Morphometric Response of Galaxias maculatus (Jenyns) to Lake Colonization in Chile
Abstract
1. Introduction
2. Materials and Methods
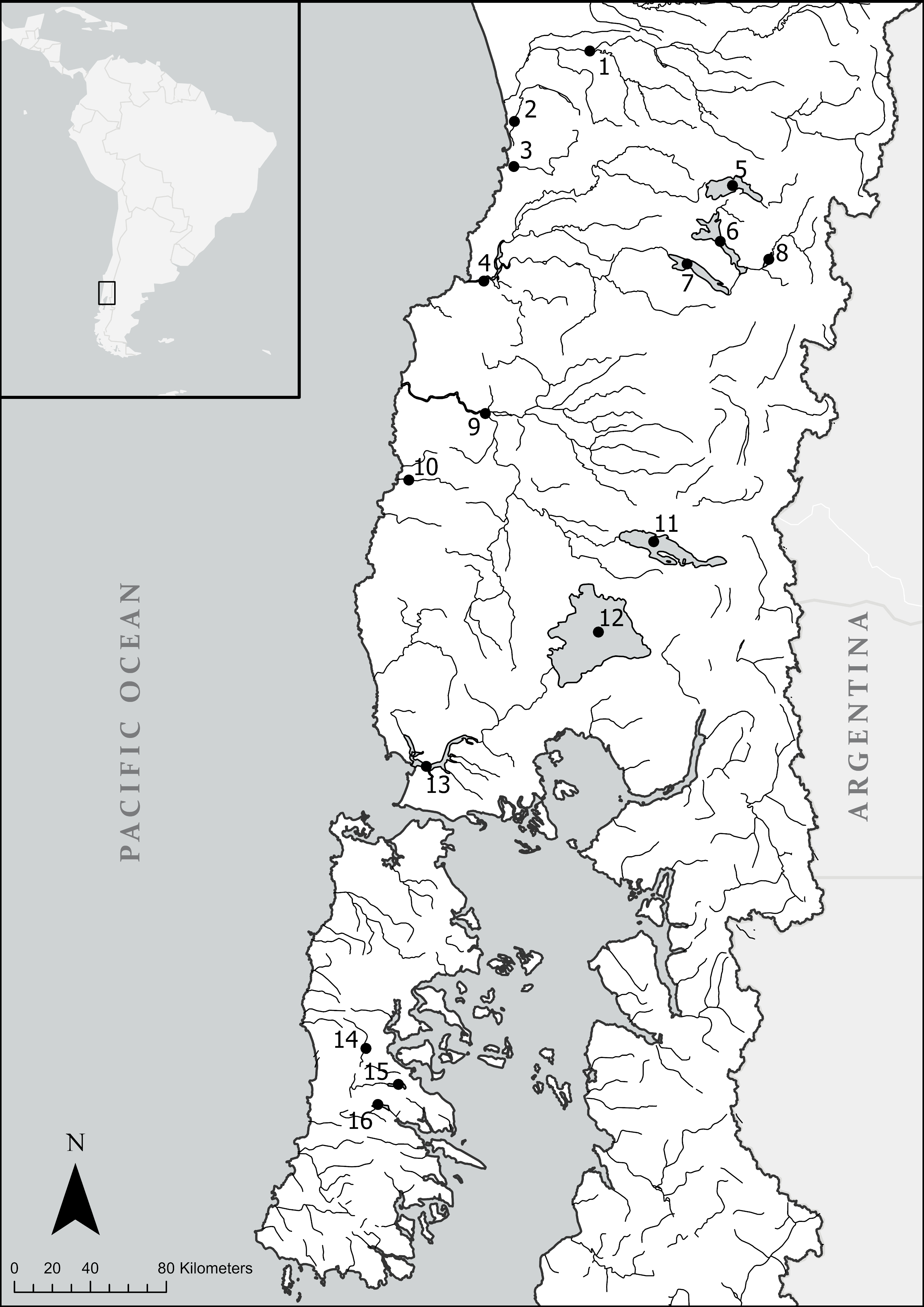
2.1. Study Site and Collection
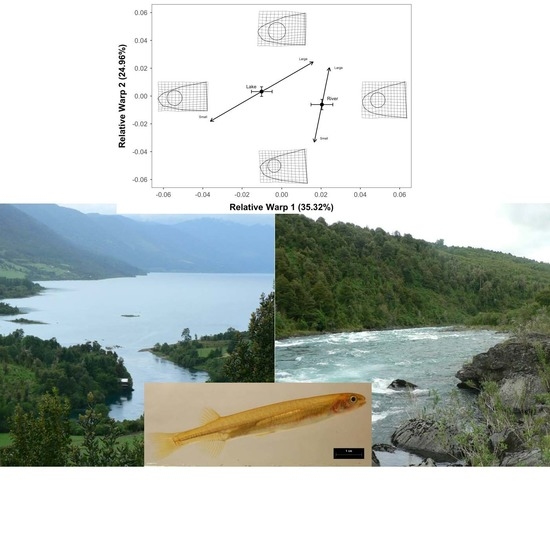
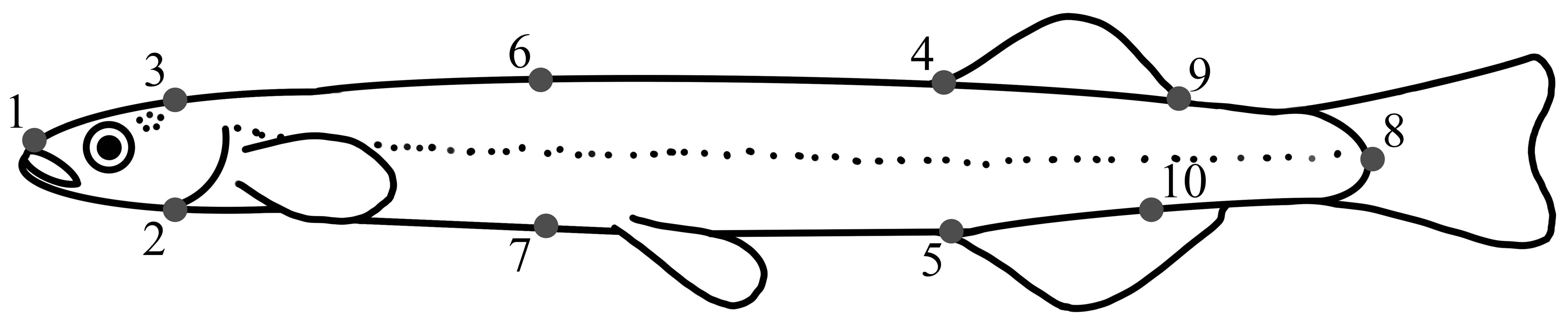
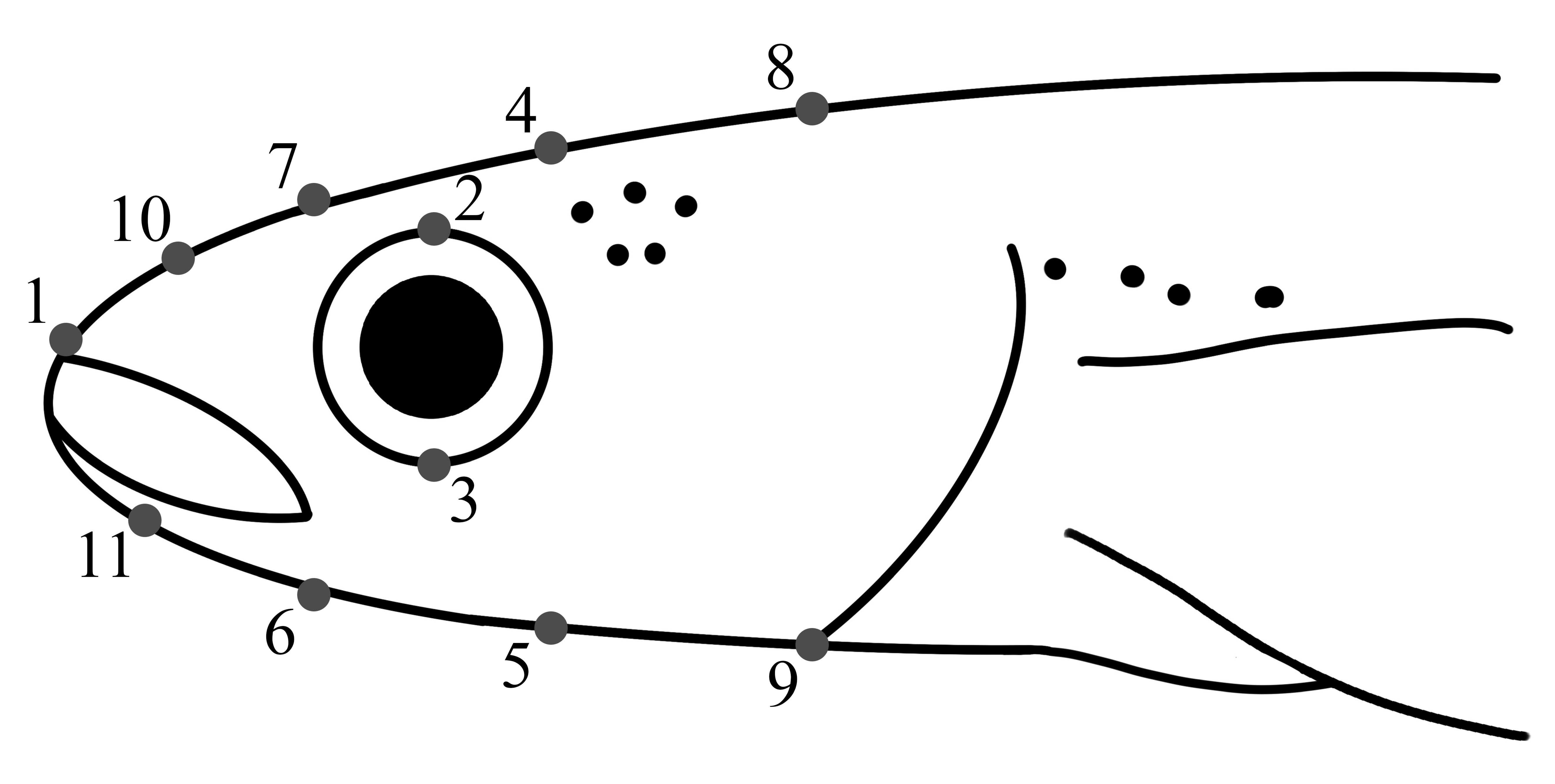
2.2. Geometric Morphometrics
2.3. Statistical Analysis
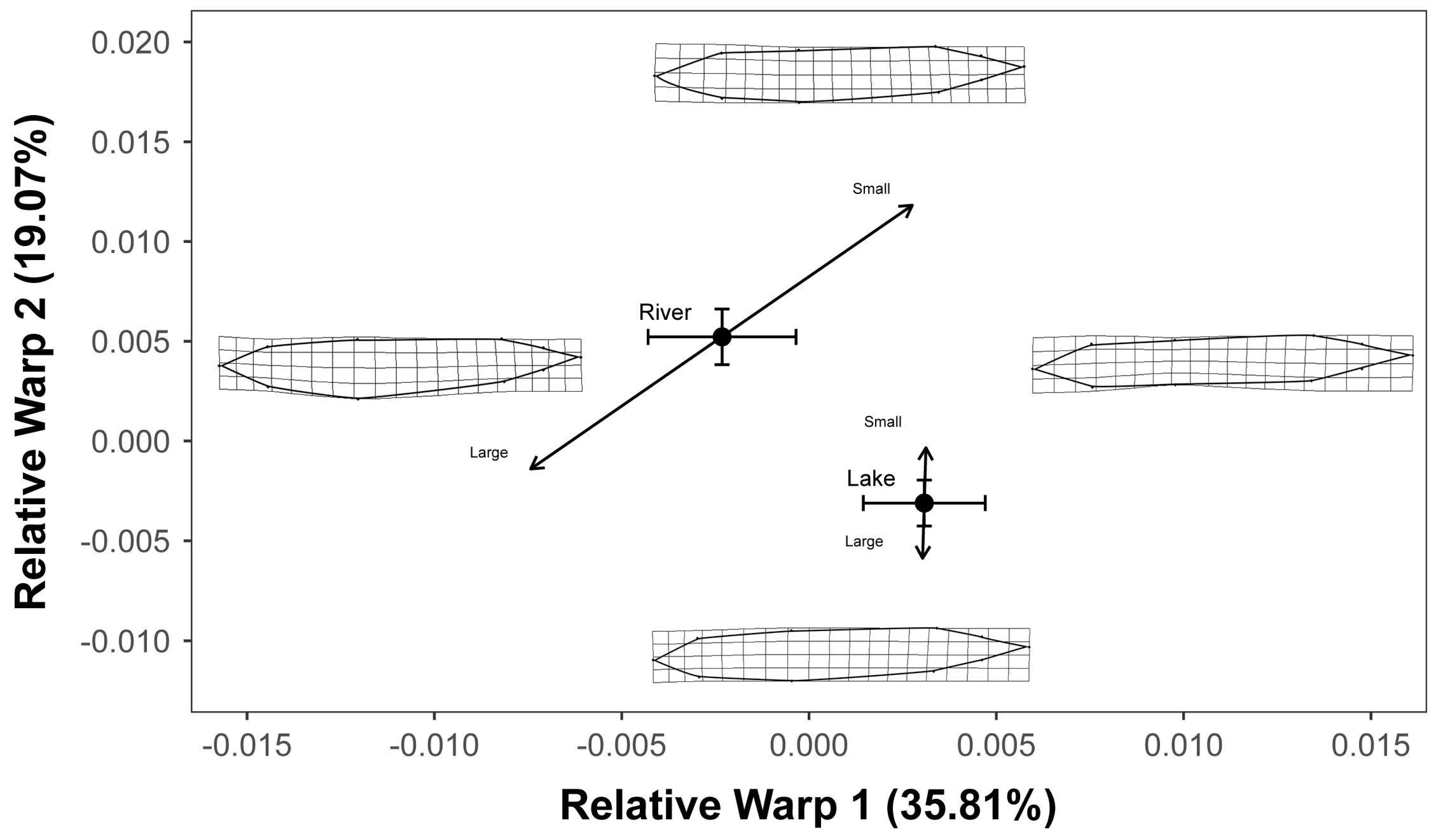
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Villaluz, A.C.; Maccrimmon, H.R. Meristic variation in milkfish Chanos chanos from Philippine waters. Mar. Biol. 1988, 97, 145–150. [Google Scholar] [CrossRef]
- Douglas, M.E.; Matthews, W.J. Does morphology predict ecology-hypothesis-testing within a fresh-water stream fish assemblage. Oikos 1992, 65, 213–224. [Google Scholar] [CrossRef]
- Blake, R.W. Fish functional design and swimming performance. J. Fish Biol. 2004, 65, 1193–1222. [Google Scholar] [CrossRef]
- Casatti, L.; Castro, R.M.C. Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the rio Sao Francisco, southeastern Brazil. Neotrop. Ichthyol. 2006, 4, 203–214. [Google Scholar] [CrossRef]
- Bock, W.J. Concepts and methods in ecomorphology. J. Biosci. 1994, 19, 403–413. [Google Scholar] [CrossRef]
- McLaughlin, R.L.; Grant, J.W.A. Morphological and behavioural differences among recently-emerged brook charr, Salvelinus fontinalis, foraging in slow- vs. fast-running water. Environ. Biol. Fishes 1994, 39, 289–300. [Google Scholar] [CrossRef]
- Pakkasmaa, S.; Piironen, J. Water velocity shapes juvenile salmonids. Evol. Ecol. 2000, 14, 721–730. [Google Scholar] [CrossRef]
- Brinsmead, J.; Fox, M.G. Morphological variation between lake- and stream-dwelling rock bass and pumpkinseed populations. J. Fish Biol. 2002, 61, 1619–1638. [Google Scholar] [CrossRef]
- Hendry, A.P.; Taylor, E.B.; McPhail, J.D. Adaptive divergence and the balance between selection and gene flow: Lake and stream stickleback in the misty system. Evolution 2002, 56, 1199–1216. [Google Scholar] [CrossRef]
- Langerhans, R.B. Predictability of phenotypic differentiation across flow regimes in fishes. Integr. Comp. Biol. 2008, 48, 750–768. [Google Scholar] [CrossRef]
- Meyers, P.J.; Belk, M.C. Shape variation in a benthic stream fish across flow regimes. Hydrobiologia 2014, 738, 147–154. [Google Scholar] [CrossRef]
- Nagel, L.; Schluter, D. Body size, natural selection, and speciation in sticklebacks. Evolution 1998, 52, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ryder, R.A.; Pesendorfer, J. Large rivers are more than flowing lakes: A comparative review. In Proceedings of the International Large River Symposium, Honey Harbour, ON, Canada, 14–21 September 1989. [Google Scholar]
- Naspleda, J.; Vila-Gispert, A.; Fox, M.G.; Zamora, L.; Ruiz-Navarro, A. Morphological variation between non-native lake- and stream-dwelling pumpkinseed Lepomis gibbosus in the Iberian Peninsula. J. Fish Biol. 2012, 81, 1915–1935. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, I.J.; Aho, J.; Vornanen, M.; Huuskonen, H. Phenotypic plasticity and predator effects on morphology and physiology of crucian carp in nature and in the laboratory. J. Fish Biol. 1997, 50, 781–798. [Google Scholar] [CrossRef]
- Chivers, D.P.; Zhao, X.X.; Brown, G.E.; Marchant, T.A.; Ferrari, M.C.O. Predator-induced changes in morphology of a prey fish: The effects of food level and temporal frequency of predation risk. Evol. Ecol. 2008, 22, 561–574. [Google Scholar] [CrossRef]
- Sass, G.G.; Gille, C.M.; Hinke, J.T.; Kitchell, J.F. Whole-lake influences of littoral structural complexity and prey body morphology on fish predator-prey interactions. Ecol. Freshw. Fish 2006, 15, 301–308. [Google Scholar] [CrossRef]
- Williams, T.J.; Johnson, J.B.; Belk, M.C. Interaction between predation environment and diet constrains body shape in Utah chub, Gila atraria (Cypriniformes: Cyprinidae). Biol. J. Linnean Soc. 2017, 122, 147–156. [Google Scholar] [CrossRef]
- Hugueny, B.; Pouilly, M. Morphological correlates of diet in an assemblage of West African freshwater fishes. J. Fish Biol. 1999, 54, 1310–1325. [Google Scholar] [CrossRef]
- Karpouzi, V.S.; Stergiou, K.I. The relationships between mouth size and shape and body length for 18 species of marine fishes and their trophic implications. J. Fish Biol. 2003, 62, 1353–1365. [Google Scholar] [CrossRef]
- Koene, J.P.; Crotti, M.; Elmer, K.R.; Adams, C.E. Differential selection pressures result in a rapid divergence of donor and refuge populations of a high conservation value freshwater fish Coregonus lavaretus (L.). Evol. Ecol. 2019, 33, 533–548. [Google Scholar] [CrossRef]
- Etheridge, E.C.; Bean, C.W.; Maitland, P.S.; Adams, C.E. Morphological and ecological responses to a conservation translocation of powan (Coregonus lavaretus) in Scotland. Aquat. Conserv.-Mar. Freshw. Ecosyst. 2010, 20, 274–281. [Google Scholar] [CrossRef]
- Meyer, A. Phenotypic plasticity and heterochrony in Cichlasoma managuense (Pisces, Cichlidae) and their implications for speciation in Cichlid fishes. Evolution 1987, 41, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Spoljaric, M.A.; Reimchen, T.E. 10,000 years later: Evolution of body shape in Haida Gwaii three-spined stickleback. J. Fish Biol. 2007, 70, 1484–1503. [Google Scholar] [CrossRef]
- Spoljaric, M.A.; Reimchen, T.E. Habitat-dependent reduction of sexual dimorphism in geometric body shape of Haida Gwaii threespine stickleback. Biol. J. Linnean Soc. 2008, 95, 505–516. [Google Scholar] [CrossRef]
- Spoljaric, M.A.; Reimchen, T.E. Habitat-Specific Trends in Ontogeny of Body Shape in Stickleback From Coastal Archipelago: Potential for Rapid Shifts in Colonizing Populations. J. Morphol. 2011, 272, 590–597. [Google Scholar] [CrossRef]
- Ali, M.Y.; Lindsey, C.C. Heritable and temperature-induced meristic variation in the medaka, Oryzias latipes. Can. J. Zool.-Rev. Can. Zool. 1974, 52, 959–976. [Google Scholar] [CrossRef]
- Nonaka, E.; Svanback, R.; Thibert-Plante, X.; Englund, G.; Brannstrom, A. Mechanisms by Which Phenotypic Plasticity Affects Adaptive Divergence and Ecological Speciation. Am. Nat. 2015, 186, E126–E143. [Google Scholar] [CrossRef]
- Langerhans, R.B.; Reznick, D.N. Ecology and evolution of swimming performance in fishes: Predicting evolution with biomechanics. In Fish Locomotion: An Eco Ethological Perspective; Domenici, P., Kapoor, B.G., Eds.; Science Publisher: Enfield, CT, USA, 2009; pp. 200–248. [Google Scholar]
- McDowall, R.M. The species problem in freshwater fishes and the taxonomy of diadromous and lacustrine populations of Galaxias maculatus (Jenyns). J. R. Soc. New Zealand 1972, 2, 325–367. [Google Scholar] [CrossRef]
- Barriga, J.P.; Battini, M.A.; Macchi, P.J.; Milano, D.; Cussac, V.E. Spatial and temporal distribution of landlocked Galaxias maculatus and Galaxias platei (Pisces: Galaxiidae) in a lake in the South American Andes. N. Z. J. Mar. Freshw. Res. 2002, 36, 345–359. [Google Scholar] [CrossRef]
- Cussac, V.E.; Barrantes, M.E.; Boy, C.C.; Górski, K.; Habit, E.; Lattuca, M.E.; Rojo, J.H. New Insights into the Distribution, Physiology and Life Histories of South American Galaxiid Fishes, and Potential Threats to this Unique Fauna. Diversity 2020, 12, 178. [Google Scholar] [CrossRef]
- McDowall, R.M.; Frankenburg, R.S. The Galaxiid Fishes of Australia. In Australian Museum Scientific Publications; Australian Museum: Sydney, Australia, 1981; pp. 443–605. [Google Scholar]
- Delgado, M.L.; Gorski, K.; Habit, E.; Ruzzante, D.E. The effects of diadromy and its loss on genomic divergence: The case of amphidromous Galaxias maculatus populations. Mol. Ecol. 2019, 5217–5231. [Google Scholar] [CrossRef] [PubMed]
- McDowall, R.M. Galaxias Maculatus (Jenyns), the New Zealand Whitebait; Fisheries Research Division: Wellington, NZ, USA, 1968. [Google Scholar]
- McDowall, R.M. On amphidromy, a distinct form of diadromy in aquatic organisms. Fish. Fish. 2007, 8, 1–13. [Google Scholar] [CrossRef]
- Pollard, D.A. The biology of a landlocked form of the normally catadromous salmoniform fish Galaxias maculatus (Jenyns). Mar. Freshw. Res. 1971, 22, 91–124. [Google Scholar] [CrossRef]
- Zattara, E.E.; Premoli, A.C. Genetic structuring in Andean landlocked populations of Galaxias maculatus: Effects of biogeographic history. J. Biogeogr. 2004, 32, 5–14. [Google Scholar] [CrossRef]
- Rojo, J.H.; Fernandez, D.A.; Figueroa, D.E.; Boy, C.C. Phenotypic and genetic differentiation between diadromous and landlocked puyen Galaxias maculatus. J. Fish Biol. 2020, 96, 956–967. [Google Scholar] [CrossRef]
- Torres, A.G.; Bailly, N. Galaxias maculatus (Jenyns, 1842) Inanga. In FishBase. Available online: https://www.fishbase.se/search.php (accessed on 7 November 2019).
- Rohlf, F.J. tpsRelw, RWs Analysis. Available online: https://life.bio.sunysb.edu/morph/ (accessed on 15 October 2019).
- Bookstein, F.L. Principal warps-thin-plate splines and the decomposition of deformations. IEEE Trans. Pattern Anal. Mach. Intell. 1989, 11, 567–585. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Slice, D. Extensions of the procrustes method for the optimal superimposition of landmarks. Syst. Zool. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Marcus, L.F. A revolution in morphometrics. Trends Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Wesner, J.S.; Billman, E.J.; Meier, A.; Belk, M.C. Morphological convergence during pregnancy among predator and nonpredator populations of the livebearing fish Brachyrhaphis rhabdophora (Teleostei: Poeciliidae). Biol. J. Linnean Soc. 2011, 104, 386–392. [Google Scholar] [CrossRef]
- Anderson, T.W. An Introduction to Multivariate Statistical Analysis, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Ingley, S.J.; Billman, E.J.; Belk, M.C.; Johnson, J.B. Morphological Divergence Driven by Predation Environment within and between Species of Brachyrhaphis Fishes. PLoS ONE 2014, 9, 11. [Google Scholar] [CrossRef]
- Heins, D.C.; Baker, J.A. Stream-flow environment predicts divergent life history phenotypes among populations of the Blacktail Shiner Cyprinella venusta: Temporal stability of a large-scale pattern. Ecol. Freshw. Fish 2018, 27, 453–459. [Google Scholar] [CrossRef]
- Roth-Monzón, A.; Belk, M.C.; Zúñiga-Vega, J.J.; Johnson, J.B. Beyond pairwise interactions: Multispecies character displacement in Mexican freshwater fish communities. American Naturalist. Am. Nat. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kenward, M.G.; Roger, J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef]
- Zelditch, M.; Swiderski, D.; Sheets, H. Geometric Morphometrics for Biologists: A Primer, 2nd ed.; Academic Press: Waltham, MA, USA, 2012. [Google Scholar]
- Langerhans, R.B.; Layman, C.A.; Langerhans, A.K.; Dewitt, T.J. Habitat-associated morphological divergence in two Neotropical fish species. Biol. J. Linnean Soc. 2003, 80, 689–698. [Google Scholar] [CrossRef]
- Sidlauskas, B.; Chernoff, B.; Machado-Allison, A. Geographic and environmental variation in Bryconops sp cf. melanurus (Ostariophysi: Characidae) from the Brazilian Pantanal. Ichthyol. Res. 2006, 53, 24–33. [Google Scholar] [CrossRef]
- Krabbenhoft, T.J.; Collyer, M.L.; Quattro, J.M. Differing evolutionary patterns underlie convergence on elongate morphology in endemic fishes of Lake Waccamaw, North Carolina. Biol. J. Linnean Soc. 2009, 98, 636–645. [Google Scholar] [CrossRef]
- Franssen, N.R.; Stewart, L.K.; Schaefer, J.F. Morphological divergence and flow-induced phenotypic plasticity in a native fish from anthropogenically altered stream habitats. Ecol. Evol. 2013, 3, 4648–4657. [Google Scholar] [CrossRef]
- Robinson, B.W.; Wilson, D.S. Genetic variation and phenotypic plasticity in a trophically polymorphic population of pumpkinseed sunfish (Lepomis gibbosus). Evol. Ecol. 1996, 10, 631–652. [Google Scholar] [CrossRef]
- Hjelm, J.; van de Weerd, G.H.; Sibbing, F.A. Functional link between foraging performance, functional morphology, and diet shift in roach (Rutilus rutilus). Can. J. Fish. Aquat. Sci. 2003, 60, 700–709. [Google Scholar] [CrossRef]
- Willacker, J.J.; Von Hippel, F.A.; Wilton, P.R.; Walton, K.M. Classification of threespine stickleback along the benthic-limnetic axis. Biol. J. Linnean Soc. 2010, 101, 595–608. [Google Scholar] [CrossRef]
- Schluter, D.; McPhail, J.D. Ecological character displacement and speciation in sticklebacks. Am. Nat. 1992, 140, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, C.C.; Kirk, E.C. Visual acuity in mammals: Effects of eye size and ecology. Brain Behav. Evol. 2014, 83, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Caves, E.M.; Sutton, T.T.; Johnsen, S. Visual acuity in ray-finned fishes correlates with eye size and habitat. J. Exp. Biol. 2017, 220, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.K.; Dean, T.L. Effects of turbidity on the feeding ability of the juvenile migrant stage of six New Zealand freshwater fish species. N. Z. J. Mar. Freshw. Res. 1998, 32, 21–29. [Google Scholar] [CrossRef]
- Bauer, T.; Kredler, M. Morphology of the compound eyes as an indicator of life-style in carabid beetles. Can. J. Zool.-Rev. Can. Zool. 1993, 71, 799–810. [Google Scholar] [CrossRef]
- Lisney, T.J.; Stecyk, K.; Kolominsky, J.; Schmidt, B.K.; Corfield, J.R.; Iwaniuk, A.N.; Wylie, D.R. Ecomorphology of eye shape and retinal topography in waterfowl (Aves: Anseriformes: Anatidae) with different foraging modes. J. Comp. Physiol. A -Neuroethol. Sens. Neural Behav. Physiol. 2013, 199, 385–402. [Google Scholar] [CrossRef]
- Brandon, C.S.; James, T.; Dudycha, J.L. Selection on incremental variation of eye size in a wild population of Daphnia. J. Evol. Biol. 2015, 28, 2112–2118. [Google Scholar] [CrossRef]
- Hodge, J.R.; Alim, C.; Bertrand, N.G.; Lee, W.; Price, S.A.; Tran, B.; Wainwright, P.C. Ecology shapes the evolutionary trade-off between predator avoidance and defence in coral reef butterflyfishes. Ecol. Lett. 2018, 21, 1033–1042. [Google Scholar] [CrossRef]
- Beston, S.M.; Walsh, M.R. Natural selection favours a larger eye in response to increased competition in natural populations of a vertebrate. Funct. Ecol. 2019, 33, 1321–1331. [Google Scholar] [CrossRef]
- Leaver, S.D.; Reimchen, T.E. Abrupt changes in defence and trophic morphology of the giant threespine stickleback (Gasterosteus sp.) following colonization of a vacant habitat. Biol. J. Linnean Soc. 2012, 107, 494–509. [Google Scholar] [CrossRef]
- Beston, S.M.; Wostl, E.; Walsh, M.R. The evolution of vertebrate eye size across an environmental gradient: Phenotype does not predict genotype in a Trinidadian killifish. Evolution 2017, 71, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Svanback, R.; Johansson, F. Predation selects for smaller eye size in a vertebrate: Effects of environmental conditions and sex. Proc. R. Soc. B-Biol. Sci. 2019, 286, 8. [Google Scholar] [CrossRef] [PubMed]
- McPhail, J.D. Ecology and evolution of sympatric sticklebacks (Gasterosteus) morphological and genetic evidence for a species pair in Enos Lake, British-Columbia. Can. J. Zool.-Rev. Can. Zool. 1984, 62, 1402–1408. [Google Scholar] [CrossRef]
- Hulsey, C.D.; Mims, M.C.; Streelman, J.T. Do constructional constraints influence cichlid craniofacial diversification? Proc. R. Soc. B-Biol. Sci. 2007, 274, 1867–1875. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Bellwood, D.R. Morphological structure in a reef fish assemblage. Coral Reefs 2009, 28, 449–457. [Google Scholar] [CrossRef]
- Schmitz, L.; Wainwright, P.C. Ecomorphology of the eyes and skull in zooplanktivorous labrid fishes. Coral Reefs 2011, 30, 415–428. [Google Scholar] [CrossRef]
- McDowall, R.M. New Zealand Freshwater Fishes: A Guide and Natural History; Heinemann Reed: Aukland, Australia, 1990; p. 232. [Google Scholar]
- Modenutti, B.E.; Balseiro, E.G.; Cervellini, P.M. Effect of the selective feeding of Galaxias maculatus (Salmoniformes, Galaxiidae) on zooplankton of a South Andes lake. Aquat. Sci. 1993, 55, 65–75. [Google Scholar] [CrossRef]
- Ferriz, A. Alimentación del puyen Galaxias maculatus (Jenyns) en el río Limay, Provincia de Neuquén. Physica B 1984, 42, 29–32. [Google Scholar]
- Vamosi, S.M. The presence of other fish species affects speciation in threespine sticklebacks. Evol. Ecol. Res. 2003, 5, 717–730. [Google Scholar]
- Knudsen, R.; Klemetsen, A.; Amundsen, P.A.; Hermansen, B. Incipient speciation through niche expansion: An example from the Arctic charr in a subarctic lake. Proc. R. Soc. B-Biol. Sci. 2006, 273, 2291–2298. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Polymorphism and speciation in Arctic charr. J. Fish Biol. 2001, 58, 605–638. [Google Scholar] [CrossRef]
- Ostbye, K.; Amundsen, P.A.; Bernatchez, L.; Klemetsen, A.; Knudsen, R.; Kristoffersen, R.; Naesje, T.F.; Hindar, K. Parallel evolution of ecomorphological traits in the European whitefish Coregonus lavaretus (L.) species complex during postglacial times. Mol. Ecol. 2006, 15, 3983–4001. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.S.; Krueger, C.C.; Eshenroder, R.L. Morphological and ecological differences between shallow- and deep-water lake trout in Lake Mistassini, Quebec. J. Gt. Lakes Res. 2007, 33, 156–169. [Google Scholar] [CrossRef]
- Kautt, A.F.; Machado-Schiaffino, G.; Meyer, A. Lessons from a natural experiment: Allopatric morphological divergence and sympatric diversification in the Midas cichlid species complex are largely influenced by ecology in a deterministic way. Evol. Lett. 2018, 2, 323–340. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.D.; Schluter, D.; Wainwright, P.C. Functional basis of ecological divergence in sympatric stickleback. BMC Evol. Biol. 2013, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Swanson, B.O.; Gibb, A.C.; Marks, J.C.; Hendrickson, D.A. Trophic polymorphism and behavioral differences decrease intraspecific competition in a cichlid, Herichthys minckleyi. Ecology 2003, 84, 1441–1446. [Google Scholar] [CrossRef]
- Vanderpham, J.P.; Nakagawa, S.; Closs, G.P. Habitat-related patterns in phenotypic variation in a New Zealand freshwater generalist fish, and comparisons with a closely related specialist. Freshw. Biol. 2013, 58, 396–408. [Google Scholar] [CrossRef]
- Belk, M.C.; Habit, E.; Ortiz-Sandoval, J.J.; Sobenes, C.; Combs, E.A. Ecology of Galaxias platei in a depauperate lake. Ecol. Freshw. Fish 2014, 23, 615–621. [Google Scholar] [CrossRef]
- Ruiz, V. Ictiofauna del Río Andalién (Concepción, Chile). Gayana 1993, 57, 109–278. [Google Scholar]
- Ferriz, R.A.; Aramburu, W.S. Relaciones troficas de los peces de un embalse patagónico, Provincia del Neuquén, Argentina. Bioikos 1994, 8, 7–19. [Google Scholar]
- Vega, R.; Dantagnan, P.; Mardones, A.; Valdebenito, I.; Zamorano, J.; Encina, F. Bases biológicas para el cultivo del puye Galaxias maculatus (Jenyns, 1842): Una revisión. Lat. Am. J. Aquat. Res. 2013, 41, 369–386. [Google Scholar]
- Tagliaferro, M.; Arismendi, I.; Lancelotti, J.; Pascual, M. A natural experiment of dietary overlap between introduced Rainbow Trout (Oncorhynchus mykiss) and native Puyen (Galaxias maculatus) in the Santa Cruz River, Patagonia. Environ. Biol. Fishes 2015, 98, 1311–1325. [Google Scholar] [CrossRef]
- Hall, E.S.; Martin, B.E.; Brubaker, K.; Grant, C.J. Latitudinal variation in the geometric morphology of the largemouth bass. Mar. Freshw. Res. 2018, 69, 1480–1485. [Google Scholar] [CrossRef]
- Recknagel, H.; Elmer, K.R.; Meyer, A. Crater lake habitat predicts morphological diversity in adaptive radiations of cichlid fishes. Evolution 2014, 68, 2145–2155. [Google Scholar] [CrossRef]

| Source | Degrees of Freedom | F-Value | p-Value |
|---|---|---|---|
| Body shape | |||
| River/Lake (RL) | 1, 1375 | 3.00 | 0.0834 |
| Index | 8, 1726 | 14.96 | <0.0001 |
| Centroid size (CS) | 1, 1665 | 43.38 | <0.0001 |
| CS*Index | 8, 1726 | 15.19 | <0.0001 |
| River/Lake*Index | 8, 1726 | 7.01 | <0.0001 |
| CS*RL*Index | 9, 1710 | 5.78 | <0.0001 |
| Head shape | |||
| River/Lake (RL) | 1, 1055 | 10.35 | 0.0013 |
| Index | 8, 684 | 49.47 | <0.0001 |
| Centroid Size (CS) | 1, 1055 | 186.08 | <0.0001 |
| CS*Index | 8, 684 | 49.28 | <0.0001 |
| RL*Index | 8, 684 | 7.42 | <0.0001 |
| CS*RL*Index | 9, 675 | 6.44 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercer, M.; Searle, P.C.; Cifuentes, R.; Habit, E.; Belk, M.C. Morphometric Response of Galaxias maculatus (Jenyns) to Lake Colonization in Chile. Diversity 2020, 12, 219. https://doi.org/10.3390/d12060219
Mercer M, Searle PC, Cifuentes R, Habit E, Belk MC. Morphometric Response of Galaxias maculatus (Jenyns) to Lake Colonization in Chile. Diversity. 2020; 12(6):219. https://doi.org/10.3390/d12060219
Chicago/Turabian StyleMercer, Margaret, Peter C. Searle, Roberto Cifuentes, Evelyn Habit, and Mark C. Belk. 2020. "Morphometric Response of Galaxias maculatus (Jenyns) to Lake Colonization in Chile" Diversity 12, no. 6: 219. https://doi.org/10.3390/d12060219
APA StyleMercer, M., Searle, P. C., Cifuentes, R., Habit, E., & Belk, M. C. (2020). Morphometric Response of Galaxias maculatus (Jenyns) to Lake Colonization in Chile. Diversity, 12(6), 219. https://doi.org/10.3390/d12060219