Conservation Lessons from the Study of North American Boreal Birds at Their Southern Periphery
Abstract
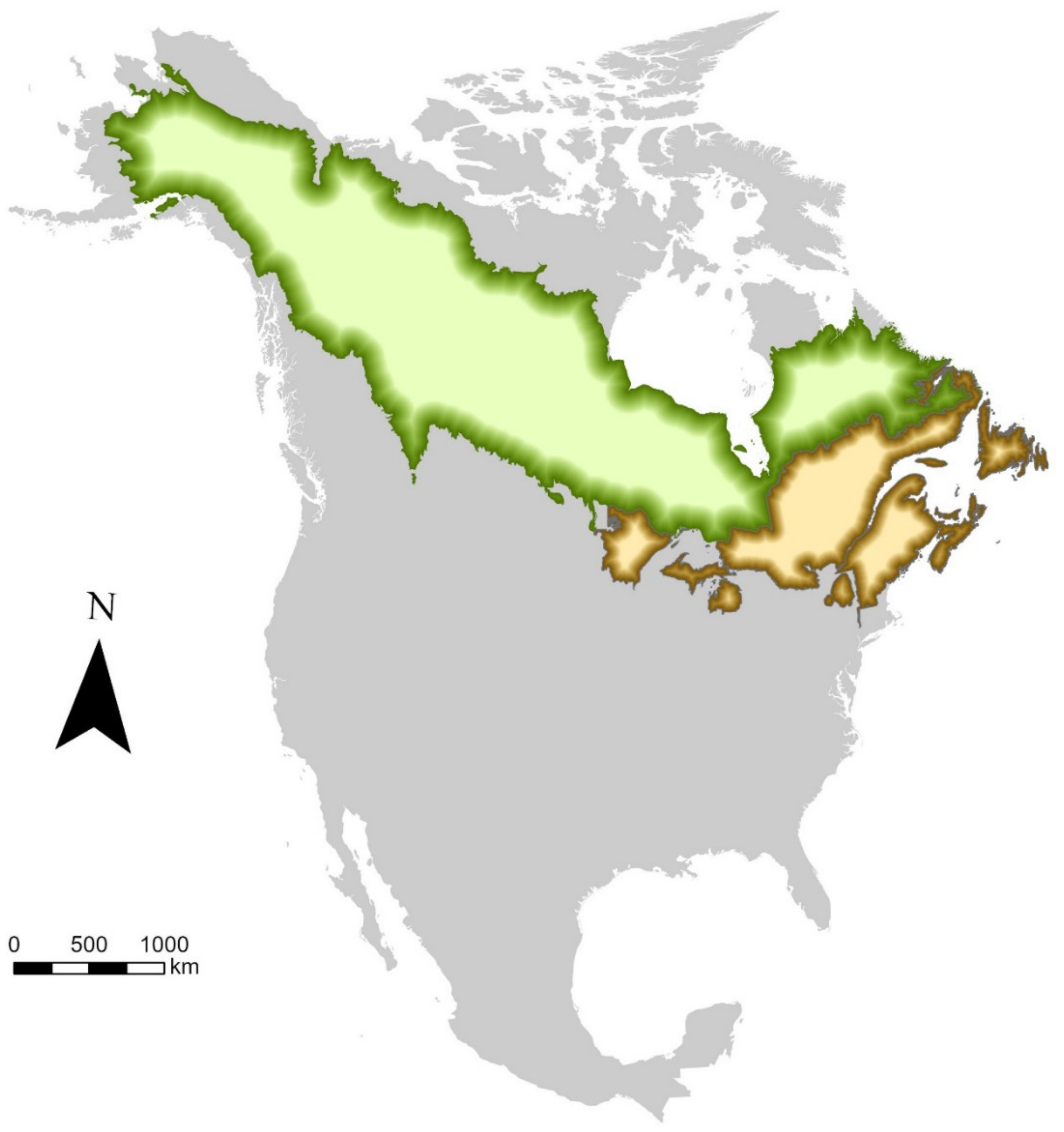
1. Introduction
2. Peripheral Populations of Boreal Birds Are Genetically Unique, Threatened by Climate Change, and Declining
3. Species Differ in Their Responses to Changing Environments, Creating a Challenge for Community Level Conservation
4. As Communities Reshuffle, Species Face New Biotic Interactions—Some of Which May Have Unexpected Outcomes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wells, J.; Stralberg, D.; Childs, D. Boreal Forest Refuge: Conserving North America’s Bird Nursery in the Face of Climate Change; Boreal Songbird Initiative: Seattle, WA, USA, 2018. [Google Scholar]
- Blancher, P.; Wells, J. The Boreal Forest Region: North. America’s Bird Nursery; Canadian Boreal Initiative and Boreal Songbird Initiative: Ottawa, ON, Canada, 2005; pp. 1–12. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; 1535p. [Google Scholar]
- Walker, X.J.; Baltzer, J.L.; Cumming, S.G.; Day, N.J.; Ebert, C.; Goetz, S.; Johnstone, J.F.; Potter, S.; Rogers, B.M.; Schuur, E.A.; et al. Increasing wildfires threaten historic carbon sink of boreal forest soils. Nature 2019, 572, 520–525. [Google Scholar] [CrossRef]
- Nelson, T.A.; Coops, N.C.; Wulder, M.A.; Perez, L.; Fitterer, J.; Powers, R.; Fontana, F. Predicting climate change impacts to the Canadian boreal forest. Diversity 2014, 6, 133–157. [Google Scholar] [CrossRef]
- Stralberg, D.; Berteaux, D.; Drever, C.R.; Drever, M.; Naujokaitis-Lewis, I.; Schmiegelow, F.K.A.; Tremblay, J.A. Conservation planning for boreal birds in a changing climate: A framework for action. Avian Conserv. Ecol. 2019, 14, 13. [Google Scholar] [CrossRef]
- Bateman, B.L.; Wilsey, C.; Taylor, L.; Wu, J.; LeBaron, G.S.; Langham, G.M. North American birds require mitigation and adaptation to reduce vulnerability to climate change. Conserv. Sci. Pract. 2019, in press. [Google Scholar]
- Damman, A.W.H. An ecological subdivision of the island of Newfoundland. In Biogeography and Ecology of the Island of Newfoundland; South, G.R., Ed.; Dr. W. Junk Publishers: The Hague, The Netherlands, 1983; pp. 163–206. [Google Scholar]
- Anderson, M.G.; Clark, M.; Ferree, C.E.; Jospe, A.; Olivero Sheldon, A.; Weaver, K.J. Northeast. Habitat Guides: A Companion to the Terrestrial and Aquatic Habitat Maps; The Nature Conservancy, Eastern Conservation Science: Boston, MA, USA, 2013. [Google Scholar]
- Zukerburg, B.; Woods, A.M.; Porter, W.F. Poleward shufts in breeding bird distributions in New York State. Glob. Chang. Biol. 2009, 15, 1866–1883. [Google Scholar] [CrossRef]
- Ralston, J.; Kirchman, J.J. Predicted range shifts in North American boreal forest birds and the effect of climate change on genetic diversity in blackpoll warblers (Setophaga striata). Conserv. Genet. 2013, 14, 543–555. [Google Scholar] [CrossRef]
- DeLuca, W.V.; King, D.I. Montane birds shift downslope despite recent warming in the northern Appalachian Mountains. J. Ornithol. 2017, 158, 493–505. [Google Scholar] [CrossRef]
- Crowl, T.A.; Crist, T.O.; Parmenter, R.R.; Belovsky, G.; Lugo, A.E. The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Environ. 2008, 6, 238–246. [Google Scholar] [CrossRef]
- Fuller, T.; Bensch, S.; Müller, I.; Novembre, J.; Pérez-Tris, J.; Ricklefs, R.E.; Smith, T.B.; Waldenström, J. The ecology of emerging infectious diseases in migratory birds: An assessment of the role of climate change and priorities for future research. EcoHealth 2012, 9, 80–88. [Google Scholar] [CrossRef]
- Whitaker, D.M. The colonization of Newfoundland by red squirrels (Tamasciurus hudsonicus). Osprey Nat. J. Nfld. Labrador 2015, 46, 23–29. [Google Scholar]
- Imbeau, L.; Mönkkönen, M.; Desrochers, A. Long-term effects of forestry on birds of the eastern Canadian boreal forests: A comparison with Fennoscandia. Conserv. Biol. 2001, 15, 1151–1162. [Google Scholar] [CrossRef]
- Rimmer, C.C.; McFarland, K.P.; Evers, D.C.; Miller, E.K.; Aubry, Y.; Busby, D.; Taylor, R.J. Mercury concentrations in Bicknell’s Thrush and other insectivorous passerines in montane forests of Northeastern North America. Ecotoxicology 2005, 14, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Venier, L.A.; Thompson, I.D.; Fleming, R.; Malcolm, J.; Aubin, I.; Trofymow, J.A.; Langor, D.; Sturrock, R.; Patry, C.; Outerbridge, R.O.; et al. Effects of natural resource development on the terrestrial biodiversity of Canadian boreal forest. Environ. Rev. 2014, 22, 457–490. [Google Scholar] [CrossRef]
- Zlonis, E.J.; Niemi, G.J. Avian communities of managed and wilderness hemiboreal forests. For. Ecol. Manag. 2014, 328, 26–34. [Google Scholar] [CrossRef]
- DeLuca, W.V.; King, D.I. Influence of hiking trails on montane birds. J. Wildlife Manag. 2014, 78, 494–502. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Kirchman, J.J.; Ralston, J. The Adirondack archipelago. Adirondack J. Environ. Stud. 2015, 20, 5. [Google Scholar]
- Ralston, J.; FitzGerald, A.M.; Burg, T.M.; Starkloff, N.C.; Whitaker, D.M.; Warkentin, I.G.; Norris, D.R.; Kirchman, J.J. Comparative phylogeographic analysis suggests a shared history among eastern North American boreal forest birds. Glob. Ecol. Biogeog. Under review.
- Kirchman, J.J.; Ross, A.M.; Johnson, G. Historical decline of genetic diversity in a range-periphery population of Spruce Grouse (Falcipennis canadensis) inhabiting the Adirondack Mountains. Conserv. Genet. 2020, 21, 373–380. [Google Scholar] [CrossRef]
- Van Els, P.; Cicero, C.; Klicka, J. High latitude and high genetic diversity: Phylogeography of a widespread boreal bird, the gray jay (Perisoreus candensis). Mol. Phylogenet. Evol. 2012, 63, 456–465. [Google Scholar] [CrossRef]
- Dohms, K.M.; Graham, B.A.; Burg, T.M. Multilocus genetic analyses and spatial modeling reveal complex population structure and history in a widespread resident North American passerine (Perisoreus canadensis). Ecol. Evol. 2017, 7, 9869–9889. [Google Scholar] [CrossRef] [PubMed]
- Lait, L.A.; Burg, T.M. When east meets west: Population structure of a high-latitude resident species, the boreal chickadee (Poecile hudsonicus). Heredity 2013, 111, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ralston, J.; Kirchman, J.J. Continent-scale genetic structure in a boreal forest migrant, the blackpoll warbler (Steophaga striata). Auk 2012, 129, 467–478. [Google Scholar]
- FitzGerald, A.M.; Weir, J.; Ralston, J.; Warkentin, I.G.; Whitaker, D.M.; Kirchman, J.J. Genetic structure and biogeographical history of the Bicknell’s Thrush/Gray-cheeked Thrush species complex. Auk 2020, 137, 1–20. [Google Scholar] [CrossRef]
- Zink, R.M. The geography of mitochondrial DNA variation, population structure, hybridization, and species limits in the fox sparrow (Passerella iliaca). Evolution 1994, 48, 96–111. [Google Scholar]
- Townsend, J.M.; McFarland, K.P.; Rimmer, C.C.; Ellison, W.G.; Goetz, J.E. Bicknell’s Thrush (Catharus bicknelli), version 1.0. In Birds of the World; Rodewald, P.G., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- FitzGerald, A.M.; Whitaker, D.M.; Ralston, J.; Kirchman, J.J.; Warkentin, I.G. Taxonomy and distribution of the imperiled Newfoundland Gray-cheeked Thrush, Catharus minimus minimus. Avian Conserv. Ecol. 2017, 12, 10. [Google Scholar] [CrossRef]
- FitzGerald, A.M. Division within the North American boreal forest: Ecological niche divergence between the Bicknell’s Thrush (Cathatus bicknelli) and Gray-cheeked Thrush (C. minimus). Ecol. Evol. 2017, 7, 5285–5295. [Google Scholar] [CrossRef] [PubMed]
- Designing Sustainable Landscapes: Modeling Focal Species. Report to the North Atlantic Conservation Cooperative, US Fish and Wildlife Service, Northeast Region. Available online: http://jamba.provost.ads.umass.edu/web/lcc/dsl/technical/DSL_documentation_species.pdf (accessed on 22 May 2020).
- Loman, Z.G.; DeLuca, W.V.; Harrison, D.; Loftin, C.S.; Wood, P.B. Landscape capability models as a tool to predict fine-scale forest bird occupancy and abundance. Landsc. Ecol. 2018, 33, 77–91. [Google Scholar] [CrossRef]
- Able, K.P.; Noon, B.R. Avian community structure along elevational gradients in the Northeastern United States. Oecologia 1979, 26, 275–294. [Google Scholar] [CrossRef]
- Kirchman, J.J.; Van Keuren, A.E. Altitudinal range shifts of birds at the southern periphery of the boreal forest: 40 years of change in the Adirondacks Mountains. Wilson J. Ornithol. 2017, 129, 742–753. [Google Scholar] [CrossRef]
- Beckage, B.; Osborne, B.; Gavin, D.G.; Pucko, C.; Siccama, T.; Perkins, T. A rapid upward shift of the forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proc. Natl. Acad. Sci. USA 2008, 105, 4197–4202. [Google Scholar] [CrossRef] [PubMed]
- Elsen, P.R.; Tingley, M.W. Global mountain topography and the fate of montane species under climate change. Nat. Clim. Chang. 2015, 5, 772–776. [Google Scholar] [CrossRef]
- Foster, J.R.; D’Amato, A.W. Montane forest ecotones moved downslope in northeastern USA in spite of warming between 1984 and 2011. Glob. Chang. Biol. 2015, 21, 4497–4507. [Google Scholar] [CrossRef] [PubMed]
- Hanowski, J.A.; Niemi, G.J. A comparison of on- and off-road bird counts: Do you need to go off road to count birds accurately? J. Field Ornithol. 1995, 66, 469–483. [Google Scholar]
- Ralston, J.; King, D.I.; DeLuca, W.V.; Niemi, G.J.; Glennon, M.J.; Scarl, J.C.; Lambert, J.D. Analysis of combined data sets yields trend estimates for vulnerable spruce-fir birds in northern United States. Biol. Conserv. 2015, 187, 270–278. [Google Scholar] [CrossRef]
- Sólymos, P.; Toms, J.D.; Matsuoka, S.M.; Cumming, S.G.; Barker, N.K.; Thogmartin, W.E.; Stralberg, D.; Crosby, A.D.; Dénes, F.V.; Haché, S.; et al. Lessons learned from comparing spatially explicit models and the Partners in Flight approach to estimate population sizes of boreal birds in Alberta, Canada. Condor 2020. [Google Scholar] [CrossRef]
- Partners in Fight Science Committee: Partners in Flight Landbird Conservation Plan: 2016 Revision for Canada and Continental United States. Available online: https://www.partnersinflight.org/wp-content/uploads/2016/08/pif-continental-plan-final-spread-single.pdf. (accessed on 22 May 2020).
- Howe, R.H.; Roberts, L.J. Sixteen Years of Habitat-Based Bird Monitoring in the Nicolet National Forest; USDA Forest Service General Technical Report PSW-GTR-191; USDA Forest Service: Washington, DC, USA, 2005.
- US Forest Service. White Mountain National Forest, Monitoing and Valuation Report; US Department of Argiculture: Washinginton, DC, USA, 2006.
- Johnson, B. The Ottawa National Forest Breeding Bird Census, an Analysis of Twenty Years of Data, 1992–2011; Report Submitted to Ottawa National Forest; USDA Forest Service: Ironwood, MI, USA, 2012.
- Zlonis, E.J.; Grinde, A.; Bednar, J.; Niemi, G.J. Summary of Breeding Bird Trends in the Chippewa and Superior National Forests of Minnesota- 1995–2013; NRRI Technical Report NRRI/TR-2013/36; University of Minnnesota: Duluth, MN, USA, 2013. [Google Scholar]
- Faccio, S.D.; Mitchell, B.R. Breeding Landbird Monitoring in the Northeast; Temperate Network, 2013 Summary Report; Natural Resource Data Series NPS/NETN/NRDS-2014/630; National Park Service: Fort Collins, CO, USA, 2014. [Google Scholar]
- Glennon, M.J.; Langdon, S.F.; Rubenstein, M.A.; Cross, M.S. Temporal changes in avian community composition in lowland conifer habitats at the southern edge of the boreal zone in the Adirondack Park, NY. PLoS ONE 2019, 14, e0220927. [Google Scholar] [CrossRef]
- Hill, J.M. The State of the Mountain Birds Report: 2020; Vermont Center for Ecostudies: White River Junction, VT, USA, 2020. [Google Scholar]
- Duclos, T.R.; DeLuca, W.V.; King, D.I. Direct and indirect effects of climate on bird abundance along elevational gradients in the Northern Appalachian Mountains. Divers. Distrib. 2019, 25, 1670–1683. [Google Scholar] [CrossRef]
- Ralston, J.; FitzGerald, A.M.; Scanga, S.E.; Kirchman, J.J. Observations of habitat associations in boreal forest birds and the geographic variation in bird community composition. Wilson J. Ornithol. 2019, 131, 12–23. [Google Scholar] [CrossRef]
- Glennon, M.J.; Langdon, S.F.; Rubenstein, M.A.; Cross, M.S. Relative contribution of climate and non-climate drivers in determining dynamic rates of boreal birds at the edge of their range. PLoS ONE 2019, 14, e0224308. [Google Scholar] [CrossRef]
- Niemi, G.J.; Hanowski, J.M.; Lima, A.R.; Nicholls, T.; Weiland, N. A critical analysis on the use of indicator species in management. J. Wildlife Manag. 1997, 61, 1240–1252. [Google Scholar] [CrossRef]
- Aubry, Y.; Desrochers, A.; Seutin, G. Regional patterns of habitat use by a threatened forest bird, the Bicknell’s Thrush (Catharus bicknelli), in Quebec. Can. J. Zool. 2016, 94, 301–309. [Google Scholar] [CrossRef]
- Chisholm, S.E.; Leonard, M.L. Effect of forest management on a rare habitat specialist, the Bicknell’s Thrush (Catharus bicknelli). Can. J. Zool. 2008, 86, 217–223. [Google Scholar] [CrossRef]
- Keith, S.A.; Newton, A.C.; Herbert, R.J.H.; Morecroft, M.D.; Bealey, C.E. Non-analogous community formation in response to climate change. J. Nat. Conserv. 2009, 17, 228–235. [Google Scholar] [CrossRef]
- MacArthur, R.H. Geographical Ecology: Patterns in the Distribution of Species; Harper and Row: New York, NY, USA, 1972. [Google Scholar]
- Brown, J.H.; Lomolino, M.V. Biogeography, 2nd ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 1998. [Google Scholar]
- Freeman, B.G.; Montgomery, G. Interspecific aggression by the Swainson’s Thrush (Catharus ustulatus) may limit the distribution of the threatened Bicknell’s Thrush (Catharus bicknelli) in the Adirondack Mountains. Condor 2016, 118, 169–178. [Google Scholar] [CrossRef]
- Noon, B.R. The distribution of an avian guild along a temperate elevational gradient: The importance and expression of competition. Ecol. Monogr. 1981, 51, 105–124. [Google Scholar] [CrossRef]
- MacArthur, R.H. Population ecology of some warblers of northeastern coniferous forests. Ecology 1958, 39, 599–619. [Google Scholar] [CrossRef]
- Gill, F.B. Local cytonuclear extinction of the golden-winged warbler. Evolution 1997, 51, 519–525. [Google Scholar] [CrossRef]
- Vallender, R.; Van Wilgenburg, S.L.; Bulluck, L.P.; Roth, A.; Canterbury, R.; Larkin, J.; Fowlds, R.M.; Lovette, I.J. Extensive rangewide mitochondrial introgression indicated substantial cryptic hybridization in the golden-winged warbler (Vermivora chrysoptera). Avian Conserv. Ecol. 2009, 4, 4. [Google Scholar] [CrossRef]
- Kearns, A.M.; Restani, M.; Szabo, I.; Schrøder-Nielsen, A.; Kim, J.A.; Richardson, H.M.; Marzluff, J.M.; Fleischer, R.C.; Johnsen, A.; Omland, K.E. Genomic evidence of speciation reversal in ravens. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Rhymer, J.M.; Simberloff, D. Extinction by hybridization and introgression. Annu. Rev. Ecol. Syst. 1996, 27, 83–109. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Leary, R.F.; Spurell, P.; Wenburg, J.K. The problems with hybrids: Setting conservation guidelines. Trends Ecol. Evol. 2001, 16, 613–622. [Google Scholar] [CrossRef]
- Marshall, J.T. The Gray-Cheeked Thrush, Catharus minimus, and Its New England Subspecies, Bicknell’s thrush, Catharus minimus bicknelli. No. 28; Nuttall Ornithological Club: Cambridge, MA, USA, 2001. [Google Scholar]
- Morelli, T.L.; Hallworth, M.T.; Duclos, T.; Siren, A.; Ells, A.; Faccio, S.; McFarland, K.; Nislow, K.; Ralston, J.; Ratnaswamy, M.; et al. Habitat causes lags in response to climate change for a climate-vulnerable species. Glob. Ecol. Biogeog. Under review.
- Hallworth, M.; Siren, A.; DeLuca, W.V.; Duclos, T.; McFarland, K.P.; Hill, J.; Rimmer, C.C.; Morelli, T.L. Boom and bust: Pulsed resources drive distribution dynamics in forested food webs. Glob. Ecol. Biogeog. Under review.
- Whitaker, D.M.; Taylor, P.D.; Warkentin, I.G. Gray-cheeked Thrush (Catharus minimus minimus) distribution and habitat use in a montane forest landscape of western Newfoundland, Canada. Avian Conserv. Ecol. 2015, 10, 4. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ralston, J.; DeLuca, W.V. Conservation Lessons from the Study of North American Boreal Birds at Their Southern Periphery. Diversity 2020, 12, 257. https://doi.org/10.3390/d12060257
Ralston J, DeLuca WV. Conservation Lessons from the Study of North American Boreal Birds at Their Southern Periphery. Diversity. 2020; 12(6):257. https://doi.org/10.3390/d12060257
Chicago/Turabian StyleRalston, Joel, and William V. DeLuca. 2020. "Conservation Lessons from the Study of North American Boreal Birds at Their Southern Periphery" Diversity 12, no. 6: 257. https://doi.org/10.3390/d12060257
APA StyleRalston, J., & DeLuca, W. V. (2020). Conservation Lessons from the Study of North American Boreal Birds at Their Southern Periphery. Diversity, 12(6), 257. https://doi.org/10.3390/d12060257



