Knowledge Gaps or Change of Distribution Ranges? Explaining New Records of Birds in the Ecuadorian Tumbesian Region of Endemism
Abstract
1. Introduction
2. Materials and Methods
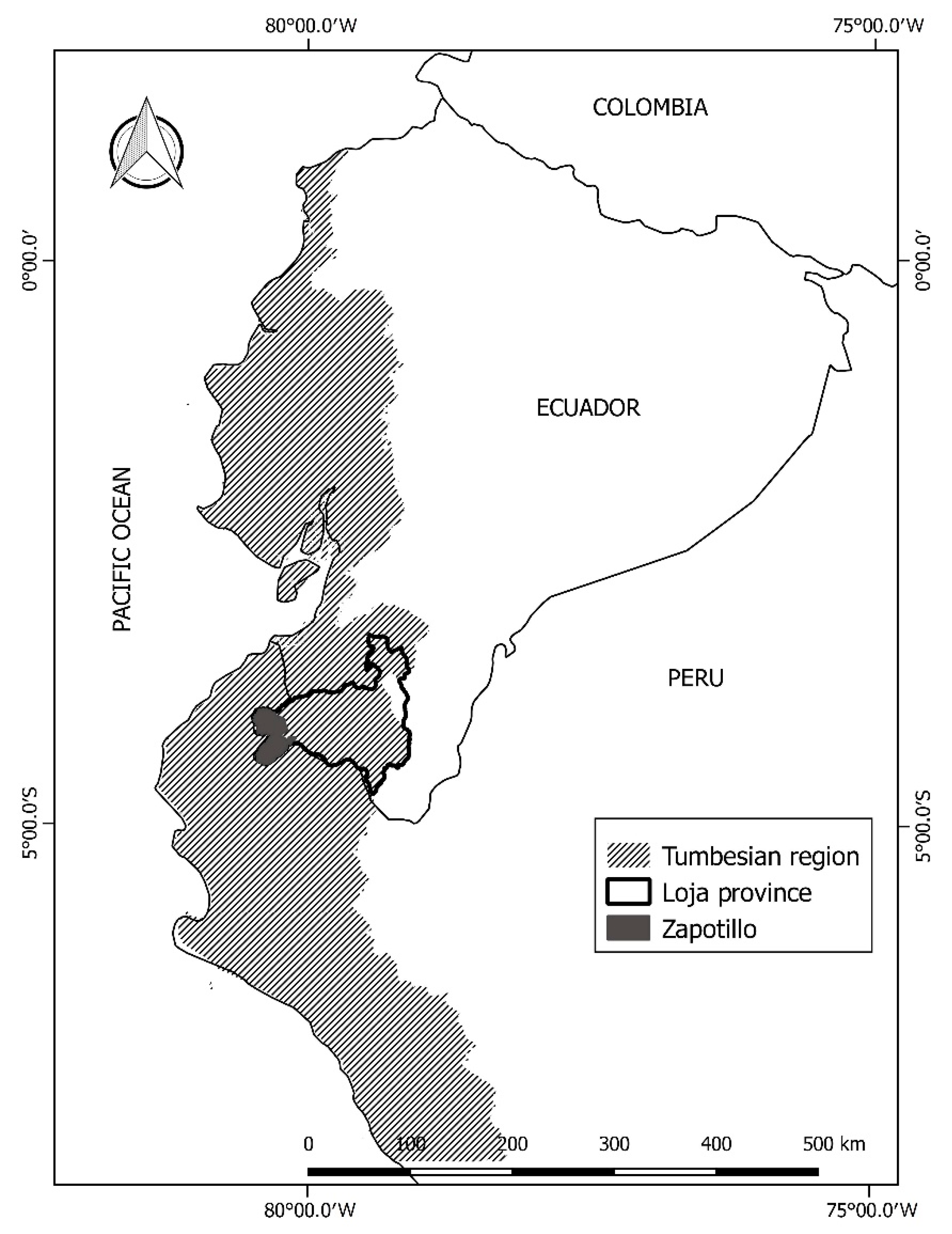
2.1. Study Area
2.2. Sampling and Data Collection
2.3. Category Assignment
2.4. Validation
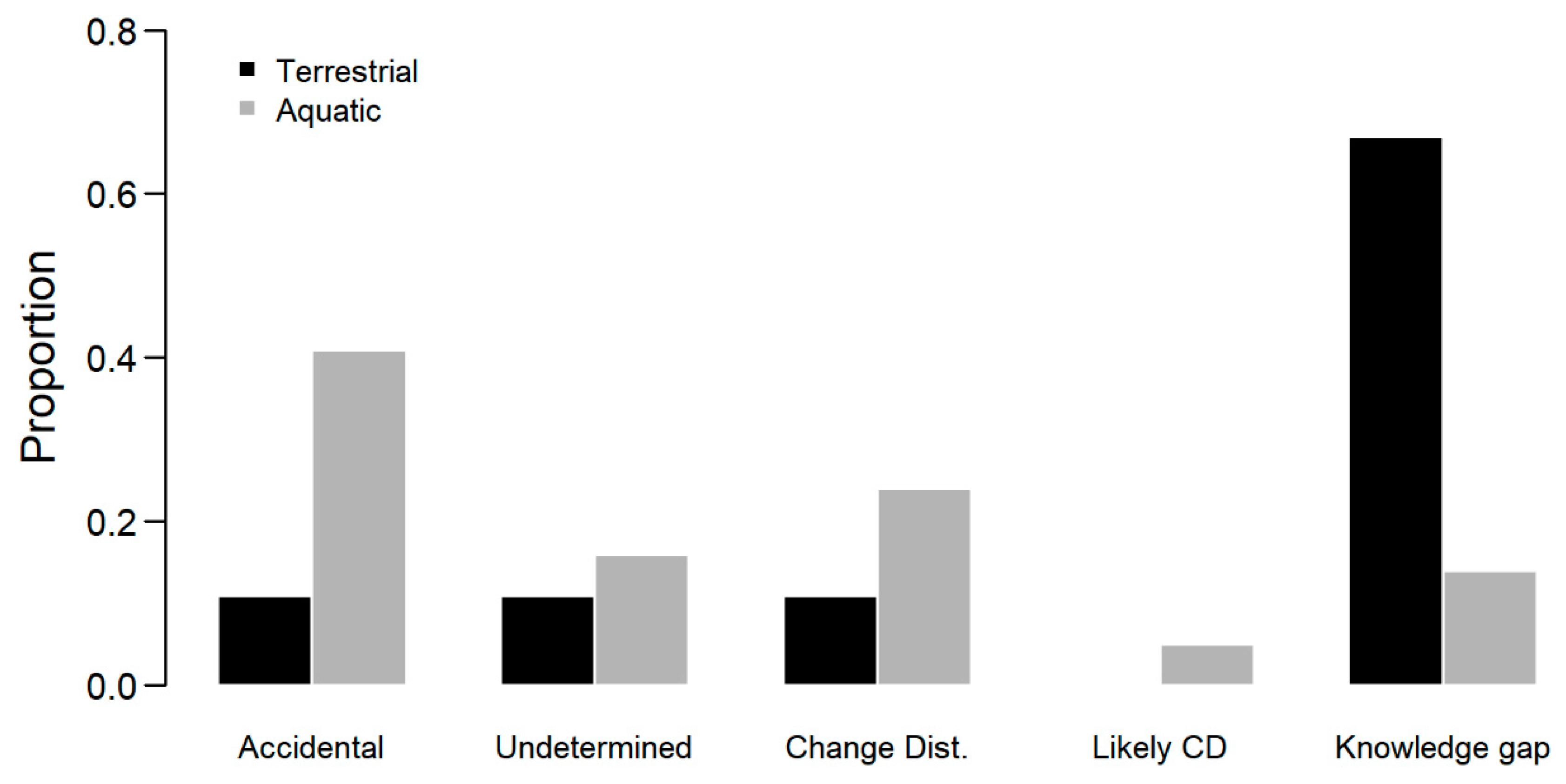
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Distribution Area | Conspicuousness | Recording Dynamics | |||||
|---|---|---|---|---|---|---|---|
| Species | Habitat | Distribution | Abundance | Detectability | Dynamic | Category | Source |
| Spatula cyanoptera | Usual | Atypical | Rare | High | Increasing | Change of distribution range | [67,68] |
| Spatula clypeata | Usual | Atypical | Rare | High | Scarce | Accidental | [67,68] |
| Netta erythrophthalma | Usual | Atypical | Rare | High | Scarce | Accidental | [67] |
| Aythya collaris | Usual | Atypical | Rare | High | Scarce | Accidental | [67] |
| Aythya affinis | Usual | Atypical | Rare | High | Increasing | Change of distribution range | [67] |
| Anas carolinensis | Usual | Atypical | Rare | High | Scarce | Accidental | [69] |
| Anas bahamensis++ | Unusual | Normal | Abundant | High | Increasing | Change of distribution range | Field work |
| Anas discors++ | Unusual | Normal | Abundant | High | Scarce | Accidental | Field work |
| Phoenicopterus chilensis++ | Unusual | Normal | Rare | High | Increasing | Change of distribution range | Field work |
| Podiceps major | Usual | Atypical | Rare | High | Increasing | Change of distribution range | [67] |
| Phaethornis griseogularis+ | Usual | Normal | Abundant | Low | Scarce | Knowledge gap | Field work |
| Pardirallus maculatus+ | Usual | Normal | Rare | Low | Scarce | Knowledge gap | Field work |
| Numenius americanus | Usual | Atypical | Rare | High | Scarce | Accidental | [68] |
| Limosa fedoa | Usual | Atypical | Rare | Low | Increasing | Likely change of distribution range | [67,68,74] |
| Haematopus ater | Usual | Atypical | Rare | High | Scarce | Accidental | [68] |
| Chroicocephalus cirrocephalus+ | Unusual | Normal | Abundant | High | Scarce | Accidental | Field work |
| Chroicocephalus philadelphia | Usual | Atypical | Rare | Low | Scarce | Undetermined | [68] |
| Larus delawarensis | Usual | Atypical | Rare | Low | Scarce | Undetermined | [68] |
| Hydroprogne caspia | Usual | Atypical | Rare | Low | Increasing | Likely change of distribution range | [67,68] |
| Stercorarius chilensis | Usual | Atypical | Rare | High | Scarce | Accidental | [69] |
| Tringa solitaria | Usual | Normal | Abundant | Low | Scarce | Knowledge gap | [42] |
| Tringa melanoleuca++ | Usual | Normal | Abundant | Low | Scarce | Knowledge gap | Field work |
| Calidris bairdii+ | Unusual | Normal | Rare | Low | Scarce | Undetermined | Field work |
| Calidris himantopus++ | Unusual | Normal | Rare | Low | Scarce | Undetermined | Field work |
| Calidris melanotos++ | Usual | Normal | Rare | Low | Scarce | Knowledge gap | Field work |
| Calidris alpina | Usual | Atypical | Rare | Low | Scarce | Undetermined | [69] |
| Phalaropus tricolor+ | Unusual | Normal | Abundant | High | Scarce | Accidental | Field work |
| Oceanites oceanicus | Usual | Normal | Rare | Low | Scarce | Knowledge gap | [67,68] |
| Oceanodroma hornbyi | Usual | Atypical | Rare | Low | Scarce | Undetermined | [67,68] |
| Thalassarche bulleri | Usual | Atypical | Rare | High | Scarce | Accidental | [69] |
| Platalea ajaja | Unusual | Normal | Rare | High | Increasing | Change of distribution range | [42] |
| Sula leucogaster | Usual | Atypical | Rare | High | Increasing | Change of distribution range | [67,68,69] |
| Ardea herodias | Usual | Atypical | Rare | High | Increasing | Change of distribution range | [67,68] |
| Egretta rufescens | Usual | Atypical | Rare | High | Scarce | Accidental | [67,68,73] |
| Egretta caerulea+ | Unusual | Normal | Abundant | High | Scarce | Accidental | Field work |
| Aramus guarauna++ | Usual | Atypical | Rare | High | Scarce | Accidental | Field work |
| Plegadis falcinellus | Usual | Atypical | Rare | High | Increasing | Change of distribution range | [67,68,74] |
| Eudocimus albus++ | Unusual | Normal | Abundant | High | Scarce | Accidental | Field work |
| Elanus leucurus++ | Usual | Atypical | Abundant | High | Increasing | Change of distribution range | Field work |
| Ciccaba nigrolineata | Usual | Normal | Abundant | Low | Scarce | Knowledge gap | [42] |
| Falco femoralis+ | Unusual | Atypical | Rare | High | Scarce | Accidental | Field work |
| Turdus maculirostris+ | Usual | Normal | Abundant | Low | Scarce | Knowledge gap | Field work |
| Stelgidopteryx ruficollis+ | Usual | Normal | Abundant | Low | Scarce | Knowledge gap | Field work |
| Tumbezia salvini | Usual | Normal | Rare | Low | Scarce | Knowledge gap | [67] |
| Tyrannus dominicensis | Usual | Atypical | Rare | Low | Scarce | Undetermined | [72,74] |
| Sicalis taczanowskii | Usual | Normal | Rare | Low | Scarce | Knowledge gap | [42] |
| Passer hispaniolensis | Usual | Atypical | Abundant | High | Increasing | Change of distribution range | Example, [75] |
| Elanus ceruleus | Usual | Atypical | Abundant | High | Increasing | Change of distribution range | Example, [76] |
| Columba palumbus | Unusual | Atypical | Abundant | High | Increasing | Change of distribution range | Example, [77] |
| Streptopelia decaocto | Usual | Atypical | Abundant | High | Increasing | Change of distribution range | Example, [78] |
References
- Tscharntke, T.; Klein, A.M.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity – ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Flynn, D.F.; Gogol-Prokurat, M.; Nogeire, T.; Molinari, N.; Richers, B.T.; Lin, B.B.; DeClerck, F. Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 2009, 12, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.E.; Shanahan, D.F.; Di Marco, M.; Allan, J.; Laurance, W.F.; Sanderson, E.W.; Venter, O. Catastrophic declines in wilderness areas undermine global environment targets. Curr. Biol. 2016, 26, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Armijos, M.F.; Homeier, J.; Munt, D.D. Spatio-temporal analysis of the human footprint in South Ecuador: Influence of human pressure on ecosystems and effectiveness of protected areas. Appl. Geogr. 2017, 78, 22–32. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Kappelle, M.; Van Vuuren, M.M.; Baas, P. Effects of climate change on biodiversity: a review and identification of key research issues. Biodivers. Conserv. 1999, 8, 1383–1397. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Heller, N.E.; Zavaleta, E.S. Biodiversity management in the face of climate change: a review of 22 years of recommendations. Biol. Conserv. 2009, 142, 14–32. [Google Scholar] [CrossRef]
- Melo, I.; Ochoa-Quintero, J.M.; de Oliveira Roque, F.; Dalsgaard, B. A review of threshold responses of birds to landscape changes across the world. J. Field Ornithol. 2018, 89, 303–314. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.G.; Scholer, M.N.; Ruiz-Gutierrez, V.; Fitzpatrick, J.W. Climate change causes upslope shifts and mountaintop extirpations in a tropical bird community. Proc. Natl. Acad. Sci. USA 2018, 115, 201804224. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.C.; Sekercioglu, C.H.; Sodhi, N.S.; Fordham, D.A.; Paton, D.C.; Brook, B.W. The tropical frontier in avian climate impact research. Ibis 2011, 153, 877–882. [Google Scholar] [CrossRef]
- Sekercioglu, C.H.; Primack, R.B.; Wormworth, J. The effects of climate change on tropical birds. Biol. Conserv. 2012, 148, 1–18. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Freeman, B.G.; Class-Freeman, A.M. Rapid upslope shifts in New Guinean birds illustrate strong distributional responses of tropical montane species to global warming. Proc. Natl. Acad. Sci. USA 2014, 111, 4490–4494. [Google Scholar] [CrossRef]
- Beckage, B.; Osborne, B.; Gavin, D.G.; Pucko, C.; Siccama, T.; Perkins, T. A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proc. Natl. Acad. Sci. USA 2008, 105, 4197–4202. [Google Scholar] [CrossRef]
- Zuckerberg, B.; Woods, A.M.; Porter, W.F. Poleward shifts in breeding bird distributions in New York State. Glob. Chang. Biol. 2009, 15, 1866–1883. [Google Scholar] [CrossRef]
- Tingley, M.W.; Koo, M.S.; Moritz, C.; Rush, A.C.; Beissinger, S.R. The push and pull of climate change causes heterogeneous shifts in avian elevational ranges. Glob. Chang. Biol. 2012, 18, 3279–3290. [Google Scholar] [CrossRef]
- DeLuca, W.V.; King, D.I. Montane birds shift downslope despite recent warming in the northern Appalachian Mountains. J. Ornithol. 2017, 158, 493–505. [Google Scholar] [CrossRef]
- Kirchman, J.J.; Van Keuren, A.E. Altitudinal Range Shifts of Birds At the Southern Periphery of the Boreal Forest: 40 Years of Change In the Adirondack Mountains. Wilson J. Ornithol. 2017, 129, 742–753. [Google Scholar] [CrossRef]
- Thomas, C.D.; Lennon, J.J. Birds extend their ranges northwards. Nature 1999, 399, 213. [Google Scholar] [CrossRef]
- Archaux, F. Breeding upwards when climate is becoming warmer: no bird response in the French Alps. Ibis 2004, 146, 138–144. [Google Scholar] [CrossRef]
- Gregory, R.D.; Willis, S.G.; Jiguet, F.; Vorˇíšek, P.; Klvanˇ ová, A.; van Strien, A.; Huntley, B.; Collingham, Y.C.; Couvet, D.; Green, R.E. An indicator of the impact of climatic change on European bird populations. PLoS ONE 2009, 4, e4678. [Google Scholar] [CrossRef]
- Devictor, V.; Julliard, R.; Couvet, D.; Jiguet, F. Birds are tracking climate warming, but not fast enough. Proc. R. Soc. Lond. B Biol. Sci. 2008, 275, 2743–2748. [Google Scholar] [CrossRef]
- Roth, T.; Plattner, M.; Amrhein, V. Plants, Birds and Butterflies: Short-Term Responses of Species Communities to Climate Warming Vary by Taxon and with Altitude. PLoS ONE 2014, 9, e82490. [Google Scholar] [CrossRef]
- Pounds, J.A.; Fogden, M.P.L.; Campbell, J.H. Biological response to climate change on a tropical mountain. Nature 1999, 398, 611. [Google Scholar] [CrossRef]
- Forero-Medina, G.; Terborgh, J.; Socolar, S.J.; Pimm, S.L. Elevational ranges of birds on a tropical montane gradient lag behind warming temperatures. PLoS ONE 2011, 6, e28535. [Google Scholar] [CrossRef] [PubMed]
- Freile, J.F.; Greeney, H.F.; Bonacorsso, E. Current Neotropical ornithology: Research progress 1996–2011. Condor Ornithol. Appl. 2014, 116, 84–96. [Google Scholar] [CrossRef]
- Wormworth, J.; Sekercioglu, C.H. Winged Sentinels: Birds and Climate Change, 1st ed.; Cambridge University Press: New York, NY, USA, 2008; pp. 42–63. [Google Scholar]
- Giorgi, F. Climate change hot-spots. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
- Hermes, C.; Keller, K.; Nicholas, R.E.; Segelbacher, G.; Schaefer, H.M. Projected impacts of climate change on habitat availability for an endangered parakeet. PLoS ONE 2018, 13, e0191773. [Google Scholar] [CrossRef]
- McCain, C.M. Vertebrate range sizes indicate that mountains may be ‘higher’ in the tropics. Ecol. Lett. 2009, 12, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.H.; Parra, J.L.; Rahbek, C.; McGuire, J.A. Phylogenetic structure in tropical hummingbird communities. Proc. Natl. Acad. Sci. USA 2009, 106, 19673–19678. [Google Scholar] [CrossRef] [PubMed]
- Best, B.J.; Kessler, M. Biodiversity and Conservation in Tumbesian Ecuador and Peru; BirdLife Internacional: Cambridge, UK, 1995. [Google Scholar]
- Portillo-Quintero, C.A.; Sánchez-Azofeifa, G.A. Extent and conservation of tropical dry forests in the Americas. Biol. Conserv. 2010, 143, 144–155. [Google Scholar] [CrossRef]
- Jara-Guerrero, A.; Maldonado-Riofrío, D.; Espinosa, C.I.; Duncan, D. Beyond the Blame Game : A Restoration Pathway Reconciles Ecologists and Local Leaders Divergent Models of Seasonally Dry Tropical Forest Degradation. Ecol. Soc. 2019, 24, 22. [Google Scholar] [CrossRef]
- Tapia-Armijos, M.F.; Homeier, J.; Espinosa, C.I.; Leuschner, C.; de la Cruz, M. Deforestation and forest fragmentation in South Ecuador since the 1970s–losing a hotspot of biodiversity. PLoS ONE 2015, 10, e0133701. [Google Scholar] [CrossRef]
- Prieto-Torres, D.A.; Nori, J.; Rojas-Soto, O.R. Identifying priority conservation areas for birds associated to endangered Neotropical dry forests. Biol. Conserv. 2018, 228, 205–214. [Google Scholar] [CrossRef]
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; Gordon, J.E. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- Escribano-Avila, G.; Cervera, L.; Ordóñez-Delgado, L.; Jara-Guerrero, A.; Amador, L.; Paladines, B.; Espinosa, C.I. Biodiversity patterns and ecological processes in Neotropical dry forest: the need to connect research and management for long-term conservation. Neotrop. Biodiv. 2017, 3, 107–116. [Google Scholar] [CrossRef]
- Ordóñez-Delgado, L.; Tomás, G.; Armijos-Ojeda, D.; Jara-Guerrero, A.; Cisneros, R.; Espinosa, C.I. Nuevos aportes al conocimiento de avifauna en la región Tumbesina; implicaciones para la conservación de la Reserva de Biosfera del Bosque Seco, Zapotillo, Ecuador. Ecosistemas 2016, 25, 13–23. [Google Scholar] [CrossRef]
- Benítez, V.; Sánchez, T. Evaluación ecológica rápida de la avifauna en los bosques secos de La Ceiba y Cordillera Arañitas, provincia de Loja, Ecuador. In Biodiversidad en los bosques secos del suroccidente de la provincia de Loja: un reporte de las evaluaciones ecológicas y socioeconómicas rápidas, 1st ed.; Vázquez, M.A., Larrea, M., Suárez, L., Eds.; EcoCiencia, Ministerio del Ambiente, Herbario Loja y Proyecto Bosque Seco: Quito, Ecuador, 2001; pp. 47–65. [Google Scholar]
- Freile, J.F.; Bonaccorso, E.; Santander, T. First nesting report of the West Peruvian Screech-owl (Otus roboratus). Ornitol. Neotrop. 2003, 14, 107–111. [Google Scholar]
- Freile, J.F.; Moreano, M.; Bonaccorso, E.; Santander, T.; Chaves, J. Notas sobre la historia natural, distribución y conservación de algunas especies de aves amenazadas del suroccidente de Ecuador. Cotinga 2004, 21, 18–24. [Google Scholar]
- Bonaccorso, E.; Santander, T.; Freile, J.F.; Tinoco, B.; Rodas, F. Avifauna and conservation of the Cerro Negro-Cazaderos area, Tumbesian Ecuador. Cotinga 2007, 27, 61–66. [Google Scholar]
- Aguilar, Z. Guía de Vida Silvestre del Área de Conservación y Desarrollo: La Ceiba; Naturaleza y Cultura Internacional: Quito, Ecuador, 2008. [Google Scholar]
- Tinoco, B. Estacionalidad de la comunidad de aves en un bosque deciduo tumbesino en el sur occidente de Ecuador. Ornitol. Neotrop. 2009, 20, 157–170. [Google Scholar]
- Pratolongo, F.A.; Flanagan, J.N.; Vellinga, W.P.; Durand, N. Notes on the birds of Laquipampa Wildlife Refuge, Lambayeque, Peru. Bull. Br. Ornithol. Club 2012, 132, 162–174. [Google Scholar]
- Stager, M.; Lopresti, E.; Pratolongo, F.A.; Ardia, D.R.; Caceres, D.; Cooper, C.B.; Winkler, D.W. Reproductive biology of a narrowly endemic Tachycineta swallow in dry, seasonal forest in coastal Peru. Ornitol. Neotrop 2012, 23, 95–112. [Google Scholar]
- LoPresti, E.; Angulo, F. New bird distribution records for Lambayeque, Peru: Nomonyx dominicus (Linneaus, 1766) (Anatidae) and Incaspiza pulchra (Sclater, 1886) (Emberizidae). Check List 2014, 10, 618–620. [Google Scholar] [CrossRef][Green Version]
- Solano-Ugalde, A.; Pérez, V.; Ahlman, R. Primeros registros de la Reinita Gorrinegra (Wilsonia pusilla) en Ecuador. Boletín SAO 2007, 17, 59–62. [Google Scholar]
- Athanas, N.; Davies, A.; Miller, R. Discovery of Tumbes Tyrant Tumbezia salvini in Ecuador. Cotinga 2009, 31, 137. [Google Scholar]
- Pozo-Zamora, G.M.; Garzón, C.; Echeverría-Vaca, G.; León, K. Nuevos datos de distribución del colibrí Pico Lanza Frentiverde Doryfera ludovicae (Trochilidae) y del Pinzón Oliváceo Arremon castaneiceps (Emberizidae) en la provincia de El Oro, Ecuador. Avances en Ciencias e Ingenierías 2014, 6, 9–12. [Google Scholar] [CrossRef]
- Sánchez, J.E.; Zook, J.R.; Carman, E.; Sandoval, L. Information on abundance and occurrence of two recently recorded species of ducks for Costa Rica. Check List 2014, 10, 420–422. [Google Scholar] [CrossRef][Green Version]
- Cisneros-Heredia, D.F. Notes on breeding, behaviour and distribution of some birds in Ecuador. Bull. Br. Ornithol. Club 2006, 126, 153. [Google Scholar]
- Jahn, O.; Cosgrove, P.; Cosgrove, C.; Mueses, T.; Santander, T. First record of Brown Pelican Pelecanus occidentalis from the Ecuadorian highlands. Cotinga 2010, 32, 108. [Google Scholar]
- Santander, T.; Terán, K.; Mueces, T.; Lara, A.; Llumiquinga, C.; Guevara, E. Registros inusuales de aves costeras en lagunas Altoandinas de Ecuador. Cotinga 2011, 33, 105–107. [Google Scholar]
- Guevara, E.A.; Santander, T.; Duivenvoorden, J.F. Seasonal Patterns in Aquatic Bird Counts at Five Andean Lakes of Ecuador. Waterbirds 2013, 35, 636–641. [Google Scholar] [CrossRef]
- Bahamonde-Vinueza, D.; Cadena-Ortiz, H.; Cajas-Bermeo, C.; Bonaccorso, E. Unusual records of Cochlearius cochlearius (Linnaeus, 1766) (Aves: Ardeidae) in the Andes of Ecuador. Check List 2014, 10, 687–688. [Google Scholar] [CrossRef]
- Ordóñez-Delgado, L.; Reyes-Bueno, F.; Orihuela-Torres, A.; Armijos-Ojeda, D. Registros inusuales de aves en la hoya de Loja, Andes sur del Ecuador. Avances en Ciencias e Ingenierías 2016, 8, 26–36. [Google Scholar] [CrossRef]
- Ordóñez-Delgado, L.; González, I.; Armijos-Ojeda, D.; Orihuela-Torres, A. Primer registro de Ardea cocoi (Pelecaniformes: Ardeidae) en la región Andina del sur de Ecuador. Revista CEDAMAZ 2017, 7, 10–15. [Google Scholar]
- Holbrook, N.M.; Whitbeck, J.L.; Mooney, H.A. Drought responses of Neotropical deciduous forest trees. In Tropical Deciduous Forests, 1st ed.; Mooney, H.A., Medina, E., Bullock, S.H., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 243–276. [Google Scholar]
- ICBP. Putting Biodiversity on the Map: Priority Areas for Global Conservation; International Council for Bird Preservation: Cambridge, UK, 1992. [Google Scholar]
- Stattersfield, A.J.; Crosby, M.J.; Long, A.J.; Wege, O.E. Endemic Bird Areas of the World: Priorities for Biodiversity Bonservation; BirdLife Internarional Conservation Series No. 7; BirdLife Internarional: Cambridge, UK, 1998. [Google Scholar]
- Cerón, C.; Palacios, W.; Valencia, R.; Sierra, R. Las formaciones naturales de la Costa del Ecuador. In Propuesta Preliminar de un Sistema de Clasificación de Vegetación Para el Ecuador Continental; Sierra, R., Ed.; Proyecto INEFAN/GEF-BIRF y EcoCiencia: Quito, Ecuador, 1999. [Google Scholar]
- Freile, J.; Ahlman, R.; Brinkuizen, D.; Greenfield, P.; Solano-Ugalde, A.; Navarrete, L.; Ridgely, R. Rare birds in Ecuador: first annual report of the Committee of Ecuadorian Records in Ornithology (CERO). In Avances en Ciencias e Ingenierías; 2013; Volume 5, pp. 24–41. [Google Scholar] [CrossRef]
- Nilsson, J.; Freile, J.F.; Ahlman, R.; Brinkhuizen, D.M.; Greenfield, P.J.; Solano-Ugalde, A. Rare birds in Ecuador: second annual report of the Committee for Ecuadorian Records in Ornithology (CERO). Avances en Ciencias e Ingenierías 2014, 6, 38–50. [Google Scholar] [CrossRef]
- Freile, J.F.; Solano-Ugalde, A.; Brinkhuizen, D.M.; Greenfield, P.J.; Lysinger, M.; Nilsson, J.; Navarrete, L.; Ridgely, R.S. Rare Birds in Ecuador: Third Report of the Committee for Ecuadorian Records in Ornithology (CERO). Revista Ecuatoriana de Ornitología, 27. [CrossRef]
- Ridgely, R.; Greenfield, P.J. The Birds of Ecuador. Status, Ddistribution and Taxonomy, 1st ed.; Cornell University Press: Ithaca, NY, USA, 2001. [Google Scholar]
- Del Hoyo, J.; Elliott, A.; Sargatal, J.; Christie, D.A.; de Juana, E. (Eds.) Handbook of the Birds of the World Alive; Lynx Edicions: Barcelona, Spain, 2019; Available online: https://www.hbw.com (accessed on 21 March 2019).
- Ridgely, R.; Greenfield, P.J. Aves del Ecuador. Guía de Campo, 2nd ed.; Academia de Ciencias Naturales de Filadelfia. Fundación de Conservación Jocotoco: Quito, Ecuador, 2006. [Google Scholar]
- McMullan, M.; Navarrete, L. Fieldbook of the Birds of Ecuador Including the Galapagos Islands and Common Mammals, 2nd ed.; Ratty Ediciones: Quito, Ecuador, 2017. [Google Scholar]
- eBird Home Page. Available online: https://ebird.org/home (accessed on 10 June 2019).
- Cramp, S.; Perrins, C.M. The Birds of the Western Palearctic. Crows to Finches; Handbook of the Birds of Europe, the Middle East and North Africa; Oxford University Press: Oxford & New York, USA, 1994. [Google Scholar]
- Balbontin, J.; Negro, J.J.; Sarasola, J.H.; Ferrero, J.J.; Rivera, D. Land-use changes may explain the recent range expansion of the Black-shouldered Kite Elanus caeruleus in southern Europe. Ibis 2008, 150, 707–716. [Google Scholar] [CrossRef]
- BirdLife International. European Red List of Birds; Office for Official Publications of the European Communities: Luxembourg, 2015. [Google Scholar]
- Bonter, D.N.; Zuckerberg, B.; Dickinson, J.L. Invasive birds in a novel landscape: habitat associations and effects on established species. Ecography 2010, 33, 494–502. [Google Scholar] [CrossRef]
- Henry, P.Y. Distributional and altitudinal range extensions for birds from Ecuador. Boletín SAO 2012, 20, 89–106. [Google Scholar]
- Félix, F.; Haase, B. A new record of the Blackish Oystercatcher, Haematopus ater ater (Vieillot and Oudart, 1825), in the Gulf of Guayaquil, Ecuador. Check List 2016, 12, 1857. [Google Scholar] [CrossRef]
- Sandoval, L.; Acosta-Chaves, V.J.; Ocampo, D.; Mora, C.; Camacho, A.; Martinez, D.; Sanchez, C. Unusual records of waterbirds in Costa Rice: possible connection to El Niño 2015–2016. Mar. Ornithol. 2016, 44, 167–169. [Google Scholar]
- Sandoval, L.; Martínez, D.; Ocampo, D.; Pizarro, M.V.; Araya-H, D.; Carman, E.; García-Rodríguez, A. Range expansion and noteworthy records of Costa Rican birds (Aves). Check List 2018, 14, 141. [Google Scholar] [CrossRef]
- Bierregaard, R.O., Jr.; Marks, J.S.; Boesman, P.; Kirwan, G.M. White-tailed Kite (Elanus leucurus). In Handbook of the Birds of the World Alive; Del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Lynx Edicions: Barcelona, Spain, 2019; Available online: https://www.hbw.com/node/52968 (accessed on 20 June 2019).
- Bonaccorso, E.; Arzuza, D.; Buitrón-Jurado, G.; Lucía, A.; Charpentier, M.J.; Piedrahita, P.; Freile, J.F. Range extensions and other noteworthy bird records from the Ecuadorian Andes. Bull. Br. Ornithol. Club 2011, 131, 261–265. [Google Scholar]
- Schuchmann, K.L. First record of the Grey-chinned Hermit (Phaethornis griseogularis) west of the Colombian Andes, with notes on the displays of the species. Wilson Bull. 1987, 99, 122–124. [Google Scholar]
- Taylor, B. Spotted Rail (Pardirallus maculatus). In Handbook of the Birds of the World Alive; Del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., De Juana, E., Eds.; Lynx Edicions: Barcelona, Spain, 2020; Available online: https://www.hbw.com/node/53674 (accessed on 13 January 2020).
- Gerhardt, R.P.; Gerhardt, D.M.; Bonilla, N.; Flatten, C.J. Black-and-white Owl. In Neotropical Birds of Prey: Biology and Ecology of a Forest Raptor Community; Whitacre, D.F., Ed.; Cornell University Press: Ithaca, NY, USA, 2012; p. 320327. [Google Scholar]
- Ordóñez-Delgado, L.; Freile, J.F. First records of Koepcke’s Screech-Owl Megascops koepckeae (Aves: Strigidae) in Ecuador. Revista Ecuatoriana de Ornitología 2019, 5. [Google Scholar] [CrossRef]
- Ordóñez-Delgado, L.; Erazo, S.; González, I.; Armijos-Ojeda, D.; Rosado, D. Pyroderus scutatus masoni (Shaw, 1792) (Aves, Cotingidae): A subspecies of Red-ruffed Fruit crow newly confirmed for Ecuador. Check List 2018, 14, 281. [Google Scholar] [CrossRef]
- Sornoza-Molina, F.; Freile, J.F.; Nilsson, J.; Krabbe, N.; Bonaccorso, E. A striking, critically endangered, new species of hillstar (Trochilidae: Oreotrochilus) from the southwestern Andes of Ecuador. Auk Ornithol. Adv. 2018, 135, 1146–1171. [Google Scholar] [CrossRef]

| Indicator Criteria | Description | Groups |
|---|---|---|
| Distribution area | ||
| Habitat | Habitat type in which the new records appear | Usual: When the new record appears in a habitat known to be used by the species Unusual: When the new record appears in a rare or unusual habitat for the species |
| Distribution | Spatial location of the new records | Normal: When the new record is located within latitudinal, longitudinal and altitudinal boundaries of the distribution range reported for the species Atypical: When new record is located at an altitude, longitude or latitude outside the boundaries of the distribution range reported for the species |
| Conspicuousness | ||
| Abundance | Abundance of the species in the country | Rare: When the species is considered as unusual, rare, incidental, accidental or hypothetical for the country Abundant: When the species is considered as quite common, common or abundant for the country |
| Detectability | The ease with which the species can be detected and/or the possibility of misidentifications | High: When the species is easy to detect and/or with low probability of being confused with other species that occur in the habitat where it was recorded. A species is highly detectable when it has behaviors or colors that facilitate visualization, and is easy to observe or hear (e.g., Phoenicopterus chilensis, Aramus guarauna or Elanus leucurus) Low: When the species is difficult to detect and/or has high probability of being confused with other species that occur in the habitat where it was recorded. These are species which usually remain hidden and are difficult to observe or hear. In other cases, species with similar morphology or vocalizations occur at the location where it was recorded, and may be misidentified (e.g., Calidris sp. or many tiranids) |
| Dynamics of records | ||
| Dynamic | Number of records of the species at the new location | Scarce: When the species has few records in the area/habitat where new records have been reported and records are not increasing over time Increasing: When records have been increasing in the area/habitat where the species has been found in recent years |
| Criteria | Indicator | |
|---|---|---|
| Habitat | Distribution | Distribution area |
| Usual | Normal | Normal |
| Usual | Atypical | Unusual |
| Unusual | Normal | Unusual |
| Unusual | Atypical | Unusual |
| Abundance | Detectability | Conspicuousness |
| Abundant | High | High |
| Abundant | Low | High |
| Rare | High | High |
| Rare | Low | Low |
| Distribution Area | Conspicuousness | Recording Dynamics | Category |
|---|---|---|---|
| Unusual | High | Scarce | Accidental |
| Unusual | High | Increasing | Change of distribution range |
| Unusual | Low | Scarce | Undetermined |
| Unusual | Low | Increasing | Likely Change of distribution range |
| Normal | High | Increasing | Does not apply, unlikely |
| Normal | Low | Increasing | Knowledge gap |
| Normal | High | Scarce | Knowledge gap |
| Normal | Low | Scarce | Knowledge gap |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orihuela-Torres, A.; Tinoco, B.; Ordóñez-Delgado, L.; Espinosa, C.I. Knowledge Gaps or Change of Distribution Ranges? Explaining New Records of Birds in the Ecuadorian Tumbesian Region of Endemism. Diversity 2020, 12, 66. https://doi.org/10.3390/d12020066
Orihuela-Torres A, Tinoco B, Ordóñez-Delgado L, Espinosa CI. Knowledge Gaps or Change of Distribution Ranges? Explaining New Records of Birds in the Ecuadorian Tumbesian Region of Endemism. Diversity. 2020; 12(2):66. https://doi.org/10.3390/d12020066
Chicago/Turabian StyleOrihuela-Torres, Adrian, Boris Tinoco, Leonardo Ordóñez-Delgado, and Carlos Ivan Espinosa. 2020. "Knowledge Gaps or Change of Distribution Ranges? Explaining New Records of Birds in the Ecuadorian Tumbesian Region of Endemism" Diversity 12, no. 2: 66. https://doi.org/10.3390/d12020066
APA StyleOrihuela-Torres, A., Tinoco, B., Ordóñez-Delgado, L., & Espinosa, C. I. (2020). Knowledge Gaps or Change of Distribution Ranges? Explaining New Records of Birds in the Ecuadorian Tumbesian Region of Endemism. Diversity, 12(2), 66. https://doi.org/10.3390/d12020066






