A Snap-Shot of Domatial Mite Diversity of Coffea arabica in Comparison to the Adjacent Umtamvuna Forest in South Africa
Abstract
1. Introduction
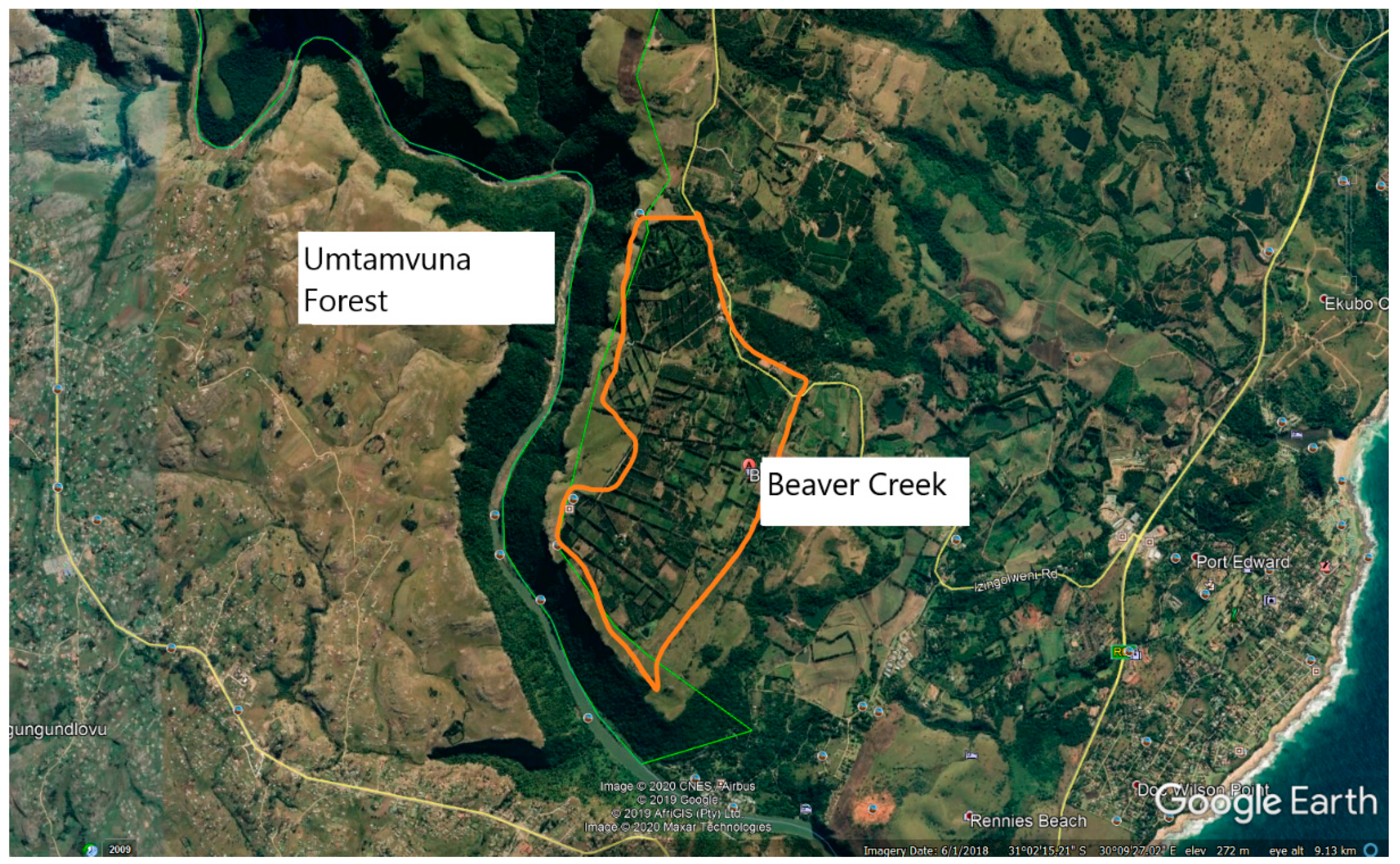
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vega, F.E.; Rosenquist, E.; Collins, W. Global project needed to tackle coffee crisis. Nature 2003, 425, 343. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.E.; Ochoa, R.; Astorga, C.; Walter, D.E. Mites (Arachnida: Acari) inhabiting coffee domatia: A short review and recent findings from Costa Rica. Int. J. Acarol. 2007, 33, 291–295. [Google Scholar] [CrossRef]
- O’Dowd, D.J. Mite association with the leaf domatia of coffee (Coffea arabica) in North Queensland, Australia. Bull. Ent. Res. 1994, 84, 361–366. [Google Scholar] [CrossRef]
- Agrawal, A. Do leaf domatia mediate a plant–mite mutualism? An experimental test of the effects on predators and herbivores. Ecol. Entomol. 1997, 22, 371–376. [Google Scholar] [CrossRef]
- Norton, A.P.; English-Loeb, G.; Gadoury, D.; Seem, R.C. Mycophagous mites and foliar pathogens: Leaf domatia mediate tritrophic interactions in grapes. Ecology 2000, 81, 490–499. [Google Scholar] [CrossRef]
- Onzo, A.; Hanna, R.; Zannou, I.; Sabelis, M.W.; Yaninek, J.S. Dynamics of refuge use: Diurnal, vertical migration by predatory and herbivorous mites within cassava plants. Oikos 2003, 101, 59–69. [Google Scholar] [CrossRef]
- Vega, F.E.; Infante, F.; Castillo, A.; Jaramillo, J. The coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae): A short review, with recent findings and future research directions. Terr. Arthrop. Rev. 2009, 2, 129–147. [Google Scholar]
- Oliveira, C.M.; Ferreira, J.A.M.; Olivereira, R.M.; Santo, F.O.; Pallini, A. Ricoseius loxocheles, a phytoseiid mite that feeds on coffee leaf rust. Exp. Appl. Acarol. 2014, 64, 223–233. [Google Scholar] [CrossRef]
- English-loeb, G.; Norton, A.P.; Gadoury, D.M.; Seem, R.C.; Wilcox, W.F. Control of Powdery Mildew in Wild and Cultivated Grapes by a Tydeid Mite. Bio. Con. 1999, 14, 97–103. [Google Scholar] [CrossRef]
- Ferreira, J.A.M.; Eshuis, B.; Janssen, A.; Sabelis, M.W. Domatia reduce larval cannibalism in predatory mites. Ecol. Entomol. 2008, 33, 374–379. [Google Scholar] [CrossRef]
- Ferreira, J.A.M.; Cunha, D.F.S.; Pallini, A.; Sabelis, M.W.; Janssen, A. Leaf domatia reduce intraguild predation among predatory mites. Ecol. Entomol. 2011, 36, 435–441. [Google Scholar] [CrossRef]
- Mineiro, J.L.D.; Sato, M.E.; Raga, A.; Arthur, V. Population dynamics of phytophagous and predaceous mites on coffee in Brazil, with emphasis on Brevipalpus phoenicis (Acari: Tenuipalpidae). Exp. Appl. Acarol. 2008, 44, 277–291. [Google Scholar] [CrossRef]
- Fahl, J.I.; Queiroz-Voltan, R.B.; Carrelli, M.L.C.; Schiavinato, M.A.; Pradro, A.K.S.; Souza, J.C. Alterations in leaf anatomy and physiology caused by the red mite (Oligonychus ilicis) in plants of Coffea arabica. Bra. J. Pl. Physiol. 2007, 19, 61–86. [Google Scholar] [CrossRef]
- Romero, G.Q.; Daud, R.D.; Salomão, A.T.; Martins, L.F.; Feres, R.J.F.; Benson, W.W. Mites and leaf domatia: No evidence of mutualism in Coffea arabica plants. Bio. Neo. 2011, 11, 27–33. [Google Scholar] [CrossRef]
- Pemberton, R.W.; Turner, C.E. Occurrence of predatory and fungivorous mites in leaf domatia. Amer. J. Bot. 1989, 76, 105–112. [Google Scholar] [CrossRef]
- Agricultural Reseach Council. Available online: http://www.arc.agric.za/arc-itsc/Pages/Coffee-Information.aspx (accessed on 20 November 2019).
- Situngu, S. An Investigation of the Leaf Domatia—Mite Mutualism in South Africa: Insights from Ecological Studies. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, 2017. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Matos, C.H.C.; Pallini, A.; Chaves, F.F.; Schoereder, J.S.; Janssen, A. Do domatia mediate mutualistic interactions between coffee plants and predatory mites? Ent. Exp. Et. App. 2006, 118, 185–192. [Google Scholar] [CrossRef]
- Mineiro, J.L.C.; Sato, M.E.; Berton, J.H.C.; Raga, A. Mites (Arachnida: Acari) on coffee plants in forest fragment and conventional plantation in Monte Alegre do Sul, Atate of São Paulo, Brazil. Divulgação Scientífica 2019, 81, 1–30. [Google Scholar]
- Agrawel, A.A.; Karban, R.; Colfer, R.G. How leaf domatia and induced plant resistance affect herbivores, natural enemies and plant performance. Oikos 2000, 89, 70–80. [Google Scholar] [CrossRef]
- O’Connell, D.M.; Lee, W.G.; Monks, A.; Dickinson, K.J.M. Does microhabitat structure affect foliar mite assemblages? Ecol. Entomol. 2010, 35, 317–328. [Google Scholar] [CrossRef]
- English-Loeb, G.; Norton, A.; Walker, M.A. Behavioral and population consequences of acarodomatia in grapes on phytoseiid mites (Acari: Mesostigmata) and implications for plant breeding. Ent. Exp. Et. Appl. 2002, 104, 307–319. [Google Scholar] [CrossRef]
- Melidossian, H.S.; Seem, R.C.; English-Loeb, G.; Wilcox, W.F.; Gadoury, D.M. Suppression of grapevine powdery mildew by a mycophagous mite. Plant Dis. 2005, 89, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C.M.; Kitajima, E.W.; Rodrigues, J.C.V. Coffee ringspot virus vectored by Brevipalpus phoenicis (Acari: Tenuipalpidae) in coffee. Exp. Appl. Acarol. 2003, 30, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.; Navia, D.; dos Santos, L.R.; Rideiqui, P.J.S.; Silva, E.S. Mites associated with sugarcane crop and with nativetrees from adjacent Atlantic forest fragment in Brazil. Exp. Appl. Acarol. 2015, 66, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Demite, P.R.; Feres, R.J.F.; Lofego, A.C. Influence of agricultural environment on the plant mite community in forest fragments. Braz. J. Biol. 2015, 75, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Murcia, C. Edge effects in fragmented forests: Implications for conservation. Trends Ecol. Evol. 1995, 10, 58–62. [Google Scholar] [CrossRef]
- Harper, K.A.; Macdonald, S.E.; Burton, P.J.; Chen, J.; Brosofske, K.D.; Saunders, S.C.; Euskirchen, E.S.; Roberts, D.; Jaiteh, M.S.; Esseen, P. Edge influence on forest structure and composition in fragmented landscapes. Con. Biol. 2005, 19, 768–782. [Google Scholar] [CrossRef]
- Ries, L.; Sisk, T.D. A predictive model of edge effects. Ecology 2004, 85, 2917–2926. [Google Scholar] [CrossRef]
- Tian, C.; Yang, X.; Liu, Y. Edge effect and its impacts on forest ecosystem: A review. Chin. J. Appl. Ecol. 2011, 22, 2184–2192. [Google Scholar]
- Lacasella, F.; Gratton, C.; Felici, S.D.; Sbordoni, V. Asymmetrical responses of forest and ‘‘beyond edge’’ arthropod communities across a forest–grassland ecotone. Bio. Con. 2015, 24, 447–465. [Google Scholar] [CrossRef]
- Chen, J.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, L.; Franklin, J.F. Microclimate in Forest Ecosystem and Landscape Ecology: Variations in local climate can be used to monitor and compare the effects of different management regimes. BioScience 1999, 49, 288–297. [Google Scholar] [CrossRef]
- Fukui, A. Indirect interactions mediated by leaf shelters in animal-plant communities. Pop. Ecol. 2001, 43, 31–40. [Google Scholar] [CrossRef]
- Situngu, S.; Barker, N.P. Position, position, position: Mites occupying leaf domatia are not uniformly distributed in the tree canopy. SA J. Bot. 2016, 108, 23–28. [Google Scholar] [CrossRef]
- Croft, B.A.; Messing, R.H.; Dunley, J.E.; Strong, W.B. Effects of humidity on eggs and immatures of Neoseiulus fallacis, Amblyseieus andersoni, Metaseiulus occidentalis and Typhlodromus pyri (Phytoseiidae): Implications for biological control on apple, caneberry, strawberry and hop. Exp. App. Acarol. 1993, 17, 451–459. [Google Scholar] [CrossRef]


| Mite Species | Feeding Guild | Coffee Edge: Abundance (% Frequency) | Coffee Middle: Abundance (% Frequency) | Natural Forest: Abundance (% Frequency) | Total: Abundance (% Frequency) |
|---|---|---|---|---|---|
| Cheyletidae Prosocheyla hepburni (Lawrence) | Predacious | 14 (7%) | 14 (3%) | ||
| Cunaxidae Bunaxella quini Den Heyer Rubroscirus sp. | Predacious Predacious | 15 (25%) | 28 (30%) | 37 (15%) 5 (4%) | 80 (24%) 5 (1%) |
| Eriophyidae Eriophyid sp. | Herbivorous | 104 (19%) | 104 (7%) | ||
| Eupodidae Eupodes sp. | Algiphagus | 17 (15%) | 17 (6%) | ||
| Oribatida Oribatid mite | Mycophagous/Saprophytic | 2 (10%) | 30 (30%) | 32 (12%) | |
| Phytoseiidae Amblyseius anomalus van der Merwe Euseius addoensis (van der Merwe and Ryke) Ueckermannseius sp1 Ueckermannseius sp2 Ueckermannseius munsteriensisvan der Merwe Typhlodromus microbullatus van der Merwe Typhlodromus crassusvan der Merwe | Predacious Predacious Predacious Predacious Predacious Predacious Predacious | 212 (95%) 2 (10%) 77 (50%) | 136 (30%) 59 (25%) 35 (35%) 78 (85%) | 227 (56%) 9 (4%) 18 (26%) 73 (41%) 7 (7%) 4 (4%) | 227 (22%) 357 (38 %) 59 (7%) 20 (13%) 73 (16%) 7 (3%) 35 (10%) 159 (41%) |
| Stigmaeidae Agistemus tranatalensisMeyer Agistemus sp. (probably new) Mullederia centrata (Meyer) | Predacious Predacious Predacious | 2 (5%) | 15 (20%) | 5 (4%) | 5 (1%) 15 (6%) 2 (1%) |
| Tenuipalpidae Brevipalpus sp. (probably new) | Herbivorous | 8 (11%) | 8 (4%) | ||
| Tetranychidae Oligonychus sp. (probably new) Tetranychus sp. Tetranychus nymph | Herbivorous Herbivorous Herbivorous | 6 (4%) 33 (25%) 83 (25%) | 6 (1%) 33 (10%) 83 (10%) | ||
| Triophtydeidae Tetratriophtydeus myacanthus Ueckermann | Predacious | 5 (4%) | 45 (1%) | ||
| Tydeidae Tydeus munsteri Meyer and Ryke | Predacious | 46 (48%) | 46 (19%) | ||
| Winterschmidtiidae Saproglyphus sp. | Mycophagous | 11 (15%) | 11 (6%) |
| Site | Average within Group Similarity (%) | Contribution of Species (%) |
|---|---|---|
| Natural forest | 31.21 | Phytoseiidae Amblyseius anomalus (23.6) Ueckermannseius munsteriensis (21.5) Oribatida Oribatid mite (16.8) |
| Middle of coffee plantation | 68.27 | Phytoseiidae Euseius addoensis (81.1) Typhlodromus crassus (16.0) |
| Edge of coffee plantation | 46.17 | Phytoseiidae Typhlodromus crassus (70.2) Cunaxidae Bunaxella quini (8.7) Phytoseiidae Euseius addoensis (8.2) |
| Site | Average between Group Dissimilarity (%) | Contribution of Species (%) |
|---|---|---|
| Natural forest vs. middle of plantation | 97.97 | Phytoseiidae Euseius addoensis (17.5) Amblyseius anomalus (10.1) Ueckermannseius Munsteriensis (9.7) |
| Middle of coffee plantation vs. edge of plantation | 62.48 | Phytoseiidae Euseius addoensis (29.0) Typhlodromus crassus (23.3) Cunaxidae Bunaxella quini (16.1) |
| Edge of coffee plantation vs. natural forest | 89.64 | Phytoseiidae Typhlodromus crassus (15.0) Amblyseius anomalus (9.9) Phytoseius sp. (9.4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Situngu, S.; Barker, N.P.; Vetter, S. A Snap-Shot of Domatial Mite Diversity of Coffea arabica in Comparison to the Adjacent Umtamvuna Forest in South Africa. Diversity 2020, 12, 79. https://doi.org/10.3390/d12020079
Situngu S, Barker NP, Vetter S. A Snap-Shot of Domatial Mite Diversity of Coffea arabica in Comparison to the Adjacent Umtamvuna Forest in South Africa. Diversity. 2020; 12(2):79. https://doi.org/10.3390/d12020079
Chicago/Turabian StyleSitungu, Sivuyisiwe, Nigel P. Barker, and Susanne Vetter. 2020. "A Snap-Shot of Domatial Mite Diversity of Coffea arabica in Comparison to the Adjacent Umtamvuna Forest in South Africa" Diversity 12, no. 2: 79. https://doi.org/10.3390/d12020079
APA StyleSitungu, S., Barker, N. P., & Vetter, S. (2020). A Snap-Shot of Domatial Mite Diversity of Coffea arabica in Comparison to the Adjacent Umtamvuna Forest in South Africa. Diversity, 12(2), 79. https://doi.org/10.3390/d12020079






