA, the δ13C and δ15N values were all significantly different between the two species (δ13C: F = 54.040, P < 0.001; δ15N: F = 31.897, P < 0.001). The δ13C range (CR), δ15N range (NR), corrected standard ellipse area (SEAc), and total area of the convex hull encompassing the data points (TA) of H. leucisculus and H. bleekeri at Mudong were all different (Table 3). The CRs of H. leucisculus and H. bleekeri were 2.280 and 3.882, respectively. The NRs of H. leucisculus and H. bleekeri were 1.223 and 3.444, respectively. The SEAcs of H. leucisculus and H. bleekeri were 0.789 and 2.999, respectively. The TAs of H. leucisculus and H. bleekeri were 1.905 and 7.064, respectively (Table 3).

Table 3.
δ13C range (CR), δ15N range (NR), corrected standard ellipse area (SEAc), and total area of the convex hull encompassing the data points (TA) of H. leucisculus and H. bleekeri at the Mudong site.
4. Discussion
4.1. Morphological Characteristics and Ecological Niches
Morphological characteristics provide evidence for important ecological characteristics, which might be indicators of life habits, resource utilization types or environmental adaptations of species [24,26,33,43]. Since recent studies have confirmed the close relationship between morphological features and ecological niches, it has proven useful to infer ecological information from morphological characteristics [25,26,33,34,43]. Morphological variations are tightly related to niche segregation.
First, body depth (BD) is related to swimming behavior. According to the hydrodynamic theory, fishes in lotic habitats have more slender bodies than those living in lentic habitats, as they reduce drag during steady swimming while searching for prey and during fast acceleration while attacking prey [54,64]. Generally, the location of the dorsal fin (DPrD) is associated with the fish’s vertical position in the water. Fishes with posteriorly located dorsal fins adapt more easily to surface habitats in non-flowing water, and have the capacity to stabilize and brake when accelerating [56]. Eye diameter (ED) is assumed to be related to the visual sensitivity and foraging position of the fish in the water column [33]. Head depth (HD) is generally associated with food size, as fishes with larger heads always consume larger prey items [25,26,33]. Anal fin base length (LAb) is associated with maneuverability capacity and movement stabilization [65]. Fishes in lotic habitats usually have wider anal fins to improve their swimming and stability in a current [56].
In the present study, we found a clear morphological difference and strong relationship between morphology and ecology in these two species. The PCA results showed that H. leucisculus has a posteriorly located dorsal fin, whereas H. bleekeri has a more slender body, bigger eyes, a longer anal fin base, and a higher head than H. leucisculus (Table 2). Combining all of the above characters and their functions, we conclude that H. bleekeri can consume larger prey than H. leucisculus, and the former inhabits a part of the water column closer to the surface. Moreover, H. bleekeri swims better than H. leucisculus, and is better suited to living in a flowing water environment. As expected, morphological variation between these two species is indeed a reliable predictor of differences in their habitats and diet preferences [25,27,33,34].
4.2. Niche Segregation and Species Coexistence
Spatial niche segregation may be expressed in the form of habitat segregation [66]. A fish’s habitat preference can be influenced by many factors: environment variables, like water temperature [67,68,69], salinity [69], depth [69,70], turbidity [71], altitude [70], current velocity [71], vegetation characteristic, pH and dissolved oxygen [67]; and biological variables like competition, food availability and predation [72,73].
In terms of the large distribution area, H. leucisculus and H. bleekeri, in the upper reaches of the Yangtze River, are only distributed in the lower and middle reaches of the main stream and tributaries at low altitudes, but it is difficult for them to live in the source and upper reaches. This may be related to the following factors: (1) Body size. Both H. leucisculus and H. bleekeri are compressiform, and fish with this body shape mostly inhabit areas with slow currents [25]. However, the river source and upstream areas have fast currents, high altitudes and large slopes [74,75,76]. Fishes living here are mostly anguilliform (e.g., Coreius guichenoti and Rhinogobio cylindricus) or depressiform (e.g., Homaloptera and Gastromyzon) [76]. (2) Feeding mode. The eutrophication degree of the water in the source and upstream areas is low. Most of the phytoplankton are sessile diatoms [77], and most of the aquatic arthropods and crustaceans are benthic. Therefore, the fish in these areas can either stabilize their bodies in the water flow and search for crustaceans in the bottom sand with their snouts, like Rhynchocypris does, or develop scraping habits [74], like Onychostoma and Schizothorax do. Therefore, fish in source and upstream areas are mainly adapted to the rapid current and benthic life, while H. leucisculus and H. bleekeri are pelagic fishes that struggle in these areas.
In the current study, H. bleekeri was usually distributed in areas of the upper Yangtze River basin with low altitude and slope, like habitat types 4 and 5. H. leucisculus, however, was distributed in habitat types 2, 4, 5 and 6, including the low altitude and slope areas (types 4 and 5)—which H. bleekeri preferred—and the slightly higher altitude and slope areas (types 2 and 6), which were difficult for H. bleekeri to occupy. Therefore, H. leucisculus is more widely distributed than H. bleekeri in the Yangtze River. Yibin had the highest H. leucisculus and H. bleekeri biomass in spring and autumn, followed by Hejiang and Mudong. Yibin is more upstream in the upper Yangtze River, Mudong is more downstream, and Hejiang is in between the two. This also shows that H. leucisculus and H. bleekeri are more suited to living in the lower reaches, with a lower altitude and slope.
When they coexist in a specific place, H. bleekeri is significantly more abundant than H. leucisculus. The results of the trophic niche analyses at the Mudong site showed that the food source (CR), trophic level (NR) and trophic niche width (TA and SEAc) of H. bleekeri were all greater than those of H. leucisculus, which indicates that H. bleekeri uses more resources and is more competitive than H. leucisculus when the two are sympatric. Therefore, when H. leucisculus and H. bleekeri are sympatric, to reduce the disadvantage of competition, H. leucisculus is forced to move to regions without H. bleekeri. According to the literature, H. leucisculus can survive in various habitats, such as reservoirs, lakes and rivers, even in other countries [78,79,80]. Some studies have also shown that the spawning habits of H. leucisculus are different in different regions. For example, it spawns adhesive eggs in Erhai [81], Fenhe Reservoir [82] and Dalai Lake [83], but pelagic eggs in Heilongjiang [84] and Erlonghu Reservoir [85]. In addition, H. leucisculus is invasive in some places (e.g., Erhai) [81]. All the above factors indicate that H. leucisculus has a strong adaptability, and this explains why it is more widely distributed in the upper Yangtze River.
5. Conclusions
In summary, niche segregation occurs between H. leucisculus and H. bleekeri in the upper Yangtze River, and such niche segregation and partitioning likely allows these two closely related species to co-exist. Of the three important dimensions of niche partitioning—space, time and food—food and space are the principal mechanisms of niche segregation between H. leucisculus and H. bleekeri in the upper Yangtze River, whereas we found no robust evidence of temporal segregation. H. bleekeri is more competitive, while H. leucisculus is more adaptive. In addition, there are morphological variations between H. leucisculus and H. bleekeri, and morphological characteristics are good indicators of a species’ niche.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/12/7/284/s1, Table S1: Comparison of the range of morphometric characteristics between H. leucisculus and H. bleekeri from reference “Fauna Sinica – Osteichthyes Cyperiniformes II” and this study, Table S2: Sampling location and sample information (body length, body weight, and their mean values) that used in stable isotope analysis, and the mean of all samples.
Author Contributions
Conceptualization, W.J.L., X.G. and H.Z.L.; methodology, W.J.L. and H.Z.L.; investigation, W.J.L.; data curation, W.J.L.; writing–original draft preparation, W.J.L.; writing—review and editing, W.J.L., X.G., H.Z.L., W.X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Key R&D Program of China (2018YFD0900806), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB31040000), and the Sino BON—Inland Water Fish Diversity Observation Network.
Acknowledgments
We thank Ning Qiu, Lin Chen, Dan Yu and other colleagues for their assistance in field sampling and taking photos. We are indebted to HuanShan Wang and Xi Wang (institution: Museum of Hydrobiological Sciences, Institute of hydrology, Chinese academy of sciences) for their assistance in examining the specimens. The authors also thank Noah Last of Third Draft Editing for his English language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carniatto, N.; Fugi, R.; Thomaz, S.M. Highly segregated trophic niche of two congeneric fish species in Neotropical floodplain lakes. J. Fish Biol. 2016, 90, 1118–1125. [Google Scholar] [CrossRef]
- Mouillot, D. Niche-assembly vs. dispersal-assembly rules in coastal fish metacommunities: Implications for management of biodiversity in brackish lagoons. J. Appl. Ecol. 2007, 44, 760–767. [Google Scholar] [CrossRef]
- Albrecht, M.; Gotelli, N.J. Spatial and temporal niche partitioning in grassland ants. Oecologia 2001, 126, 134–141. [Google Scholar] [CrossRef]
- Gause, G.F. The struggle for existence. Soil Sci. 1934, 41, 159. [Google Scholar] [CrossRef]
- Knickle, D.C.; Rose, G.A. Examination of fine-scale spatial-temporal overlap and segregation between two closely related congeners Gadus morhua and Gadus ogac in coastal Newfoundland. J. Fish Biol. 2014, 85, 713–735. [Google Scholar] [CrossRef]
- Knickle, D.C. Niche Partitioning in Sympatric Greenland Cod (Gadus ogac) and Atlantic Cod (Gadus morhua) in Coastal Newfoundland. Ph.D. Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2013. [Google Scholar]
- Neves, M.P.; Da Silva, J.C.; Baumgartner, D.; Baumgartner, G.; Delariva, R.L. Is resource partitioning the key? The role of intra-interspecific variation in coexistence among five small endemic fish species (Characidae) in subtropical rivers. J. Fish Biol. 2018, 93, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.T. Resource partitioning in fish assemblages: A review of field studies. Copeia 1986, 352–388. [Google Scholar] [CrossRef]
- Maci, S.; Basset, A. Composition, structural characteristics and temporal patterns of fish assemblages in non-tidal Mediterranean lagoons: A case study. Estuar. Coast. Shelf Sci. 2009, 83, 602–612. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource partitioning in ecological communities. Science 1974, 185, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerken, I.; Velde, G.V.D.; Verberk, W.C.E.P.; Dorenbosch, M. Segregation along multiple resource axes in a tropical seagrass fish community. Mar. Ecol. Prog. Ser. 2006, 308, 79–89. [Google Scholar] [CrossRef]
- Randler, C.; Teichmann, C.; Pentzold, S. Breeding habitat preference and foraging of the Cyprus Wheatear Oenanthe cypriaca and niche partitioning in comparison with migrant Oenanthe species on Cyprus. J. Ornithol. 2010, 151, 113–121. [Google Scholar] [CrossRef]
- Richardson, M.L.; Hanks, L.M. Partitioning of niches among four species of orb-Weaving spiders in a grassland habitat. Environ. Entomol. 2009, 38, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.E.; David, L.A.; Gevers, D.; Preheim, S.P.; Alm, E.J.; Polz, M.F. Resource partitioning and sympatric differentiation among closely related bacterioplankton. Science 2008, 320, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Sampayo, E.M.; Franceschinis, L.; Hoegh-Guldberg, O.; Dove, S. Niche partitioning of closely related symbiotic dinoflagellates. Mol. Ecol. 2007, 16, 3721–3733. [Google Scholar] [CrossRef]
- Peterson, M.L.; Rice, K.J.; Sexton, J.P. Niche partitioning between close relatives suggests trade-offs between adaptation to local environments and competition. Ecol. Evol. 2013, 3, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Munday, P.L.; Jones, G.P.; Caley, M.J. Interspecific competition and coexistence in a guild of coral-dwelling fishes. Ecology 2001, 82, 2177–2189. [Google Scholar] [CrossRef]
- Silva, N.C.D.S.; Costa, A.J.L.d.; Louvise, J.; Soares, B.E.; Reis, V.C.E.S.; Albrecht, M.P.; Caramaschi, É.P. Resource partitioning and ecomorphological variation in two syntopic species of Lebiasinidae (Characiformes) in an Amazonian stream. Acta Amaz. 2016, 46, 25–36. [Google Scholar] [CrossRef]
- Cloyed, C.; Eason, P. Niche partitioning and the role of intraspecific niche variation in structuring a guild of generalist anurans. R. Soc. Open Sci. 2017, 4, 170060. [Google Scholar] [CrossRef]
- Lehtinen, R.M.; Glf, C. Habitat selection, the included niche, and coexistence in plant-specialist frogs from Madagascar. Biotropica 2011, 43, 58–67. [Google Scholar] [CrossRef]
- Rossier, O. Spatial and temporal separation of littoral zone fishes of Lake Geneva (Switzerland-France). Hydrobiologia 1995, 300–301, 321–327. [Google Scholar] [CrossRef]
- Leray, M.; Alldredge, A.L.; Yang, J.Y.; Meyer, C.P.; Holbrook, S.L.; Schmitt, R.J.; Knowlton, N.; Brooks, A.J. Dietary partitioning promotes the coexistence of planktivorous species on coral reefs. Mol. Ecol. 2019, 28, 2694–2710. [Google Scholar] [CrossRef]
- Peres-Neto, P.R. Alguns métodos e estudos em ecomorfologia de peixes de riachos. Oecologia Aust. 1999, 6. [Google Scholar] [CrossRef]
- Oliveira, E.F.; Goulart, E.; Breda, L.; Minte-Vera, C.V.; Paiva, L.R.D.S.; Vismara, M.R. Ecomorphological patterns of the fish assemblage in a tropical floodplain: Effects of trophic, spatial and phylogenetic structures. Neotrop. Ichthyol. 2010, 8, 569–586. [Google Scholar] [CrossRef]
- Sampaio, A.L.A.; Pagotto, J.P.A.; Goulart, E. Relationships between morphology, diet and spatial distribution: Testing the effects of intra and interspecific morphological variations on the patterns of resource use in two Neotropical Cichlids. Neotrop. Ichthyol. 2013, 11, 351–360. [Google Scholar] [CrossRef]
- Costa, C.; Cataudella, S. Relationship between shape and trophic ecology of selected species of Sparids of the Caprolace coastal lagoon (Central Tyrrhenian Sea). Environ. Biol. Fishes 2007, 78, 115–123. [Google Scholar] [CrossRef]
- Eklöv, P.; Svanbäck, R. Predation risk influences adaptive morphological variation in fish populations. Am. Nat. 2006, 167, 440–452. [Google Scholar] [CrossRef]
- Pakkasmaa, S.; Piironen, J. Water velocity shapes juvenile salmonids. Evol. Ecol. 2000, 14, 721–730. [Google Scholar] [CrossRef]
- Helland, I.P.; Vøllestad, L.A.; Freyhof, J.; Mehner, T. Morphological differences between two ecologically similar sympatric fishes. J. Fish Biol. 2009, 75, 2756–2767. [Google Scholar] [CrossRef]
- Svanbäck, R.; Eklöv, P.; Fransson, R.; Holmgren, K. Intraspecific competition drives multiple species resource polymorphism in fish communities. Oikos 2008, 117, 114–124. [Google Scholar] [CrossRef]
- Cochran-Biederman, J.L.; Winemiller, K.O. Relationships among habitat, ecomorphology and diets of cichlids in the Bladen River, Belize. Environ. Biol. Fishes 2010, 88, 143–152. [Google Scholar] [CrossRef]
- Gatz, A.J., Jr. Community organization in fishes as indicated by morphological features. Ecology 1979, 60, 711–718. [Google Scholar] [CrossRef]
- Leitão, R.P.; Sánchez-Botero, J.I.; Kasper, D.; Trivério-Cardoso, V.; Araújo, C.M.; Zuanon, J.; Caramaschi, É.P. Microhabitat segregation and fine ecomorphological dissimilarity between two closely phylogenetically related grazer fishes in an Atlantic Forest stream, Brazil. Environ. Biol. Fishes 2015, 98, 2009–2019. [Google Scholar] [CrossRef]
- Tan, X.; Li, X.; Lek, S.; Li, Y.F.; Wang, C.; Li, J.; Luo, J.R. Annual dynamics of the abundance of fish larvae and its relationship with hydrological variation in the Pearl River. Environ. Biol. Fishes 2010, 88, 217–225. [Google Scholar] [CrossRef]
- IHB (Institute of Hydrobiology). Fishes in the Yangtze River; Science Press: Beijing, China, 1976. (In Chinese) [Google Scholar]
- Bonato, K.O.; Burress, E.D.; Fialho, C.B.; Armbruster, J. Resource partitioning among syntopic Characidae corroborated by gut content and stable isotope analyses. Hydrobiologia 2017, 805, 1–14. [Google Scholar] [CrossRef]
- Mol, J.H. Ontogenetic diet shifts and diet overlap among three closely related Neotropical armoured catfishes. J. Fish Biol. 1995, 47, 788–807. [Google Scholar] [CrossRef]
- Mooney, K.A.; Jones, P.; Agrawal, A.A. Coexisting congeners: Demography, competition and interactions with cardenolides for two milkweed-feeding aphids. Oikos 2008, 117, 450–458. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Lek-Ang, S.; Lek, S. Predicting assemblages and species richness of endemic fish in the upper Yangtze River. Sci. Total Environ. 2010, 408, 4211–4220. [Google Scholar] [CrossRef]
- Dong, Z.R. Diversity of river morphology and diversity of biocommunities. J. Hydraul. Eng. 2003, 11, 1–6. [Google Scholar] [CrossRef]
- Ding, R. The Fishes of Sichuan, China; Sichuan Publishing House of Science and Technology: Chengdu, China, 1994. (In Chinese) [Google Scholar]
- Wang, M.; Liu, F.; Lin, P.; Yang, S.; Liu, H. Evolutionary dynamics of ecological niche in three rhinogobio fishes from the upper Yangtze River inferred from morphological traits. Ecol. Evol. 2015, 5, 567–577. [Google Scholar] [CrossRef]
- Chen, Y.Y. (Ed.) Fauna Sinica—Osteichthyes Cyperiniformes II; Science Press: Beijing, China, 1998; pp. 225–228. [Google Scholar]
- Kai, Y.; Nakabo, T. Morphological differences among three color morphotypes of sebastes inermis (scorpaenidae). Ichthyol. Res. 2002, 49, 260–266. [Google Scholar] [CrossRef]
- Chuang, L.C.; Lin, Y.S.; Liang, S.H. Ecomorphological comparison and habitat preference of 2 cyprinid fishes, varicorhinus barbatulus and candidia barbatus, in Hapen Creek of Northern Taiwan. Zool. Stud. 2006, 45, 114–123. [Google Scholar]
- Motta, P.J.; Clifton, K.B.; Hernandez, P.; Eggold, B.T. Ecomorphological correlates in ten species of subtropical seagrass fishes: Diet and microhabitat utilization. Environ. Biol. Fishes 1995, 44, 37–60. [Google Scholar] [CrossRef]
- Kassam, D.D.; Adams, D.C.; Hori, M.; Yamaoka, K. Morphometric analysis on ecomorphologically equivalent cichlid species from Lakes Malawi and Tanganyika. J. Zool. 2002, 260, 153–157. [Google Scholar] [CrossRef]
- Kong, W.; Sun, O.J.; Chen, Y.; Yu, Y.; Tian, Z. Patch-level based vegetation change and environmental drivers in Tarim River drainage area of West China. Landscape Ecol. 2010, 25, 1447–1455. [Google Scholar] [CrossRef]
- Higgins, J.V.; Bryer, M.T.; Khoury, M.L.; Fitzhugh, T.W. A freshwater classification approach for biodiversity conservation planning. Conserv. Biol. 2005, 19, 432–445. [Google Scholar] [CrossRef]
- Cohen, S.; Wan, T.; Islam, M.T.; Syvitski, J.P.M. Global river slope: A new geospatial dataset and global- scale analysis. J. Hydrol. 2018, 563, 1057–1067. [Google Scholar] [CrossRef]
- Allan, J.D.; Castillo, M.M. Stream Ecology: STRUCTURE and Function of Running Waters, 2nd ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–436. [Google Scholar] [CrossRef]
- Yao, X.Y.; Huang, G.T.; Xie, P.; Xu, J. Trophic niche differences between coexisting omnivores silver carp and bighead carp in a pelagic food web. Ecol. Res. 2016, 31, 831–839. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Jagsch, A. Changes in phenotype and genotype of Austrian Salmo trutta populations during the last century. Environ. Biol. Fishes 2005, 74, 51–65. [Google Scholar] [CrossRef]
- Brosse, S.; Giraudel, J.; Lek, S. Utilisation of non-supervised neural networks and principal component analysis to study fish assemblages. Ecol. Model. 2001, 146, 159–166. [Google Scholar] [CrossRef]
- Samaee, S.M.; Mansour, N. Morphological differentiation within the population of Siah Mahi, Capoeta capoeta gracilis, (Cyprinidae, Teleostei) in a river of the south Caspian Sea basin: A pilot study. J. Appl. Ichthyol. 2009, 25, 583–590. [Google Scholar] [CrossRef]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Gao, X.; Zeng, Y.; Wang, J.; Liu, H. Immediate impacts of the second impoundment on fish communities in the three gorges reservoir. Environ. Biol. Fishes 2010, 87, 163–173. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Jackson, M.C.; Donohue, I.; Jackson, A.L.; Britton, J.R.; Harper, D.M.; Grey, J. Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PLoS ONE 2012, 7, e31757. [Google Scholar] [CrossRef]
- Syväranta, J.; Lensu, A.; Marjomäki, T.J.; Oksanen, S.; Jones, R.I. An Empirical Evaluation of the Utility of Convex Hull and Standard Ellipse Areas for Assessing Population Niche Widths from Stable Isotope Data. PLoS ONE 2013, 8, e56094. [Google Scholar] [CrossRef]
- Parnell, A.; Jackson, A. Siar: Stable Isotope Analysis in R. R Package Version 4.2. 2013. Available online: https://cran.r-project.org/web/packages/siar/ (accessed on 7 July 2020).
- U.S. Geological Survey; U.S. Department of Agriculture; Natural Resources Conservation Service. Federal Standards and Procedures for the National Watershed Boundary Dataset (WBD), 4th ed.Techniques and Methods 11–A3; 2013; 63 p. Available online: https://pubs.usgs.gov/tm/11/a3/ (accessed on 12 June 2020).
- Brinsmead, J.; Fox, M.G. Morphological variation between lake- and stream-dwelling rock bass and pumpkinseed populations. J. Fish Biol. 2002, 61, 1619–1638. [Google Scholar] [CrossRef]
- Standen, E.M.; Lauder, G.V. Dorsal and anal fin function in bluegill sunfish Lepomis macrochirus: Three-dimensional kinematics during propulsion and maneuvering. J. Exp. Biol. 2005, 208, 2753–2763. [Google Scholar] [CrossRef]
- Scott, D.M.; Dunstone, N. Environmental determinants of the composition of desert-living rodent communities in the northeast Badia region of Jordan. J. Zool. 2000, 251, 481–494. [Google Scholar] [CrossRef]
- Cech, J.J.; Mitchell, S.J.; Castleberry, D.T.; Mcenroe, M. Distribution of California stream fishes: Influence of Environmental temperature and hypoxia. Environ. Biol. Fishes 1990, 29, 95–105. [Google Scholar] [CrossRef]
- Matis, P.A.; Donelson, J.M.; Bush, S.; Fox, R.J.; Booth, D.J. Temperature influences habitat preference of coral reef fishes: Will generalists become more specialised in a warming ocean? Glob. Chang. Biol. 2018, 24, 3158–3169. [Google Scholar] [CrossRef] [PubMed]
- Vogler, R.; Milessi, A.C.; Quinones, R.A. Influence of environmental variables on the distribution of Squatina Guggenheim (Chondrichthyes, Squatinidae) in the Argentine-Uruguayan Common Fishing Zone. Fish. Res. 2008, 91, 212–221. [Google Scholar] [CrossRef]
- Srinivasan, M. Depth distributions of coral reef fishes the influence of microhabitat structure, settlement, and post-settlement processes. Oecologia 2003, 137, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Imai, T.; Ukita, M. A model of fish distribution in rivers according to their preference for environmental factors. Ecol. Model. 1997, 104, 215–230. [Google Scholar] [CrossRef]
- Infante, D.M.; Allan, J.D.; Linke, S.; Norris, R.H. Relationship of fish and macroinvertebrate assemblages to environmental factors: Implications for community concordance. Hydrobiologia 2009, 623, 87–103. [Google Scholar] [CrossRef]
- Schlosser, I.J. Environmental variation, life history attributes, and community structure in stream fish: Implications for environmental management and assessment. Environ. Manag. 1990, 14, 621–628. [Google Scholar] [CrossRef]
- Liu, F. Fish Community Ecology in the Chishui River. Ph.D. Thesis, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China, 2013. [Google Scholar]
- Liu, F.; Lin, P.; Li, M.; Gao, X.; Wang, C.; Liu, H. Situations and conservation strategies of fish resources in the Yangtze River basin. Acta Hydrobiol. Sin. 2019, 43, 144–156. [Google Scholar]
- Liu, J.; Cao, W. Fish resources of the Yangtze River basin and the tactics for their conservation. Resour. Environ. Yangtze River Val. 1992, 1, 17–23. [Google Scholar]
- Yin, D.; Xu, J.; Jin, Y.; Xu, Z. Characteristics of Phytoplankton assemblage and distribution in the source regions of the Yangtze River and Lancang River. J. Yangtze River Sci. Res. Inst. 2017, 34, 61–66. [Google Scholar]
- Chen, W.; Zhong, Z.; Dai, W.; Fan, Q.; He, S. Phylogeographic structure, cryptic speciation and demographic history of the sharpbelly (Hemiculter leucisculus), a freshwater habitat generalist from southern china. BMC Evol. Biol. 2017, 17, 216. [Google Scholar] [CrossRef]
- Coad, B.W.; Hussain, N.A. First record of the exotic species Hemiculter leucisculus (Actinopterygii: Cyprinidae) in Iraq. Zool. Middle East 2007, 40, 107–109. [Google Scholar] [CrossRef]
- Patimar, R.; Abdoli, A.; Kiabi, B.H. Biological characteristics of the introduced sawbelly, Hemiculter leucisculus (Basilewski, 1855), in three wetlands of northern Iran: Alma-Gol, Adji-Gol and Ala-Gol. J. Appl. Ichthyol. 2008, 24, 617–620. [Google Scholar] [CrossRef]
- Wang, T.; Jakovlic, I.; Huang, D.; Wang, J.G.; Shen, J.Z. Reproductive strategy of the invasive sharpbelly, Hemiculter leucisculus (Basilewsky 1855), in Erhai Lake, China. J. Appl. Ichthyol. 2016, 32, 324–331. [Google Scholar] [CrossRef]
- Xie, Z.Y.; Wu, X.F.; Zhuang, L.H.; Li, D.S. Investigations of the biology of Hemiculter leucisculus in Fenhe Reservoir. J. Shandong Collage Oceanol. 1986, 16, 54–69. [Google Scholar]
- Li, B.L.; Wang, Y.T. Biology of H. leucisculus in Dalai Lake. Chin. J. Fish. 1995, 8, 46–49. [Google Scholar]
- Wu, Q.J.; Yi, B.L. Fishes of Hemiculter and preliminary ecological monitoring of Hemiculter in Heilongjiang River basin. Acta Hydrobiol. Sin. 1959, 2, 157–168. [Google Scholar]
- Sun, Z.H. Biological characteristic of Hemiculter leucisculus in the Erlongshan Reservoir. J. Jilin Agric. Univ. 1987, 9, 66–69. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).