Two Seas for One Great Diversity: Checklist of the Marine Heterobranchia (Mollusca; Gastropoda) from the Salento Peninsula (South-East Italy)
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dainelli, G. Appunti Geologici sulla Parte Meridionale del Capo di Leuca; Tipografia della Pace di Filippo Cuggiani: Rome, Italy, 1901. [Google Scholar]
- De Giorgi, C. La Serie Geologica dei Terreni nella Penisola Salentina; Tipografia della Pace di Filippo Cuggiani: Rome, Italy, 1903. [Google Scholar]
- Biasutti, R. Note morfologiche ed idrografiche sulla Terra d’Otranto. Riv. Geogr. It 1911, 18, 508–531. [Google Scholar]
- Sacco, F. La geotettonica dell’Appennino meridionale. Boll. Soc. Geol. Ital. 1912, 31, 379–387. [Google Scholar]
- Colamonico, C. La Geografia della Puglia: Profilo Monografico Regionale:(Con una Carta Geografica a Colori); Cressati: Bari, Italy, 1926. [Google Scholar]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Notes Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Bianchi, C.N. Proposta di suddivisione dei mari italiani in Settori Biogeografici. Not. Soc. Biol. Mar. 2004, 46, 57–59. [Google Scholar]
- Taviani, M.; Angeletti, L.; Beuck, L.; Campiani, E.; Canese, S.; Foglini, F.; Freiwald, A.; Montagna, P.; Trincardi, F. Reprint of ‘On and off the beaten track: Megafaunal sessile life and Adriatic cascading processes’. Mar. Geol. 2016, 375, 146–160. [Google Scholar] [CrossRef]
- Savini, A.; Corselli, C. High-resolution bathymetry and acoustic geophysical data from Santa Maria di Leuca Cold Water Coral province (Northern Ionian Sea—Apulian continental slope). Deep Sea Res. Part II Trop. Stud. Oceanogr. 2010, 57, 326–344. [Google Scholar] [CrossRef]
- Goddard, J.H.R.; Gosliner, T.M.; Pearse, J.S. Impacts associated with the recent range shift of the aeolid nudibranch Phidiana hiltoni (Mollusca, Opisthobranchia) in California. Mar. Biol. 2011, 158, 1095–1109. [Google Scholar] [CrossRef]
- Goddard, J.H.R.; Treneman, N.; Pence, W.E.; Mason, D.E.; Dobry, P.M.; Green, B.; Hoover, C. Nudibranch Range Shifts associated with the 2014 Warm Anomaly in the Northeast Pacific. Bull. South. Calif. Acad. Sci. 2016, 115, 15–40. [Google Scholar] [CrossRef]
- Goddard, J.H.R.; Treneman, N.; Prestholdt, T.; Hoover, C.; Green, B.; Pence, W.E.; Douglas, E.; Mason, D.E.; Dobry, P.L.; Sones, J.L.; et al. Heterobranch Sea Slugs range shifts in the northeast Pacific Ocean associated with the 2015–16 El Niño. Proc. Calif. Acad. Sci. 2018, 4, 107–131. [Google Scholar]
- Nimbs, M.J.; Larkin, M.; Davis, T.R.; Harasti, D.; Willan, R.C.; Smith, D.A. Southern range extensions for 661 twelve heterobranch sea slugs (Gastropoda: Heterobranchia) on the eastern coast of Australia. Mar. Biodiv. Rec. 2016, 9, 27. [Google Scholar] [CrossRef]
- Eisenbarth, J.H.; Undap, N.; Papu, A.; Schillo, D.; Dialao, J.; Reumschüssel, S.; Kaligis, F.; Bara, R.; Schäberle, T.F.; König, M.G.; et al. Marine Heterobranchia (Gastropoda, Mollusca) in Bunaken National Park, North Sulawesi, Indonesia—A Follow-Up Diversity Study. Diversity 2018, 10, 127. [Google Scholar] [CrossRef]
- Cimino, G.; Ghiselin, M.T. Chemical defense and the evolution of opisthobranch gastropods. Proc. Calif. Acad. Sci. 2009, 60, 175–422. [Google Scholar]
- Carbone, M.; Gavagnin, M.; Haber, M.; Guo, Y.-W.; Fontana, A.; Manzo, E.; Genta-Jouve, G.; Tsoukatou, G.; Rudman, W.B.; Cimino, G.; et al. Packaging and Delivery of Chemical Weapons: A Defensive Trojan Horse Stratagem in Chromodorid Nudibranchs. PLoS ONE 2013, 8, e62075. [Google Scholar] [CrossRef] [PubMed]
- Cheney, K.L.; White, A.; Mudianta, I.W.; Winters, A.E.; Quezada, M.; Capon, R.J.; Mollo, E.; Garson, M.J. Choose your weaponry: Selective storage of a single toxic compound, Latrunculin A, by closely related Nudibranch Molluscs. PLoS ONE 2016, 11, e0145134. [Google Scholar] [CrossRef]
- Winters, A.E.; White, A.M.; Dewi, A.S.; Mudianta, W.I.; Wilson, N.G.; Forster, L.C.; Garson, M.J.; Cheney, K.L. Distribution of defensive metabolites in Nudibranch Molluscs. J. Chem. Ecol. 2018, 44, 384–396. [Google Scholar] [CrossRef]
- Winters, A.E.; Wilson, N.G.; van den Berg, C.P.; How, M.J.; Endler, J.A.; Marshall, N.J.; White, A.M.; Garson, M.J.; Cheney, K.L. Toxicity and taste: Unequal chemical defences in a mimicry ring. Proc. R. Soc. B 2018, 285, 20180457. [Google Scholar] [CrossRef]
- Borsa, P. Allozyme, mitochondrial-DNA, and morphometric variability indicate cryptic species of anchovy (Engraulis encrasicolus). Biol. J. Linnean Soc. 2002, 75, 261–269. [Google Scholar] [CrossRef][Green Version]
- Calvo, M.; Templado, J.; Oliverio, M.; Machordom, A. Hidden Mediterranean biodiversity: Molecular evidence for a cryptic species complex within the reef building vermetid gastropod Dendropoma petraeum (Mollusca: Caenogastropoda). Biol. J. Linnean Soc. 2009, 96, 898–912. [Google Scholar] [CrossRef][Green Version]
- Claremont, M.; Reid, D.G.; Williams, S.T. Evolution of corallivory in the gastropod genus Drupella. Coral Reefs 2011, 30, 977–990. [Google Scholar] [CrossRef]
- Barco, A.; Houart, R.; Bonomolo, G.; Crocetta, F.; Oliverio, M. Molecular data reveal cryptic lineages within the northeastern Atlantic and Mediterranean small mussel drills of the Ocinebrina edwardsii complex (Mollusca: Gastropoda: Muricidae). Zool. J. Linnean Soc. 2013, 169, 389–407. [Google Scholar] [CrossRef]
- Carmona, L.; Gosliner, T.M.; Pola, M.; Cervera, J.L. A Molecular approach to the Phylogenetic status of the Aeolid genus Babakina Roller, 1973 (Nudibranchia). J. Molluscan Stud. 2011, 77, 417–422. [Google Scholar] [CrossRef]
- Furfaro, G.; Mariottini, P.; Modica, M.V.; Trainito, E.; Doneddu, M.; Oliverio, M. Sympatric sibling species: The case of Caloria elegans and Facelina quatrefagesi (Gastropoda: Nudibranchia). Sci. Mar. 2016, 80, 511–520. [Google Scholar] [CrossRef]
- Furfaro, G.; Modica, M.V.; Oliverio, M.; Mariottini, P. A DNA-barcoding approach to the phenotypic diversity of Mediterranean species of Felimare Ev. Marcus and Er. Marcus, 1967 (Mollusca: Gastropoda), with a preliminary phylogenetic analysis. Ital. J. Zool. 2016, 83, 195–207. [Google Scholar] [CrossRef]
- Furfaro, G.; Salvi, D.; Mancini, E.; Mariottini, P. A multilocus view on Mediterranean aeolid nudibranchs (Mollusca): Systematics and cryptic diversity of Flabellinidae and Piseinotecidae. Mol. Phylogenet. Evol. 2018, 118, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, G.; Mariottini, P. Less rare than we thought: Two new localities for Piseinotecus soussi Tamsouri, Carmona, Moukrim, Cervera, 2014 along the Tyrrhenian coast. Turk. J. Zool. 2019, 43, 287–289. [Google Scholar] [CrossRef]
- Martín-Hervás, M.R.; Carmona, L.; Jensen, K.R.; Licchelli, C.; Vitale, F.; Cervera, J.L. Description of a new pseudocryptic species of Elysia Risso, 1818 (Heterobranchia, Sacoglossa) in the Mediterranean Sea. Bull. Mar. Sci. 2019, 96, 127–144. [Google Scholar] [CrossRef]
- Perrone, A.S. Nudibranchi del genere Taringa Marcus, 1955 dal Golfo di Taranto (Heterobranchia: Nudibranchia). Boll. Malacol. 1992, 28, 207–220. [Google Scholar]
- Perrone, A.S. Opistobranchi (Aplysiomorpha, Pleurobrancomorpha, Sacoglossa, Nudibranchia) del litorale salentino (Mar Jonio) (Elenco—contributo primo). Thalassia Salentina 1983, 13, 118–144. [Google Scholar]
- Perrone, A.S. Opistobranchi (Aplysiomorpha, Pleurobrancomorpha, Sacoglossa, Nudibranchia) del litorale salentino (Mare Jonio) (Elenco—Contributo secondo). Thalassia Salentina 1986, 16, 19–42. [Google Scholar]
- Perrone, A.S. Primo rinvenimento di Elysia flava Verrill, 1901 per le coste italiane (Heterobranchia: Sacoglossa). Atti Soc. Ital. Sci. Nat. 1988, 129, 483–488. [Google Scholar]
- Perrone, A.S. Una nuova specie di Elysiidae, Elysia hetta nov. sp. dal litorale salentino (Mediterraneo—Golfo di Taranto) (Heterobranchia: Sacoglossa). Atti Soc. Ital. Sci. Nat. 1990, 130, 249–252. [Google Scholar]
- Perrone, A.S. Una nuova specie di Nudibranchi dal Golfo di Taranto: Rostanga anthelia nov. sp. (Heterobranchia: Nudibranchia). Boll. Malacol. 1990, 26, 179–188. [Google Scholar]
- Perrone, A.S. Una nuova specie di Nudibranchi Doridiani, Peltodoris sordii nov. sp., dalla biocenosi a Anadiomene stellata, Geodia cydonium e Holothuria impatiens. Boll. Malacol. 1989, 25, 295–300. [Google Scholar]
- Perrone, A.S. Una specie di Nudibranchi nuova per le coste italiane: Ridescrizione di Geitodoris (Verrilia) bonosi Ortea & Ballesteros, 1981 (Heterobranchia: Nudibranchia). Boll. Malacol. 1992, 28, 27–34. [Google Scholar]
- Onorato, M.; Belmonte, G. Submarine caves of the Salento peninsula: Faunal aspects. Thalassia Salentina 2017, 39, 47–72. [Google Scholar]
- Micaroni, V.; Strano, F.; Di Franco, D.; Crocetta, F.; Grech, D.; Piraino, S.; Boero, F. Project “Biodiversity MARE Tricase”: A biodiversity inventory of the coastal area of Tricase (Ionian Sea, Italy)—Mollusca: Heterobranchia. Eur. Zool. J. 2018, 85, 180–193. [Google Scholar] [CrossRef]
- Colucci, G.; Strafella, R.; Trainito, E.; Doneddu, M. First record of the genus Dermatobranchus van Hasselt, 1824, in the Mediterranean Sea (Nudibranchia: Arminidae). Mediterr. Mar. Sci. 2015, 16, 331–333. [Google Scholar] [CrossRef][Green Version]
- Pola, M.; Paz-Sedano, S.; Macali, A.; Minchin, D.; Marchini, A.; Vitale, F.; Licchelli, C.; Crocetta, F. What is really out there? Review of the genus Okenia Menke, 1830 (Nudibranchia: Goniodorididae) in the Mediterranean Sea with description of two new species. PLoS ONE 2019, 14, e0215037. [Google Scholar] [CrossRef]
- Araujo, A.K.; Pola, M.; Malaquias, M.A.E.; Cervera, J.L. To be or not to be? What molecules say about Runcina brenkoae Thompson, 1980 (Gastropoda: Heterobranchia: Runcinida). Sci. Mar. 2019, 83, 223–235. [Google Scholar] [CrossRef]
- Golestani, H.; Crocetta, F.; Padula, V.; Camacho-García, Y.; Langeneck, J.; Poursanidis, D.; Pola, M.; Yokeş, M.B.; Cervera, J.L.; Jung, D.W.; et al. The little Aplysia coming of age: From one species to a complex of species complexes in Aplysia parvula (Mollusca: Gastropoda: Heterobranchia). Zool. J. Linnean Soc. 2019, 187, 279–330. [Google Scholar] [CrossRef]
- Korshunova, T.; Malmberg, K.; Prkić, J.; Petani, A.; Fletcher, K.; Lundin, K.; Martynov, A. Fine-scale species delimitation: Speciation in process and periodic patterns in nudibranch diversity. ZooKeys 2020, 917, 15–50. [Google Scholar] [CrossRef] [PubMed]
- Nimbs, M.J.; Smith, S.D. An illustrated inventory of the sea slugs of New South Wales, Australia (Gastropoda: Heterobranchia). Proc. R. Soc. Vic. 2016, 128, 44–113. [Google Scholar] [CrossRef]
- Smith, S.D.; Davis, T.R. Slugging it out for science: Volunteers provide valuable data on the diversity and distribution of heterobranch sea slugs. Molluscan Res. 2019, 39, 214–223. [Google Scholar] [CrossRef]
- Smith, S.D.; Nimbs, M.J. Quantifying temporal variation in heterobranch (Mollusca: Gastropoda) sea slug assemblages: Tests of alternate models. Molluscan Res. 2017, 37, 140–147. [Google Scholar] [CrossRef]
- iNaturalist. Available online: https://www.inaturalist.org/ (accessed on 19 April 2020).
- Word Register of Marine Species. Available online: http://www.marinespecies.org/ (accessed on 21 April 2020).
- Cattaneo-Vietti, R.; Chemello, R.; Giannuzzi-Savelli, R. Atlante dei Nudibranchi del Mediterraneo; Editrice La Conchiglia: Rome, Italy, 1990. [Google Scholar]
- Trainito, E.; Doneddu, M. Nudibranchi del Mediterraneo; Il Castello: Milan, Italy, 2014. [Google Scholar]
- OPK Opistobranquis. Available online: https://opistobranquis.info/en/ (accessed on 19 April 2020).
- Sea Slug Forum. Available online: http://www.seaslugforum.net/ (accessed on 19 April 2020).
- Furfaro, G.; Picton, B.; Martynov, A.; Mariottini, P. Diaphorodoris alba Portmann & Sandmeier, 1960 is a valid species: Molecular and morphological comparison with D. luteocincta (M. Sars, 1870) (Gastropoda: Nudibranchia). Zootaxa 2016, 4193, 304–316. [Google Scholar]
- Mastrototaro, F.; Panetta, P.; D’Onghia, G. Further records of Melibe viridis (Mollusca, Nudibranchia) in the Mediterranean Sea, with observations on the spawning. Vie Milieu 2004, 54, 251–253. [Google Scholar]
- Ballesteros, M.; Madrenas, E.; Pontes, M. “Doto Fragaria” in OPK-Opistobranquis (2012–2020). Available online: https://opistobranquis.info/en/hejyE (accessed on 21 April 2020).
- Tamsouri, N.; Carmona, L.; Moukrim, A.; Cervera, J.L. Description of a new species of Piseinotecus (Castropoda, Heterobranchia, Piseinotecidae) from the northeastern Atlantic Ocean. Bull. Mar. Sci. 2014, 90, 991–997. [Google Scholar] [CrossRef]
- Crocetta, F.; Colamonaco, G. Percnon gibbesi (Crustacea: Decapoda) and Aplysia dactylomela (Mollusca: Gastropoda) in the Taranto Gulf (Italy, Ionian Sea): New populations incoming. Mar. Biodiv. Rec. 2010, 3, e88. [Google Scholar] [CrossRef]
- Wernberg, T.; Russell, B.D.; Moore, P.J.; Ling, S.D.; Smale, D.A.; Campbell, A.; Coleman, M.A.; Steinberg, P.D.; Kendrick, G.A.; Connell, S.D. Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. J. Exp. Mar. Biol. Ecol. 2011, 400, 7–16. [Google Scholar] [CrossRef]
- Wernberg, T.; Smale, D.A.; Thomsen, M.S. A decade of climate change experiments on marine organisms: Procedures, patterns and problems. Glob. Change Biol. 2012, 18, 1491–1498. [Google Scholar] [CrossRef]
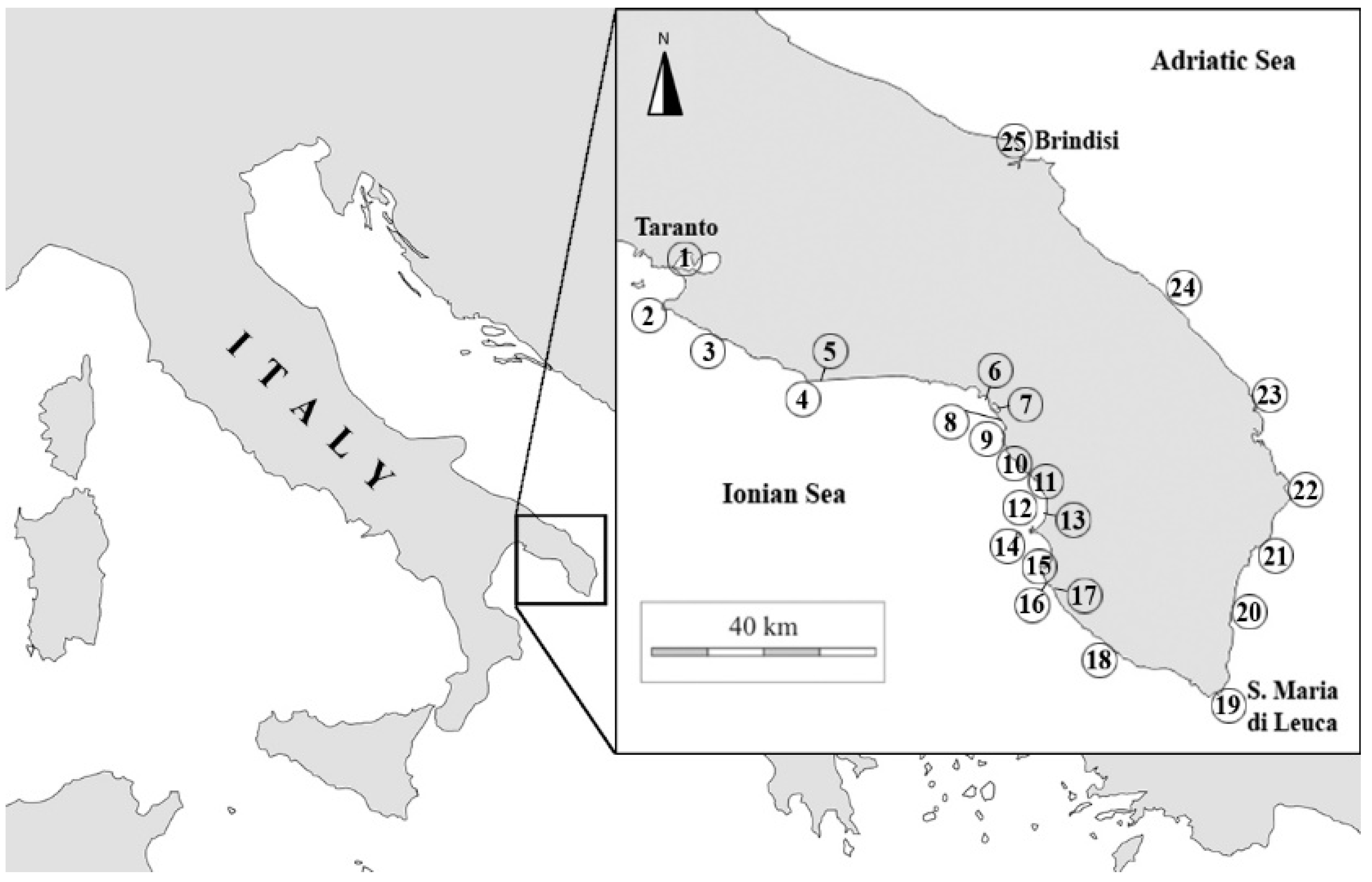





| N° | Station | Latitude | Longitude | Depth |
|---|---|---|---|---|
| 1 | Mar Piccolo, Taranto (Ionian Sea) | 40°28′53.62′′N | 17°16′00.91′′E | 1–10 m |
| 2 | Capo S. Vito, Taranto (Ionian Sea) | 40°24′35.85′′N | 17°12′06.07′′E | 1–20 m |
| 3 | Porto Pirrone, Taranto (Ionian Sea) | 40°21′27.79′′N | 17°19′40.93′′E | 4–20 m |
| 4 | Torre Ovo, Taranto (Ionian Sea) | 40°17′29.18′′N | 17°30′11.20′′E | 1–20 m |
| 5 | Campomarino, Taranto (Ionian Sea) | 40°17′34.76′′N | 17°31′41.48′′E | 1–20 m |
| 6 | Porto Cesareo I. Conigli, Lecce (Ionian Sea) | 40°15′32.15′′N | 17°52′57.59′′E | 1–15 m |
| 7 | Porto Cesareo, Lecce (Ionian Sea) | 40°14′51.48′′N | 17°54′33.51′′E | 0–1 m |
| 8 | S. Isidoro, Lecce (Ionian Sea) | 40°13′15.51′′N | 17°55′29.27′′E | 1–5 m |
| 9 | Torre Inserraglio, Lecce (Ionian Sea) | 40°10′41.44′′N | 17°55′51.53′′E | 1–20 m |
| 10 | Santa Caterina di Nardò, Lecce (Ionian Sea) | 40°08′05.87′′N | 17°59′15.53′′E | 1–15 m |
| 11 | Santa Maria al Bagno, Lecce (Ionian Sea) | 40°07′30.58′′N | 17°59′46.43′′E | 0–6 m |
| 12 | O.R. Gallipoli, Lecce (Ionian Sea) | 40°06′04.68′′N | 17°58′04.22′′E | 36 m |
| 13 | Gallipoli, Lecce (Ionian Sea) | 40°04′34.70′′N | 17°59′50.25′′E | 1–20 m |
| 14 | Gallipoli - Isola S. Andrea, Lecce (Ionian Sea) | 40°02′36.77′′N | 17°57′01.64′′E | 5–20 m |
| 15 | Gallipoli Pizzo, Lecce (Ionian Sea) | 39°59′57.64′′N | 17°59′27.36′′E | 1–15 m |
| 16 | Marina Mancaversa, Lecce (Ionian Sea) | 39°58′58.04′′N | 18°00′18.58′′E | 1–5 m |
| 17 | Torre Suda, Lecce (Ionian Sea) | 39°56′27.95′′N | 18°02′17.36′′E | 1–5 m |
| 18 | Ugento, Lecce (Ionian Sea) | 39°52′36.70′′N | 18°05′25.14′′E | 5–30 m |
| 19 | Santa Maria di Leuca, Lecce (Ionian Sea) | 39°48′18.38′′N | 18°22′42.56′′E | 1–20 m |
| 20 | Tricase, Lecce (Adriatic Sea) | 39°55′51.31′′N | 18°23′47.92′′E | 1–30 m |
| 21 | Porto Miggiano, Lecce (Adriatic Sea) | 40°01′46.72′′N | 18°27′01.30′′E | 1–25 m |
| 22 | Otranto, Lecce (Adriatic Sea) | 40°08′18.52′′N | 18°30′47.65′′E | 1–40 m |
| 23 | Roca, Lecce (Adriatic Sea) | 40°17′50.18′′N | 18°24′49.69′′E | 1–6 m |
| 24 | Frigole, Lecce (Adriatic Sea) | 40°26′20.52′′N | 18°14′46.56′′E | 0–1 m |
| 25 | Brindisi (Adriatic Sea) | 40°39′54.11′′N | 17°57′34.81′′E | 1–8 m |
| Taxonomy | A | B | C | D | Vouchers |
|---|---|---|---|---|---|
| Pleurobranchida | |||||
| Family Pleurobranchidae Gray, 1827 | |||||
| Pleurobranchus membranaceus (Montagu, 1803) | 16 | ||||
| Pleurobranchus testudinarius Cantraine, 1835 | 1,17 | ||||
| Berthellina cf. edwardsii | 1,4,6,10,13,15 | RM3_1865 | |||
| Berthella aurantiaca (Risso, 1818) | 8,9,19,23 | ||||
| Berthella elongata (Cantraine, 1836) | 16 | ||||
| Berthella ocellata (delle Chiaje, 1830) | 8 | 5,6 | |||
| Berthella plumula (Montagu, 1803) | 16 | ||||
| Berthella stellata (Risso, 1826) | 9 | 22 | |||
| Berthellina citrina (Rüppell & Leuckart, 1828) | 18 | ||||
| Family Pleurobranchaeidae Pilsbry, 1896 | |||||
| Pleurobranchaea meckeli (Blainville, 1825) | 16 | 11 | 5,6 | ||
| Nudibranchia - Doridina | |||||
| Family Calycidorididae Roginskaya, 1972 | |||||
| Diaphorodoris luteocincta (M. Sars, 1870) | 6 | ||||
| Diaphorodoris papillata Portmann & Sandmeier, 1960 | 11 | 5,6,10 | |||
| Family Onchidorididae Gray, 1927 | |||||
| Adalaria proxima (Alder & Hancock, 1854) | 21 | ||||
| Knoutsodonta albonigra (Pruvot-Fol, 1951) | 20,24 | ||||
| Family Goniodorididae H. & A. Adams, 1854 | |||||
| Goniodoris castanea Alder & Hancock, 1845 | 20 | 1 | |||
| Okenia longiductis Pola M, Paz-Sedano S, Macali A, Minchin D, Marchini A, Vitale F, 2019 [41] | 1,4 | ||||
| Okenia mediterranea (von Ihering, 1886) [41] | 6 | ||||
| Okenia problematica Pola M, Paz-Sedano S, Macali A, Minchin D, Marchini A, Vitale F, 2019 [41] | 7 | ||||
| Trapania lineata Haefelfinger, 1960 | 4–6,10,13,15 | RM3_1042, RM3_1048, RM3_1077 | |||
| Trapania maculata Haefelfinger, 1960 | 4–6,10,13,15 | RM3_1076 | |||
| Family Polyceridae Alder & Hancock, 1845 | |||||
| Crimora papillata Alder & Hancock, 1862 | 5,6,11 | ||||
| Kaloplocamus ramosus (Cantraine, 1835) | 11 | ||||
| Polycera elegans (Bergh, 1894) | 1 | ||||
| Polycera hedgpethi Marcus, 1964 | 1 | ||||
| Polycera quadrilineata (O. F. Müller, 1776) | 3,16 | 11 | 1,3–6,10,13,15 | RM3_1065 | |
| Thecacera pennigera (Montagu, 1815) | 1 | ||||
| Family Aegiridae P. Fischer, 1883 | |||||
| Aegires punctilucens (d’Orbigny, 1837) | 4 | ||||
| Family Cadlinidae Bergh, 1891 | |||||
| Aldisa banyulensis Pruvot-Fol, 1951 | 23,24 | 6 | |||
| Family Chromodorididae Bergh, 1891 | |||||
| Felimare fontandraui (Pruvot-Fol, 1951) | 6,8 | RM3_1039, RM3_1040, RM3_1099, RM3_1100 | |||
| Felimare orsinii (Vérany, 1846) | 8,12,13,15 | ||||
| Felimare picta (Philippi, 1836) | 19 | 13 | 11 | 1,4–6, 8,10,12,13,15 | RM3_1041, RM3_1052, RM3_1053 |
| Felimare tricolor (Cantraine, 1835) | 5 | 11 | 1,4–6, 8,10,12,13,15 | RM3_1074, RM3_1075 | |
| Felimare villafranca (Risso, 1818) | 16 | 11 | 1,5,6 | RM3_1231, RM3_1232 | |
| Felimida binza (Ev. Marcus & Er. Marcus, 1963) | 15 | ||||
| Felimida krohni (Vérany, 1846) | 13 | 11 | 4–6,13 | RM3_1061, RM3_1068 | |
| Felimida luteorosea (Rapp, 1827) | 16 | 11 | 1,6 | ||
| Felimida purpurea (Risso, 1831) | 4 | 6 | |||
| Family Dorididae Rafinesque, 1815 | |||||
| Doris ocelligera (Bergh, 1881) | 11 | 3 | |||
| Doris pseudoargus Rapp, 1827 | 19 | ||||
| Doris verrucosa Linnaeus, 1758 | 19 | 1 | |||
| Family Discodorididae Bergh, 1891 | |||||
| Atagema rugosa Pruvot-Fol, 1951 | 19 | ||||
| Baptodoris cinnabarina Bergh, 1884 | 8,20 | 22, 6 | |||
| Discodoris stellifera (Vayssière, 1903) | 9,19 | 15 | |||
| Gargamella perezi (Llera & Ortea, 1982) | 19,24 | ||||
| Geitodoris bonosi Ortea & Ballesteros, 1981 | 3 | ||||
| Geitodoris portmanni (Schmekel, 1972) | 20 | ||||
| Jorunna tomentosa (Cuvier, 1804) | 3,10,20 | 1, 13 | |||
| Paradoris indecora (Bergh, 1881) | 3,24 | ||||
| Peltodoris atromaculata Bergh, 1880 | 8 | 11 | ALL | RM3_1054, RM3_1056, RM3_1057 | |
| Peltodoris sordii Perrone, 1989 | 3 | ||||
| Platydoris argo (Linnaeus, 1767) | 8 | 11 | 5,6,10,13 | ||
| Rostanga anthelia Perrone, 1991 | 4 | ||||
| Rostanga rubra (Risso, 1818) | 20,23 | ||||
| Taringa armata Swennen, 1961 | 22 | ||||
| Taringa pinoi Perrone, 1985 | 20,24 | ||||
| Tayuva lilacina (Gould, 1852) | 3 | 1,22 | |||
| Family Phyllidiidae Rafinesque, 1814 | |||||
| Phyllidia flava Aradas, 1847 | 19 | 11 | 4–6,9,10,13 | RM3_1049, RM3_1055, RM3_1058 | |
| Family Dendrodorididae O’Donoghue, 1924 | |||||
| Dendrodoris grandiflora (Rapp, 1827) | 16 | 11 | 1,3,5,6 | ||
| Dendrodoris limbata (Cuvier, 1804) | 1,16, 19 | 1,3,5,6 | |||
| Doriopsilla areolata Bergh, 1880 | 17,23 | 3,4,6 | |||
| Nudibranchia - Cladobranchia | |||||
| Family Tritoniidae Lamarck, 1809 | |||||
| Marionia blainvillea (Risso, 1818) | 9 | 19 | |||
| Tritonia manicata Deshayes, 1853 | 8,16 | 11 | 5,6 | ||
| Tritonia nilsodhneri Marcus Ev., 1983 | 9 | ||||
| Tritonia striata Haefelfinger, 1963 | 16 | 5,6 | |||
| Family Hancockiidae MacFarland, 1923 | |||||
| Hancockia uncinata (Hesse, 1872) | 16 | ||||
| Family Scyllaeidae Alder & Hancock, 1855 | |||||
| Scyllaea pelagica Linnaeus, 1758 | 19 | ||||
| Family Tethydidae Rafinesque, 1815 | |||||
| Melibe viridis Kelaart, 1858 [55] | 1,5,6 | ||||
| Tethys fimbria Linnaeus, 1767 | 16 | 6,13 | |||
| Family Dotidae Gray, 1853 | |||||
| Doto acuta Schmekel & Kress, 1977 | 11 | ||||
| Doto cervicenigra Ortea & Bouchet, 1989 | 3,6 | ||||
| Doto floridicola Simroth, 1888 | 13 | ||||
| Doto fragaria Ortea & Bouchet, 1989 [56] | 6 | ||||
| Doto koenneckeri Lemche, 1976 | 11 | 13 | |||
| Doto paulinae Trinchese, 1881 | 11 | 6 | |||
| Doto pygmaea Bergh, 1871 | 11 | ||||
| Family Proctonotidae Gray, 1853 | |||||
| Antiopella cristata (Delle Chiaje, 1841) | 9 | 1,3,5,6 | |||
| Family Arminidae Iredale & O’Donoghue, 1841 | |||||
| Armina tigrina Rafinesque, 1814 | 18 | 5 | |||
| Dermatobranchus cf. rubidus (Gould, 1852) [40] | 2 | ||||
| Family Coryphellidae Bergh, 1889 | |||||
| Fjordia lineata (Lovén, 1846) | 16 | 6,7 | |||
| Family Flabellinidae Bergh, 1889 | |||||
| Calmella cavolini (Vérany, 1846) | 8 | 11 | 5,6,13,15 | RM3_354, RM3_481, RM3_482, RM3_484, RM3_1079, RM3_1080, RM3_1081, RM3_1082, RM3_1083, RM3_1084, RM3_1085, RM3_1086, RM3_1087, RM3_1088, RM3_1089, RM3_1090, RM3_1091, RM3_1092, RM3_1093, RM3_1095 | |
| Edmundsella pedata (Montagu, 181) | 8 | 13 | ALL | RM3_1046, RM3_1047, RM3_1059, RM3_1067 | |
| Flabellina affinis (Gmelin, 1791) | 8 | 13 | 11 | ALL | RM3_1050, RM3_1043, RM3_1060, RM3_1063, RM3_1064, RM3_1070, RM3_1071, RM3_1073 |
| Paraflabellina gabinierei (Vicente, 1975) | 1,4,5,13 | ||||
| Paraflabellina ischitana (Hirano & T. E. Thompson, 1990) | 13 | 2,4–6,9,10,13,15 | RM3_345, RM3_346, RM3_532, RM3_533 | ||
| Family Samlidae Korshunova, Martynov, Bakken, Evertsen, Fletcher, Mudianta, Saito, Lundin, Schrödl & Picton, 2017 | |||||
| Luisella babai (Schmekel, 1972) | 13 | 11 | 13 | RM3_1069 | |
| Family Piseinotecidae Edmunds, 1970 | |||||
| Piseinotecus soussi Tamsouri, Carmona, Moukrim & Cervera, 2014 [57] | 6 | RM3_862, RM3_863, RM3_1236 | |||
| Family Aeolidiidae Gray, 1827 | |||||
| Aeolidiella alderi (Cocks, 1852) | 16,20 | 1,3 | |||
| Aeolidiella glauca (Alder & Hancock, 1845) | 16 | ||||
| Berghia coerulescens (Laurillard, 1832) | 16 | 11 | 5,6,13,15,18 | ||
| Berghia verrucicornis (A. Costa, 1867) | 19 | 13,6 | |||
| Limenandra nodosa Haefelfinger & Stamm, 1958 | 2 | ||||
| Spurilla neapolitana (Delle Chiaje, 1841) | 16 | 11 | 1,3,4 | ||
| Family Facelinidae Berg, 1889 | |||||
| Caloria elegans (Alder & Hancock, 1845) | 4,5,6 | RM3_1051 | |||
| Cratena peregrina (Gmelin, 1791) | 11 | ALL | RM3_1038, RM3_1045, RM3_1062, RM3_1066, RM3_1072, RM3_1078, RM3_1094 | ||
| Dicata odhneri (Schmekel, 1967) | 1,4,6 | ||||
| Dondice banyulensis Portmann & Sandmeier, 1960 | 6,8,13,19 | ||||
| Facelina annulicornis (Chamisso & Eysenhardt, 1821) | 11 | 5,6,13–15 | |||
| Facelina fusca Schmekel, 1966 | 3 | RM3_1201, RM3_1235 | |||
| Facelina rubrovittata (A. Costa, 1866) | 2,4–6,12,13,15 | ||||
| Facelina vicina (Bergh, 1882) | 6 | RM3_1202 | |||
| Favorinus branchialis (Rathke, 1806) | 16,20 | 1,3,5,6,13,15 | |||
| Family Eubranchidae Odhner, 1934 | |||||
| Eubranchus andra (Korshunova, Malmberg, Prkić, Petani, Fletcher, Lundin, Martynov, 2020) [44] | 1, 3, 6 | ||||
| Eubranchus cf. farrani (Alder & Hancock, 1844) | 1,3,10,13,15,21 | ||||
| Eubranchus cf. linensis Garcia-Gomez, Cervera & Garcia, 1990 | 1,6 | ||||
| Eubranchus cf. exiguus (Alder & Hancock, 1848) | 11 | 10,6 | |||
| Family Fionidae Gray, 1857 | |||||
| Fiona pinnata (Eschscholtz, 1831) | 11 | RM3_1097, RM3_1098 | |||
| Family Trinchesiidae F. Nordsieck, 1972 | |||||
| Catriona maua Ev. Marcus & Er. Marcus, 1960 | 4 | ||||
| Rubramoena amoena (Alder & Hancock, 1845) | 13 | ||||
| Trinchesia genovae (O’Donoghue, 1926) | 3,5,6,13 | ||||
| Trinchesia morrowae Korshunova, Picton, Furfaro, Mariottini, Pontes, Prkić, Fletcher, Malmberg, Lundin & Martynov, 2019 | 6 | ||||
| Trinchesia cf. miniostriata Schmekel, 1968 | 13 | ||||
| Trinchesia ocellata Schmekel, 1966 | 5 | ||||
| Umbraculida | |||||
| Family Tylodinidae Gray, 1847 | |||||
| Tylodina perversa (Gmelin, 1791) | 4,16 | 5,6 | |||
| Family Umbraculidae Dall, 1889 | |||||
| Umbraculum umbraculum (Lightfoot, 1786) | 16 | 13 | 11 | 3,5,6,13,15 | RM3_1037 |
| Runcinida | |||||
| Family Runcinidae H. & A. Adams, 1854 | |||||
| Runcina adriatica T. E. Thompson, 1980 [42] | 11 | 3,5,6,13 | |||
| Runcina brenkoae T. E. Thompson, 1980 | 11 | ||||
| Runcina cf. ferruginea Kress, 1977 | 13 | ||||
| Runcina cf. ornata (Quatrefages, 1844) | 11 | ||||
| Cephalaspidea | |||||
| Family Philinidae Gray, 1850 | |||||
| Philine punctata(J. Adams, 1800) | 4 | ||||
| Philine quadripartita Ascanius, 1772 | 1 | ||||
| Family Aglajidae Pilsbry, 1895 | |||||
| Aglaja tricolorata Renier, 1807 | 11 | 5,6 | |||
| Camachoaglaja africana (Pruvot-Fol, 1953) | 3,5,6,13 | ||||
| Philinopsis depicta (Renier, 1807) | 5,6 | ||||
| Melanochlamys wildpretii Ortea, Bacallado & Moro, 2003 | 3 | ||||
| Family Bullidae Gray, 1827 | |||||
| Bulla striata Bruguière, 1792 | 11 | 1, 4 | |||
| Family Haminoeidae Pilsbry, 1895 | |||||
| Haminoea cf. orteai Talavera, Murillo & Templado, 1987 | 3,13 | ||||
| Weinkauffia turgidula (Forbes, 1844) | 11 | 4 | |||
| Aplysiida | |||||
| Family Aplysiidae Lamarck, 1809 | |||||
| Aplysia dactylomela Rang, 1828 [58] | 5,6,13 | ||||
| Aplysia depilans Gmelin, 1791 | 16 | 13 | 11 | ALL | |
| Aplysia fasciata Poiret, 1789 | 16 | 11 | ALL | ||
| Aplysia parvula Mörch, 1863 | 16 | 11 | ALL | ||
| Aplysia punctata (Cuvier, 1803) | 16 | 13 | ALL | ||
| Bursatella leachii Blainville, 1817 | 2,16,19 | 1 | |||
| Notarchus punctatus Philippi, 1836 | 16 | ||||
| Petalifera petalifera (Rang, 1828) | 16,23 | 5,13 | |||
| Phyllaplysia lafonti (P. Fischer, 1870) | 19 | ||||
| Sacoglossa | |||||
| Family Oxynoida Stoliczka, 1868 | |||||
| Lobiger serradifalci (Calcara, 1840) | 2 | ||||
| Oxynoe olivacea Rafinesque, 1814 | 2 | ||||
| Family Plakobranchidae Gray, 1840 | |||||
| Bosellia mimetica Trinchese, 1891 | 8,16,19 | 11 | 3,6,10 | ||
| Elysia flava Verrill, 1901 | 19,20 | 6 | |||
| Elysia gordanae T. E. Thompson & Jaklin, 1988 | 3,6,10,13,15 | ||||
| Elysia hetta Perrone, 1990 | 9,19 | 15 | |||
| Elysia margaritae Fez, 1962 | 6 | ||||
| Elysia timida (Risso, 1818) | 16 | 11 | ALL | ||
| Elysia translucens Pruvot-Fol, 1957 | 19 | ||||
| Elysia viridis (Montagu, 1804) | 16 | 11 | 1,22 | RM3_1096 | |
| Elysia rubeni Martín-Hervás, Carmona, Jensen, Licchelli, Vitale & Cervera, 2019 [29] | 5,6,13 | ||||
| Thuridilla hopei (Vérany, 1853) | 16,19,20 | 11 | ALL | RM3_1044 | |
| Family Hermaeidae H. & A. Adams, 1854 | |||||
| Aplysiopsis elegans Deshayes, 1853 | 1 | ||||
| Cyerce cristallina (Trinchese, 1881) | 19 | 2, 13 | |||
| Cyerce graeca T. E. Thompson, 1988 | 6 | ||||
| Hermaea bifida (Montagu, 1816) | 1 | RM3_1165, RM3_1166, RM3_1167 | |||
| Hermaea paucicirra Pruvot-Fol, 1953 | 3,6 | ||||
| Hermaea variopicta (A. Costa, 1869) | 24 | 6 | |||
| Family Limapontiidae Gray, 1847 | |||||
| Calliopaea bellula d’Orbigny, 1837 | 16,19 | ||||
| Ercolania coerulea Trinchese, 1892 | 3 | ||||
| Ercolania viridis (A. Costa, 1866) | 11 | 3,14 | |||
| Limapontia capitata (O. F. Müller, 1774) | 25 | ||||
| Placida cremoniana (Trinchese, 1892) | 1,6 | ||||
| Placida dendritica (Alder & Hancock, 1843) | 9 | 3 | |||
| Species Name | Figure | Ecological Notes | Phenotypical Variability | Abundance | |
|---|---|---|---|---|---|
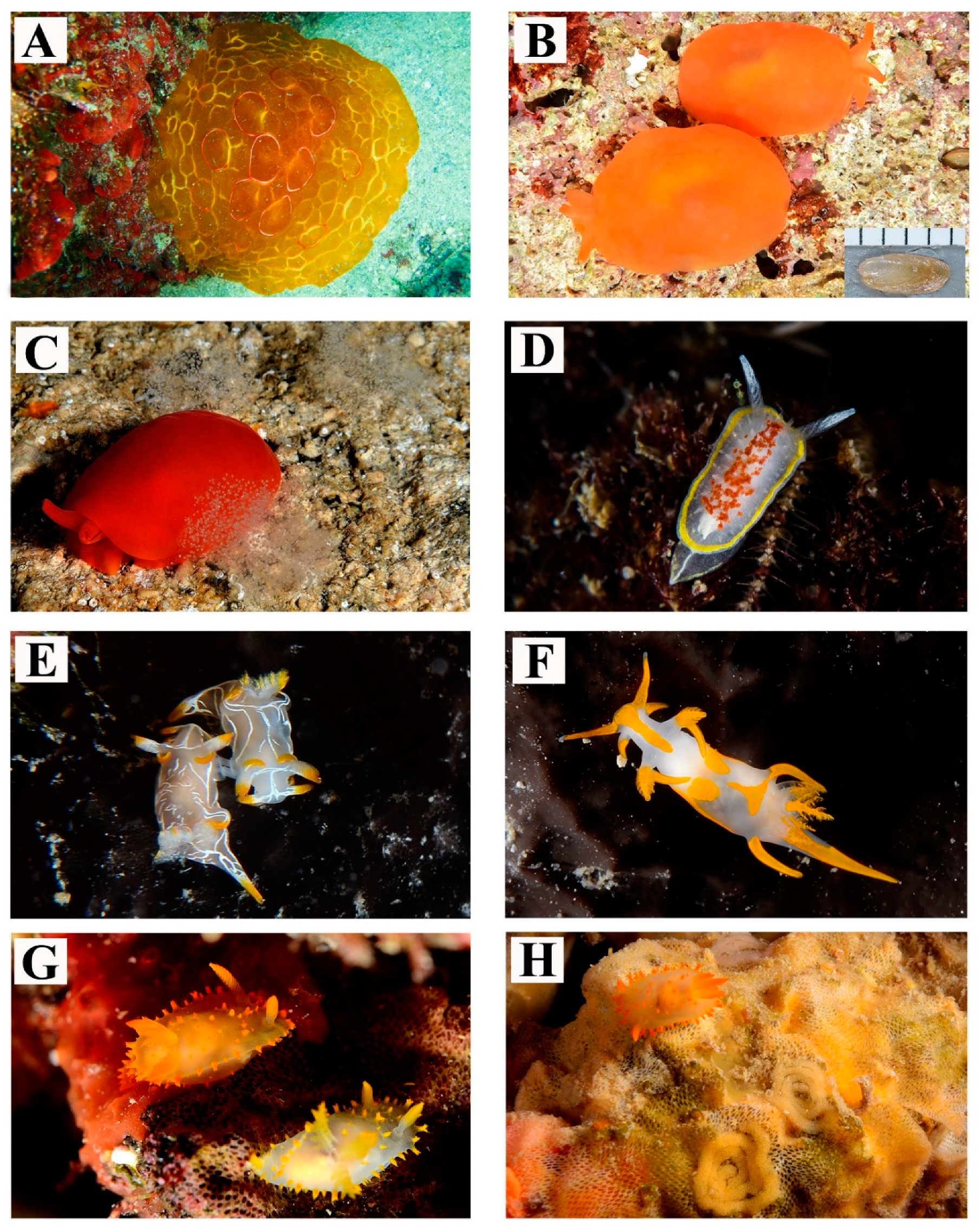
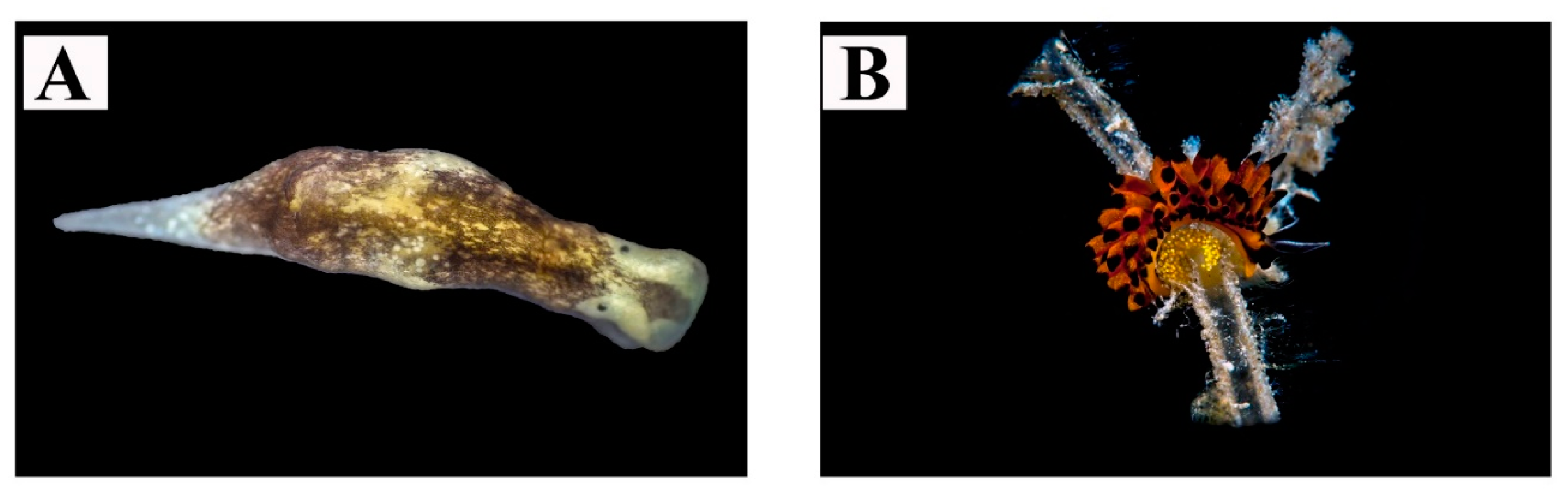
| 1 | Pleurobranchus testudinarius Cantraine, 1835 | Figure 2A | On soft bottom during diurnal dive. Depth: 25 m | - | 1–2 |
| 2 | Berthellina cf. edwardsii (Vayssière, 1897) | Figure 2B,C | Usually living singularly or in groups under stones, in small shaded crevices or in dark caves. Depth: 0–15 m | Body color ranging from light creamy-yellow to reddish-orange | >100 |
| 3 | Diaphorodoris luteocincta (M. Sars, 1870) | Figure 2D | This sedentary species usually lives near bryozoan colonies (cf. Nolella stipata Gosse, 1855). Depth: 0–3 m | The specimens of this species show the red spot on the dorsum which varies in shape and size | 3–10 |
| 4 | Trapania lineata Haefelfinger, 1960 | Figure 2E | Often in clusters, feeding on Entoprocta spp. covering black sponges. Recorded all year long. Depth: 0–30 m | - | >100 |
| 5 | Trapania maculata Haefelfinger, 1960 | Figure 2F | Often sympatric with the congeneric T. lineata. Depth: 0–30 m | - | >100 |
| 6 | Crimora papillata Alder & Hancock, 1862 | Figure 2G,H | Found all year long, in shady pre-coralligenous shallow waters or coastal caves. Often in association with encrusting bryozoans on which their egg masses are laid. Depth: 0–10 m | Color of the notum variable from light yellow to ochre | >100 |
| 7 | Polycera elegans (Bergh, 1894) | Figure 3A,B | Observed during the winter months, in shallow waters. Depth: 5 m | Very typical body color pattern, showing blue spots differing in size and number between individuals | 11–30 |
| 8 | Polycera hedgpethi Er. Marcus, 1964 | Figure 3C,D | Observed during winter season, in shallow waters. Depth: 5 m | - | 3–10 |
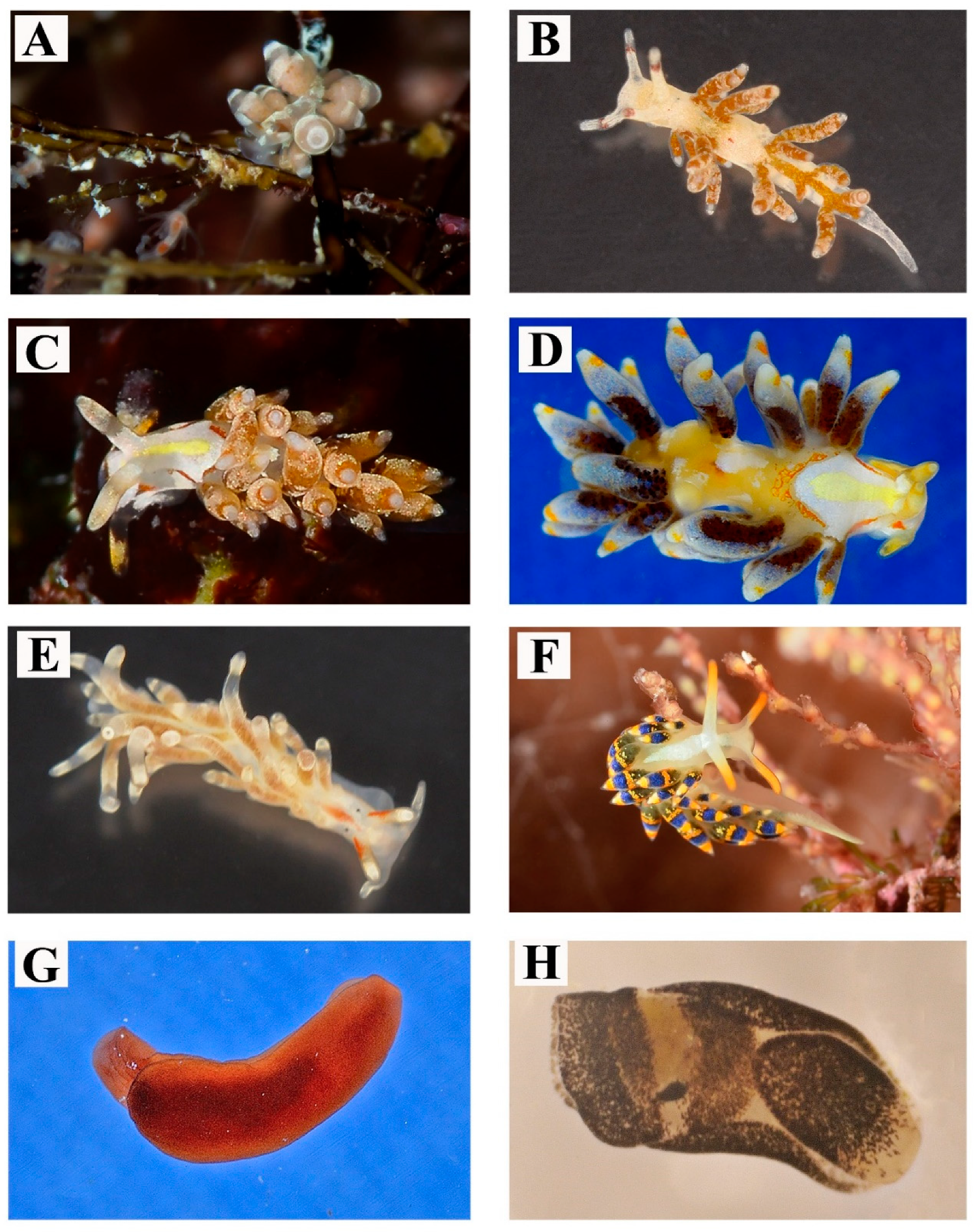
| 9 | Felimare fontandraui (Pruvot-Fol, 1951) | Figure 3E,F | Observed in large assemblages at the end of spring, associated with the sponge Dysidea avara (Schmidt, 1862). Depth: 7 m | Even if this species shows a variable phenotype [26], the Salentine specimens have constant body color pattern | 31–100 |
| 10 | Felimare orsinii (Vérany, 1846) | Figure 3G | In large groups mating and feeding on black sponges. Found between April and July. Depth: 0–15 m | - | 31–100 |
| 11 | Felimida binza (Ev. Marcus & Er. Marcus, 1963) | Figure 3H | On a rocky substrates. Found during September. Depth: 5–7 m | - | 1–2 |
| 12 | Tritonia nilsodhneri Marcus Ev., 1983 | Figure 4A | Found on the yellow gorgonian Eunicella cavolinii Koch, 1887. Depth: 30 m | Very mimetic morphotype that can consistently vary from dark brown to pale yellow or white | 1–2 |
| 13 | Doto cervicenigra Ortea & Bouchet, 1989 | Figure 4B | This small species (few millimetres) is found from winter to early spring on hydrozoans colonies of Aglaophenia Lamouroux, 1812. Depth: 0–3 m | - | 11–30 |
| 14 | Doto floridicola Simroth, 1888 | Figure 4C,D | The average size of specimens observed is ca. 5 mm. Its host hydrozoan colonies belonging to Aglaophenia. Depth: 8–12 m | - | 11–30 |
| 15 | Paraflabellina gabinierei (Vicente, 1975) | Figure 4E | On hard substrates. Depth: 0–25 m | The body colors vary from white to opaque pinkish | 3–10 |
| 16 | Limenandra nodosa Haefelfinger & Stamm, 1958 | Figure 4F | Found on Padina pavonica (Linnaeus) Thivy, 1960 in summer. Depth 6 m | - | 1–2 |
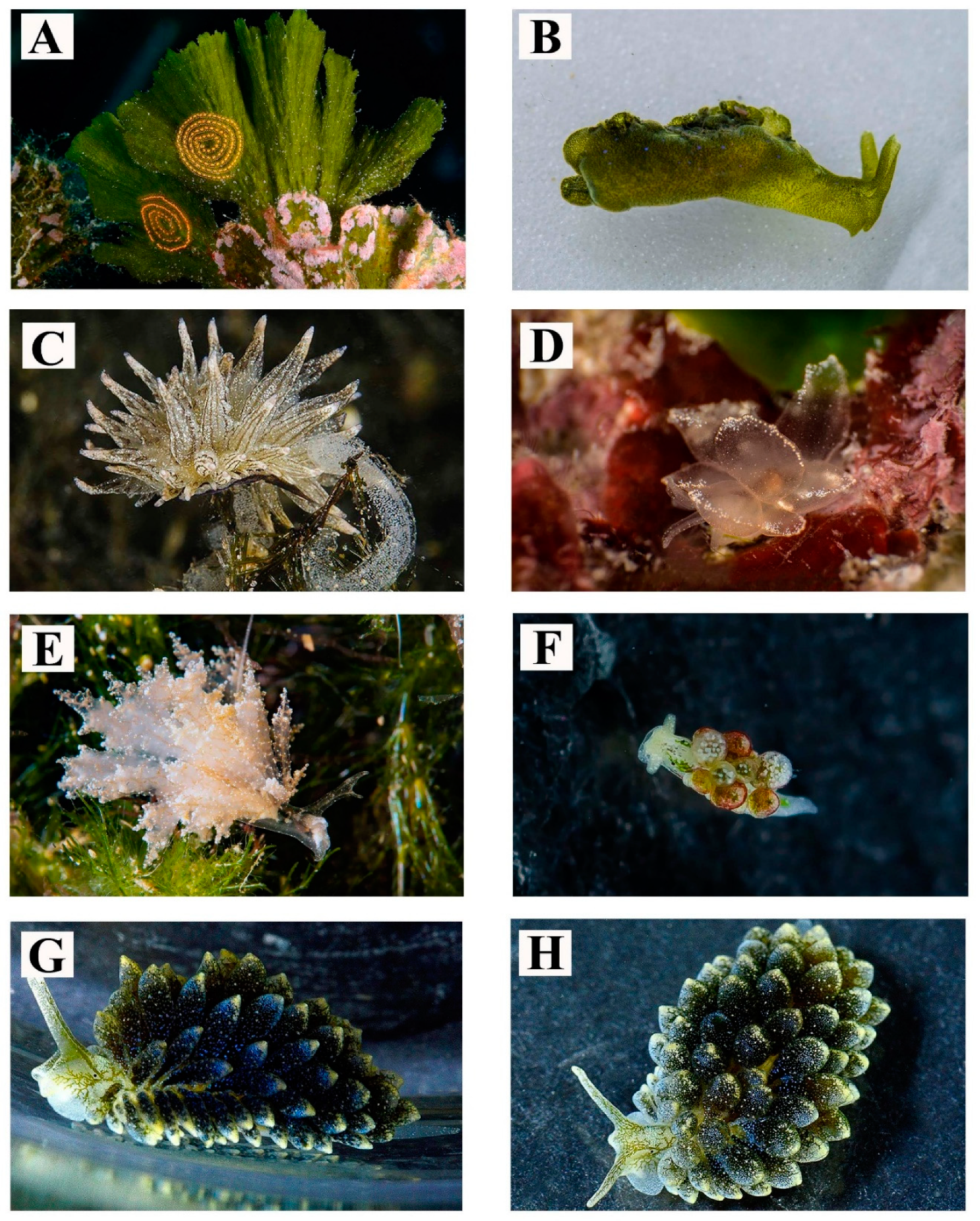
| 17 | Caloria elegans (Alder & Hancock, 1845) | Figure 4G | Usually observed on hard substrata. Depth: 0–15 m | Body pattern with cerata brightly colored from white to light orange | 11–30 |
| 18 | Dicata odhneri Schmekel, 1967 | Figure 4H | Found in shallow waters. Depth: 0–15 m | - | 11–30 |
| 19 | Dondice banyulensis Portmann & Sandmeier, 1960 | Figure 5A | Conspicuous in size and brightly coloured. Depth: 0- to more than 30 m | - | 11–30 |
| 20 | Facelina fusca Schmekel, 1966 | Figure 5B | Found in a tidal pool in association with the green algae Anadyomene stellata (Wulfen) C. Agardh, 1823. Depth: 0.5 m | - | 1–2 |
| 21 | Facelina rubrovittata (A. Costa, 1866) | Figure 5C | Observed all year long in shady pre-coralligenous habitats. Depth: 0–15 m. | - | 31–100 |
| 22 | Facelina vicina (Bergh, 1882) | Figure 5D,E | Depth: 0–15 m | The color of the digestive gland visible through cerata vary from light orange/pink to dark violet | 3–10 |
| 23 | Eubranchus cf. farrani (Alder & Hancock, 1844) | Figure 5F | Found at shallow depth on hydrozoans colonies. Depth: 0–15 m | This species shows differences in the shape and the number of the dorsal yellow spots | 11–30 |
| 24 | Eubranchus cf. linensis Garcia-Gomez, Cervera & Garcia, 1990 | Figure 5G,H | Depth: 0–5 m. | - | 11–30 |
| 25 | Catriona maua Ev. Marcus & Er. Marcus, 1960 | Figure 6A | Recorded in shallow water on hydrozoans colonies. Depth: 0.5 m | - | 1–2 |
| 26 | Rubramoena amoena (Alder & Hancock, 1845) | Figure 6B | Found in April in shallow water. Temperature 15 °C. Depth: 8 m | - | 1–2 |
| 27 | Trinchesia genovae (O’Donoghue, 1926) | Figure 6C,D | Occurs in shallow water on algae, bryozoan, and hydrozoans substrates. Depth: 0–20 m | - | 11–30 |
| 28 | Trinchesia cf. miniostriata Schmekel, 1968 | Figure 6E | On rocky substrates. Depth: 9 m | - | 1–2 |
| 29 | Trinchesia morrowae Korshunova, Picton, Furfaro, Mariottini, Pontes, Prkić, Fletcher, Malmberg, Lundin & Martynov, 2019 | Figure 6F | Common in March-April and in July when it is possible to observe several specimens living and laying eggs on hydroid of the genus Sertularella Gray, 1848. Depth: 0–15 m | The typical color of the apical portion of the rhinophores and the oral tentacles varies from orange to yellow or it could completely lack. | >100 |
| 30 | Runcina cf. ferruginea Kress, 1977 | Figure 6G | Very small species 1.5–2 mm, as expected for Runcina species. Depth: 8 m. | - | 3–10 |
| 31 | Philine punctata (J. Adams, 1800) | Figure 6H | Found only as single and very small specimen, ca. 1 mm, on Posidonia oceanica rhizomes. | - | 1–2 |
| 32 | Philine quadripartita Ascanius, 1772 | Figure 7A | Recorded on soft bottoms. Depth: 8 m | - | 1–2 |
| 33 | Camachoaglaja africana (Pruvot-Fol, 1953) | Figure 7B,C | Recorded on soft bottoms or on algae. Depth: 0–20 m | Its body color pattern varies from a dark form to another much lighter and densely covered by whitish tiny dots | 11–30 |
| 34 | Philinopsis depicta (Renier, 1807) | Figure 7D | Found on soft bottoms or on algae mainly during winter season and in shallow water. Depth: 0–15 m | - | 11–30 |
| 35 | Melanochlamys wildpretii Ortea, Bacallado & Moro, 2003 | Figure 7E | Found in winter in shallow water. Depth: 0–3 m | - | 1–2 |
| 36 | Haminoea cf. orteai Talavera, Murillo & Templado, 1987 | Figure 7F | Found on green algae. Depth: 0–3 m | - | 31–100 |
| 37 | Elysia gordanae T. E. Thompson & Jaklin, 1988 | Figure 7G,H and Figure 8A | Very mimetic species, usually in association with the green alga Flabellia petiolata (Turra) Nizamuddin, 1987. Depth: 0–15 m | - | >100 |
| 38 | Elysia margaritae Fez, 1962 | Figure 8B | On Dictyota dichotoma (Hudson) J.V. Lamouroux, 1809. Depth: 0–2 m | - | 1–2 |
| 39 | Aplysiopsis elegans Deshayes, 1853 | Figure 8C | Depth: 0–12 m | - | 3–10 |
| 40 | Cyerce graeca T. E. Thompson, 1988 | Figure 8D | Found on April. 18 °C water temperature. Depth: 3 m | The body color patter varies from withe to light yellow | 1–2 |
| 41 | Hermaea bifida(Montagu, 1816) | Figure 8E | Found mainly in spring and summer on green algae. Depth: 5–6 m | - | 3–10 |
| 42 | Hermaea paucicirra Pruvot-Fol, 1953 | Figure 8F | Found on algae in shallow waters. Depth: 0–1 m | - | 1–2 |
| 43 | Ercolania coerulea Trinchese, 1892 | Figure 8G,H | Found on algae in shallow waters. Depth: 0–5 m | - | 3–10 |
| 44 | Limapontia capitata (O. F. Müller, 1774) | Figure 9A | Found on algae in shallow waters. Depth: 0.5 m | - | 11–30 |
| 45 | Placida cremoniana (Trinchese, 1892) | Figure 9B | Depth: 0–12 m | - | 11–30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furfaro, G.; Vitale, F.; Licchelli, C.; Mariottini, P. Two Seas for One Great Diversity: Checklist of the Marine Heterobranchia (Mollusca; Gastropoda) from the Salento Peninsula (South-East Italy). Diversity 2020, 12, 171. https://doi.org/10.3390/d12050171
Furfaro G, Vitale F, Licchelli C, Mariottini P. Two Seas for One Great Diversity: Checklist of the Marine Heterobranchia (Mollusca; Gastropoda) from the Salento Peninsula (South-East Italy). Diversity. 2020; 12(5):171. https://doi.org/10.3390/d12050171
Chicago/Turabian StyleFurfaro, Giulia, Fabio Vitale, Cataldo Licchelli, and Paolo Mariottini. 2020. "Two Seas for One Great Diversity: Checklist of the Marine Heterobranchia (Mollusca; Gastropoda) from the Salento Peninsula (South-East Italy)" Diversity 12, no. 5: 171. https://doi.org/10.3390/d12050171
APA StyleFurfaro, G., Vitale, F., Licchelli, C., & Mariottini, P. (2020). Two Seas for One Great Diversity: Checklist of the Marine Heterobranchia (Mollusca; Gastropoda) from the Salento Peninsula (South-East Italy). Diversity, 12(5), 171. https://doi.org/10.3390/d12050171








