More Knot Worms: Four New Polygordius (Annelida) Species from the Pacific and Caribbean
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Specimens
2.2. List of Museum Abbreviations
2.3. Comparative Material Examined
2.3.1. Type Material
2.3.2. Nontype Material
2.4. Light and Scanning Electron Microscopy
2.5. DNA Extraction, Amplification, and Sequencing
2.6. Molecular Methods and Analysis
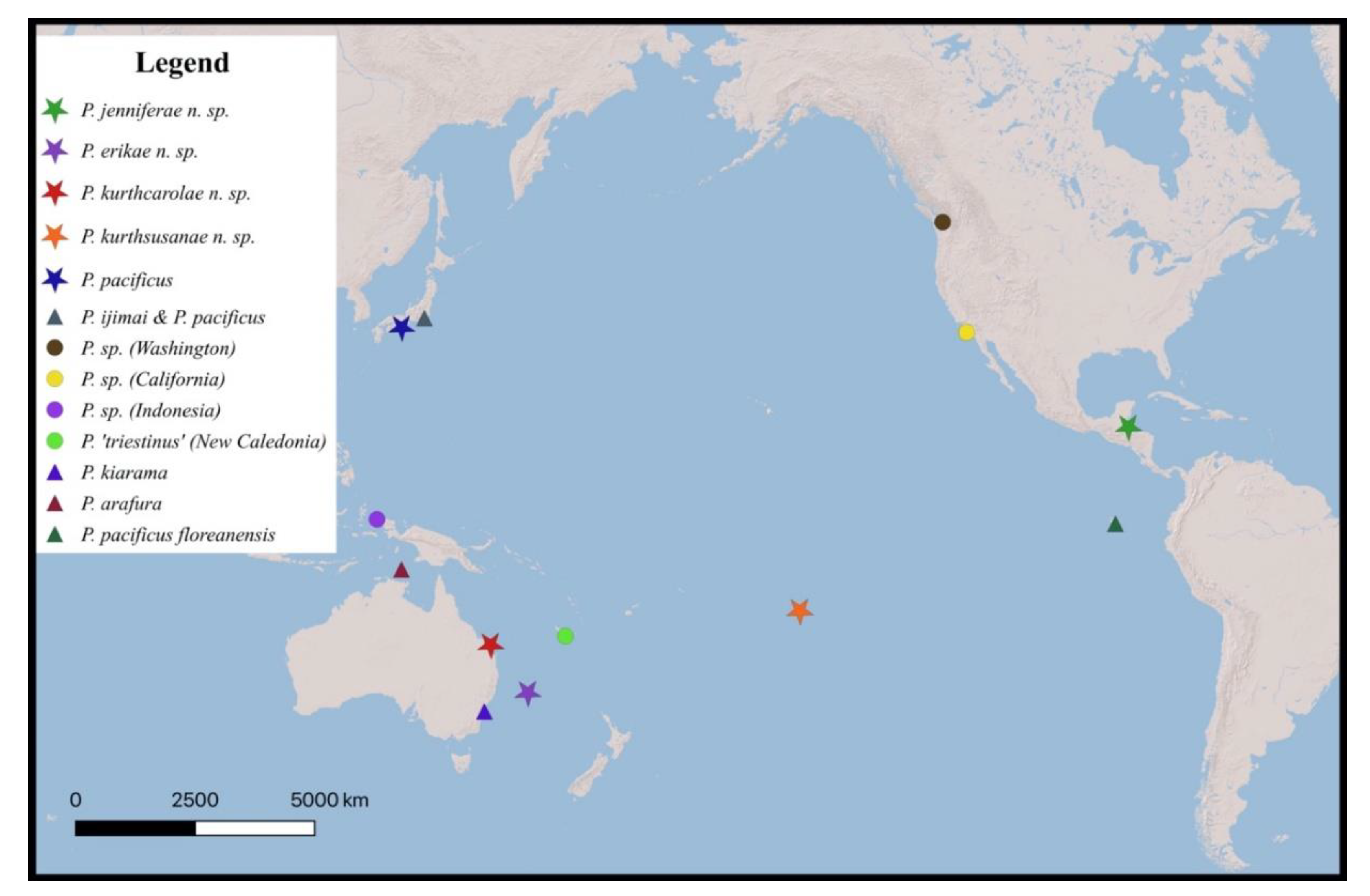
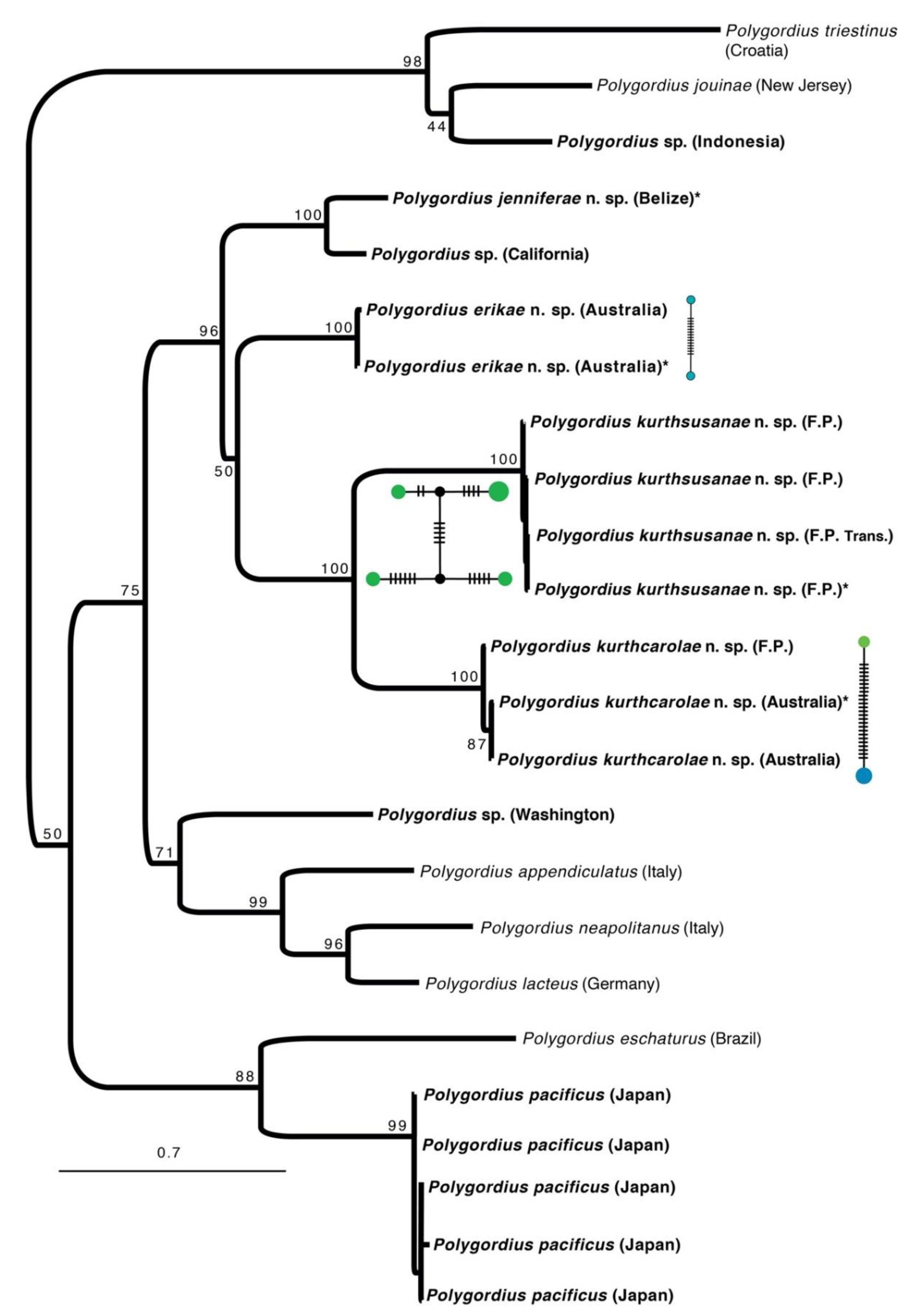
3. Results and Discussion
Species Delimitation and Phylogeny
4. Taxonomy
4.1. Polygordius jenniferae n. sp.
4.1.1. Material
4.1.2. Etymology
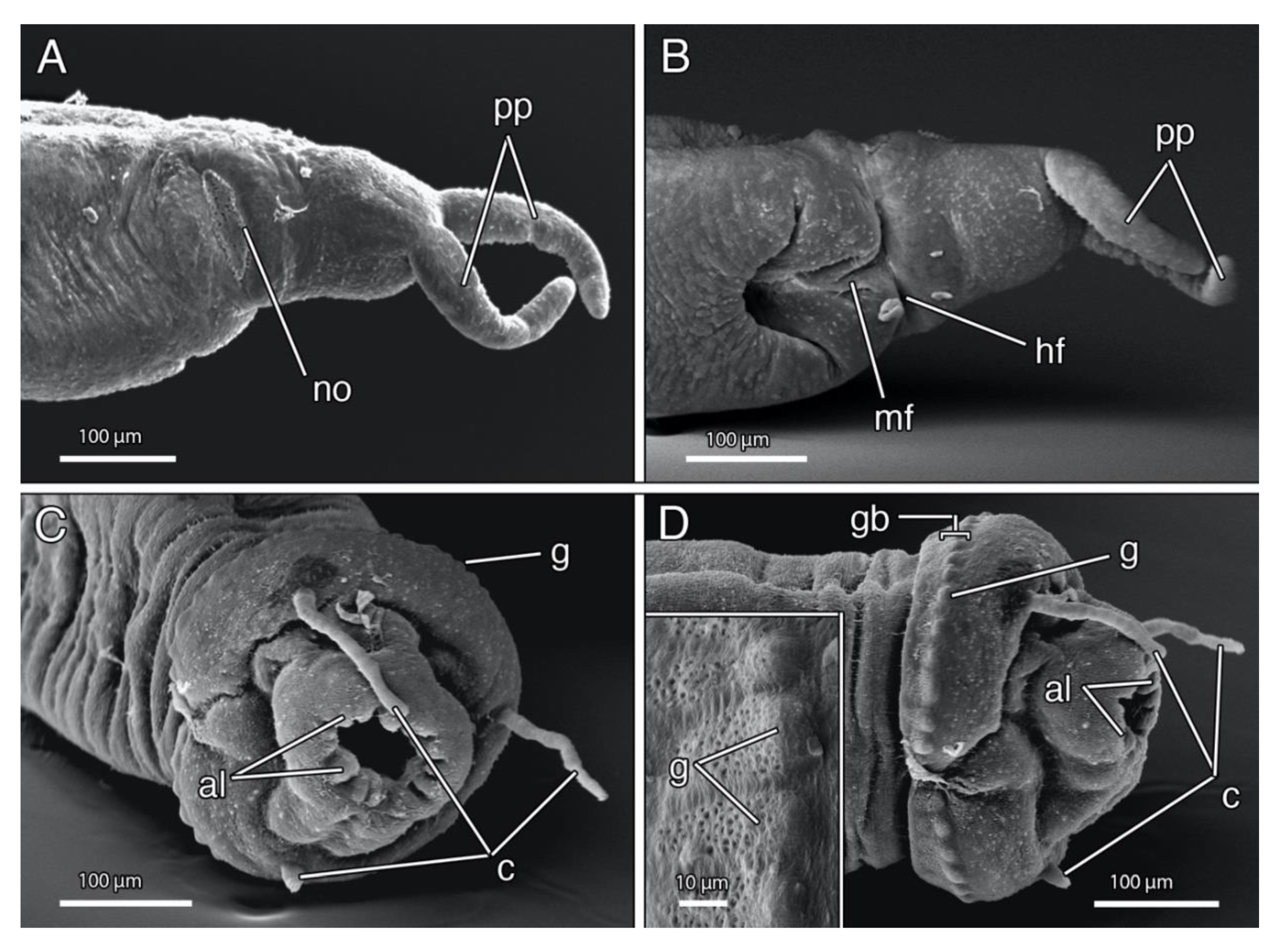
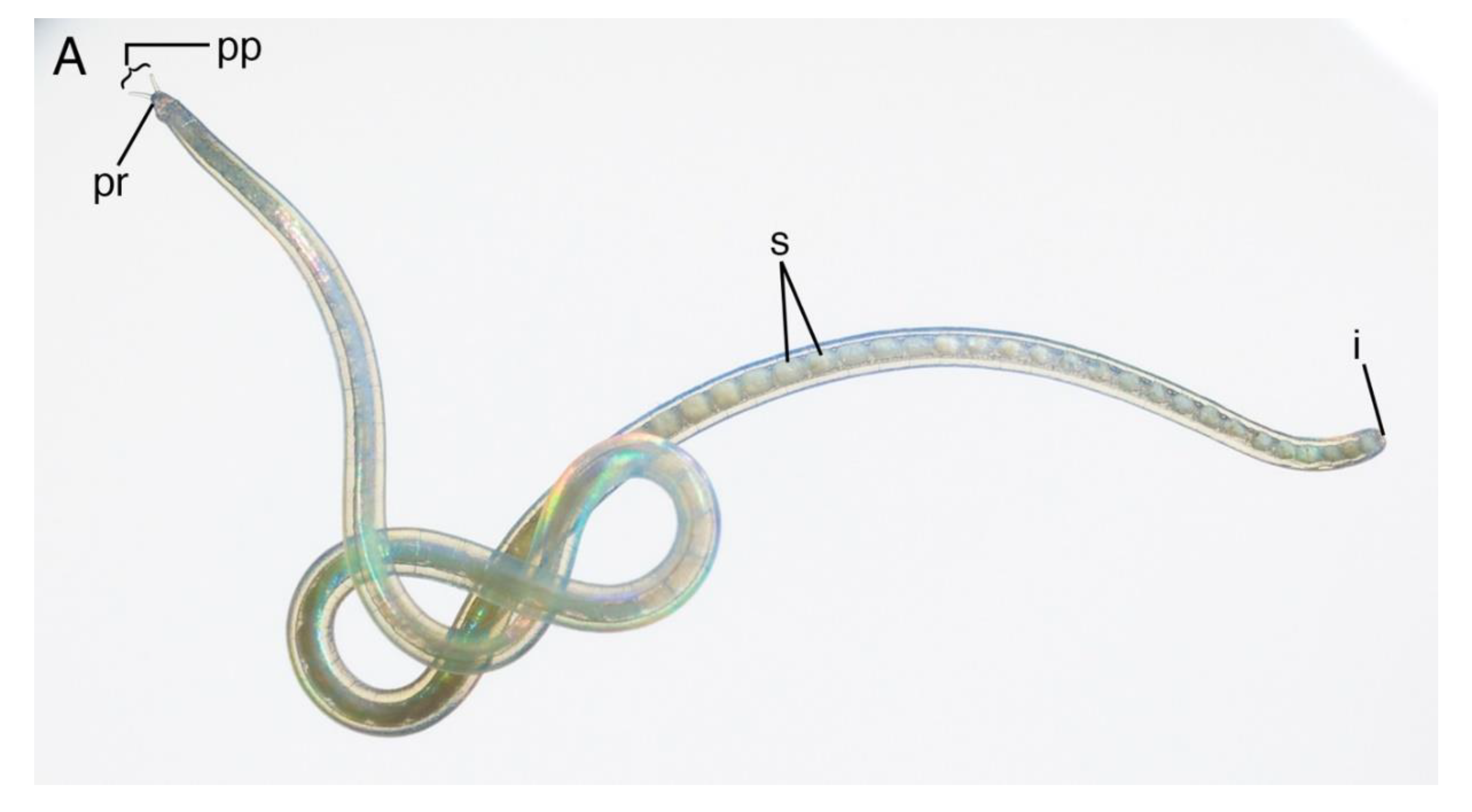
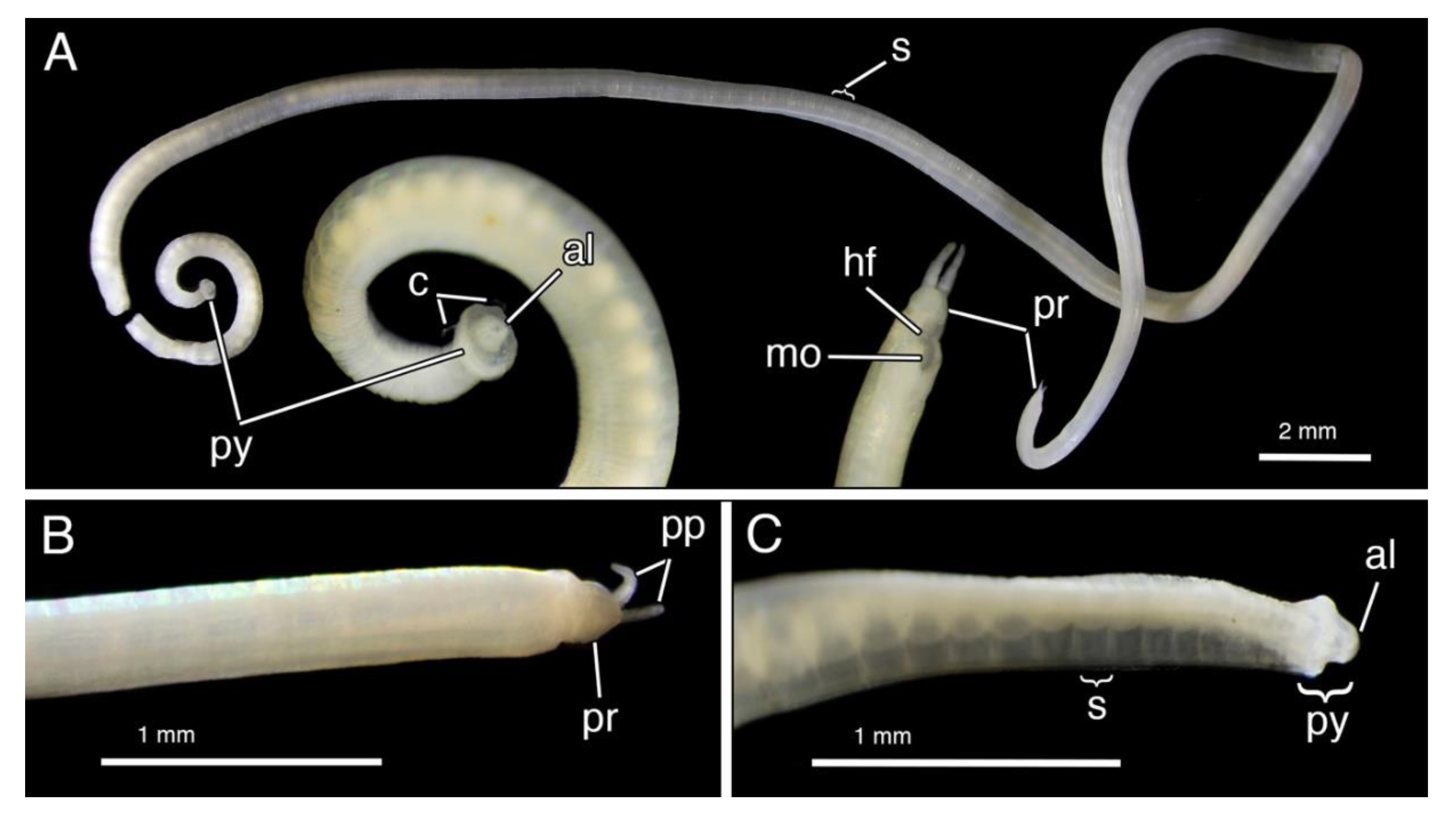
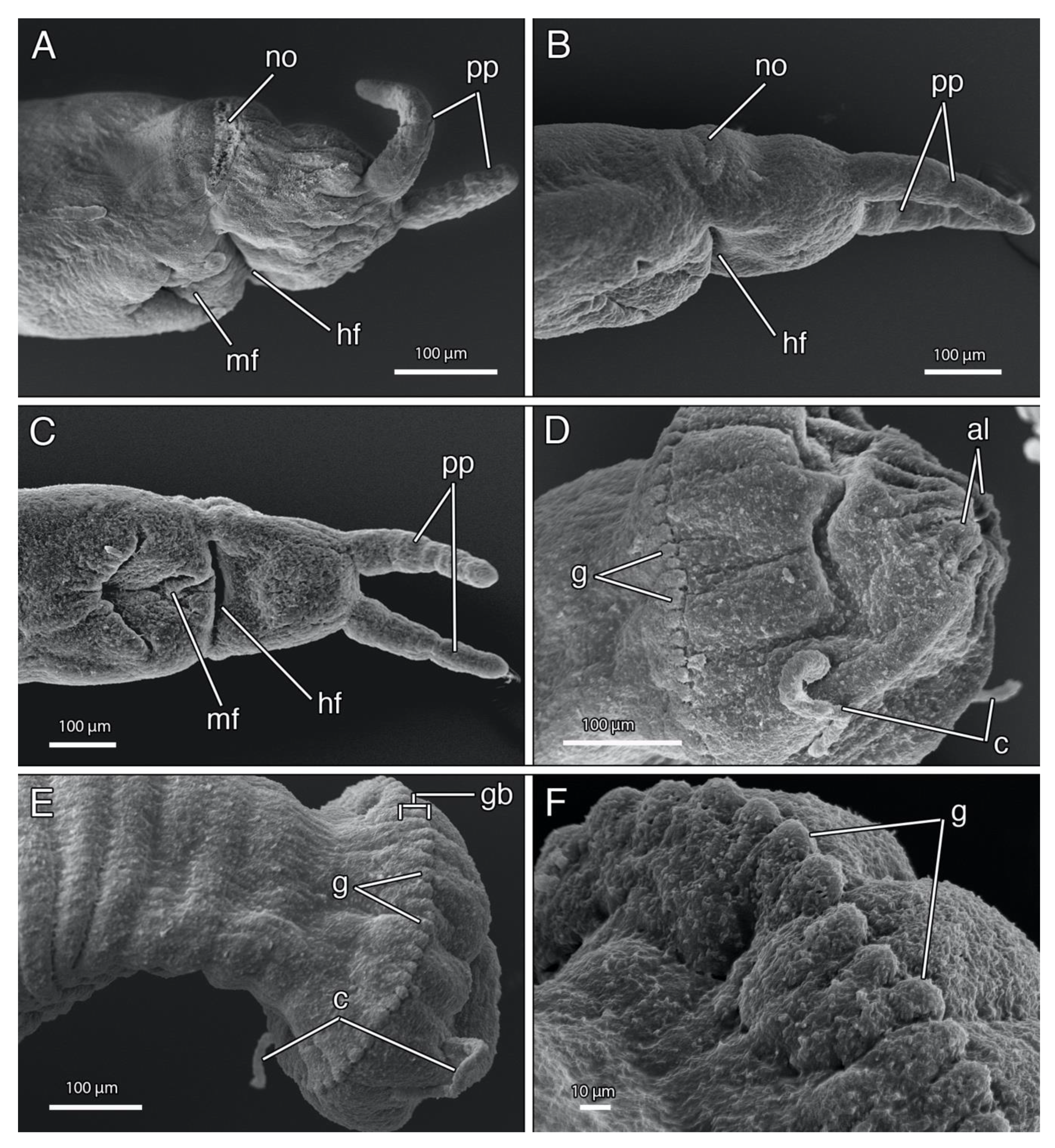
4.1.3. Description
4.1.4. Remarks
4.2. Polygordius erikae n. sp.
4.2.1. Material
4.2.2. Etymology
4.2.3. Description
4.2.4. Remarks
4.3. Polygordius kurthcarolae n. sp.
4.3.1. Material
4.3.2. Etymology
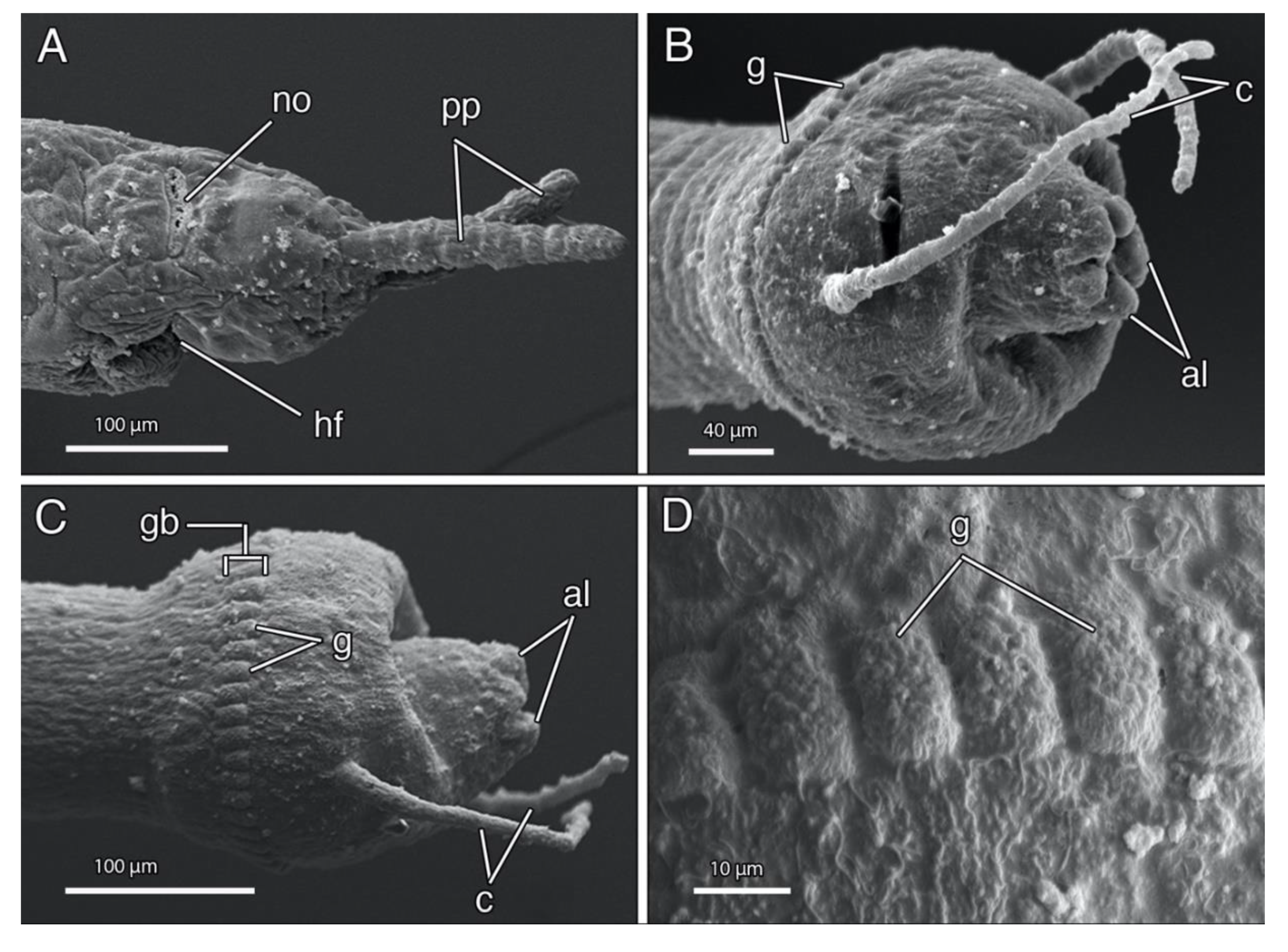
4.3.3. Description
4.3.4. Remarks
4.4. Polygordius kurthsusanae n. sp.
4.4.1. Material
4.4.2. Etymology
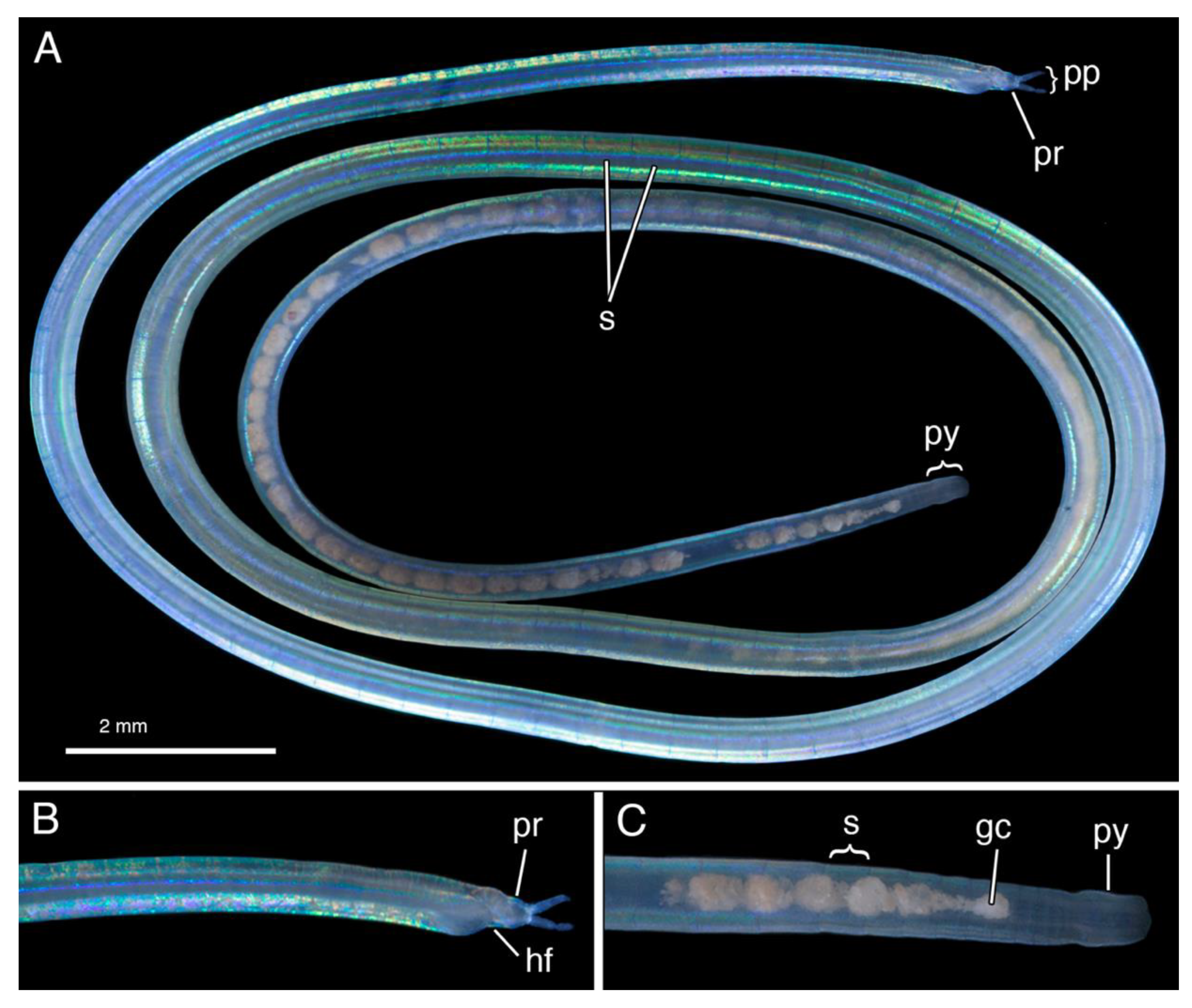
4.4.3. Description
4.4.4. Remarks
4.5. Polygordius pacificus Uchida, 1935
4.5.1. Material
4.5.2. Redescription
4.5.3. Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schneider, A. Ueber Bau und Entwicklung von Polygordius. Arch. Anat. Physiol. Med. Leipzig 1868, 1868, 51–60. [Google Scholar]
- Czerniavsky, V. Material ad zoographiam Ponticam comparatam. Fasc. III Vermes. Bulletin de la Société Impériale des naturalistes de Moscou (= Byulletin’ Moskovskogo obshchestva ispytatelei prirody) 1881, 55, 211–363. [Google Scholar]
- Ramey-Balci, P.; Purschke, G.; Fiege, D. Polygordiidae. In Handbook of Zoology; Purschke, G., Böggemann, M., Westheide, W., Eds.; Walter de Gruyter: Berlin, Germany, 2020; Volume 3, in press. [Google Scholar]
- Rouse, G.W.; Pleijel, F. Polychaetes; Oxford University Press: Oxford, UK, 2001; pp. 279–281. [Google Scholar]
- Ramey-Balcı, P.; Fiege, D.; Struck, T.H. Molecular phylogeny, morphology, and distribution of Polygordius (Polychaeta: Polygordiidae) in the Atlantic and Mediterranean. Mol. Phylogenet. Evol. 2018, 127, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Purschke, G. Ultrastructure of the nuchal organs in polychaetes (Annelida)- New results and review. Acta Zool. 1997, 78, 123–143. [Google Scholar] [CrossRef]
- Rota, E.; Carchini, G. A new Polygordius (Annelida: Polycheata) from the Terra Nova Bay, Ross Sea, Antarctica. Polar Biol. 1999, 21, 201–213. [Google Scholar] [CrossRef]
- Wilkens, V.; Purschke, G. Central nervous system and sense organs, with special reference to photoreceptor-like sensory elements, in Polygordius appendiculatus (Annelida), an interstitial polychaete with uncertain phylogenetic affinities. Invertebr. Biol. 2009, 128, 46–64. [Google Scholar] [CrossRef]
- Hempelmann, F. Zur Morphologie von Polygordius lacteus Schn. und Polygordius triestinus Woltereck nov. spec. Zeitschrift für wissenschaftliche Zoologie 1906, 84, 527–618. [Google Scholar]
- Avery, L.; Ramey, P.A.; Wilson, R.S. New Polygordiidae (Polychaeta) from the Australian region. Zootaxa 2009, 2068, 59–68. [Google Scholar] [CrossRef]
- Andrade, S.C.S.; Novo, M.; Kawauchi, G.Y.; Worsaae, K.; Pleijel, F.; Giribet, G.; Rouse, G.W. Articulating “archiannelids”: Phylogenomics and annelid relationships, with emphasis on meiofaunal taxa. Mol. Biol. Evol. 2015, 32, 2860–2875. [Google Scholar]
- Ramey, P.A.; Fiege, D.; Leander, B.S. A new species of Polygordius (Polychaeta: Polygordiidae): from the inner continental shelf and in bays and harbours of the north-eastern United States. J. Mar. Biol. Ass. U.K. 2006, 86, 1025–1034. [Google Scholar] [CrossRef]
- Hatschek, B. Studien über die Entwicklungsgeschichte der Anneliden: Ein Beitrag zur Morphologie der Bilaterien. Arbeiten aus dem Zoologischen Institute der Universität Wien und der Zoologischen Station in Triest 1878, 1, 277–404. [Google Scholar]
- Hermans, C.O. The systematic position of the Archiannelida. Syst. Zool. 1969, 18, 85–102. [Google Scholar] [CrossRef]
- Struck, T.H.; Golombek, A.; Weigert, A.; Franke, F.A.; Westheide, W.; Purschke, G.; Bleidorn, C.; Halanych, K.M. The evolution of annelids reveals two adaptive routes to the interstitial realm. Curr. Biol. 2015, 25, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T. Eine Neue Urannelidenart, Polygordius pacificus n. sp. Proceedings of the Imperial Academy 1935, 11, 119–120. [Google Scholar] [CrossRef][Green Version]
- Jouin, C.; Rao, G.C. Morphological studies on some Polygordiidae and Saccocirridae (Polychaeta) from the Indian Ocean. Cah. Biol. Mar. 1987, 28, 389–402. [Google Scholar]
- Schmidt, P.; Westheide, W. Interstitielle Fauna von Galapagos. XVII. Polygordiidae, Saccocirridae, Protodrilidae, Nerillidae, Dinophilidae (Polychaeta). Mikrofauna des Meeresbodens 1977, 62, 1–38. [Google Scholar]
- Fraipont, J. Le genre Polygordius. Fauna Flora Golf. Neapel 1887, 14, 1–130. [Google Scholar]
- Marcus, E. du B.-R.. Further Archiannelids from Brazil. Comunicaciones Zoologicas del Museo de Historia Natural de Montevideo 1948, 2, pp. 1–17. [Google Scholar]
- Murtey, M.D.; Ramasamy, P. Sample Preparations for Scanning Electron Microscopy—Life Sciences. In Modern Electron Microscopy in Physical and Life Sciences; Janecek, M., Ed.; IntechOpen: London, UK, 2016; pp. 161–185. [Google Scholar]
- Palumbi, S.R. Nucleic acids II: The polymerase chain reaction. In Molecular Systematics; Hillis, D.M., Moritz, C., Mable, B.K., Eds.; Sinauer Associates: Sunderland, Massachusetts, MA, USA, 1996; Volume 2, pp. 205–247. ISBN 9780878932825. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.R.; Lutz, R.A.; Vrijenhoek, R.C. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Carr, C.M.; Hardy, S.M.; Brown, T.M.; Macdonald, T.A.; Hebert, P.D.N. A tri-oceanic perspective: DNA barcoding reveals geographic structure and cryptic diversity in Canadian polychaetes. PLoS ONE 2011, 6, e22232. [Google Scholar] [CrossRef]
- Colgan, D.J.; McLauchlan, A.; Wilson, G.D.F.; Livingston, S.; Macaranas, J.; Edgecombe, G.D.; Cassis, G.; Gray, M.R. Molecular phylogenetics of the Arthropoda: relationships based on histone H3 and U2 snRNA DNA sequences. Aust. J. Zool. 1998, 46, 419–437. [Google Scholar] [CrossRef]
- Di Domenico, M.; Martínez, A.; Lana, P. da C.; Worsaae, K. Protodrilus (Protodrilidae, Annelida) from the southern and southeastern Brazilian coasts. Helgol. Mar. Res. 2013, 67, 733–748. [Google Scholar]
- Rousset, V.; Pleijel, F.; Rouse, G.W.; Erseus, C.; Siddall, M.E. A molecular phylogeny of annelids. Cladistics 2007, 23, 41–63. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. POPART: full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4; Sinauer Associates: Sunderland, MA, USA, 2002; Available online: https://paup.phylosolutions.com (accessed on 20 February 2020).
- Nygren, A. Cryptic polychaete diversity: a review. Zool. Scr. 2014, 43, 172–183. [Google Scholar] [CrossRef]
- Izuka, A. On a new Polygordius from Misaki (P. ijimai n. sp.). Annot. Zool. Jpn. 1903, 4, 137–139. [Google Scholar]
- Aiyar, R.G. & Alikunhi, K.H. On some Archiannelids of the Madras Coast. Proc. Nat. Inst. Sci. India. 1944, 113–140. [Google Scholar]




| Pygidium | Pygidial Appendages/ Cirri Absent | Pygidial Appendages/ Cirri Subterminal | Pygidial Appendages/ Cirri Terminal | Pygidial Appendages Undetermined |
|---|---|---|---|---|
| Pygidial glands present | P. antarcticus P. lacteus P. neapolitanus | P. appendiculatus P. kiarama P. leo P. jenniferae n. sp. P. kurthcarolae n.sp. P. erikae n.sp. | P. eschaturus P. eschaturus brevipapillosus P. madrasensis P. pacificus | P. pacificus floreanensis P. ijimai |
| Pygidial glands absent | P. arafura P. jouinae P. triestinus P. kurthsusanae n. sp. | |||
| Pygidial glands undetermined | P. uroviridis |
| Taxa | Location | Voucher | COI | 16S rRNA | Histone H3 |
|---|---|---|---|---|---|
| Protodrilus pythonius | Brazil | (Di Domenico et al.) [26] | KC763840 | KJ451298 | |
| Polygordius appendiculatus | Italy | (Ramey et al. 2018) [5] | MG603453 | MG603479 | |
| Polygordius eschaturus | Brazil | (Ramey et al. 2018) [5] | MG603404 | MG603559 | |
| Polygordius jouinae | USA, New Jersey | (Ramey et al. 2018) [5] | MG603410 | MG603509 | |
| Polygordius lacteus | Germany | (Ramey et al. 2018) [5], (Rousset et al. 2007) [27] | KT883951 | MG603540 | DQ779757 |
| Polygordius neapolitanus | Italy | (Ramey et al. 2018) [5] | MG603433 | MG603521 | |
| Polygordius triestinus | Croatia | (Ramey et al. 2018) [5] | MG603537 | ||
| Polygordius erikae n. sp. | Australia | AM W.53108, A12259 | MT263739-740 | MT260195-196 | |
| Polygordius jenniferae n. sp. | Belize | SIO-BIC A12260 | MT263733 | MT260193 | |
| Polygordius kurthcarolae n. sp. | Australia | AM W.53109, SIO-BIC A12261, USNM1434789 | MT263735-6,8 | MT260191-192 | MT263747 |
| Polygordius kurthsusanae n. sp. | French Polynesia | USNM1432890, SIO-BIC A12287, A12289 | MT263742-744 | MT260187-189 | |
| Polygordius pacificus | Japan | SIO-BIC A12402, A12265-6, A12284-6 | MT263741 | MT260197-201 | MT263748-749 |
| Polygordius sp. | Indonesia | SIO-BIC A4058 | MT263745 | MT260202 | |
| Polygordius sp. | USA, California | SIO-BIC A12290 | MT263734 | MT260194 | |
| Polygordius sp. | USA, Washington | SIO-BIC A11847 | MT263746 | KF511815 | KF511879 |
| Taxa | lacteus | appendiculatus | neapolitanus | jouinae | eschaturus | Washington | California | pacificus | Indonesia | erikae | jenniferae | kurthcarolae |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. lacteus | - | |||||||||||
| P. appendiculatus | 0.18 | - | ||||||||||
| P. neapolitanus | 0.15 | 0.20 | - | |||||||||
| P. jouinae | 0.22 | 0.21 | 0.25 | - | ||||||||
| P. eschaturus | 0.23 | 0.21 | 0.22 | 0.22 | - | |||||||
| P. Washington | 0.19 | 0.23 | 0.23 | 0.24 | 0.21 | - | ||||||
| P. California | 0.23 | 0.20 | 0.22 | 0.24 | 0.25 | 0.24 | - | |||||
| P. pacificus | 0.22 | 0.20 | 0.20 | 0.23 | 0.19 | 0.23 | 0.21 | - | ||||
| P. Indonesia | 0.24 | 0.21 | 0.23 | 0.17 | 0.22 | 0.22 | 0.23 | 0.23 | - | |||
| P. erikae n. sp. | 0.24 | 0.22 | 0.23 | 0.23 | 0.21 | 0.23 | 0.19 | 0.21 | 0.24 | - | ||
| P. jenniferae n. sp. | 0.21 | 0.20 | 0.22 | 0.22 | 0.23 | 0.24 | 0.13 | 0.22 | 0.21 | 0.20 | - | |
| P. kurthcarolae n. sp. | 0.24 | 0.23 | 0.24 | 0.25 | 0.25 | 0.24 | 0.20 | 0.22 | 0.24 | 0.22 | 0.20 | - |
| P. kurthsusanae n. sp. | 0.24 | 0.25 | 0.26 | 0.24 | 0.23 | 0.26 | 0.23 | 0.26 | 0.24 | 0.22 | 0.21 | 0.23 |
| Character/Species | P. jenniferae n. sp | P. kurthcarolae n. sp | P. erikae n. sp | P. appendiculatus Fraipont, 1887 [19] | P. kiarama Avery, Ramey, Wilson, 2009 [10] | P. ijimai Izuka, 1903 [35] |
|---|---|---|---|---|---|---|
| Prostomium shape | conical (blunt) | rounded | rounded | rounded (F, RC) | conical (blunt) | n.d. |
| Palp length (mm) | 0.18 | 0.14 {0.25} | 0.16 {0.11} | 0.24–0.41(RC) | 0.10–0.22 | 1.0 |
| Prostomium length (mm) | 0.18 | 0.15 {0.21} | 0.13 {0.11} | n.d. | 0.08–0.14 | n.d. |
| Ratio (palp:prostomium) | 1:1 | ~1:1 | 1:1 | 2:1 (F), >1:1 (RC) | 1.2–2.0:1 | n.d. |
| Pigment spots (eyespots) | absent | absent | absent | 2; 1or 2 (RC) | absent | absent |
| Head fold | deep | deep | deep | deep | n.d. | |
| Median fold shape | subtriangular with deep-cleft | subtriangular with deep-cleft | n.d. | n.d. | n.d. | |
| Pygidium shape/width (mm) | inflated/0.28 | inflated/n.d. {0.35} | inflated/0.17 | inflated/n.d. | inflated/0.12–0.40 | inflated/n.d. |
| Pygidial glands (no) | 40–54 | n.d. {54–60} | 40–52 {n.d.} | 30 (RC) | 20 | n.d. |
| Pygidial gland shape/orientation | elongate/longitudinal | n.d. {round/n.a} | oval/longitudinal | round (F, RC)/ n.a. | oval/longitudinal | elongate/n.d. |
| Glandular belt (width) (µm) | 30 | n.d. {10–13} | 17 | n.d. | n.d | n.d. |
| Gland (width) (µm) | 10–15 | n.d. {10–13} | 10 | n.d. | n.d. | |
| Gland ratio length:width | 3:1 | n.d. {1:1} | 1.7:1 | 1:1 | 2:1 | n.d. |
| Subterminal pygidial cirri (no) | 3 | n.d {2} | 2 | 2 | 2 | 3 |
| Subterminal pygidial cirri insertion point | 2 ventro-lateral, 1 dorsal median | ventro-lateral | ventro-lateral | lateral | ventro-lateral | 2 ventro-lateral, 1 dorsal median |
| Anal lobes (no.) | 6–12 | n.d. {7–8} | 5 | 5;5 (RC) | 7–8 | 8 |
| Body length (mm) | 48.4 | n.d. {42.3} | 21.4 {15.9} | max 20;20–45 (RC) | 3.8–11.6 | 70–77 |
| Body width (mm) | 0.47 | n.d.{0.50} | 0.21 {0.18} | 0.25;0.12–0.31 (RC) | 0.3–0.28 | 0.6–0.8 |
| Segment (no.) | 145 | n.d {158} | 56 {n.d.} | 95–125 | 30–74 | n.d. |
| Type locality | off Carrie Bow Cay, Caribbean Sea, Belize | One Tree Island Reef, Coral Sea, Northeastern Australia | Lord Howe, Island, Tasman Sea, Southwestern Australia | Mediterranean Sea, Gulf of Naples | S of Port Kembla, Southeastern Australia | Misaki Mar. Biol. Lab., Japan |
| Region | North Atlantic | South Pacific | South Pacific | North Atlantic | South Pacific | North Pacific |
| Latitude | 16°48’6.08’’ N | 23°29’54.50’’ S | 31°31’0.47’’ S | 35°28’0’’ S | ||
| Longitude | 88°4’47.00’’ W | 152°3’53.82’’ E | 159°3’49.67’’ E | 150°53’0’’ E | n.d. | |
| Sediment type | coarse sand | coral rubble, gravel | n.d. | coarse sand | sandy, well sorted | sand, pebbles, shell-hash |
| Depth range (m) | 18 | 5 | 3–5 | n.d. | 21–1650 | lower intertidal |
| Character/Species | P. kurthsusanae n. sp | P. triestinus Woltereck in Hempelmann, 1906 [9] | P. arafura Avery, Ramey, Wilson, 2009 [10] | P. jouinae Ramey, Fiege & Leander, 2006 [12] |
|---|---|---|---|---|
| Prostomium shape | conical {blunt} | conical (pointed) | conical (pointed) | conical (pointed) |
| Palp length (mm) | 0.30 {n.d.*} | n.d. | 0.04–0.06 | 0.11–0.15 |
| Prostomium length (mm) | 0.20 {0.25} | n.d. | 0.06–0.12 | 0.11–0.15 |
| Ratio (palp:prostomium) | 1.5:1 | n.d. | 0.5–0.86 | 1:1 |
| Pigment spots (eyespots) | absent | absent | absent | absent |
| Head fold | deep | shallow | deep | shallow |
| Median fold shape | subtriangular | n.d. | subtrian. deep cleft (F) | n.d. |
| Pygidium shape/width (mm) | not inflated to minimally inflated /0.20 {0.20} | not inflated | not inflated to minimally inflated | not inflated to minimally inflated |
| Pygidial glands (no) | absent | absent | absent | absent |
| Pygidial appendages/cirri pygidial cirri | absent | absent | absent | absent |
| Anal lobes (no.) | 4–5 | n.d. | 7–8 | 7 |
| Body length (mm) | n.d. {58.5} | max 30 | 2.4–11.6 | 13–43 |
| Body width (mm) | 0.46 {0.40} | n.d. | 0.08–0.18 | 0.23–0.38 |
| Segment (no.) | n.d. {146} | n.d. | 27–74 | 82–93 |
| Type locality | Moorea Island, French Polynesia | Northern Adriatic Sea, Italy | Australia, Gulf of Carpentaria | Beach Haven Ridge, Tuckerton, New Jersey |
| Region | South Pacific | North Atlantic | South Pacific | North Atlantic |
| Latitude | 17°28’34.0’’ S | n.d. | 9°50’6’’ S | 39°27’42’’ N |
| Longitude | 149°49’51.6’’ W | n.d. | 135°17’48’’ E | 74°15’48’’ W |
| Sediment type | coarse coral sand | mud | muddy-sand | sand |
| Depth range (m) | 10–11 | n.d. | 69–92 | 5–152 |
| Character/Species | P. pacificus | P. pacificus Uchida, 1935 [16] | P. pacificus floreanensis Schmidt & Westheide 1977 [18] | P. eschaturus Marcus, 1948 [20] | P. eschaturus brevipapillosus Jouin & Rao, 1987 [17] |
|---|---|---|---|---|---|
| Prostomium shape | rounded | n.d. | conical (F, P) | rounded (F) | rounded (F) |
| Palp length (mm) | 0.18–0.23 | n.d. | 0.10–0.225 | 0.15–0.22 | 0.20–0.25 |
| Prostomium length (mm) | 0.09–0.11 | n.d. | 0.10 (P) | n.d. | n.d |
| Ratio (palp:prostomium) | ~2:1 | n.d. | ~1:1 | n.d. | n.d. |
| Pigment spots (eyespots) | absent | absent | absent | absent | absent |
| Head fold | deep | n.d. | n.d. | deep (F) | deep |
| Median fold shape | elongate | n.d. | n.d. | n.d. | n.d. |
| Pygidium shape/width (mm) | inflated/0.35–0.38 | inflated/n.d. | inflated/0.16 | inflated/max 0.30 | inflated/0.20–0.53 |
| Pygidial glands (no) | 44–75 | n.d. | n.d. | n.d. | n.d. |
| Pygidial gland shape/orientation | elongate/ longitudinal | elongate/ ongitudinal | elongate/longitudinal | elongate/longitudinal | elongate/longitudinal |
| Glandular belt (width) (µm) | 100 | 150 (F) | 30 (F) | n.d. | 108 |
| Gland (width) (µm) | 10–15 | n.d. | n.d. | n.d. | n.d. |
| Gland ratio length:width | 7–10:1 | 5:1 (F) | 5:1 (F) | n.d. | n.d. |
| Terminal pygidial appendages/cirri | present | absent | n.d. | present | present |
| Anal lobes (total no.) | 6 | 6–8 | n.d. | n.d. | 6–8 |
| Elongate & enlarged anal lobes no./length (µm) | 2-3/40 | n.d. | n.d. | 2/50–70 | 2–3/80 |
| Body length (mm) | 18.15-20.75 | 30–35 | 4.9–23 | max 40 | 18–50 |
| Body width (mm) | 0.25–0.46 | 0.40–0.50 | 0.11 | 0.21 | 0.15–0.50 |
| Segment (no.) | 90-108 | n.d | 35–75 | 120-180 | 110–160 |
| Type locality | Seto Mar. Biol. Lab., Japan* | Misaki Mar. Biol. Lab., Japan | Floreana Isl., Galapagos | São Sebastião Isl., Brazil | Andaman Islands, India |
| Region | NW Pacific | NW Pacific | SE Pacific | SW Atlantic | Indian Ocean |
| Latitude | 33°41’29.04’’ N | n.d. | n.d. | n.d. | 12°58’06’’ N |
| Longitude | 135°20’11.04’’ W | n.d. | n.d. | n.d. | 92°59’17’’ E |
| Sediment type | pebbles | fine sand | coarse sand | coarse sand | coarse sand & shell-hash |
| Depth range (m) | intertidal | lower intertidal | lower intertidal | intertidal | lower intertidal |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tustison, C.A.; Ramey-Balci, P.A.; Rouse, G.W. More Knot Worms: Four New Polygordius (Annelida) Species from the Pacific and Caribbean. Diversity 2020, 12, 146. https://doi.org/10.3390/d12040146
Tustison CA, Ramey-Balci PA, Rouse GW. More Knot Worms: Four New Polygordius (Annelida) Species from the Pacific and Caribbean. Diversity. 2020; 12(4):146. https://doi.org/10.3390/d12040146
Chicago/Turabian StyleTustison, Chrissy A., Patricia A. Ramey-Balci, and Greg W. Rouse. 2020. "More Knot Worms: Four New Polygordius (Annelida) Species from the Pacific and Caribbean" Diversity 12, no. 4: 146. https://doi.org/10.3390/d12040146
APA StyleTustison, C. A., Ramey-Balci, P. A., & Rouse, G. W. (2020). More Knot Worms: Four New Polygordius (Annelida) Species from the Pacific and Caribbean. Diversity, 12(4), 146. https://doi.org/10.3390/d12040146






