Plant Diversity and Composition Changes along an Altitudinal Gradient in the Isolated Volcano Sumaco in the Ecuadorian Amazon
Abstract
1. Introduction
2. Materials and Methods
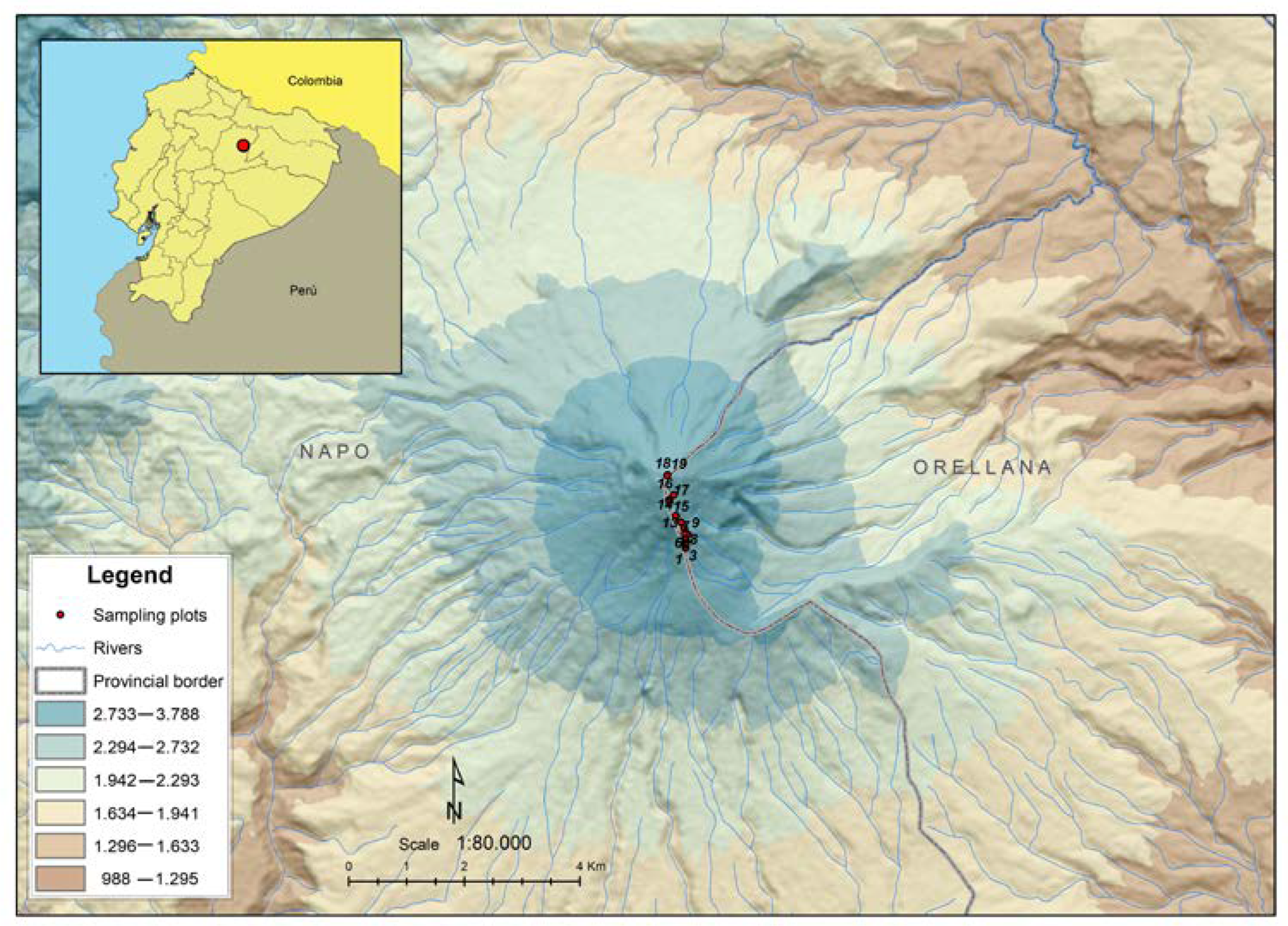
2.1. Study Area and Geological History of the Sumaco Volcano
2.2. Data Sampling and Processing
2.3. Statistical Analyses of Plant Diversity and Species Assemblages
2.4. Geographic Plant Distribution
3. Results
3.1. Plant Diversity
3.2. Species Assemblages and Floristic Groups along Gradient
3.3. Life Forms and Species Turnover
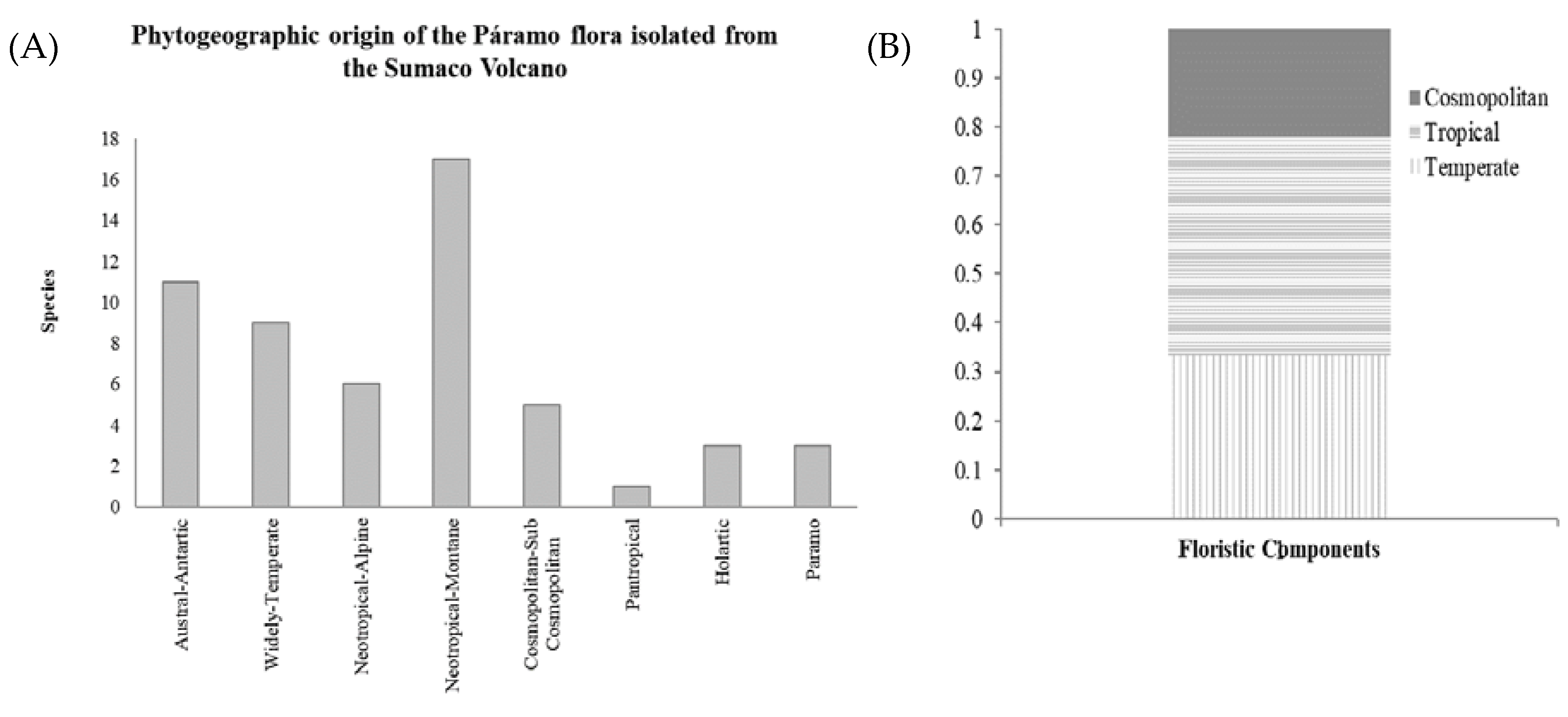
3.4. Plant Geography
4. Discussion
4.1. Diversity and Main Life Form
4.2. Phytogeography and Distribution Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luteyn, J.L.; Churchill, S.P.; Griffin, D.; Gradstein, S.R.; Sipman, H.J.; Mauricio, R. Gavilanes A. Paramos: A Checklist of Plant Diversity, Geographical Distribution, and Geo Botanical Literature; New York Botanical Garden Press: Bronx, NY, USA, 1999; Volume 84. [Google Scholar]
- García, V.J.; Márquez, C.O.; Isenhart, T.M.; Rodríguez, M.; Crespo, S.D.; Cifuentes, A.G. Evaluating the conservation state of the páramo ecosystem: An object-based image analysis and CART algorithm approach for central Ecuador. Heliyon 2019, 5, e02701. [Google Scholar] [CrossRef] [PubMed]
- Christmann, T.; Oliveras, I. Nature of Alpine Ecosystems in Tropical Mountains of South America; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Lozano, P.; Cleef, A.M.; Yangora, J.; Torres, B.; Carrión, J.; Abril, R. Páramos Aislados del Volcán Sumaco, Amazonía del Ecuador; Universidad Estatal Amazónica: Puyo, Ecuador, 2013; p. 120. [Google Scholar]
- Guayasamin, J.M.; Bonaccorso, E. (Eds.) Evaluación Ecológica Rápida de la Biodiversidad de los Tepuyes de la Cuenca Alta del Río Nangaritza, Cordillera del Cóndor, Ecuador; Conservación Internacional: Quito, Ecuador, 2013; p. 135. [Google Scholar]
- Quizhpe, W.; Benítez, Á.; Cuenca, K.; Uvidia, H.; Huamantupa-Chuquimaco, I.; Muñoz, J.; Cabrera, O. Forest Diversity and Structure in the Amazonian Mountain Ranges of Southeastern Ecuador. Diversity 2019, 11, 196. [Google Scholar] [CrossRef]
- Hodge, A.-M.C.; Arbogast, B.S. Carnivore diversity at a montane rainforest site in Ecuador’s Gran Sumaco Biosphere Reserve. Oryx 2015, 50, 474–479. [Google Scholar] [CrossRef]
- Torres, B.; Vasco, C.; Günter, S.; Knoke, T. Determinants of Agricultural Diversification in a Hotspot Area: Evidence from Colonist and Indigenous Communities in the Sumaco Biosphere Reserve, Ecuadorian Amazon. Sustainability 2018, 10, 1432. [Google Scholar] [CrossRef]
- Ramsay, P.M.; Oxley, E.R.B. The growth form composition of plant communities in the ecuadorian páramos. Plant Ecol. 1997, 131, 173–192. [Google Scholar] [CrossRef]
- Sklenar, P. Advance of plant species on slopes of the Chimborazo volcano (Ecuador) calculated based on unreliable data. Proc. Natl. Acad. Sci. USA 2016, 113, E407–E408. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, D.A.; Antonelli, A. A network approach for identifying and delimiting biogeographical regions. Nat. Commun. 2015, 6, 6848. [Google Scholar] [CrossRef] [PubMed]
- Sklenar, P.; Duskova, E.; Balslev, H. Tropical and Temperate: Evolutionary History of Páramo Flora. Bot. Rev. 2010, 77, 71–108. [Google Scholar] [CrossRef]
- Hofstede, R.; Segarra, P.; Mena Vásconez, P. (Eds.) Los Páramos del Mundo. Proyecto Atlas Mundial de los Páramos; Global Peatland Initiative: Quito, Ecuador, 2003; p. 299. [Google Scholar]
- Jiménez-Rivillas, C.; García, J.J.; Quijano-Abril, M.A.; Daza, J.M.; Morrone, J.J. A new biogeographical regionalization of the Paramo biogeographic province. Aust. Syst. Bot. 2018, 31, 296–310. [Google Scholar] [CrossRef]
- Cuesta, F.; Muriel, P.; Llambí, L.D.; Halloy, S.; Aguirre, N.; Beck, S.; Carilla, J.; Meneses, R.I.; Cuello, S.; Grau, A.; et al. Latitudinal and altitudinal patterns of plant community diversity on mountain summits across the tropical Andes. Ecography 2017, 40, 1381–1394. [Google Scholar] [CrossRef]
- Lozano, P. Plant Biodiversity of Ecuador: A Neotropical Megadiverse Country. In Global Biodiversity: Selected Countries in the Americas and Australia; Apple Academic Press: Waretown, NJ, USA, 2018; Volume 4, pp. 185–211. [Google Scholar]
- Harden, C.P.; Hartsig, J.; Farley, K.A.; Lee, J.; Bremer, L.L. Effects of Land-Use Change on Water in Andean Páramo Grassland Soils. Ann. Assoc. Am. Geogr. 2013, 103, 375–384. [Google Scholar] [CrossRef]
- Buytaert, W.; Tobón, C.; Cuesta-Camacho, F. Potential impacts of climate change on the environmental services of humid tropical alpine regions. Glob. Ecol. Biogeogr. 2010, 20, 19–33. [Google Scholar] [CrossRef]
- León-Yánez, S. La Flora de los Páramos Ecuatorianos. En: La Biodiversidad de los Páramos; Serie Páramo; GTP/AbyaYala: Quito, Ecuador, 2000; Volume 7, pp. 5–21. [Google Scholar]
- Vásquez, D.L.A.; Balslev, H.; Sklenar, P. Human impact on tropical-alpine plant diversity in the northern Andes. Biodivers. Conserv. 2015, 24, 2673–2683. [Google Scholar] [CrossRef]
- Webster, P.X.A.; Barros, S.; Siddons, D.C.; Zárate, E. Influence of habitat modification by livestock on páramo bird abundance in southern Andes of Ecuador. Stud. Neotrop. Fauna Environ. 2017, 53, 29–37. [Google Scholar]
- Espinosa, J.; Rivera, D. Variations in water resources availability at the Ecuadorian páramo due to land-use changes. Environ. Earth Sci. 2016, 75, 1173. [Google Scholar] [CrossRef]
- Borrelli, P.; Armenteras, D.; Panagos, P.; Modugno, S.; Schütt, B. The Implications of Fire Management in the Andean paramo: A Preliminary assessment using Satellite Remote Sensing. Remote Sens. 2015, 7, 11061–11082. [Google Scholar] [CrossRef]
- Acosta Solís, M. Divisiones Fitogeográficas y Formaciones Geobotánicas del Ecuador; Casa de la Cultura Ecuatoriana: Quito, Ecuador, 1968; No. 581.9866 A26. [Google Scholar]
- Harling, G. The Vegetation Types of Ecuador: A Brief Survey; Academic Press: London, UK, 1979. [Google Scholar]
- Cañadas, L. El Mapa Bioclimático del Ecuador; Ediciones del Banco Central del Ecuador: Quito, Ecuador, 1983. [Google Scholar]
- Løjtnant, B.; Molau, U. Analysis of virgin paramo plant community on Volcán Sumaco, Ecuador. Nord. J. Bot. 1982, 2, 567–574. [Google Scholar] [CrossRef]
- Sierra, R. Propuesta Preliminar de un Sistema de Clasificación de Vegetación Para el Ecuador Continental; Proyecto Inefan/Gef-Birf; EcoCiencia: Quito, Ecuador, 1999. [Google Scholar]
- Josse, C.; Cuesta, F.; Navarro, G.; Barrena, V.; Cabrera, E.; Chacón-Moreno, E.; Tovar, A. Ecosistemas de los Andes del Norte y Centro. Bolivia, Colombia, Ecuador, Perú y Venezuela. In Secretaría General de la Comunidad Andina, Programa Regional ECOBONA-Intercooperation, CONDESAN-Proyecto Páramo Andino, Programa BioAndes, EcoCiencia, NatureServe, IAvH, LTA-UNALM, ICAE-ULA, CDC-UNALM, RUMBOL SRL; Universidad de Los Andes: Lima, Peru, 2009; pp. 7–9. [Google Scholar]
- Sklenář, P. Vegetation Ecology and Phytogeography of Ecuadorian Super Paramos. Ph.D. Thesis, Charles University, Prague, Czech, 2000. [Google Scholar]
- Ramsay, P.M. The Paramo Vegetation of Ecuador: The Community Ecology, Dynamics and Productivity of Tropical Grasslands in the Andes. Ph.D. Thesis, University of Wales UCNW, Bangor, UK, 1992. [Google Scholar]
- Peyre, G. Plant Diversity and Vegetation of the Andean Paramo. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, March 2015. [Google Scholar]
- Peyre, G.; Balslev, H.; Font, X. Phytoregionalisation of the Andean páramo. PeerJ 2018, 6, e4786. [Google Scholar] [CrossRef] [PubMed]
- Fiallos, L.; Herrera, R.S.; Velázquez, R. Flora diversity in the Ecuadorian Paramo grassland ecosystem. Revista Cubana de Ciencia Agrícola 2015, 49, 399–405. [Google Scholar]
- Lozano, P.; Delgado, T.; Aguirre, Z. La Flora Endémica del Occidente del Parque Nacional Podocarpus. Lyonia 2003, 3, 145–308. [Google Scholar]
- Maarten, K.; Sally, H. The Paramo ecosystem of Costa Rica’s highlands. In Costa Rican Ecosystems; The University of Chicago Press: Chicago, IL, USA, 2016; pp. 492–523. [Google Scholar]
- Carrillo-Rojas, G.; Silva, B.; Córdova, M.; Célleri, R.; Bendix, J. Dynamic Mapping of Evapotranspiration Using an Energy Balance-Based Model over an Andean Páramo Catchment of Southern Ecuador. Remote Sens. 2016, 8, 160. [Google Scholar] [CrossRef]
- Dušková, E.; Sklenar, P.; Kolar, F.; Vásquez, D.L.A.; Romoleroux, K.; Fér, T.; Marhold, K. Growth form evolution and hybridization in Senecio (Asteraceae) from the high equatorial Andes. Ecol. Evol. 2017, 7, 6455–6468. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, D.L.; Calvo, J. Senecio sangayensis (Asteraceae, Senecioneae): A striking new species from the Ecuadorian highlands. Phytotax 2016, 268, 102. [Google Scholar]
- Cleef, A.M.; Van Der Hammen, T. El elemento de origen sabanero en la flora de los páramos. Pérez-Abelaezia 1989, 2, 417–427. [Google Scholar]
- Cleef, A.M.; van der Hammen, T.; Hooghiemstra, H. The savanna relationship in the Andean paramo flora. Opera Bot. 1993, 121, 285–290. [Google Scholar]
- Steyermark, J.A.; Huber, O. Flora del Ávila: Flora y vegetación de las Montañas del Ávila, de la Silla y del Naiguatá; Sociedad Venezolana de Ciencias Naturales bajo los auspicios de “Vollmer Foundation” y Ministerio del Ambiente y de los Recursos Naturales Renovables: Caracas, Venezuela, 1978; p. 971. [Google Scholar]
- Lozano, P.; Cleef, A.; Bussmann, R. Phytogeography of the vascular paramo flora of Podocarpus National Park, south Ecuador. Arnaldoa 2009, 16, 59–85. [Google Scholar]
- Lozano, P.; Duque, J.; Balslev, H.; Ollgaard, B.; Peyre, G.; Vallejo, S.; Valverde, V.; López, P. Flora Paramuna de la Base del Cono del Volcan Sangay—Amazonía del Ecuador; Universidad Estatal Amazónica: Puyo, Ecuador, 2018; p. 135. [Google Scholar]
- Colony, R.J.; Sinclair, J.H. The lavas of the Volcano Sumaco, eastern Ecuador, South America. Am. J. Sci. 1928, 94, 299–312. [Google Scholar] [CrossRef]
- Sheppard, G. Igneous and Associated Rocks from the Andes of Eastern Ecuador. Geol. Mag. 1930, 67, 361–371. [Google Scholar] [CrossRef]
- Tschopp, H.J. Oil Explorations in the Oriente of Ecuador, 1938-1950. AAPG Bull. 1953, 37, 2303–2347. [Google Scholar]
- Toulkeridis, T. Volcanes Activos: Ecuador; Santa Rita: Quito, Ecuador, 2013; p. 165. [Google Scholar]
- Barragan, R.; Geist, D.; Hall, M.; Larson, P.; Kurz, M. Subduction controls on the compositions of lavas from the Ecuadorian Andes. Earth Planet. Sci. Lett. 1998, 154, 153–166. [Google Scholar] [CrossRef]
- Garrison, J.M.; Sims, K.W.W.; Yogodzinski, G.M.; Escobar, R.D.; Scott, S.; Mothes, P.; Hall, M.L.; Ramón, P. Shallow-level differentiation of phonolitic lavas from Sumaco Volcano, Ecuador. Contrib. Miner. Pet. 2017, 173, 6. [Google Scholar] [CrossRef]
- Bryant, J.A.; Yogodzinski, G.M.; Hall, M.L.; Lewicki, J.L.; Bailey, D.G. Geochemical Constraints on the Origin of Volcanic Rocks from the Andean Northern Volcanic Zone, Ecuador. J. Pet. 2006, 47, 1147–1175. [Google Scholar] [CrossRef]
- Ancellin, M.-A.; Samaniego, P.; Vlastélic, I.; Nauret, F.; Gannoun, A.; Hidalgo, S. Across-arc versus along-arc Sr-Nd-Pb isotope variations in the Ecuadorian volcanic arc. Geochem. Geophys. Geosystems 2017, 18, 1163–1188. [Google Scholar] [CrossRef]
- Tibaldi, A.; Ferrari, L.; Pasquarè, G. Landslides triggered by earthquakes and their relations with faults and mountain slope geometry: An example from Ecuador. Geomorphology 1995, 11, 215–226. [Google Scholar] [CrossRef]
- Schuster, R.L.; NietoThomas, A.S.; O’Rourke, T.D.; Crespo, E.; Plaza-Nieto, G. Mass wasting triggered by the 5 March 1987 ecuador earthquakes. Eng. Geol. 1996, 42, 1–23. [Google Scholar] [CrossRef]
- Toulkeridis, T.; Zach, I. Wind directions of volcanic ash-charged clouds in Ecuador – implications for the public and flight safety. Geomat. Nat. Hazards Risk 2016, 8, 242–256. [Google Scholar] [CrossRef]
- Toulkeridis, T.; Zak, V.; Delgado, F.; León-Reyes, A.; Silva, X.; Burbano, F.; Rodriguez, A. Sumaco Ecoroute Guide; Corporación Andina de Fomento (CAF): Caracas, Venezuela, 2009. [Google Scholar]
- Braun Blanquet, J. Fito Sociología-Bases Para el Estudio de las Comunidades Vegetales; Blume: Madrid, Spain, 1979; p. 820. [Google Scholar]
- Peyre, G.; Balslev, H.; Font, X.; Tello, J.S. Fine-Scale Plant Richness Mapping of the Andean Páramo According to Macroclimate. Front. Ecol. Evol. 2019, 7, 377. [Google Scholar] [CrossRef]
- Cleef, A.M. The Vegetation of the Paramos of the Colombian Cordillera Oriental; Lubrecht & Cramer Ltd.: New York, NY, USA, 1981; Volume 61, pp. 1–321. [Google Scholar]
- Cuello, A.N.L.; Cleef, A.M. The paramo vegetation of Ramal de Guaramacal, Trujillo State, Venezuela. Zonal vegetation. Phytocoenologia 2009, 39, 295–320. [Google Scholar] [CrossRef]
- Ramsay, P.M. The zonal paramo vegetation of Volcán Chiles. In The Ecology of Volcán Chiles: High-Altitude Ecosystems on the Ecuador-Colombia Border; Pebble & Shell: Plymouth, UK, 2001; pp. 27–38. [Google Scholar]
- Zomer, M.A.; Ramsay, P.M. Post-fire changes in plant growth form composition in Andean páramo grassland. BioRxiv 2020. [Google Scholar] [CrossRef]
- Bremer, L.L.; Farley, K.A.; DeMaagd, N.; Suárez, E.; Tandalla, D.C.; Tapia, S.V.; Vásconez, P.M. Biodiversity outcomes of payment for ecosystem services: Lessons from páramo grasslands. Biodivers. Conserv. 2019, 28, 885–908. [Google Scholar] [CrossRef]
- Minaya, V.; Corzo, G.; Romero-Saltos, H.; van der Kwast, J.; Lantinga, E.; Galárraga-Sánchez, R.; Mynett, A. Altitudinal analysis of carbon stocks in the Antisana páramo, Ecuadorian Andes. J. Plant Ecol. 2016, 9, 553–563. [Google Scholar] [CrossRef]
- Asanza, M.; Vargas, J.C.; Neil, D.; Gutiérrez, D.; Vázquez, A.; Ramírez, W.; Scalvenzi, L. Herbario Amazónico del Ecuador ECUAMZ; Universidad Estatal Amazónica: Pastaza, Ecuador, 2017; Volume 2, pp. 44–54. [Google Scholar]
- Huamantupa-Chuquimaco, I.; Neill, D.A. Vochysia condorensis (Vochysiaceae), a new species from the Cordillera del Cóndor, Ecuador. Phytotaxa 2018, 340, 79–85. [Google Scholar] [CrossRef]
- Holm-Nielsen, L.B.; Harling, G. Flora of Ecuador; Department of Systematic Botany, University of Göteborg, Section for Botany, Riksmuseum: Amsterdam, The Netherlands, 1986; pp. 1–92. [Google Scholar]
- Peet, R.K. Relative Diversity Indices. Ecology 1975, 56, 496–498. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, Y.; Dong, W.; Liang, Y.; Fan, F.; Zhang, X.; Zhang, X.; Niu, J.; Ma, L.; She, S.; et al. The complicated substrates enhance the microbial diversity and zinc leaching efficiency in sphalerite bioleaching system. Appl. Microbiol. Biotechnol. 2015, 99, 10311–10322. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- González, Y.; Aragón, G.; Benítez, A.; Prieto, M. Changes in soil cryptogamic communities in tropical Ecuadorean páramos. Community Ecol. 2017, 18, 11–20. [Google Scholar] [CrossRef]
- Cabrera, O.; Benítez, Á.; Cumbicus, N.; Naranjo, C.; Ramón, P.; Tinitana, F.; Escudero, A. Geomorphology and Altitude Effects on the Diversity and Structure of the Vanishing Montane Forest of Southern Ecuador. Diversity 2019, 11, 32. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Beals, E.W. Bray-Curtis Ordination: An Effective Strategy for Analysis of Multivariate Ecological Data. In Food Webs: From Connectivity to Energetics; Elsevier BV: Amsterdam, The Netherlands, 1984; Volume 14, pp. 1–55. [Google Scholar]
- Ricotta, C.; Podani, J. On some properties of the Bray-Curtis dissimilarity and their ecological meaning. Ecol. Complex. 2017, 31, 201–205. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L.F. Numerical ecology, In Developments in Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 24, pp. 1–990. [Google Scholar]
- Oksanen, J. Vegan: Community Ecology Package R package version 2.0-2. Available online: http://CRAN.R-project.org/package=vegan (accessed on 1 September 2019).
- Anderson, M.; Gorley, R.N.; Clarke, R.K. Permanova+ for Primer: Guide to Software and Statistical Methods; Primer-E Limited: Plymouth, UK, 2008. [Google Scholar]
- Gibert, C.; Escarguel, G. PER-SIMPER—A new tool for inferring community assembly processes from taxon occurrences. Glob. Ecol. Biogeogr. 2018, 28, 374–385. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer; Primer-E: Plymouth, UK, 2006. [Google Scholar]
- Cleef, A.M. The phytogeographical position of the neotropical vascular páramo flora with special reference to the Colombian Cordillera Oriental. In Tropical Botany; Larsen, K., Holm-Nielsen, L.B., Eds.; Academic Press: London, UK, 1979; pp. 175–184. [Google Scholar]
- Cleef, A.M. Phytogeography of the generic vascular páramo flora of Tatamá (Western Cordillera), Colombia. In La Cordillera Occidental Colombiana—Transecto Tatamá. Studies on Tropical Andean Ecosystems; van der Hammen, T., Rangel, J.O., Cleef, A.M., Eds.; J. Cramer, Berlin-Stuttgart: Stutgart, Germany, 2005; Volume 6, pp. 661–668. [Google Scholar]
- Miranda-Esquivel, D.R.; Romero-Alarcon, L.V.; Diaz, C. Páramos Neotropicales como unidades biogeográficas. Revista de Biología Tropical 2020, 68, 2. [Google Scholar]
- Cleef, A.M.; Chaverri, A. Phytogeography of the páramo flora of Cordillera Talamanca, Costa Rica. In Páramo: An Andean Ecosystem under Human Influence; Balslev, H., Luteyn, J.L., Eds.; Academic Press: London, UK, 1992; pp. 45–60. [Google Scholar]
- Testoni, D.; Linder, H.P. Synoptic taxonomy of Cortaderia Stapf (Danthonioideae, Poaceae). PhytoKeys 2017, 76, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Grytnes, J.A.; Halloy, S.R.P.; Kluge, J.; Krömer, T.; León, B.; Macía, M.J.; Young, K.R. Gradientes de diversidad vegetal: Patrones y procesos locales. In Cambio Climático y la Biodiversidad en los Andes Tropicales; Herzog, S.K., Martínez, R., Jørgensen, P.M., Tiessen, H., Eds.; Instituto Interamericano para la Investigación del Cambio Global (IAI), São José dos Campos, Comité Científico sobre Problemas del Medio Ambiente (SCOPE): Paris, France, 2012; pp. 235–253. [Google Scholar]
- McCain, C.M. Elevational gradients in diversity of small mammals. Ecology 2005, 86, 366–372. [Google Scholar] [CrossRef]
- Arzac, A.; Llambí, L.D.; Dulhoste, R.; Olano, J.M.; Moreno, E.J.C. Modelling the effect of temperature changes on plant life-form distribution across a treeline ecotone in the tropical Andes. Plant Ecol. Divers. 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Campos, P.V.; Villa, P.M.; Nunes, J.A.; Schaefer, C.E.; Porembski, S.; Neri, A.V. Plant diversity and community structure of Brazilian Páramos. J. Mt. Sci. 2018, 15, 1186–1198. [Google Scholar] [CrossRef]
- Richter, M.; Moreira-Muñoz, A. Heterogeneidad climática y diversidad de la vegetación en el sur de Ecuador: Un método de fitoindicación. Revista Peruana de Biología 2005, 12, 217–238. [Google Scholar]
- Øllgaard, B. New neotropical Lycopodiaceae. Phytotaxa 2016, 277, 266–274. [Google Scholar] [CrossRef]
- González, F.; Seas, C.; Barrientos, Z.; Quesada-Acuña, S.G.; Amador, R.A.M. Poás Volcano Biodiversity. In Poás Volcano; Springer: Cham, Switzerland, 2019; pp. 309–317. [Google Scholar]
- Morueta-Holme, N.; Engemann, K.; Sandoval-Acuña, P.; Jonas, J.D.; Segnitz, R.M.; Svenning, J.C. Strong upslope shifts in Chimborazo’s vegetation over two centuries since Humboldt. Proc. Natl. Acad. Sci. USA 2015, 112, 12741–12745. [Google Scholar] [CrossRef]
- Eguiguren, P.; Ojeda, T.; Aguirre, N. Diversidad Florística del Ecosistema Páramo del Parque Nacional Podocarpus para el Monitoreo del Cambio Climático; Universidad Nacional de Loja: Loja, Ecuador, 2010; p. 14. [Google Scholar]
- Balslev, H. Distribution pattern of Ecuadorian plant species. Taxon 1988, 37, 567–577. [Google Scholar] [CrossRef]
- Keating, P.L. Changes in Paramo Vegetation Along an Elevation Gradient in Southern Ecuador. J. Torrey Bot. Soc. 1999, 126, 159–175. [Google Scholar] [CrossRef]
- Cuello, N.L.; Cleef, A.M.; Aymard, G. Phytogeography of the vascular páramo flora of Ramal de Guaramacal (Andes, Venezuela) and its ties to other páramo floras. Anales del Jardín Botánico de Madrid 2010, 67, 177–193. [Google Scholar] [CrossRef]
- Ricardi, M.; Gaviria, J.; Estrada, J. La flora del super páramo venezolano y sus relaciones fitogeográficas a lo largo de los Andes. Plantula 1997, 1, 171–187. [Google Scholar]
- Sklenar, P.; Jørgensen, P.M. Distribution patterns of paramo plants in Ecuador. J. Biogeogr. 1999, 26, 681–691. [Google Scholar] [CrossRef]
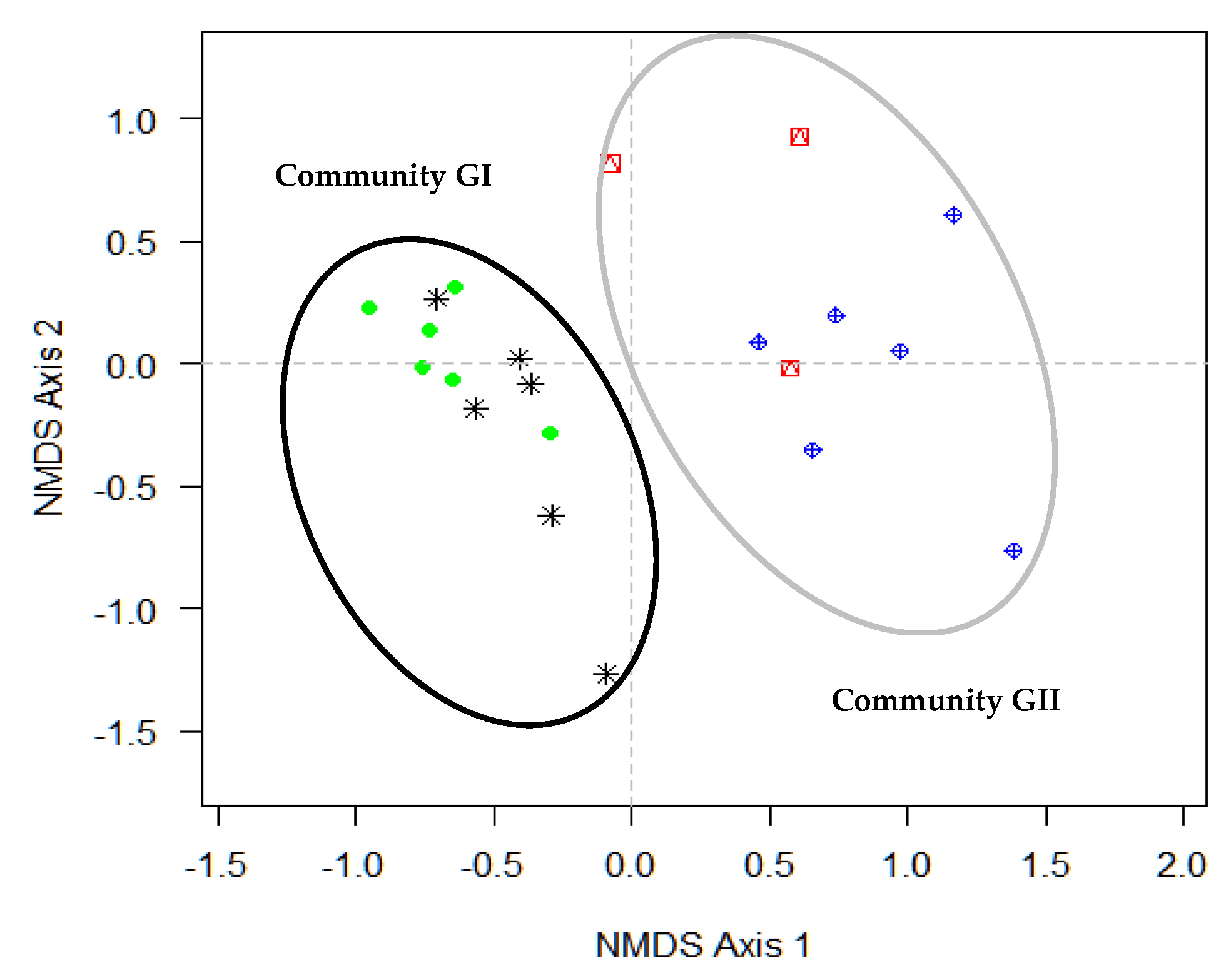
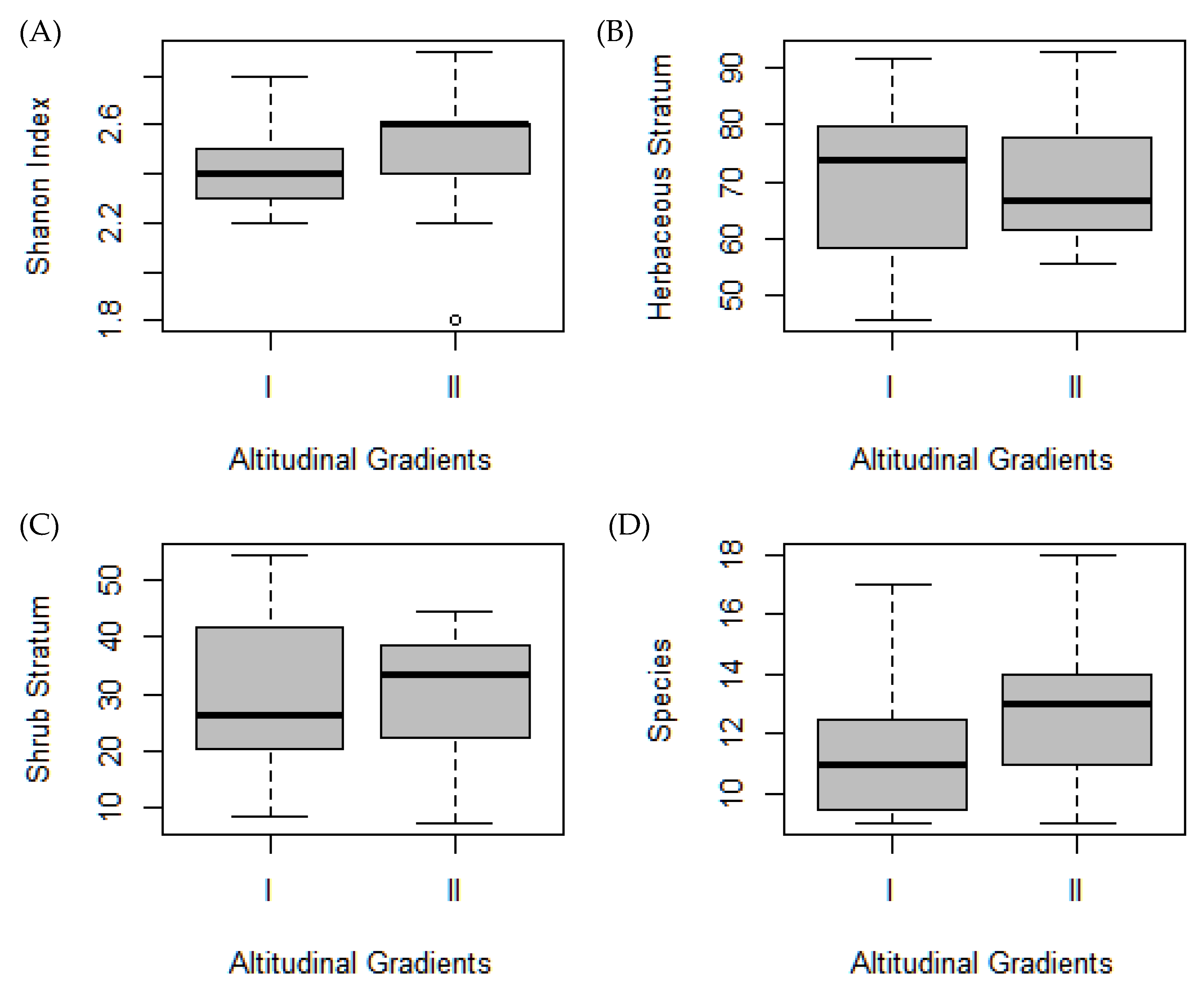

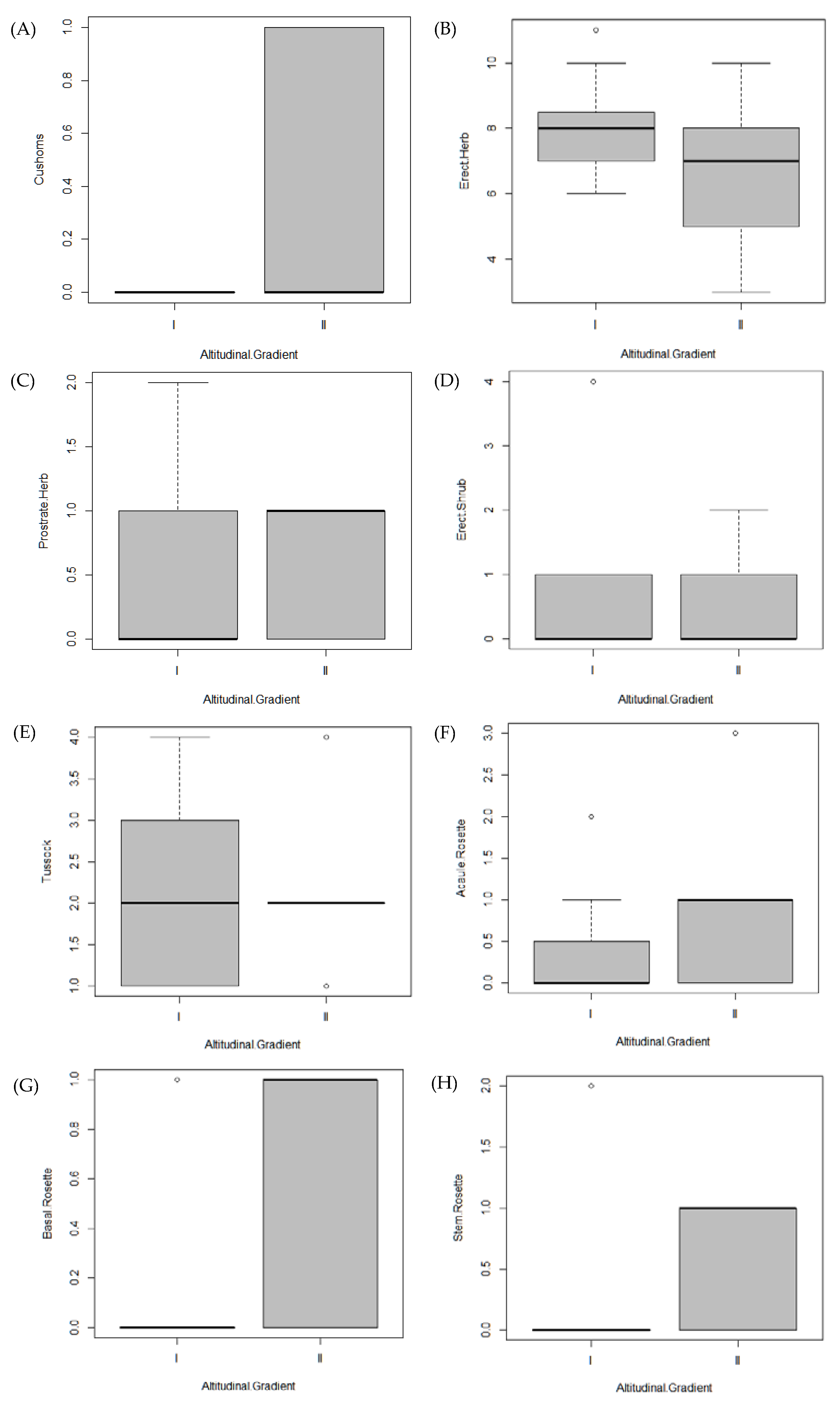
| Variables | NMDS1 | NMDS2 | r2 | Pr(>r) |
|---|---|---|---|---|
| Altitude | −0.68090 | 0.73237 | 0.83 | 0.001 *** |
| Herbaceous stratum | 0.04777 | 0.99886 | 0.53 | 0.001 *** |
| Shrub stratum | −0.04777 | −0.99886 | 0.53 | 0.001 *** |
| Slope | 0.87744 | −0.47969 | 0.34 | 0.023 * |
| Temperature | 0.13639 | −0.99066 | 0.23 | 0.087 |
| Lifeform | GI | GII | X-Squared | df | p |
|---|---|---|---|---|---|
| Cushion | 0 | 3 | 4.4 | 1 | 0.03 * |
| Erect Shrub | 8 | 5 | 0.1 | 1 | 0.9 |
| Erect Herb | 97 | 61 | 1.9 | 1 | 0.1 |
| Prostrate Herb | 6 | 5 | 0.16 | 1 | 0.68 |
| Tussock | 25 | 20 | 0.05 | 1 | 0.8 |
| Acaulescent Rosette | 4 | 8 | 3 | 1 | 0.08 |
| Basal Rosette | 1 | 5 | 5.3 | 1 | 0.02 * |
| Stem Rosette | 2 | 6 | 6.01 | 1 | 0.01 * |
| Level I to Level II Average Dissimilarity = 54.1 | |||||
|---|---|---|---|---|---|
| Species | Level I Average Abundance | Level II Average Abundance | Average Dissimilarity | Contribution (%) | Cumulative (%) |
| Macleania rupestris | 0.8 | 0.2 | 3.2 | 5.9 | 5.9 |
| Agrostis perennans | 0.2 | 0.8 | 3.1 | 5.7 | 11.6 |
| Epidendrum elleanthoides | 0.2 | 0.7 | 2.7 | 4.9 | 16.5 |
| Elaphoglossum muscosum | 0.3 | 0.8 | 2.6 | 4.87 | 21.4 |
| Elaphoglossum dendriculum | 0.7 | 0.5 | 2.2 | 4.07 | 25.4 |
| Level I to Level III Average Dissimilarity = 77.9 | |||||
| Species | Level I Average Abundance | Level III Average Abundance | Average Dissimilarity | Contribution (%) | Cumulative (%) |
| Disterigma codonanthum | 0.8 | 0 | 3.2 | 4.1 | 4.1 |
| Macleania rupestris | 0.8 | 0 | 3.2 | 4.1 | 8.2 |
| Gaultheria amoena | 0.2 | 1.0 | 3.2 | 4.1 | 12.3 |
| Elleanthus aurantiacus | 0.7 | 0 | 2.7 | 3.5 | 15.7 |
| Huperzia hystrix | 0 | 0.7 | 2.6 | 3.3 | 19.1 |
| Level I to Level IV Average Dissimilarity = 81.9 | |||||
| Species | Level I Average Abundance | Level IV Average Abundance | Average Dissimilarity | Contribution (%) | Cumulative (%) |
| Elaphoglossum antisanae | 0.8 | 0 | 3.7 | 4.5 | 4.5 |
| Macleania rupestris | 0.8 | 0 | 3.5 | 4.3 | 8.7 |
| Carex microglochin | 0 | 0.8 | 3.5 | 4.2 | 13.0 |
| Lachemila hispidula | 0 | 0.8 | 3.3 | 4.1 | 17.0 |
| Elleanthus aurantiacus | 0.7 | 0 | 2.9 | 3.6 | 20.6 |
| Level II to Level III Average Dissimilarity = 76.1 | |||||
| Species | Level II Average Abundance | Level III Average Abundance | Average Dissimilarity | Contribution (%) | Cumulative (%) |
| Elleanthus aurantiacus | 1.0 | 0 | 4.1 | 5.4 | 5.4 |
| Elaphoglossum muscosum | 0.8 | 0 | 3.4 | 4.5 | 9.9 |
| Gaultheria amoena | 0.2 | 1 | 3.4 | 4.5 | 14.4 |
| Disterigma codonanthum | 0.8 | 0 | 3.4 | 4.4 | 18.8 |
| Huperzia hystrix | 0 | 0.67 | 2.7 | 3.6 | 22.4 |
| Level II to Level IV Average Dissimilarity = 84.8 | |||||
| Species | Level II Average Abundance | Level IV Average Abundance | Average Dissimilarity | Contribution (%) | Cumulative (%) |
| Elleanthus aurantiacus | 1.0 | 0 | 4.5 | 5.2 | 5.2 |
| Elaphoglossum antisanae | 0.8 | 0 | 3.7 | 4.4 | 9.7 |
| Elaphoglossum muscosum | 0.8 | 0 | 3.7 | 4.4 | 14.1 |
| Carex microglochin | 0 | 0.83 | 3.7 | 4.4 | 18.4 |
| Agrostis perennans | 0.8 | 0 | 3.7 | 4.3 | 22.8 |
| Level III - Level IV Average Dissimilarity = 67.7 | |||||
| Species | Level III Average Abundance | Level IV Average Abundance | Average Dissimilarity | Contribution (%) | Cumulative (%) |
| Huperzia hystrix | 0.7 | 0 | 2.7 | 4.0 | 4.0 |
| Gaultheria amoena | 1.0 | 0.3 | 2.7 | 3.9 | 7.9 |
| Elaphoglossum dendriculum | 0.7 | 0.3 | 2.3 | 3.4 | 11.3 |
| Carex microglochin | 0.3 | 0.8 | 2.3 | 3.4 | 14.7 |
| Hieracium frigidum | 0.3 | 0.7 | 2.3 | 3.3 | 18.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, P.; Cabrera, O.; Peyre, G.; Cleef, A.; Toulkeridis, T. Plant Diversity and Composition Changes along an Altitudinal Gradient in the Isolated Volcano Sumaco in the Ecuadorian Amazon. Diversity 2020, 12, 229. https://doi.org/10.3390/d12060229
Lozano P, Cabrera O, Peyre G, Cleef A, Toulkeridis T. Plant Diversity and Composition Changes along an Altitudinal Gradient in the Isolated Volcano Sumaco in the Ecuadorian Amazon. Diversity. 2020; 12(6):229. https://doi.org/10.3390/d12060229
Chicago/Turabian StyleLozano, Pablo, Omar Cabrera, Gwendolyn Peyre, Antoine Cleef, and Theofilos Toulkeridis. 2020. "Plant Diversity and Composition Changes along an Altitudinal Gradient in the Isolated Volcano Sumaco in the Ecuadorian Amazon" Diversity 12, no. 6: 229. https://doi.org/10.3390/d12060229
APA StyleLozano, P., Cabrera, O., Peyre, G., Cleef, A., & Toulkeridis, T. (2020). Plant Diversity and Composition Changes along an Altitudinal Gradient in the Isolated Volcano Sumaco in the Ecuadorian Amazon. Diversity, 12(6), 229. https://doi.org/10.3390/d12060229





